Abstract
Heart valves are presumed to remodel their extracellular matrix upon application of mechanical strains. In this study, we investigated the effect of cyclic tensile strain on valvular interstitial cells’ synthesis of glycosaminoglycans (GAGs) and proteoglycans (PGs), which are altered during myxomatous degeneration. Interstitial cells were isolated from mitral valve leaflets and chordae and seeded separately within three dimensional collagen gels. Cell-seeded collagen gels were then subjected to cyclic strains of 2, 5 or 10% at 1.16 Hz for 48 hours using a custom-built stretching device. The application of cyclic strains reduced the total GAGs retained within collagen gels in a magnitude dependent manner for both leaflet and chordal cells. With increasing strain magnitude, however, secretion of total GAGs into the medium was reduced for leaflet cells and elevated for chordal cells. Retention of 4-sulfated GAGs increased with increasing strain magnitude for both cell types; for the chordal samples, retention of 6-sulfated GAGs was reduced at higher strain magnitudes. Compared to statically constrained or unconstrained conditions, the application of cyclic strain reduced the secretion of 6-sulfated GAGs by both cell types, and elevated secretion of 4-sulfated GAGs by leaflet cells only. Retention of the PG biglycan and secretion of the PG decorin was significantly reduced at 10% strain compared to 2% strain. In addition, there were numerous differences in the strain-dependent retention and secretion of GAGs and PGS within the leaflet and chordal groups. These results demonstrate that GAG and PG synthesis by VICs is regulated by cyclic stretching conditions.
Keywords: glycosaminoglycan, proteoglycan, mechanical strain, collagen gels, valvular interstitial cells
1. Introduction
The application of mechanical strains has been shown to induce remodeling of the extracellular matrix (ECM) in many tissues, including heart valves [1, 2]. Heart valves are maintained in vivo under a range of strain magnitudes during opening and closing of the valve and during relaxed or stressed conditions of the heart, such as exercise or pathological conditions. Correspondingly, the valvular interstitial cells (VICs) within heart valves are mechanoresponsive to tensile strains in two-dimensional (2D) and three-dimensional (3D) cell cultures [3, 4] and to pressure and shear forces in organ culture [5, 6], resulting in altered ECM synthesis. However, the effect of varying magnitudes of strain and frequencies, such as those found in vivo, have not been widely investigated in the context of VICs in 3D cultures. One study of VICs did examine the effect of strain magnitudes on ECM synthesis, but in 2D culture [3].
Nevertheless, investigations of other types of cardiovascular cells have shown that their synthesis of ECM is significantly influenced by strain. To provide several examples, cardiac fibroblasts subjected to either uniaxial or equibiaxial strains tend to express more collagen III mRNA at lower strains and less at higher strains compared to unstretched controls [7]. Smooth muscle cells (SMCs) have also been shown to synthesize more collagen when subjected to 10% or higher magnitudes of strain [8]. Moreover, the secretion of transforming growth factor-b [9] and fibroblast growth factor-2 [10] by SMCs was found to be proportional to strain. The release of heparan sulfate glycosaminoglycans (GAGs) and fibronectin by vascular endothelial cells was decreased when shear rates [11] and biaxial strains [12], respectively, were elevated. Therefore, strain demonstrably but variably affects ECM synthesis in different types of cardiovascular cells and its effect on VICs should be investigated in order to improve our understanding of valve mechanobiology.
GAGs and proteoglycans (PGs) are integral ECM components of valve tissues and play an important role in defining the material and structural behavior of valves. PGs consist of GAG chains (linear molecules of repeating disaccharides) attached to a core protein; these molecules perform many biological functions in tissues [13]. The sulfation pattern of GAG chains, meaning whether they are 4-sulfated or 6-sulfated, characteristically varies according to the biological and biomechanical needs of the tissue [14]. Dermatan sulfate PGs such as decorin and biglycan, which contain mostly 4-sulfated GAGs, regulate collagen fibril diameter and fibrous tissue organization [15, 16]. In contrast, the GAG hyaluronan (HA), which does not bind to a protein core but tends to aggregate with large PGs such as versican (mainly containing 6-sulfated GAGs), entraps large amounts of aqueous solvent to provide compression resistance [17]. Previous studies of mitral valves from our laboratory showed that 4-sulfated GAGs and the PGs decorin and biglycan were abundant in tensile loading regions such as chordae tendineae, while regions experiencing compression such as the leaflet free edges contained more hyaluronan, 6-sulfated GAGs, and versican [18]. In pathological conditions such as myxomatous mitral valve disease, the valve tissues experience altered tissue loading along with an overabundance of GAGs and PGs [19, 20].
GAG and PG synthesis has recently been shown to be regulated by static mechanical strains applied to VICs grown in 3D cell cultures [4, 21]. Although VICs have been investigated under cyclic straining conditions (in 2D culture), no study has examined the effect of strain on GAG and PG synthesis by VICs [3, 22]. Determining how GAG and PG synthesis responds to different strains is important since the patterns of strain and distribution of GAGs and PGs are evidently altered during myxomatous valve degeneration. In this study, VICs seeded within 3D collagen gels were stretched at different strains within a custom designed bioreactor. We chose to use a 3D collagen matrix, as opposed to a 2D cell culture approach, in order to provide a more biologically and anatomically appropriate model, since a 3D environment for the cells is closer to in vivo conditions [23]. Furthermore, previous studies have shown that VICs seeded on top of or within 3D collagen scaffolds retain their native phenotype and secrete GAGs and PGs comparable to those found in vivo [24–27]. Therefore, the purpose of this study was to determine if the GAG and PG synthesis by VICs isolated from distinct regions of the mitral valve is regulated by different cyclic strain magnitudes.
2. Materials and methods
2.1. Cell culture and seeding in collagen gels
Porcine mitral valves were obtained from an abattoir and VICs were isolated using a previously described protocol [4]. Briefly, the tissues were first soaked in Dulbecco’s Modified Eagles Medium (DMEM, Mediatech, Herndon, VA), containing 2 mg/mL collagenase type II (Worthington, Lakewood, NJ), within an incubated shaker for 20 minutes (140 rpm, 37°C). The endothelial cells were then brushed from the valve surface using cotton swabs and all chordae tendineae were dissected from the leaflet. The separated leaflet and chordal tissues were minced and dissociated with DMEM containing 1 mg/mL collagenase type III and 0.1 mg/mL hyaluronidase (both from Worthington) for 4 hours in an incubated shaker. Leaflet and chordal VICs were cultured separately in DMEM:F12 medium (1:1, containing low glucose with HEPES, Mediatech) with 10% bovine growth serum (BGS, HyClone, Logan, UT) and 1% antibiotic-antimycotic solution (Mediatech). Our laboratory has previously shown that these resulting cell populations demonstrate phenotypic characteristics that are non-endothelial, and that are slightly different between leaflet VICs and chordal VICs [28].
VICs were seeded within collagen gel scaffolds to provide them with an in vivo like 3D environment. The collagen gels were prepared using a protocol from Eastwood et al. [29]. Briefly, 8 parts rat-tail collagen type I at 2.28 mg/mL (BD Biosciences, Bedford, MA) in 0.02 M acetic acid, 1 part 10X DMEM and 1 part cells suspended in 1X DMEM (1 million cells per mL of gel) were mixed together and brought to physiologic pH using 5 M NaOH dropwise. The resulting gel solution was immediately poured into the mold (with or without anchors) within the cyclic stretching device. To minimize batch-to-batch variability, the same type of cells (from the same primary culture harvest date and passage number) and collagen lot number were used in the various samples of engineered tissues. VICs from passage numbers 6–7 were used to prepare all the collagen gels; VICs cultured in tissue culture flasks have been shown to maintain a consistent phenotype until late passage number [30].
2.2. Stretching device
A stretching device developed in our laboratory was used to apply cyclic mechanical strains to collagen gels seeded with VICs [31]. The details of this device have been published previously [31], but briefly, the main components of the device consisted of an aluminum base, stretching cam and culture lid. The collagen gel anchors were connected to roller bearings, which were displaced by the stretching cam. The stretching cam was shaped as a 4-cycle sinusoidal waveform superimposed around the circumference of a circle and caused the collagen gel to be stretched four times with each cam rotation. The cam was designed to impose 2, 5 or 10% strain in the two orthogonal directions of the gel. The cam rotation was performed using a 24 V DC gearhead motor (Barber Colman, Rockford, IL) and controlled by an external power supply (Jameco Electronics, Belmont, CA). The whole bioreactor assembly, except for the power supply, was maintained within the incubator.
A cross-shaped silicone rubber mold that fit snugly within the culture lid was designed as described previously [4]. The collagen gels within the stretching device were incubated statically at 37°C and 5% CO2 for 24 hours to let the collagen gel attach firmly to the anchors. After 24 hours, the medium from the collagen gels was collected for GAG/PG analysis and replaced with fresh medium. The collagen gels seeded with leaflet and chordal cells separately were then subjected to cyclic mechanical strains of 2% (n=5 for each cell type), 5% (n=4 each) or 10% (n=4 each) strain at 1.16 Hz for 48 hours. Additional molds were also prepared to expose the collagen gels to conditions of statically constrained (with anchors, n=2 for each cell type) and unconstrained (without anchors, n=2 for each cell type). In the statically constrained gels, the action of the cells contracting the gel against the anchors created a strain condition. This strain was only developed within the gels, as opposed to being generated through an external imposed action. In the no stretch gels, the cells contracted the freely-floating gel almost completely [4, 21].
2.3. Glycosaminoglycan analysis
Various GAG classes (chondroitin/dermatan sulfates and HA) were analyzed using fluorophore–assisted carbohydrate electrophoresis (FACE) [4, 32]. Briefly, collagen gels were harvested intact from the bioreactors, lyophilized overnight, weighed and then digested with proteinase-K (EMD Pharmaceutical, Durham, NC). The conditioned medium aliquots were also digested with proteinase and then subjected to ion exchange chromatography to remove the glucose from the medium samples and to isolate the GAG chains. Conditioned medium and collagen gel samples were then analyzed using FACE after digestion with chondroitinases ABC and ACII (Associates of Cape Cod, Falmouth, MA) to cleave the GAG chains into disaccharides as described previously [4, 18]. The fluorescent disaccharide bands within the FACE gels were imaged with a Kodak Gel Logic 100 imaging system (Kodak, Eastman, MA) and analyzed using Gel-Pro Analyzer (Media Cybernetics, Silver Spring, MD). GAG classes were identified by correspondence to bands in a disaccharide standard lane and quantified by comparison to a fluorescence standard curve. Total GAGs were calculated as the sum of the different classes of GAG disaccharides. The ratios of 4-sulfated (4-S) to 6-sulfated (6-S) GAGs and sulfated GAGs (4-S and 6-S) to unsulfated GAGs (HA) were also calculated.
2.4. DNA measurement
The DNA assay was performed on aliquots of the proteinase-K digested collagen gel samples [19]. Total cells retained within the collagen gels after the stretching protocol were measured using the Hoechst dye (Molecular Probes, Eugene, OR) DNA assay [33].
2.5. Proteoglycan analysis
PGs within the collagen gel samples were extracted using 4 mol/L guanidine HCl with protease inhibitors overnight at 4°C as described previously [18]. The extracted samples were dialyzed into 7 mol/L urea (pH 7.5). Dialyzed collagen gel samples and conditioned medium samples were then purified by ion exchange chromatography. The purified PGs were precipitated from solution using 95% ethanol (containing 1.3% potassium acetate) and digested with chondroitinase ABC to remove the GAG chains from the core proteins. The samples were vacuum dried, dissolved in SDS buffer at 100°C, separated on a 4–12% SDS-polyacrylamide gel, and blotted for western analyses. The nitrocellulose membrane was blotted with antibodies for the specific PGs (anti-decorin LF-122 and anti-biglycan LF-104, courtesy of Larry Fisher, NIH; anti-versican 2-B-1, Associates of Cape Cod) [34, 35]. The band intensities were analyzed using Gel-Pro Analyzer software (Media Cybernetics, Silver Spring, MD) and normalized to band intensity of PGs from unconstrained collagen gels run on the same western blot.
2.6. Data analysis
The absolute amounts of GAGs secreted into the conditioned medium were analyzed after accounting for the GAG contents of fresh medium and multiplying by total medium volume collected during the culture period. The proportions of specific GAGs synthesized by leaflet and chordal cells were determined by normalizing to total GAG abundance for each sample. The samples for PG analysis were run on electrophoresis gels in duplicate and their band intensities were averaged. Data were expressed as mean ± standard deviation. A two-way analysis of variance (ANOVA) was performed to analyze the effect of cell type (factor one: leaflet and chordal) and strain (factor two: unconstrained, statically constrained, 2%, 5% and 10%). When the ANOVA indicated a significant effect of one of the factors, post-hoc Tukey tests were used for pair-wise comparisons. In all analyses, the level of significance was set at 0.05. Grouped data (leaflet and chordal samples combined) from the parent ANOVA analysis was useful to examine the overall effects of strain on mitral valve cells, whereas the post-hoc analysis of the individual, ungrouped data was able to determine the differences between leaflet and chordal cells. Univariate regressions were performed for further analyses of significant strain-dependent trends.
3. Results
3.1. DNA content within collagen gels
There was no significant difference in the final DNA content (at the end of the stretching protocol) between the cyclic stretching conditions as compared to statically constrained or unconstrained conditions (Fig. 1). Though the application of 10% strain showed a trend (not significantly different) of reduced DNA content, the effect of cyclic strain magnitude on cellularity was negligible.
Figure 1.
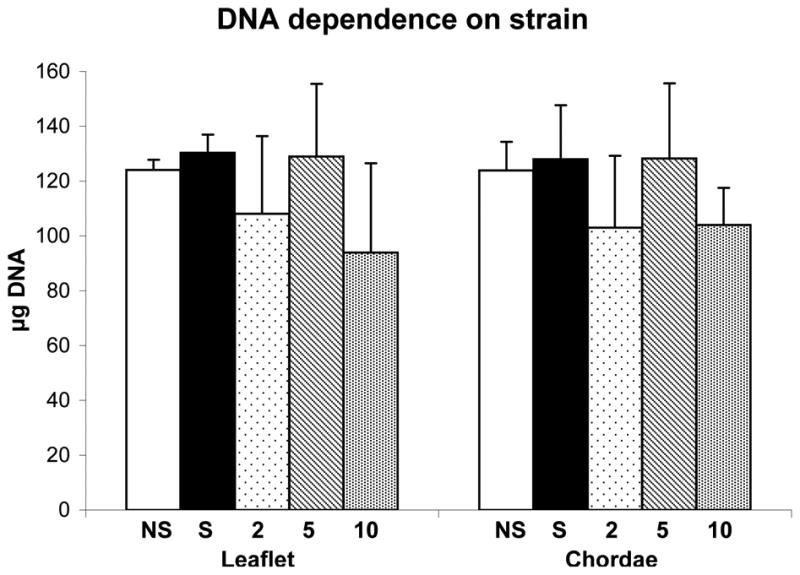
Strain dependency of DNA content within collagen gels seeded with leaflet or chordal cells. Data are mean ± standard deviation. NS-no strain or unconstrained (n=2), S-statically constrained (n=2), 2-cyclic 2% (n=5), 5-cyclic 5% (n=4), 10-cyclic 10% (n=4). Sample sizes (n) indicate the number of gels prepared for each cell type.
3.2. Glycosaminoglycans retained within collagen gels
Leaflet and chordal cells synthesized various GAG classes that were retained within the collagen gels. As shown in Table 1, total GAGs, calculated as the sum of the different GAG disaccharides, were significantly lower in the collagen gels subjected to cyclic strains of 2%, 5%, or 10% (for chordae only 5% and 10%) compared to statically constrained (p<0.01) and unconstrained gels (p<0.02, for 5% and 10% only). Under 2% cyclic strain conditions, total GAG retention was significantly lower for the leaflet group than in the chordal group (p<0.04), but otherwise there were no significant differences in total GAG retention between cell types. When the cyclic strain groups were compared to each other, the decrease in total GAGs was found to be negatively correlated with the magnitude of strain,(0, 2, 5, and 10%) with R2= 0.52 (p<0.003) for the leaflet group and R2=0.84 (p<0.001) for the chordal group. For the chordal cells alone and for all cells grouped together, the application of higher cyclic strains decreased the retention of GAGs (p<0.005). These effects of cyclic strains, particularly the strong negative correlation between retained GAGs and strain magnitude are intriguing given that myxomatous mitral valve leaflets and chordae, which are subjected to reduced loading magnitudes, contain elevated concentrations of GAGs [20].
Table 1.
Strain dependence of total GAGs (nmol/mg dry weight), ratio of 4-S to 6-S, and ratio of S-GAGs to HA calculated from amount of total GAGs retained within collagen gels seeded with leaflet or chordal cells.
| Unconstrained | Statically constrained | Cyclic 2% | Cyclic 5% | Cyclic 10% | ||
|---|---|---|---|---|---|---|
| Leaflet | Total GAGs (nmol/mg) | 12.0±0.1 | 13.7±2.7x | 8.7±1.9*a | 7.0±1.6*‡x | 5.8±1.0*‡x |
| 4-S/6-S | 5.2±0.8 | 6.7±1.8a | 5.8±2.0a | 6.5±1.5y | 5.5±0.5 | |
| S-GAGs/HA | 6.9±0.4 | 7.9±1.1 | 3.6±1.9a | 5.3±3.5a | 5.8±1.3a | |
| Chordae | Total GAGs (nmol/mg) | 12.5±0.5 | 15.3±1.4x | 11.9±2.1φ | 7.5±1.8*‡x | 4.0±0.6*‡x |
| 4-S/6-S | 3.2±0.1 | 3.8±0.2 | 4.1±0.6 | 6.1±0.6y | 6.0±0.8 | |
| S-GAGs/HA | 7.1±0.5 | 7.7±1.1 | 37.7±31.4 | 27.4±10.9 | 39.0±8.9 |
Data are mean ± standard deviation.
p<0.01 vs. statically constrained,
p<0.02 vs. unconstrained,
p<0.004 vs. 5% or 10%,
p<0.04 vs. chordae.
When leaflet and chordal data were grouped together,
p<0.005 vs. 2%,
p<0.05 vs. unconstrained.
Both unsulfated (HA) and sulfated GAGs (4- and 6-sulfated chondroitin/dermatan sulfate) were synthesized by valve cells and retained within the collagen gels (Table 2). The presence of cyclic strain, regardless of strain magnitude, significantly affected the proportions of HA, 4-S, and 6-S retained within the gels (p<0.05). In the collagen gels seeded with leaflet cells, cyclic strains of 2% caused significantly higher proportions of HA (p<0.04) and lower proportions of 4-S (p<0.03) compared to the statically constrained condition. Collagen gels seeded with chordal cells retained higher proportions of 4-S at 5% or 10% strain than in the statically constrained (p<0.03) and unconstrained condition (p<0.01). In addition, there was a trend of retaining an increasingly greater proportion of 4-S within the chordal cell-seeded gels with increasing strain magnitude (0–10%, R2=0.59, p<0.001). A strain magnitude of 10% increased the proportions of 4-S compared to 2% strain when leaflet and chordal groups were considered together (p<0.02). In the collagen gels seeded with chordal cells, the highest magnitudes of cyclic strain (5% and 10%) reduced the proportion of 6-S from the levels found in the 2% cyclic strain (p<0.04) and unconstrained condition (p<0.01). Collagen gels seeded with leaflet cells contained significantly higher proportions of HA (p<0.003 for 2%, 5%, and 10%) and lower proportions of 4-S (p<0.003, for 2%, 5%, and 10%) and 6-S (p<0.001, for 2%, statically constrained, and unconstrained) than gels seeded with chordal cells. These trends correspond to GAG patterns observed in vivo, since chordal tissues experience high tensile strains and have higher proportions of 4-sulfated GAGs, whereas leaflets experience a range of tensile strains and contain abundant HA [18].
Table 2.
Strain dependence of specific proportions of GAGs retained within collagen gels seeded with leaflet or chordal cells.
| Unconstrained | Statically constrained | Cyclic 2% | Cyclic 5% | Cyclic 10% | ||
|---|---|---|---|---|---|---|
| Leaflet | % HA | 12.6±0.7 | 11.3±1.4† | 24.7±8.7* | 19.3±8.4* | 15.0±2.7* |
| % 6-S | 14.3±2.0* | 11.8±2.5* | 12.0±4.1* | 11.0±1.2 y | 13.1±1.4 y | |
| % 4-S | 73.1±1.3 | 77.0±4.0† | 63.4±6.6* | 69.7±9.5* | 71.9±1.7*x | |
| Chordae | % HA | 12.4±0.7 | 11.6±1.5 | 4.9±4.0 | 4.0±1.6 | 2.6±0.5 |
| % 6-S | 18.4±1.1 | 18.4±1.1 | 18.8±1.8 | 13.7±1.3†‡y | 14.0±1.7†‡ y | |
| % 4-S | 66.6±0.2 | 70.0±0.5φ | 76.3±4.5 | 82.3±1.5‡ | 83.4±1.7‡x |
Data are mean percentage ± standard deviation.
p<0.007 vs. chordae,
p<0.04 vs. 2%,
p<0.01 vs. unconstrained,
p<0.03 vs. 10%.
When leaflet and chordal data were grouped together,
p<0.02 vs. 2%,
p<0.04 vs. unconstrained.
The ratio of 4-S to 6-S GAGs and the ratio of sulfated GAGs (sum of 4-S and 6-S) to unsulfated GAGs (HA) were calculated to gather more information about the mechanical behavior of the cell-seeded collagen gels. On average, the 4-S/6-S ratio was higher in collagen gels seeded with leaflet cells than in gels seeded with chordal cells (p<0.03 for 2% and statically constrained condition, Table 1). In contrast, the sulfated GAGs/HA ratio was higher for chordal cells than for leaflet cells for all cyclic strain magnitudes (p<0.04). As above, these ratios reflect the greater proportions of sulfated GAGs (particularly 4-sulfated moeities) in chordal tissues, which bear high cyclic tensile loads [18].
3.3. Glycosaminoglycans secreted into culture medium
Although many GAGs were retained within the collagen gels, some of the GAGs synthesized by the valve leaflet and chordal cells were secreted into the surrounding culture medium. On average, the amounts of total GAGs retained within the gel were 2–3 times higher than the amounts secreted into the medium (data not shown). In addition, we did not find significant differences in the volume of medium collected from the different strain conditions, indicating that evaporation of medium was comparable between groups. On average, more total GAGs were secreted into the medium by leaflet cells than by chordal cells (p<0.007); this result may be related to the in vivo condition, in which native heart valve leaflets have a greater concentration of GAGs than do chordae [18]. When exposed to increasing strain magnitudes, GAG secretion was progressively decreased for leaflet cells (2–10%, R2=0.55, p<0.005) and increased for chordal cells (0–10%, R2=0.61, p<0.001), respectively. Cyclic strains of only 2% applied to collagen gels seeded with leaflet cells caused a substantially greater secretion of total GAGs into the medium compared to 10% cyclic strain (p<0.001) and the unconstrained condition (p<0.02, Table 3). Also, significantly more GAGs were secreted by chordal cells in the 10% cyclic strain condition compared to the unconstrained condition (p<0.04). Interestingly, compared to chordal cells, leaflet cells secreted more GAGs into the medium at 2% (p<0.001) and 5% (p<0.05) strains and less GAGs at 10% strain (p<0.02).
Table 3.
Strain dependence of total GAGs (nmol), ratio of 4-S to 6-S, and ratio of S-GAGs to HA calculated from amounts of GAGs secreted into the medium surrounding collagen gels seeded with leaflet or chordal cells.
| Unconstrained | Statically constrained | Cyclic 2% | Cyclic 5% | Cyclic 10% | ||
|---|---|---|---|---|---|---|
| Leaflet | Total GAGs (nmol) | 92.4±7.7* x | 125.2±10.6 | 174.7±48.0† | 131.4±37.1† | 86.4±12.0*† |
| 4-S/6-S | 3.2±0.1y | 3.1±0.1y | 3.9±0.2 | 3.9±0.2 | 3.4±0.4 | |
| S-GAGs/HA | 0.7±0.2 | 0.9±0.5† | 1.0±0.2 | 1.0±0.2 | 0.7±0.1† | |
| Chordae | Total GAGs (nmol) | 65.2±19.5φx | 83.6±17.5 | 84.8±20.1 | 90.2±10.9 | 137.6±19.8 |
| 4-S/6-S | 3.0±0.3y | 2.6±0.0y | 3.5±0.7 | 4.0±1.3 | 3.5±0.4 | |
| S-GAGs/HA | 1.0±0.5 | 1.6±0.4 | 1.1±0.2 | 1.4±0.3 | 1.3±0.6 |
Data are mean ± standard deviation.
p<0.02 vs. 2%,
p<0.04 vs. 10%,
p<0.05 vs. chordae.
When leaflet and chordal data were grouped together,
p<0.04 vs. 2%,
p<0.03 vs. 5%.
With respect to the proportions of specific GAGs, higher proportions of 4-S were secreted by leaflet cells at 5% cyclic strain compared to the unconstrained condition (p<0.01, Table 4). Chordal cells secreted lower proportions of 6-S GAGs at 10% strain compared to the statically constrained condition (p<0.04). Leaflet cells secreted more HA than did chordal cells at both the 10% cyclic strain and statically constrained conditions (p<0.05). Compared with the leaflet cell group, the secretion of 6-S GAGs was greater in the chordal group at 5% cyclic strain and under statically constrained (both p<0.04), whereas the secretion of 4-S GAGs was significantly greater for the chordal group at 10% cyclic strain.
Table 4.
Strain dependence of specific proportions of GAGs secreted into culture medium surrounding collagen gels seeded with leaflet or chordal cells.
| Unconstrained | Statically constrained | Cyclic 2% | Cyclic 5% | Cyclic 10% | ||
|---|---|---|---|---|---|---|
| Leaflet | % HA | 56.3±8.1 | 51.5±13.5* | 48.2±5.6 | 49.5±4.1 | 60.1±5.6* |
| % 6-S | 11.1±1.4 | 12.4±2.6* | 10.4±1.2 | 8.9±0.3*x | 10.6±1.0 | |
| % 4-S | 27.0±6.4 | 32.9±9.3 | 38.7±3.2 | 39.4±3.9† | 29.8±5.3* | |
| Chordae | % HA | 49.8±15.1 | 36.2±4.6 | 46.8±4.8 | 40.9±5.8 | 43.1±10.8 |
| % 6-S | 14.7±0.6 | 19.2±0.3 | 12.9±3.3 | 12.9±4.9x | 12.0±3.1‡ | |
| % 4-S | 30.9±11.3 | 39.6±4.9 | 37.2±4.9 | 43.3±4.3 | 38.5±6.3 |
Data are mean ± standard deviation.
p<0.05 vs. chordae,
p<0.04 vs. statically constrained,
p<0.01 vs. unconstrained.
When leaflet and chordal data were grouped together,
p<0.05 vs. statically constrained.
The ratio of secreted 4-S to 6-S GAGs was significantly affected by strain conditions when leaflet and chordal data were analyzed together. The secreted 4-S/6-S GAG ratio tended to be greater in the 5% cyclically strained gels compared to statically constrained and unconstrained conditions (p<0.03). Although the secreted S-GAG to HA ratios were quite consistent and far lower than those corresponding to the GAGs retained within the collagen gels, on average the chordal cells produced greater secreted S-GAG/HA ratios than did the leaflet cells (p<0.003), particularly at the 10% cyclic strain (p<0.02) and statically constrained conditions (p<0.04).
3.4. Proteoglycans retained within collagen gels
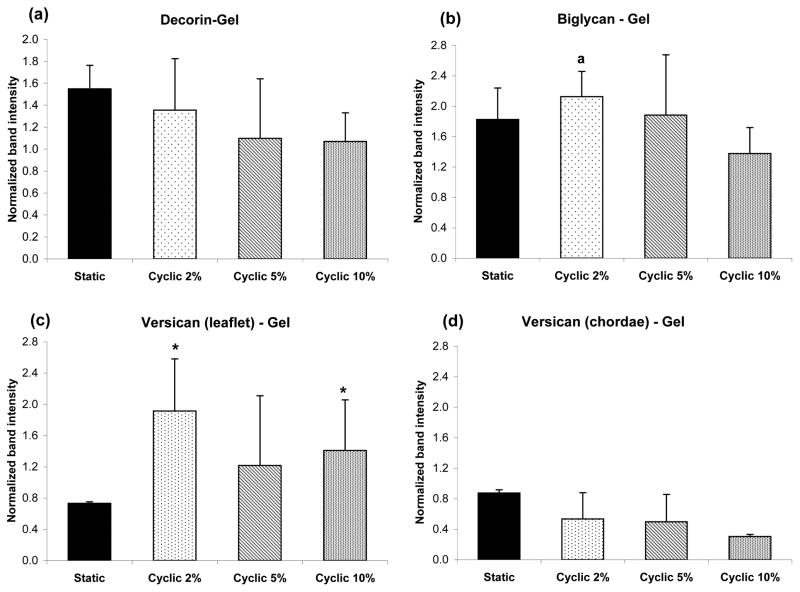
All three PGs of interest (decorin, biglycan and versican) were found within collagen gels subjected to the different stretching protocols. Retention of decorin, biglycan and versican was not found to be significantly affected by strain magnitude despite some trends that appeared promising (Fig. 2). When the leaflet and chordal data was grouped together, the retention of biglycan within collagen gels was greater at 2% than 10% cyclic strain (p<0.03). There was no significant effect of strain on retention of the other PGs, even for grouped leaflet and chordal data. However, versican retention within the collagen gels containing leaflet cells was significantly greater than in those containing chordal cells for the lowest and highest cyclic strains (p<0.01).
Figure 2.
Western blot detection of various PGs synthesized by valve cells, and retained within the collagen gels, at different strains. Leaflet and chordal data were not significantly different and thus were grouped together for (a) decorin and (b) biglycan, ap<0.03 vs. 10%. Versican data are shown separately for (c) leaflet and (d) chordae, *p<0.01 vs. chordae. Data are mean ± standard deviation.
3.5. Proteoglycans secreted into culture medium
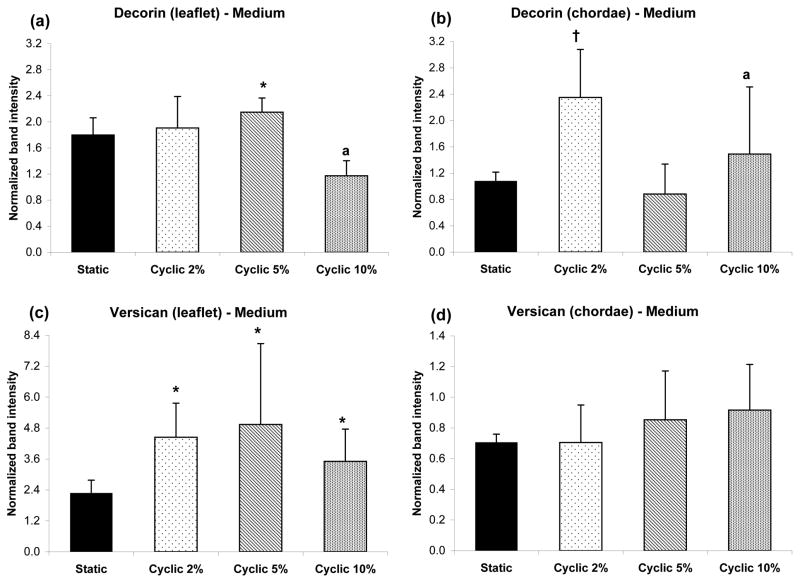
Similar to GAG secretion, PGs were also secreted into the surrounding medium (Fig. 3). At 5% cyclic strain, the secretion of decorin by gels seeded with chordal cells was lower than that of chordal gels strained 2% (p<0.006), and lower than the leaflet gels strained 5% (p<0.01). When leaflet and chordal cell data was grouped together, the secretion of decorin was higher at 2% strain compared to 10% strain (p<0.03). As with the PGs retained within the collagen gel, versican secretion by leaflet cells was greater than secretion by chordal cells for all cyclic strains (p<0.02). The secretion of biglycan was not affected by strain (data not shown).
Figure 3.
PGs secreted into culture medium surrounding the collagen gels, seeded with leaflet and chordal cells that were subjected to various strains. Decorin secretion is shown for (a) leaflet and (b) chordae, *p<0.01 vs. chordae, †p<0.006 vs. 5%, ap<0.03 vs. 2% (when leaflet and chordal data were grouped together). Similarly, versican secretion is shown for (c) leaflet and (d) chordae, *p<0.02 vs. chordae. Data are mean ± standard deviation.
4. Discussion
In this study, we used a custom built stretching device to show that the synthesis of specific GAGs and PGs by VICs is strain dependent. Total GAGs retained within collagen gels decreased under cyclic strain; this decrease was strain magnitude dependent with the least GAGs produced at 10% cyclic strain. Endothelial cells have likewise been shown to decrease GAG synthesis with increasing strain magnitudes [12]. Not all GAGs and PGs showed this trend; the retention of 4-sulfated GAGs within collagen gels increased with strain magnitude and the secretion of decorin into the culture medium was lowest at the medium strain magnitude (5%). Variable effects of strain magnitudes have been shown previously for collagen synthesis by other cardiovascular cell types [7, 8, 36].
Cyclic strains were applied in this study because it was believed that these would elicit ECM synthesis patterns that more closely resembled the in vivo behavior of the specific cell types. Overall, the application of cyclic mechanical strains to the valve cell-seeded collagen gels reduced the overall synthesis of GAGs, although the proportions of 4-sulfated GAGs and total sulfated GAGs tended to increase while the 6-sulfated GAGs and the unsulfated GAG HA tended to decrease. This trend was previously reported for SMCs, which synthesized more sulfated GAGs and less HA when exposed to centrifugal mechanical forces [37]. In contrast, other studies have reported an increase in versican (more associated with 6-sulfated GAGs and HA) and decrease in decorin (associated with 4-sulfated GAGs) retained within the cell layer as well as secreted into the medium upon the application of cyclic strains on SMCs [38]; these differences may be due to the specific magnitudes of strain or frequency applied in the various investigations.
Leaflet and chordal tissues experience distinctive strain patterns in vivo and in our 3D model the cells derived from these tissues showed different trends with cyclic strain. GAGs that were secreted into the medium by chordal cells (as opposed to those being retained within the collagen gels) increased with cyclic strain in a magnitude dependent manner, whereas the GAGs secreted by the leaflet cells showed the opposite trend. In general, the chordal cells tended to produce greater proportions of 6-sulfated GAGs (statically constrained, unconstrained) and 4-sulfated GAGs (cyclic strain) than the leaflet cells, which produced greater proportions of HA. Compared to the profile of GAGs produced by the cells under statically constrained conditions [4], the application of cyclic strains to the cell-seeded collagen gels brought the profile of GAGs synthesized by valve cells closer to what is observed in vivo. This recapitulation of in vivo behavior was also demonstrated by the synthesis of the PG versican, which was upregulated by cyclically strained leaflet VICs. Versican and its associated 6-sulfated GAGs were decreased by chordal VICs under cyclic strain, which again match the GAG and PG patterns found in native mitral valve tissues under continuous cyclic strains [18].
Because this study was the first to examine the variable effect of strain on VICs’ synthesis of GAGs and PGs, our study parameters were guided by investigations of other cardiovascular cell types. Our selection of 48 hour periods of stretching was justified by previous studies showing that the application of mechanical strains causes changes in PG and collagen synthesis within a few hours [38, 39]. Other cardiovascular cell types have been examined using a wide range of strains [40]. Our applied frequency was based on heart rate (72 beats per minute), which the VICs normally experience in vivo, although this frequency certainly varies depending on health and activity. Furthermore, most cardiovascular cells experience strains at 10% [41], which was the maximum strain we were able to apply to our cell-seeded collagen gels. As noted above, the results obtained in this investigation are quite consistent with previous investigations of other cell types [7, 12, 37, 38].
These results also contribute to the growing field of heart valve mechanobiology, in which the experimental mechanical stimulation of heart valves and VICs has proven highly relevant to our understanding of the pathological remodeling of valves and to the improvement of tissue engineered heart valves. Our findings that strains modulate GAG and PG synthesis by VICs are supported by recent studies showing that mechanically strained VICs regulate their expression of other extracellular matrix (such as collagen) as well as their expression of inflammatory genes, which may play a role in the development and prevention of valve pathologies [3, 22]. In the area of tissue engineering, it has been widely shown that tissue engineered heart valves that are cyclically mechanically stimulated during their in vitro incubation and maturation will have improved matrix production, greater cell density, more compact matrix organization, and greater mechanical strength [42]. As GAGs and PGs are also structurally and biologically important ECM components, the regulation of these molecules by applying various cyclic strain regimens will aid in the development of a tissue engineered heart valves that more closely recapitulates the native valve.
This study had a number of limitations, the first being that we were only able to apply a narrow range of strains due to the delicacy of the engineered tissue and to device constraints. In vivo, the heart valves are subjected to high strain loading conditions during exercise and hypertension [43]. In vitro studies using ventricular simulators have measured the maximum principal strains in mitral chordae and posterior leaflet to be 4.3% ± 3.4% and 22.8 ± 14.1% respectively [44, 45]. However, low magnitudes of strains are observed during pathological conditions such as myxoid degeneration [46]. Although a wider range of strain conditions were pilot tested, the cell-seeded collagen gels tended to tear at high strains such as 20%. In addition, the magnitudes of the imposed strains (2, 5, and 10%) were calculated from the device dimensions and may not have been uniformly transferred to all the cells within the collagen gels, although we have shown previously that VICs grown in these collagen gels do align in the direction of stretch [4]. We did observe some structural differences in the collagen gel matrix under different stretching conditions. The matrix appeared fragile at the highest strain applied, but more robust at the lowest strain. However, there were no differences in the areal contraction of the collagen gels or the final concentration of collagen within the gels (data not shown) and only minor differences in the final density of cells between the various stretching conditions. Finally, it might be speculated that the cross-shape of the collagen gel would impose different strain patterns throughout the engineered tissue (biaxial in the center and uniaxial along the arm of the gel) that would influence the local production of GAGs or PGs. In previous studies, however, we found that the biaxial vs. uniaxial loading in these gels did not cause substantial changes in their GAG and PG synthesis; stretch alone was the primary factor influencing the synthesis of these matrix components [4]. We also believed that the use of a culture system that imposed biaxial loading would be more representative of the type of loading experienced by the leaflet cells in vivo. In the future, it will also be interesting to examine the effect of frequency on GAG and PG synthesis; our preliminary studies on this topic, however, did not reveal any significant effect of varying frequency within the narrow range of 0.83–1.5 Hz (data not shown). A wider range of frequencies may need to be examined before frequency dependent effects become apparent. Another limitation of the study was the 10–15% variability between duplicate FACE gel runs of the same sample, which may have caused high standard deviations in the data and contributed to the lack of statistical significance between different strain magnitudes, even when a trend was suggested.
5. Conclusions
Overall, we found that different strain magnitudes affected the synthesis of specific GAGs and PGs by VICs seeded within 3D collagen gels. Cyclically straining the VICs grown from mitral valve leaflets and chordae caused the upregulation of specific GAGs and PGs that are usually in abundance in these distinctive tissue regions. The controlled synthesis of specific GAGs and PGs in mechanically loaded collagen gels will be important in tissue engineering applications as these ECM molecules have diverse biological and biomechanical functionalities that are desirable to recapitulate in engineered tissues [47, 48]. This study also contributes to the growing body of knowledge about heart valve mechanobiology in normal and pathological conditions.
Acknowledgments
The authors thank Ivan Vesely, Ph.D. and Mike Allen, M.S. for their suggestions in the design of the biaxial stretching device, and Jenni Cieluch for assistance with the FACE gel analysis. This project was funded by the Whitaker Foundation and the American Heart Association Ohio Valley Affiliate Summer Undergraduate Research Fellowship.
References
- 1.Grande-Allen KJ, Borowski AG, Troughton RW, Houghtaling PL, Dipaola NR, Moravec CS, Vesely I, Griffin BP. Apparently normal mitral valves in patients with heart failure demonstrate biochemical and structural derangements: an extracellular matrix and echocardiographic study. J Am Coll Cardiol. 2005;45:54–61. doi: 10.1016/j.jacc.2004.06.079. [DOI] [PubMed] [Google Scholar]
- 2.Chiquet M, Matthisson M, Koch M, Tannheimer M, Chiquet-Ehrismann R. Regulation of extracellular matrix synthesis by mechanical stress. Biochem Cell Biol. 1996;74:737–744. doi: 10.1139/o96-080. [DOI] [PubMed] [Google Scholar]
- 3.Ku CH, Johnson PH, Batten P, Sarathchandra P, Chambers RC, Taylor PM, Yacoub MH, Chester AH. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc Res. 2006;71:548–556. doi: 10.1016/j.cardiores.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Gupta V, Werdenberg JA, Blevins TL, Grande-Allen KJ. Synthesis of glycosaminoglycans in differently loaded regions of collagen gels seeded with valvular interstitial cells. Tissue Eng. 2007;13:41–49. doi: 10.1089/ten.2006.0091. [DOI] [PubMed] [Google Scholar]
- 5.Xing Y, He Z, Warnock JN, Hilbert SL, Yoganathan AP. Effects of constant static pressure on the biological properties of porcine aortic valve leaflets. Ann Biomed Eng. 2004;32:555–562. doi: 10.1023/b:abme.0000019175.12013.8f. [DOI] [PubMed] [Google Scholar]
- 6.Xing Y, Warnock JN, He Z, Hilbert SL, Yoganathan AP. Cyclic pressure affects the biological properties of porcine aortic valve leaflets in a magnitude and frequency dependent manner. Ann Biomed Eng. 2004;32:1461–1470. doi: 10.1114/b:abme.0000049031.07512.11. [DOI] [PubMed] [Google Scholar]
- 7.Lee AA, Delhaas T, McCulloch AD, Villarreal FJ. Differential responses of adult cardiac fibroblasts to in vitro biaxial strain patterns. J Mol Cell Cardiol. 1999;31:1833–1843. doi: 10.1006/jmcc.1999.1017. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Muragaki Y, Hatamura I, Ueno H, Ooshima A. Stretch-induced collagen synthesis in cultured smooth muscle cells from rabbit aortic media and a possible involvement of angiotensin II and transforming growth factor-beta. J Vasc Res. 1998;35:93–103. doi: 10.1159/000025570. [DOI] [PubMed] [Google Scholar]
- 9.Reyna SV, Ensenat D, Johnson FK, Wang H, Schafer AI, Durante W. Cyclic strain stimulates L-proline transport in vascular smooth muscle cells. Am J Hypertens. 2004;17:712–717. doi: 10.1016/j.amjhyper.2004.03.673. [DOI] [PubMed] [Google Scholar]
- 10.Cheng GC, Briggs WH, Gerson DS, Libby P, Grodzinsky AJ, Gray ML, Lee RT. Mechanical strain tightly controls fibroblast growth factor-2 release from cultured human vascular smooth muscle cells. Circ Res. 1997;80:28–36. doi: 10.1161/01.res.80.1.28. [DOI] [PubMed] [Google Scholar]
- 11.Grimm J, Keller R, de Groot PG. Laminar flow induces cell polarity and leads to rearrangement of proteoglycan metabolism in endothelial cells. Thromb Haemost. 1988;60:437–441. [PubMed] [Google Scholar]
- 12.Gorfien SF, Winston FK, Thibault LE, Macarak EJ. Effects of biaxial deformation on pulmonary artery endothelial cells. J Cell Physiol. 1989;139:492–500. doi: 10.1002/jcp.1041390307. [DOI] [PubMed] [Google Scholar]
- 13.Raman R, Sasisekharan V, Sasisekharan R. Structural insights into biological roles of protein-glycosaminoglycan interactions. Chem Biol. 2005;12:267–277. doi: 10.1016/j.chembiol.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Hardingham TE, Fosang AJ. Proteoglycans: many forms and many functions. Faseb J. 1992;6:861–870. [PubMed] [Google Scholar]
- 15.Schonherr E, Hausser H, Beavan L, Kresse H. Decorin-type I collagen interaction. Presence of separate core protein-binding domains. J Biol Chem. 1995;270:8877–8883. doi: 10.1074/jbc.270.15.8877. [DOI] [PubMed] [Google Scholar]
- 16.Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, Bianco P, Young MF. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17:1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- 17.Wight TN, Heinegard D, Hascall VC. In: Proteoglycans: Structure and function. Cell Biology of Extracellular Matrix. 2. Hay ED, editor. Plenum Press; New York: 1991. pp. 45–78. [Google Scholar]
- 18.Grande-Allen KJ, Calabro A, Gupta V, Wight TN, Hascall VC, Vesely I. Glycosaminoglycans and proteoglycans in normal mitral valve leaflets and chordae: association with regions of tensile and compressive loading. Glycobiology. 2004;14:621–633. doi: 10.1093/glycob/cwh076. [DOI] [PubMed] [Google Scholar]
- 19.Grande-Allen KJ, Griffin BP, Calabro A, Ratliff NB, Cosgrove DM, 3rd, Vesely I. Myxomatous mitral valve chordae. II: Selective elevation of glycosaminoglycan content. J Heart Valve Dis. 2001;10:325–332. discussion 332–323. [PubMed] [Google Scholar]
- 20.Grande-Allen KJ, Griffin BP, Ratliff NB, Cosgrove DM, Vesely I. Glycosaminoglycan profiles of myxomatous mitral leaflets and chordae parallel the severity of mechanical alterations. J Am Coll Cardiol. 2003;42:271–277. doi: 10.1016/s0735-1097(03)00626-0. [DOI] [PubMed] [Google Scholar]
- 21.Gupta V, Werdenberg JA, Mendez JS, Grande-Allen KJ. Influence of strain on proteoglycan synthesis by valvular interstitial cells in three-dimensional culture. Acta Biomater. 2008;4:88–96. doi: 10.1016/j.actbio.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Warnock JN, Burgess SC, Shack A, Yoganathan AP. Differential immediate-early gene responses to elevated pressure in porcine aortic valve interstitial cells. J Heart Valve Dis. 2006;15:34–41. discussion 42. [PubMed] [Google Scholar]
- 23.Scherberich A, Beretz A. Culture of vascular cells in tridimensional (3-D) collagen: a methodological review. Therapie. 2000;55:35–41. [PubMed] [Google Scholar]
- 24.Taylor PM, Allen SP, Dreger SA, Yacoub MH. Human cardiac valve interstitial cells in collagen sponge: a biological three-dimensional matrix for tissue engineering. J Heart Valve Dis. 2002;11:298–306. discussion 306–297. [PubMed] [Google Scholar]
- 25.Butcher JT, Nerem RM. Porcine aortic valve interstitial cells in three-dimensional culture: comparison of phenotype with aortic smooth muscle cells. J Heart Valve Dis. 2004;13:478–485. discussion 485–476. [PubMed] [Google Scholar]
- 26.Rothenburger M, Volker W, Vischer P, Glasmacher B, Scheld HH, Deiwick M. Ultrastructure of proteoglycans in tissue-engineered cardiovascular structures. Tissue Eng. 2002;8:1049–1056. doi: 10.1089/107632702320934146. [DOI] [PubMed] [Google Scholar]
- 27.Dreger SA, Thomas P, Sachlos E, Chester AH, Czernuszka JT, Taylor PM, Yacoub MH. Potential for synthesis and degradation of extracellular matrix proteins by valve interstitial cells seeded onto collagen scaffolds. Tissue Eng. 2006;12:2533–2540. doi: 10.1089/ten.2006.12.2533. [DOI] [PubMed] [Google Scholar]
- 28.Blevins TL, Peterson SB, Lee EL, Bailey AM, Frederick JD, Huynh TN, Gupta V, Grande-Allen KJ. Mitral valvular interstitial cells demonstrate regional, adhesional, and synthetic heterogeneity. Cells Tissues Organs. 2008;187:113–122. doi: 10.1159/000108582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eastwood M, McGrouther DA, Brown RA. A culture force monitor for measurement of contraction forces generated in human dermal fibroblast cultures: evidence for cell-matrix mechanical signalling. Biochim Biophys Acta. 1994;1201:186–192. doi: 10.1016/0304-4165(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 30.Lester W, Rosenthal A, Granton B, Gotlieb AI. Porcine mitral valve interstitial cells in culture. Lab Invest. 1988;59:710–719. [PubMed] [Google Scholar]
- 31.Gupta V, Werdenberg JA, Lawrence BD, Mendez JS, Stephens EH, Grande-Allen KJ. Reversible secretion of glycosaminoglycans and proteoglycans by cyclically stretched valvular cells in 3D culture. Ann Biomed Eng. 2008;36:1092–1103. doi: 10.1007/s10439-008-9501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calabro A, Benavides M, Tammi M, Hascall VC, Midura RJ. Microanalysis of enzyme digests of hyaluronan and chondroitin/dermatan sulfate by fluorophore-assisted carbohydrate electrophoresis (FACE) Glycobiology. 2000;10:273–281. doi: 10.1093/glycob/10.3.273. [DOI] [PubMed] [Google Scholar]
- 33.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 34.Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem. 1990;38:1549–1563. doi: 10.1177/38.11.2212616. [DOI] [PubMed] [Google Scholar]
- 35.Isogai Z, Shinomura T, Yamakawa N, Takeuchi J, Tsuji T, Heinegard D, Kimata K. 2B1 antigen characteristically expressed on extracellular matrices of human malignant tumors is a large chondroitin sulfate proteoglycan, PG-M/versican. Cancer Res. 1996;56:3902–3908. [PubMed] [Google Scholar]
- 36.Isenberg BC, Tranquillo RT. Long-term cyclic distention enhances the mechanical properties of collagen-based media-equivalents. Ann Biomed Eng. 2003;31:937–949. doi: 10.1114/1.1590662. [DOI] [PubMed] [Google Scholar]
- 37.Merrilees MJ, Merrilees MA, Birnbaum PS, Scott PJ, Flint MH. The effect of centrifugal force on glycosaminoglycan production by aortic smooth muscle cells in culture. Atherosclerosis. 1977;27:259–264. doi: 10.1016/0021-9150(77)90034-x. [DOI] [PubMed] [Google Scholar]
- 38.Lee RT, Yamamoto C, Feng Y, Potter-Perigo S, Briggs WH, Landschulz KT, Turi TG, Thompson JF, Libby P, Wight TN. Mechanical strain induces specific changes in the synthesis and organization of proteoglycans by vascular smooth muscle cells. J Biol Chem. 2001;276:13847–13851. doi: 10.1074/jbc.M010556200. [DOI] [PubMed] [Google Scholar]
- 39.Sumpio BE, Banes AJ, Link GW, Iba T. Modulation of endothelial cell phenotype by cyclic stretch: inhibition of collagen production. J Surg Res. 1990;48:415–420. doi: 10.1016/0022-4804(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 40.Gupta V, Grande-Allen KJ. Effects of static and cyclic loading in regulating extracellular matrix synthesis by cardiovascular cells. Cardiovasc Res. 2006;72:375–383. doi: 10.1016/j.cardiores.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 41.Ono S, Waldman LK, Yamashita H, Covell JW, Ross J., Jr Effect of coronary artery reperfusion on transmural myocardial remodeling in dogs. Circulation. 1995;91:1143–1153. doi: 10.1161/01.cir.91.4.1143. [DOI] [PubMed] [Google Scholar]
- 42.Mendelson K, Schoen FJ. Heart valve tissue engineering: concepts, approaches, progress, and challenges. Ann Biomed Eng. 2006;34:1799–1819. doi: 10.1007/s10439-006-9163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Safar ME, Peronneau PA, Levenson JA, Toto-Moukouo JA, Simon AC. Pulsed Doppler: diameter, blood flow velocity and volumic flow of the brachial artery in sustained essential hypertension. Circulation. 1981;63:393–400. doi: 10.1161/01.cir.63.2.393. [DOI] [PubMed] [Google Scholar]
- 44.He Z, Ritchie J, Grashow JS, Sacks MS, Yoganathan AP. In vitro dynamic strain behavior of the mitral valve posterior leaflet. J Biomech Eng. 2005;127:504–511. doi: 10.1115/1.1894385. [DOI] [PubMed] [Google Scholar]
- 45.Ritchie J, Jimenez J, He Z, Sacks MS, Yoganathan AP. The material properties of the native porcine mitral valve chordae tendineae: an in vitro investigation. J Biomech. 2006;39:1129–1135. doi: 10.1016/j.jbiomech.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 46.Barber JE, Kasper FK, Ratliff NB, Cosgrove DM, Griffin BP, Vesely I. Mechanical properties of myxomatous mitral valves. J Thorac Cardiovasc Surg. 2001;122:955–962. doi: 10.1067/mtc.2001.117621. [DOI] [PubMed] [Google Scholar]
- 47.Allison DD, Grande-Allen KJ. Review. Hyaluronan: a powerful tissue engineering tool. Tissue Eng. 2006;12:2131–2140. doi: 10.1089/ten.2006.12.2131. [DOI] [PubMed] [Google Scholar]
- 48.Ferdous Z, Grande-Allen KJ. Utility and control of proteoglycans in tissue engineering. Tissue Eng. 2007;13:1893–1904. doi: 10.1089/ten.2006.0056. [DOI] [PubMed] [Google Scholar]