Abstract
Objective
To describe ethnic and gender differences in the prevalence and determinants of fatty liver in a multi-ethnic cohort.
Patients and Methods
We studied participants from the Multi-Ethnic Study of Atherosclerosis who underwent baseline non-contrast cardiac CT between July 2000-August 2002, and had adequate hepatic and splenic imaging for fatty liver determination (n=4088). Fatty liver was diagnosed by a liver/spleen attenuation ratio <1. We compared the prevalence and severity of fatty liver, among four ethnicities (White, Chinese, African American, Hispanic), stratifying by obesity and metabolic syndrome. Multivariable ordinal logistic regression was employed to determine the impact of cardio-metabolic risk factors on fatty liver prevalence in different ethnicities.
Results
The prevalence of fatty liver varied significantly by ethnicity (White 15%, Chinese 20%, African-American 11%, Hispanic 27%, p<0.001). Although African-Americans had the highest prevalence of obesity, a smaller percentage of obese African Americans were diagnosed with fatty liver compared to other ethnicities (African American 17%, White 31%, Chinese 37%, Hispanic 39%, p<0.001). Hispanics demonstrated the highest prevalence of fatty liver, including among the obese and metabolic syndrome population. An increase in insulin resistance predicted a two-fold increased prevalence of fatty liver in all ethnicities after multi-variable adjustment.
Conclusion
African-Americans have a lower prevalence, and Hispanic Americans a higher prevalence of fatty liver compared to other ethnicities. There are distinct ethnic variations in the prevalence of fatty liver even among patients with the metabolic syndrome or obesity, suggesting that genetic factors may play a significant role in the phenotypic expression of fatty liver.
Keywords: Non-alcoholic Fatty Liver Disease, Ethnicity, Computed Tomography
Introduction
Non-alcoholic fatty liver disease (NAFLD) is estimated to affect 20-30% of the world population (1), and approximately one in three adult Americans (2). An increasing prevalence of NAFLD has been linked with rising rates of insulin resistance, the metabolic syndrome, and diabetes mellitus (3, 4). This trend portends increased morbidity and mortality, as NAFLD has been shown to be independent associated with cardiovascular disease events (5, 6) and all-cause mortality (7-9), even among younger adults (10).
Prior research has compared ethnic differences in fatty liver with lipoprotein classes, abdominal fat, and insulin resistance (11-13). While African Americans have a similar burden of obesity, liver fat content and the prevalence of NAFLD may be lower than in other ethnicities (14). In contrast, patients of Hispanic (15) and Asian (16) ethnicities have a higher liver fat content compared to Whites. Gender differences in liver fat have been less well studied, with The Dallas Heart Study finding that white women had a much lower prevalence of hepatic steatosis compared to white men (1).
Novel accurate radiological methods for liver fat estimation have enabled study in larger populations (17-19). The Multi-Ethnic Study of Atherosclerosis (MESA), a population-based cohort that emphasizes diverse ethnic enrollment, is ideal for this study because all participants received cardiac CT scans with axial slice acquisition extending inferiorly to the liver and spleen. The aim of this study was to describe the ethnic- and gender-specific prevalence of CT-measured fatty liver in a secondary analysis of the MESA cohort, and to delineate the factors associated with fatty liver within each ethnic group.
Methods
Design Overview
The MESA is a population-based study investigating the prevalence, correlates, and progression of subclinical cardiovascular disease. The study design has been previously published (20). Briefly, 6,814 participants aged 45-84 years old representing four ethnicities (White, Chinese, African American, Hispanic) were recruited from six U.S. communities (Forsyth County, North Carolina; Northern Manhattan and Bronx, New York; Baltimore City and Baltimore County, Maryland; St. Paul, Minnesota; Chicago, Illinois; Los Angeles County, California) between July 2000 to August 2002. All participants were free of clinical cardiovascular disease at study enrollment. An approximately equal number of men and women were recruited according to pre-specified age and ethnicity strata. All participants gave informed consent, and the institutional review board at each site approved the study protocol.
Medical history, anthropometric measurements, laboratory testing, and cardiac CT scans were taken during the first examination. Waist circumference at the umbilicus was measured to the nearest 0.1 cm. Height and weight were measured, and body mass index was calculated (kg/m2). Diabetes mellitus was defined as a fasting blood glucose ≥126 mg/dl or the use of hypoglycemic medications. Hypertension was defined as a systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥ 90 mmHg, or the use of anti-hypertensive medications. The metabolic syndrome was defined by the National Cholesterol Education Program Adult Treatment Panel III criteria as 3 or more of the following: waist circumference >102 cm (men) or >88 cm (women), triglycerides ≥150 mg/dl, HDL <40 mg/dl (men) or < 50 mg/dl (women), blood pressure ≥130/85 mm Hg and a fasting blood glucose ≥ 110 mg/dl (21).
Diet was assessed based on a previously published scale quantifying adherence to a Mediterranean diet (22), which has been studied in MESA participants (23). Participants were awarded points for consuming more healthy foods (vegetables, legumes, fruits, nuts, cereal/grains, fish) and fewer detrimental foods (full-fat dairy, meat, poultry, saturated fat) than the median intake. Participants whose diet scores were above the median were defined as having a healthy diet.
Data regarding physical activity were obtained from the MESA Typical Week Physical Activity Survey (24). Participants averaging >150 minutes/week moderate intensity exercise or >75 minutes/week vigorous exercise were considered physically active based on current American Heart Association guidelines (25).
Participants with a history of heavy alcohol use [> 14 drinks/week (men), >7 drinks/week (women)], cirrhosis, or oral steroid or amiodarone use (n=285) were excluded.
Image Acquisition
Two consecutive non-enhanced cardiac CT scans were performed for coronary artery calcium scoring. Participants were scanned using electron beam CT at 3 centers (New York, Chicago, and Los Angeles: Imatron C150 [General Electric Medical Systems, Milwaukee, Wisconsin]), and using four-detector row computed tomography at 3 centers (Lightspeed [General Electric Medical Systems, Milwaukee, Wisconsin] or Volume Zoom [Siemens, Erlangen, Germany], Baltimore, Minneapolis and Winston-Salem, North Carolina centers).
Electron-beam CT used an exposure time of 100 milliseconds, peak voltage of 130 kVp, and tube current of 630 mA. The two multi-detector row CT scanners operated in axial scan mode with a gantry rotation speed of 0.5 seconds. The Lightspeed scanner employed a tube voltage of 120 kVp and tube current of 320 mA, acquiring four 2.5-mm sections simultaneously. The Volume Zoom system used a tube voltage of 140 kVp and tube current of 139 mA, acquiring four 2.5-mm sections per cardiac cycle. Prospective ECG triggering occurred at 50% and 80% of the R-R interval for multi-detector row and electron beam CT respectively. Images were interpreted at a centralized center at the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center. Further details regarding the protocol of scanner parameters have been reported previously (26).
Liver Fat Measurement
Two experienced readers measured the scans independently, blinded to demographic data. Adequate hepatic and splenic imaging were obtained in 6,587 (97%) and 4,396 (65%) individuals respectively.
Hepatic and splenic attenuation values (Hounsfield units [HU]) were measured using regions of interest (ROI) >100 mm2. Two ROIs in the right hepatic lobe and one splenic ROI were measured. Liver/spleen attenuation ratio (L/S ratio) was selected as the most stable measure of liver fat (23), and was calculated as the mean of both hepatic ROIs divided by the splenic ROI. The L/S ratio was available in 4,384 individuals (64%). The diagnosis of fatty liver was defined as an L/S ratio <1.0 (27). Liver fat severity was graded as mild (<1.0, ≥0.7), moderate (<0.7, ≥0.5) and severe (<0.5) based on previous research (28). These L/S ratios correspond to <30%, 30-41%, and >41% macrovesicular hepatic steatosis respectively (29).
Statistical Analysis
After exclusion of patients with a history of heavy alcohol use, cirrhosis, oral steroid or amiodarone use (n=285), the final study sample consisted of 4,088 participants. The Chi-square test was used for categorical variables, while continuous variables were compared using ANOVA or Kruskal-Wallis equality of proportions tests.
Liver fat prevalence and severity were compared among each gender and ethnic group. In order to assess the gender and ethnicity-based associations between obesity and liver fat, we calculated the prevalence of obesity and the prevalence of fatty liver among obese patients in each ethnic group. A similar analysis was performed to evaluate the association between the metabolic syndrome using National Cholesterol Education Program Adult Treatment Panel III criteria (21), and fatty liver.
Multivariable ordinal logistic regression was used to determine the prevalence of liver fat compared to Caucasians, and to assess the specific risk factors which correlated with an increase in liver fat prevalence in different ethnicities. Adjusted variables included age, gender, body mass index (BMI), waist circumference, high density lipoprotein cholesterol (HDL-C), log triglycerides, log high sensitivity C-reactive protein (HS-CRP), log homeostatic model assessment for insulin resistance (HOMA-IR), the presence of diabetes, diabetes treatment, diet and exercise.
In order to identify whether ethnic differences influenced fatty liver prevalence among participants with obesity and metabolic syndrome, ordinal logistic regression was conducted while stratifying patients with and without obesity and metabolic syndrome.
All statistical analyses were performed with STATA version 11 (STATA Corp., College Station, Texas).
Results
Baseline Characteristics
The average age of the population was 62±10 years old, of which 45% were men. Hypertension was more common among African-Americans (Table 1: African-Americans 61%, Whites 40%, Chinese 42%, Hispanics 43%, p<0.001). Hispanics and African-Americans had a significantly higher prevalence of diabetes mellitus, HS-CRP, LDL and HOMA-IR (Table 1). In contrast, African-Americans had higher HDL and lower triglyceride levels compared to other ethnicities.
Table 1. Baseline Characteristics of the Study Population.
| Variable | Caucasian (N=2323) | Chinese (N=791) | African-American (N=1811) | Hispanic (N=1430) | Statistical Significance |
|---|---|---|---|---|---|
| Age in years | 63.5 | 62.8 | 63.0 | 61.8 | P=0.001 |
| Male gender, % | 48.6 | 41.8 | 42.9 | 44.0 | P=0.008 |
| Mean BMI | 28.0 | 24.3 | 30.0 | 29.4 | P<0.001 |
| Waist Circumference (cm) | 98.6 | 88.3 | 101.1 | 100.4 | P<0.001 |
| Obesity (BMI>30) | 28.0 | 4.4 | 45.7 | 38.4 | P<0.001 |
| Diabetes Mellitus,%: Treated Untreated IFG | 4.8 | 8.6 | 13.9 | 14.8 | P<0.001 |
| 2.1 | 3.8 | 3.7 | 3.6 | ||
| 10.9 | 16.9 | 14.3 | 15.4 | ||
| Hypertension, % | 39.6 | 42.1 | 60.8 | 42.6 | P<0.001 |
| Mean SBP | 123.8 | 125.5 | 132.0 | 127.2 | P<0.001 |
| Smoking, % Current Former Never | 9.5 | 5.1 | 15.9 | 11.9 | P<0.001 |
| 43.4 | 15.9 | 37.2 | 31.2 | ||
| 47.2 | 79.0 | 47.0 | 56.9 | ||
| LDL, mg/dL | 116.8 | 114.7 | 117.7 | 119.7 | P=0.03 |
| HDL, mg/dL | 50.8 | 49.9 | 52.1 | 47.7 | P<0.001 |
| Triglycerides, mg/dL | 114.5 (78-166) | 128.0 (86-185) | 89.0 (66.5-12 | 136 (95-192) | P=0.001 |
| hs-CRP, mg/L | 1.7 (0.8-4.1) | 1.0 (0.5-1.9) | 2.5 (1.1-5.9) | 2.5 (1.2-5.2) | P=0.001 |
| HOMA-IR | 1.1 (0.7-1.8) | 1.2 (0.8-1.9) | 1.3 (0.8-2.2) | 1.5 (0.9-2.6) | P=0.001 |
| Metabolic Syndrome, % | 30.9 | 25.3 | 33.3 | 42.5 | P<0.001 |
| Anti-hypertensive medication, % | 34.9 | 30.0 | 52.2 | 32.7 | P<0.001 |
| Lipid lowering medication, % | 18.4 | 14.9 | 16.5 | 13.6 | P=0.01 |
| Healthy diet* | 1319 (53.4) | 249 (31.5) | 789 (48.9) | 467 (34.4) | P<0.001 |
| Regular exercise** | 606 (23.2) | 101 (12.6) | 312 (16.6) | 185 (12.4) | P<0.001 |
Values are expressed either as %, mean, or median (interquartile range). BMI= body mass index; SBP=systolic blood pressure; LDL=low density lipoprotein; HDL=high density lipoprotein; hs-CRP=high sensitivity C-reactive protein; HOMA-IR= homeostatic model assessment of insulin resistance, IFG= impaired fasting glucose.
Defined as scoring above the median on total Mediterranean diet score, calculated out of 6236 participants for whom data were available.
Defined as >150 hours/week moderate physical activity or >75 hours/week vigorous physical activity, calculated out of 6795 participants for whom data were available.
African-Americans and Hispanics were more obese, and Chinese less obese, when compared to Whites (BMI/waist circumference [cm]: White 28/99; Chinese 24/88; African-American 30/101; Hispanic 29/100; p<0.001 for BMI and waist circumference). Correspondingly, the prevalence of obesity was higher among African-Americans and Hispanics, and significantly lower among Chinese participants (p<0.001). Hispanics had the highest percentage of patients with the metabolic syndrome (p<0.001).
Prevalence of Liver Fat by Gender and Ethnicity
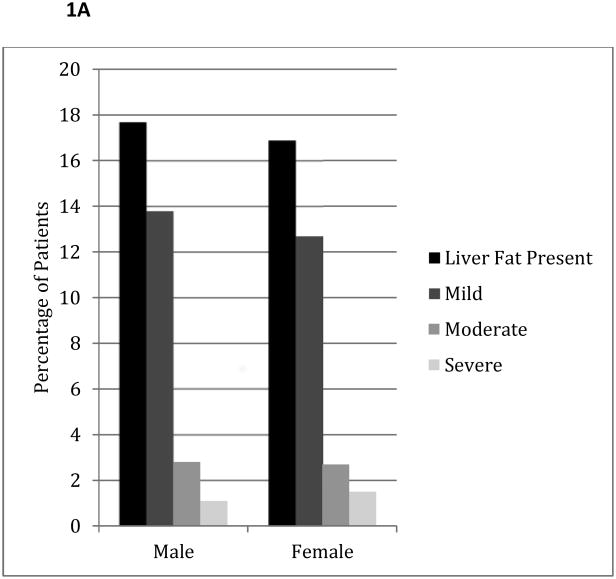
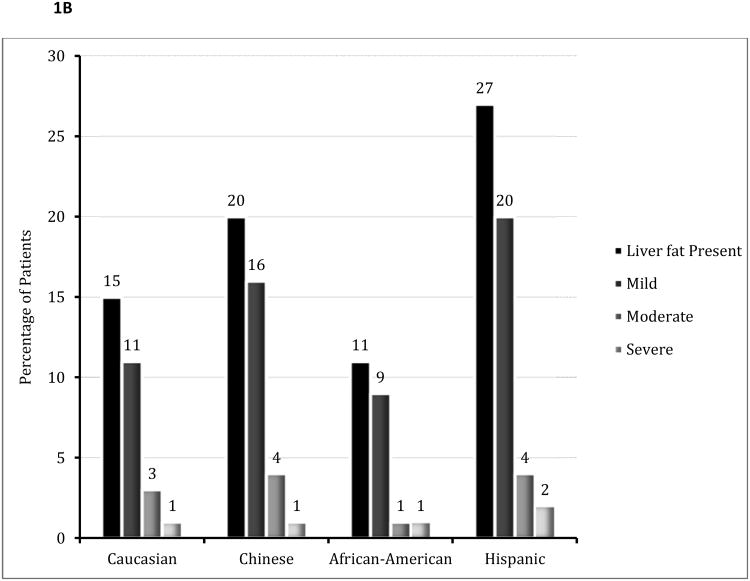
The overall prevalence of fatty liver was 17% (706/4088). There was no statistical difference in the presence of liver fat by gender (Figure 1, p=0.54). African-Americans had a significantly lower prevalence of fatty liver, and Hispanics a higher prevalence and severity of fatty liver compared to other ethnicities (p<0.001 for both prevalence and severity of fatty liver).
Figure 1.
A: Prevalence and Severity of Liver Fat by Gender
P=0.54 and P=0.48 for the presence and severity of liver fat by gender respectively.
B: Prevalence and Severity of Liver Fat by Ethnicity
P<0.001 for the presence and severity of liver fat by ethnicity.
When compared to Whites, the odds ratio for the prevalence of fatty liver was significantly lower in African-Americans, but higher in Chinese and Hispanic participants (Table 2). Multi-variable adjustment for demographic and cardiovascular risk factors did not attenuate the observed inter-ethnic differences.
Table 2. Prevalence Odds Ratio of Liver Fat Among Different Ethnicities Compared to Caucasians.
| Ethnicity | Prevalence Odds Ratio- Unadjusted | Prevalence Odds Ratio- Age/Gender Adjusted | Prevalence Odds Ratio- Multi-variable Adjusteda |
|---|---|---|---|
| Caucasian | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Chinese | 1.39 (1.05-1.84) | 1.38 (1.04-1.82) | 2.05 (1.50-2.81) |
| African-American | 0.69 (0.55-0.87) | 0.69 (0.55-0.86) | 0.58 (0.44-0.76) |
| Hispanic | 2.06 (1.68-2.51) | 2.01 (1.64-2.45) | 1.56 (1.24-1.96) |
Values are expressed as prevalence odds ratio (95% confidence interval).
Multi-variable adjustment includes: age, gender, body mass index, waist circumference, HDL, log CRP, log triglycerides, log HOMA, diabetes history and diabetes treatment, diet and physical activity.
Risk Factors Associated with Fatty Liver
Table 3 shows the relationship between a change of one standard deviation of several metabolic risk factors and the prevalence of fatty liver. With age and gender adjustment, the risk of fatty liver decreased with one standard deviation increase in HDL, and increased with one standard deviation increase in triglycerides for all ethnicities.
Table 3. Prevalence Odds Ratios of Fatty Liver Per Standard Deviation Change in Metabolic Variables in Different Ethnicities.
| Ethnicity | Metabolic Variable | Age/Gender Adjusted Prevalence Odds Ratio | Multi-variable Adjusted Prevalence Odds Ratio |
|---|---|---|---|
| Caucasian | HDL | 0.47 (0.37-0.60) | 0.86 (0.65-1.13) |
| Log Triglycerides | 1.85 (1.61-2.14) | 1.17 (0.97-1.40) | |
| Log HS-CRP | 1.42 (1.30-1.55) | 1.20 (1.09-1.33) | |
| BMI | 2.20 (1.88-2.58) | -- | |
| Waist circumference | 2.37 (2.02-2.77) | 1.39 (1.14-1.68) | |
| Log HOMA-IR | 3.07 (2.58-3.66) | 2.28 (1.80-2.89) | |
| Diabetes Mellitus | 2.88 (1.87-4.44) | 1.02 (0.49-2.14) | |
| Diabetes treatment | 2.16 (1.26-3.69) | 0.54 (0.21-1.41) | |
| Chinese | HDL | 0.50 (0.35-0.72) | 1.15 (0.79-1.67) |
| Log Triglycerides | 2.20 (1.71-2.82) | 1.65 (1.16-2.34) | |
| Log HS-CRP | 1.31 (1.12-1.54) | 1.01 (0.85-1.22) | |
| BMI | 3.24 (2.24-4.70) | -- | |
| Waist circumference | 3.10 (2.25-4.25) | 1.86 (1.19-2.91) | |
| Log HOMA-IR | 3.57 (2.48-5.15) | 2.51 (1.67-3.77) | |
| Diabetes Mellitus | 2.90 (1.42-5.94) | 1.49 (0.46-4.81) | |
| Diabetes treatment | 2.59 (1.13-5.93) | 1.06 (0.23-4.91) | |
| African-American | HDL | 0.58 (0.45-0.74) | 0.89 (0.67-1.20) |
| Log Triglycerides | 1.72 (1.41-2.12) | 1.37 (1.05-1.78) | |
| Log HS-CRP | 1.21 (1.09-1.33) | 1.08 (0.96-1.22) | |
| BMI | 1.63 (1.41-1.89) | -- | |
| Waist circumference | 1.77 (1.51-2.08) | 1.21 (0.99-1.49) | |
| Log HOMA-IR | 2.56 (2.06-3.18) | 2.06 (1.60-2.66) | |
| Diabetes Mellitus | 2.71 (1.82-4.04) | 1.70 (0.77-3.76) | |
| Diabetes treatment | 1.99 (1.27-3.13) | 0.53 (0.23-1.21) | |
| Hispanic | HDL | 0.53 (0.43-0.64) | 0.83 (0.66-1.04) |
| Log Triglycerides | 1.71 (1.48-1.98) | 1.34 (1.12-1.61) | |
| Log HS-CRP | 1.30 (1.19-1.41) | 1.16 (1.06-1.28) | |
| BMI | 1.87 (1.61-2.18) | -- | |
| Waist circumference | 1.87 (1.60-2.19) | 1.25 (1.03-1.51) | |
| Log HOMA-IR | 2.45 (2.04-2.94) | 2.11 (1.70-2.61) | |
| Diabetes Mellitus | 1.88 (1.34-2.63) | 1.04 (0.52-2.09) | |
| Diabetes treatment | 1.45 (0.99-2.10) | 0.51 (0.24-1.11) |
HDL= high density lipoprotein; HS-CRP= high sensitivity C-reactive protein; BMI= body mass index; HOMA-IR= homeostatic model assessment- insulin resistance. BMI excluded from multi-variable adjusted risk ratio due to collinearity with waist circumference. Multi-variable adjustment includes HDL, log triglycerides, log HS-CRP, log HOMA, waist circumference, presence of diabetes and treatment of diabetes.
After multi-variable adjustment, an increase in HDL was no longer inversely correlated with a decreased prevalence odds ratio of fatty liver. An increase in triglycerides correlated with an increased odds ratio of having fatty liver in all ethnicities except Whites. An increase in HS-CRP was associated with an increased prevalence odds ratio for fatty liver in Whites and Hispanics, but was not statistically significant in the Chinese and African-American groups, while diabetes lost statistical significance in all ethnic subgroups with multi-variable adjustment.
An increase in insulin resistance predicted an approximately two-fold increased prevalence of fatty liver in all ethnicities. Waist circumference remained predictive of an increased prevalence odds ratio of fatty liver in all ethnicities, except African-Americans where it achieved only borderline statistical significance (Table 3).
Ethnic and Gender Differences Across the Spectrum of Obesity/Metabolic Syndrome
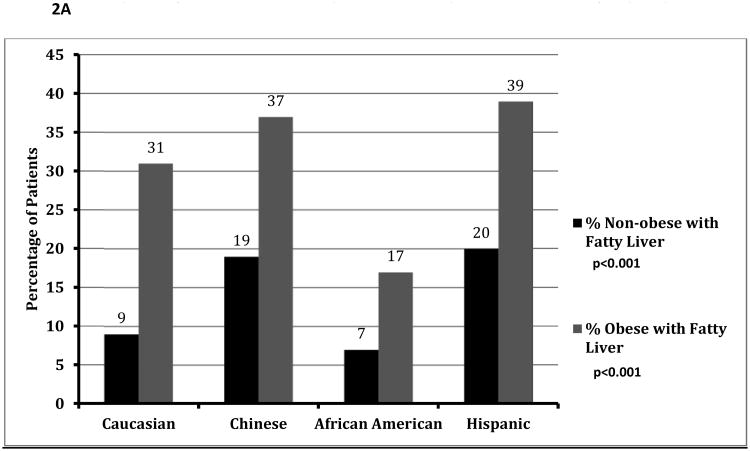
The prevalence of fatty liver among obese participants was 27%, compared with 12% of non-obese participants (p<0.001). This approximately two-fold increased prevalence of fatty liver among obese participants was a consistent finding across all ethnicities (Figure 2A).
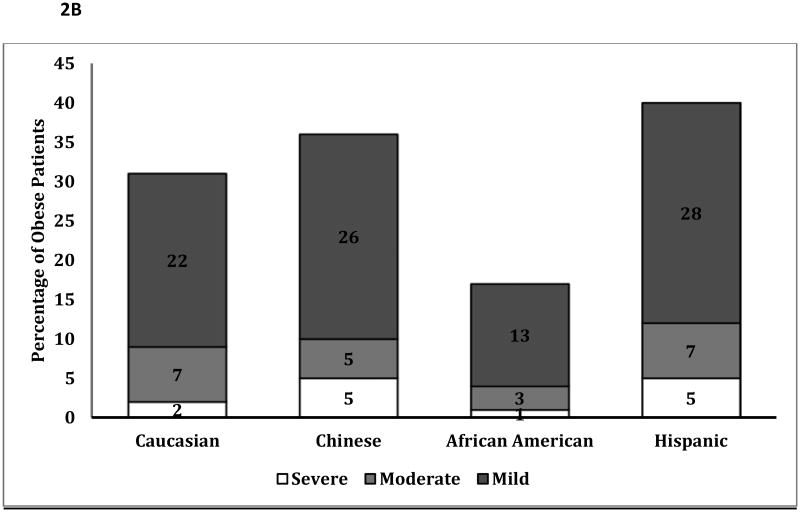
Figure 2.
A: Prevalence of Fatty Liver Among Obese and Non-Obese Patients, Stratified by Ethnicity
B: Percentage of Obese Patients with Mild, Moderate and Severe Fatty Liver
p<0.001 for differences in the severity of fatty liver among obese patients by ethnicity
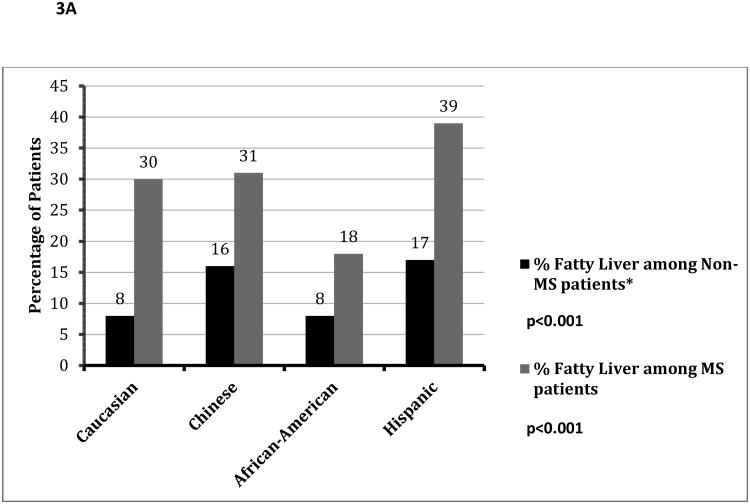
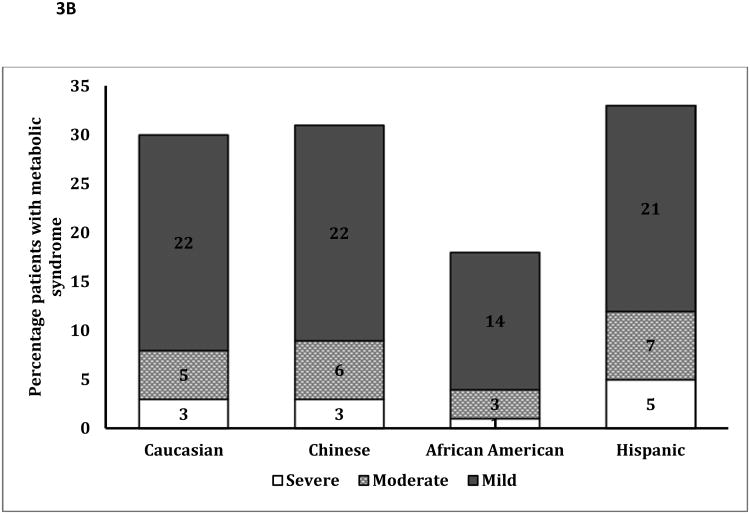
Although African-Americans had a higher prevalence of obesity, a much smaller percentage of obese African Americans were diagnosed with fatty liver compared to other ethnicities (Figure 2A: p<0.001). Obese African-Americans also had a significantly lower severity of fatty liver compared to other ethnicities (Figure 2B, p<0.001). Similar results were noted among those with and without metabolic syndrome (figure 3A/3B), with a much lower proportion of African Americans with the metabolic syndrome diagnosed with fatty liver compared to other ethnicities. There was no significant gender disparity in the proportion of obese participants diagnosed with fatty liver (p=0.16, data not shown).
Figure 3.
A: Prevalence of Fatty Liver Among Patients with and without the Metabolic Syndrome, Stratified by Ethnicity
*MS= Metabolic Syndrome.
B: Percentage of Patients with the Metabolic Syndrome With Mild, Moderate and Severe Fatty Liver
p<0.001 for differences in the severity of fatty liver in patients with the metabolic syndrome by ethnicity
African-Americans had a significantly lower likelihood of having fatty liver compared to Whites, independent of the presence of obesity or the metabolic syndrome (Table 4). When compared to obese Whites, obese Chinese and Hispanic patients had a similar prevalence odds ratio of fatty liver. Among non-obese patients however, Chinese and Hispanic patients were significantly more likely to have fatty liver than Whites. A similar trend was noted for patients with the metabolic syndrome.
Table 4. Prevalence Odds Ratio of Liver Fat Among Different Ethnicities Compared to Caucasians Across Spectrum of Obesity and Metabolic Syndrome.
| Ethnicity | Non-Obese | Obese | aMS (-) | aMS (+) |
|---|---|---|---|---|
| Caucasian | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Chinese | 2.44 (1.67-3.56) | 1.05 (0.35-3.19) | 2.58 (1.68-3.95) | 1.34 (0.83-2.17) |
| African-American | 0.60 (0.40-0.91) | 0.54 (0.38-0.76) | 0.59 (0.39-0.88) | 0.51 (0.36-0.73) |
| Hispanic | 1.61 (1.16-2.23) | 1.20 (0.88-1.65) | 1.48 (1.04-2.09) | 1.29 (0.95-1.74) |
Values are expressed as prevalence odds ratio (95% confidence interval). Adjusted for age, gender, BMI, waist circumference, log HS-CRP, log triglycerides, log HOMA, HDL, diabetes, and diabetes treatment.
indicates Metabolic Syndrome.
Discussion
In our study, a much lower prevalence of fatty liver was noted among African-Americans, a finding which persisted even after multivariable adjustment. This ethnic disparity persisted even among African-Americans with obesity and the metabolic syndrome. In contrast, Hispanic participants had a higher prevalence and severity of liver fat compared to other ethnicities.
The finding of a lower prevalence of NAFLD among African-Americans has been previously described (1, 12, 13). The Dallas Heart Study (1) noted a lower incidence of hepatic triglyceride content despite increasing total body fat among African-Americans compared to other ethnicities. In our study, MESA has highlighted this finding in a larger population specifically designed to compare ethnic differences in cardiovascular risk.
We have added to the findings of the Dallas Heart Study by comparing the Asian sub-population to Whites, African-Americans and Hispanics. Consistent with Azuma et al (16), we noted two-fold increased odds of fatty liver among Asians compared to Whites (table 2).
Hispanic Americans demonstrated the highest prevalence of fatty liver among all ethnic groups studied. In the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) study (30), increased caloric intake and less physical activity compared to Whites partially mediated this disparity. In contrast, we noted an increased prevalence odds ratio of fatty liver among Hispanics compared to Caucasians, even after adjusting for dietary and lifestyle markers. This suggests that a genetic susceptibility, perhaps mediated through insulin resistance and abdominal obesity (Table 3), may partially account for the increased liver fat prevalence among Hispanics.
Metabolic Risk Factors and Ethnic Differences in Fatty Liver
An increase in HDL was not significantly associated with a lower prevalence of fatty liver after multi-variable adjustment (Table 3). The Jackson Heart study (31) demonstrated a similar trend, with HDL becoming a less potent predictor of fatty liver after adjustment for visceral adipose tissue (P<0.001 before adjustment for adiposity, P=0.01 after adjustment). Conversely, an increase in triglycerides remained significantly associated with an increased prevalence of fatty liver after multi-variable adjustment. This is not surprising, as the pathogenesis of fatty liver is characterized by an imbalance between the rate of hepatic triglyceride synthesis and removal (32).
Increasing insulin resistance predicted a two-fold increased prevalence of fatty liver in all ethnicities studied, highlighting the fundamental role of insulin resistance in the pathogenesis of fatty liver. Insulin resistance leads to an increased availability of glucose and free fatty acids, thereby promoting intrahepatic triglyceride synthesis and liver fat accumulation (33).
We noted higher triglyceride and lower HDL levels in Chinese participants compared to Whites. Consistent with other studies comparing NAFLD among Asians and Whites (16, 34), we demonstrated that hypertriglyceridemia was significantly associated with an increased prevalence of fatty liver, despite a lower mean waist circumference among Chinese patients. These findings complement the most recent consensus definition of the metabolic syndrome (35), which recognizes the need for ethnic-specific waist circumference criteria.
Genetic Differences in Fatty Liver
Obese African-Americans or those with the metabolic syndrome maintained a lower prevalence and severity of liver fat compared to other ethnicities, despite higher indices of obesity compared to other ethnic groups (Figures 2B, 3B). Moreover, while obese Hispanic and Chinese patients demonstrated a similar prevalence odds ratio of fatty liver compared to Whites, African-Americans were significantly less likely than Whites to be diagnosed with fatty liver independent of obesity (Table 4).
Numerous genes involved in hepatic fat metabolism and inflammation have been implicated in fatty liver pathogenesis (36-38). Genes impairing phosphatidylcholine production decrease hepatic VLDL production leading to fatty liver. Specific alleles of genes involved in choline metabolism are clustered in the African-American population, potentially explaining the ethnic differences in fatty liver (39).
African Americans have been found to be insulin resistant and obese at lower triglyceride levels than Whites or Hispanics, prompting some researchers to suggest ethnic-specific cutoffs for hypertriglyceridemia (40-41). Our analysis mirrored this trend (Table 1), with the additional finding that lower triglyceride levels among African Americans correlated with a lower prevalence of liver fat.
Visceral obesity has been demonstrated to be a better predictor of metabolic syndrome and fatty liver than abdominal obesity (42). Furthermore, Despres et al demonstrated that African-Americans have less visceral adiposity for a given level of abdominal obesity compared to Caucasians (43). This finding may therefore partly explain why African-Americans, who had the highest mean BMI of all the ethnicities studied, had the lowest prevalence of fatty liver.
Liver Fat Imaging by Coronary CT
The similarity between our findings and previous liver fat studies suggests that coronary CT is a useful tool for liver fat assessment (1, 15, 16). One study demonstrated that liver fat diagnosed by coronary CT correlated with the presence of lipid-rich coronary artery plaques (44). Indeed, subclinical inflammation may be a critical shared pathophysiological pathway linking fatty liver and atherosclerosis (45).
While coronary CT correlates well with MRI measures of liver fat (17), both MRI and magnetic resonance spectroscopy are considered superior to ultrasound and CT in quantifying liver fat (46). The sensitivity of ultrasound is decreased in patients with mild fatty liver or morbidly obesity (47). It is also operator-dependent (48) and cannot accurately quantify the severity of hepatic steatosis.
Speliotes et al studied 100 participants who underwent CT scans for measurement of fatty liver (49), and found CT measures of liver fat to be highly reproducible. Coronary CT therefore provides an accurate assessment of liver fat that may incrementally improve cardiovascular risk-stratification.
Limitations
Our study's definition of excessive alcohol consumption (> 14 drinks/week for men, >7 drinks/week for women) is within the currently American College of Gastroenterology guidelines for diagnosis of NAFLD (50). However, inconsistencies in patient reporting, and the possibility of alcoholic liver disease at lower levels of alcohol consumption are possible limitations.
While coronary CT has compared favorably with other imaging modalities, histological assessment and magnetic resonance spectroscopy are the most accurate methods for diagnosing fatty liver. In spite of this, coronary CT has become an increasingly popular non-invasive mode of diagnosing and estimating the severity of non-alcoholic fatty liver disease.
Visceral obesity was not assessed in this study, but has been shown in some studies to be a better predictor of fatty liver than measures of abdominal obesity.
Conclusion
In this secondary analysis of data from the MESA, African-Americans have a much lower prevalence, and Hispanics a much higher prevalence of fatty liver than other ethnicities. There are distinct ethnic variations in the prevalence of NAFLD even amongst patients with the metabolic syndrome or obesity, suggesting that genetic factors play a significant role in the phenotypic expression of NAFLD. Coronary CT offers a reliable estimate of the burden of nonalcoholic fatty liver disease, as evidenced by the similarity between our findings and other studies.
Supplementary Material
Acknowledgments
This research was supported by R01 HL071739 and contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute.
List of Abbreviations
- MESA
Multi-Ethnic Study of Atherosclerosis
- NAFLD
Non-alcoholic fatty liver disease
- L/S ratio
Liver/Spleen attenuation ratio
- BMI
body mass index
- HDL-C
high density lipoprotein cholesterol
- HS-CRP
high sensitivity C-reactive protein
- HOMA-IR
homeostatic model assessment for insulin resistance
Footnotes
Conflict of Interest and Financial-Disclosures: Dr Matthew Budoff is on the speakers' bureau for General Electric. All other authors report no relevant financial disclosure.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loomba R, Sanyal AJ. The Global NAFLD Epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 2.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 3.Fraser A, Harris R, Sattar N, Ebrahim S, Davey SG, Lawlor DA. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: the British Women's Heart and Health Study and meta-analysis. Diabetes Care. 2009;32(4):741–750. doi: 10.2337/dc08-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamaguchi M, Kojima T, Takeda N, et al. The Metabolic Syndrome as a Predictor of Nonalcoholic Fatty Liver Disease. Ann Intern Med. 2005;143(10):722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 5.Chhabra R, O'Keefe JH, Patil H, et al. Association of coronary artery calcification with hepatic steatosis in asymptomatic individuals. Mayo Clin Proc. 2013;88(11):1259–65. doi: 10.1016/j.mayocp.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Targher G, Day CP, Bonora E. Risk of Cardiovascular Disease in Patients with Nonalcoholic Fatty Liver Disease. N Engl J Med. 2010;363(14):1341–50. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 7.Haring R, Wallaschofski H, Nauck M, Dorr M, Baumeister SE, Volzke H. Ultrasonographic Hepatic Steatosis Increases Prediction of Mortality Risk From Elevated Serum Gamma-Glutamyl Transpeptidase Levels. Hepatology. 2009;50(5):1403–1411. doi: 10.1002/hep.23135. [DOI] [PubMed] [Google Scholar]
- 8.Targher G, Bertolini L, Poli F, et al. Nonalcoholic Fatty Liver Disease and Risk of Future Cardiovascular Events Among Type 2 Diabetic Patients. Diabetes. 2005;54(12):3541–3546. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 9.Targher G, Bertolini L, Rodella S, et al. Nonalcoholic Fatty Liver Disease Is Independently Associated With an Increased Incidence of Cardiovascular Events in Type 2 Diabetic Patients. Diabetes Care. 2007;30(8):2119–2121. doi: 10.2337/dc07-0349. [DOI] [PubMed] [Google Scholar]
- 10.Dunn W, Xu R, Wingard DL, et al. Suspected Nonalcoholic Fatty Liver Disease and Mortality Risk in a Population-Based Cohort Study. Am J Gastroenterol. 2008;103(9):2263–2271. doi: 10.1111/j.1572-0241.2008.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Adamo E, Northrup V, Weiss R, et al. Ethnic differences in lipoprotein subclasses in obese adolescents: importance of liver and intraabdominal fat accretion. Am J Clin Nutr. 2010;93(3):500–508. doi: 10.3945/ajcn.2010.29270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liska D, Dufour S, Zern TL, et al. Interethnic Differences in Muscle, Liver and Abdominal Fat Partitioning in Obese Adolescents. PLoS ONE. 2007;2(6):e569. doi: 10.1371/journal.pone.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic Differences in Hepatic Steatosis: An Insulin Resistance Paradox? Hepatology. 2009;49(3):791–801. doi: 10.1002/hep.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caldwell SH, Harris DM, Patrie JT, Hespenheide EE. Is NASH Underdiagnosed Among African Americans? Am J Gastroenterol. 2002;97(6):1496–1500. doi: 10.1111/j.1572-0241.2002.05795.x. [DOI] [PubMed] [Google Scholar]
- 15.Browning JD, Kumar KS, Saboorian MH, Thiele DL. Ethnic Differences in the Prevalence of Cryptogenic Cirrhosis. Am J Gastroenterol. 2004;99(2):292–298. doi: 10.1111/j.1572-0241.2004.04059.x. [DOI] [PubMed] [Google Scholar]
- 16.Azuma K, Kadowaki T, Cetinel C, et al. for the ERA JUMP study group. Higher liver fat content among Japanese in Japan compared with non-Hispanic whites in the United States. Metabolism. 2009;58(8):1200–1207. doi: 10.1016/j.metabol.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong L, Chen JJ, Chen J, et al. Nonalcoholic fatty liver disease: Quantitative assessment of liver fat content by computed tomography, magnetic resonance imaging and proton magnetic resonance spectroscopy. J Dig Dis. 2009;10(4):315–320. doi: 10.1111/j.1751-2980.2009.00402.x. [DOI] [PubMed] [Google Scholar]
- 18.Van Werven JR, Marsman HA, Nederveen AJ, Smits NJ, et al. Assessment of Hepatic Steatosis in Patients Undergoing Liver Resection: Comparison of US, CT, T1-weighted Dual-Echo MR Imaging, and Point-resolved 1 H MR Spectroscopy. Radiology. 2010;256(1):159–168. doi: 10.1148/radiol.10091790. [DOI] [PubMed] [Google Scholar]
- 19.Matulevicius S, Huff LC, Szczepaniak LS, et al. Potential of Electron Beam Computed Tomography for Coronary Artery Calcium Screening to Evaluate Fatty Liver: Comparison With 1H Magnetic Resonance Spectroscopy in the Dallas Heart Study. J Investig Med. 2011;59(5):780–786. doi: 10.2310/JIM.0b013e318216ad1d. [DOI] [PubMed] [Google Scholar]
- 20.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 21.Grundy SM, Becker D, Clark LT, et al. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 22.Trichopoulou A, Costacou T, Bamia C, et al. Adherence to a Mediterranean diet and survival in a Greek population. New Engl J Med. 2003;348(26):2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed H, Blaha MJ, Nasir K, et al. Low-Risk Lifestyle, Coronary Calcium, Cardiovascular, Events, Mortality: Results From MESA. Am J Epidemiol. 2013 Jul 1;178(1):12–21. doi: 10.1093/aje/kws453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertoni AG, Whitt-Glover MC, Chung H, et al. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169(4):444–454. doi: 10.1093/aje/kwn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology American/Heart Association Task Force on Practice Guidelines. Circulation. 2013;00:000–000. doi: 10.1161/01.cir.0000437740.48606.d1. published online ahead of print. [DOI] [Google Scholar]
- 26.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 27.Zeb I, Li D, Nasir K, Katz R, Larijani VN, Budoff MJ. Computed Tomography Scans in the Evaluation of Fatty Liver Disease in a Population Based Study: The Multi-Ethnic Study of Atherosclerosis. Acad Radiol. 2012;19(7):811–8. doi: 10.1016/j.acra.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng MD, Fan JG, Lu LG, et al. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis. 2008;9(2):108–112. doi: 10.1111/j.1751-2980.2008.00331.x. [DOI] [PubMed] [Google Scholar]
- 29.Park SH, Kim PN, Kim KW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239(1):105–12. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 30.Bambha K, Belt P, Abraham M, et al. for the NASH CRN Research Group. Ethnicity and nonalcoholic fatty liver disease. Hepatology. 2012;55(3):769–80. doi: 10.1002/hep.24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Fox CS, Hickson D, Bidulescu A, Carr JJ, Taylor HA. Fatty liver, abdominal visceral fat, and cardiometabolic risk factors: the Jackson Heart Study. Arterioscler Thromb Vasc Biol. 2011;31(11):2715–22. doi: 10.1161/ATVBAHA.111.234062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maurantonio M, Ballestri S, Odoardi MR, Lonardo A, Loria P. Treatment of Atherogenic Liver Based on the Pathogenesis of Nonalcoholic Fatty Liver Disease: A Novel Approach to Reduce Cardiovascular Risk? Arch Med Res. 2011;42:337–353. doi: 10.1016/j.arcmed.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Kopec KL, Burns D. Nonalcoholic Fatty Liver Disease: A Review of the Spectrum of Disease, Diagnosis, and Therapy. Nutr Clin Pract. 2011;26(5):565–576. doi: 10.1177/0884533611419668. [DOI] [PubMed] [Google Scholar]
- 34.Wulana SN, Westerterpa KR, Plasquia G. Ethnic differences in body composition and the associated metabolic profile: A comparative study between Asians and Caucasians. Maturitas. 2010;65(4):315–319. doi: 10.1016/j.maturitas.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 36.Westerbacka J, Kolak M, Kiviluoto T, et al. Genes Involved in Fatty Acid Partitioning and Binding, Lipolysis, Monocyte/Macrophage Recruitment, and Inflammation Are Overexpressed in the Human Fatty Liver of Insulin-Resistant Subjects. Diabetes. 2007;56(11):2759–2765. doi: 10.2337/db07-0156. [DOI] [PubMed] [Google Scholar]
- 37.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotronen A, Johansson LE, Johansson LM, et al. A common variant in PNPLA3, which encodes adiponutrin, is associated with liver fat content in humans. Diabetologia. 2009;52(6):1056–1060. doi: 10.1007/s00125-009-1285-z. [DOI] [PubMed] [Google Scholar]
- 39.Corbin KD, Abdelmalek MF, Spencer MD, et al. Genetic signatures in choline and 1-carbon metabolism are associated with the severity of hepatic steatosis. FASEB J. 2013;27(4):1674–89. doi: 10.1096/fj.12-219097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196(2):696–703. doi: 10.1016/j.atherosclerosis.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 41.Lin SX, Carnethon M, Szklo M, Bertoni A. Racial/Ethnic Differences in the Association of Triglycerides with Other Metabolic Syndrome Components: The Multi-Ethnic Study of Atherosclerosis. Metab Syndr Relat Disord. 2011;9(1):35–40. doi: 10.1089/met.2010.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silveira LS, Monteiro PA, Antunes Bde M, et al. Intra-abdominal fat is related to metabolic syndrome and non-alcoholic fat liver disease in obese youth. BMC Pediatr. 2013;13:115. doi: 10.1186/1471-2431-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Després JP, Couillard C, Gagnon J, et al. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol. 2000;20(8):1932–8. doi: 10.1161/01.atv.20.8.1932. [DOI] [PubMed] [Google Scholar]
- 44.Akabame S, Hamaguchi M, Tomiyasu K, et al. Evaluation of vulnerable coronary plaques and non-alcoholic fatty liver disease (NAFLD) by 64-detector multislice computed tomography (MSCT) Circ J. 2008;72(4):618–25. doi: 10.1253/circj.72.618. [DOI] [PubMed] [Google Scholar]
- 45.Bieghs V, Rensen PC, Hofker MH, Shiri-Sverdlov R. NASH and Atherosclerosis are Two Aspects of a Shared Disease: Central Role for Macrophages. Atherosclerosis. 2012;220(2):287–93. doi: 10.1016/j.atherosclerosis.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 46.Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21(1):87–97. doi: 10.1007/s00330-010-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mottin CC, Moretto M, Padoin AV, et al. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes Surg. 2004;14(5):635–637. doi: 10.1381/096089204323093408. [DOI] [PubMed] [Google Scholar]
- 48.Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am J Roentgenol. 2007;189(6):W320–3. doi: 10.2214/AJR.07.2123. [DOI] [PubMed] [Google Scholar]
- 49.Speliotes EK, Massaro JM, Hoffmann U, et al. Liver fat is reproducibly measured using computed tomography in the Framingham Heart Study. J Gastoenterol Hepatol. 2008;23(6):894–9. doi: 10.1111/j.1440-1746.2008.05420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chalasani N, Younossi Z, Lavine JE, et al. Management of Non-Alcoholic Fatty Liver Disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.