Abstract
Antibody-mediated rejection (AMR) is an uncommon, but challenging type of rejection after solid organ transplantation. We review three cases of AMR in ABO- compatible liver transplant recipients. These cases were characterized by severe acute rejection resistant to steroids and antithymocyte globulin, histologic evidence of plasma cell infiltrates, C4d positivity and high serum anti-HLA donor-specific antibodies. All three patients were treated with bortezomib, a proteasome inhibitor effective in depleting plasma cells. After treatment, all patients had improved or normal liver function tests, resolution of C4d deposition and significant decline in their HLA donor-specific antibodies.
Keywords: Antibody-mediated rejection, bortezomib, crossmatch, donor-specific antibodies, HLA compatibility, liver transplantation
Introduction
Antibody-mediated rejection (AMR) is a well-established diagnosis in all solid organ transplants except liver transplant. The definition of acute AMR in renal transplantation according to the 2003 National Institutes of Health conference is acute rejection with graft dysfunction, histological evidence of acute tissue injury and C4d deposition in the presence of human leukocyte antigen (HLA) donor-specific antibodies (DSA; 1). Despite established diagnostic criteria, AMR remains a challenging complication of solid organ transplantation. Although not as common as cellular- mediated rejection, AMR is frequently treatment resistant and results in graft loss (1–4), leaving room for improvement in diagnosis and treatment options.
There has been a recent resurgence of interest in AMR in liver transplantation (5–7). The lack of interest in AMR in liver transplantation is like due to the fact that AMR in liver transplantation is uncommon and most cases have been reported in ABO-incompatible recipients (8,9). In addition, there has been a lack of consensus on the histologic and serologic criteria for the diagnosis of AMR. The occurrence of AMR in ABO-compatible liver transplants has been questioned because many believe that the liver absorbs and eliminates DSA (10,11). The ability to perform a liver transplant without hyperacute rejection despite a positive crossmatch contributes to the belief that liver a lografts are “resistant” to DSA (12). However, in the last decades, a few cases of acute AMR after ABO-compatible liver transplant have been reported (2–4,13). Furthermore, chronic rejection in liver transplantation has been associated with DSA, as it is in other solid organ transplants (7).
Treatment of AMR has been extensively studied in renal allograft recipients. Bortezomib, a proteasome inhibitor that depletes plasma cells, has recently been successful in treating AMR in kidney transplant patients (14–16). We report three cases of acute AMR in ABO-compatible liver transplant recipients treated with bortezomib.
Methods
We retrospectively evaluated all ABO-compatible liver transplant recipients with suspected acute AMR at the Baylor Simmons Transplant Institute from January 2009 to December 2011. Because there is no universally accepted definition of acute AMR in liver transplantation, this was based on the presence of graft dysfunction, pathologic evidence of acute rejection refractory to steroids and antibody treatment (Thymoglobulin or OKT-3), increased number of plasma cells in the portal infiltrate, positive C4d staining and presence of HLA DSA. All patients were subsequently treated with bortezomib (Velcade; Millenium Pharmaceuticals, Inc., Cambridge, MA, USA). Clinical findings, laboratory results, DSA mean fluorescence intensity (MFI), liver biopsies, treatments and outcomes were reviewed for each patient. Acute cellular rejection (ACR) was graded according to the Banff criteria (grade I, II, III) and the Rejection Activity Index (RAI; 17).
Patients underwent liver biopsy before and after treatment with steroids, antithymocyte globulin and bortezomib. Each liver biopsy was reviewed by two expert hepatopathologists. C4d staining was performed with rabbit antihuman C4d polyclonal antibody (BIOMEDICA Gruppe, Vienna, Austria) on formalin-fixed samples because fresh tissue was not available. C4d deposition was classified based on location (e.g. venules, portal connective tissue), extension (focal, diffuse) and intensity (from 1+ to 3+). If C4d staining was present on the vascular elastic lamina of arteries, this was considered non-specific and classified as negative (18,19). HLA DSA testing was performed on recipient serum samples before and after treatment with bortezomib. DSAs were detected with single antigen HLA class I and II beads produced by One Lambda (Canoga Park, CA, USA). Donor-recipient mismatched HLAs were compared to the recipient antibody profile to identify DSA specificities. All antibody levels were measured in MFI and trimmed mean values were considered positive if >1000 (5, 7).
Results
During the study period 370 adult patients underwent liver transplantation at Baylor Simmons Transplant Institute. Three patients developed acute rejection resistant to steroids, antithymocyte globulin and met all the diagnostic criteria for acute AMR. All three patients were treated with bortezomib. As shown in Table 1, after treatment in all patients, liver function tests (LFTs) improved, C4d stains became negative and DSA MFI declined. The details of each patient are described later. A summary chart with histologic and hematologic data for all patients is shown in Table 2.
Table 1.
Liver function tests, C4d immunostain and donor-specific antibody MFI before and after treatment with bortezomib
| Before bortezomib | After bortezomib | |||||||
|---|---|---|---|---|---|---|---|---|
| Patient | AST (U/L) |
GGT (U/L) |
C4d stain |
Mean DSA (MFI) |
AST (U/L) |
GGT (U/L) |
C4d stain |
Mean DSA (MFI) |
| 1 | 87 | 1418 | + | 7089 | 10 | 102 | − | 282 |
| 2 | 82 | 784 | + | 4268 | 27 | 242 | − | 864 |
| 3 | 170 | 1477 | + | 2886 | 100 | 646 | − | 2409 |
AST = aspartate aminotransferase; GGT = gamma glutamyl transferase; DSA = donor-specific antibody; MFI = mean fluorescence intensity.
Table 2.
Summary chart of histologic and hematologic data for each patients
| Patient (age, gender) |
Time (PTD) |
C4d intensity |
C4d site |
CD 138 intensity |
Banff score |
Hgb (g/dL) |
WBC (1000/lL) |
Plt (1000/lL) |
Treatment |
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 (62 year, F) |
8 15 |
+11 +11 |
Portal venules1 Portal venules1 |
+21 +21 |
II II |
11.6 9.7 |
13.1 14.3 |
319 590 |
Steroids OKT-3 |
| 31 | +11 | Portal venules1 | +21 | III | 10.0 | 8.0 | 127 | Thymoglobulin | |
| 41 | +21 | Portal venules1 | +31 | II | 10.1 | 9.4 | 130 | Steroids | |
| 52 | +3 | Portal venules | +3 | II | 9.6 | 6.5 | 204 | Bortezomib | |
| 84 | 0 | N/A | 0 | 0 | 11.0 | 3.9 | 169 | – | |
| 361 | 0 | N/A | 0 | 0 | 9.4 | 3.0 | 121 | – | |
| Patient 2 (28 year, F) |
452 459 |
+21 +21 |
Portal venules1 Portal venules1 |
+21 +31 |
III II |
13.8 12.7 |
3.5 5.3 |
265 347 |
Steroids Thymoglobulin |
| 469 | +2 | Portal venules | +3 | I | 13.2 | 3.7 | 304 | Rituximab | |
| 518 | +3 | Portal venules | +3 | I | 14.2 | 2.2 | 275 | Bortezomib | |
| 549 | 0 | N/A | 0 | 0 | 12.7 | 5.3 | 137 | ||
| 639 | 0 | N/A | 0 | 0 | 11.6 | 5.9 | 215 | PP + Bortezomib2 | |
| 909 | 0 | N/A | 0 | 0 | 13.4 | 7.5 | 285 | – | |
| Patient 3 (53 year, F) |
6 22 |
+31 +31 |
Portal venules1 Portal venules1 |
+31 +31 |
II I |
8.9 9.8 |
4.4 3.3 |
70 165 |
Increased prograf |
| 49 | +3 | Portal venules | +3 | I | 9.6 | 3.9 | 160 | Bortezomib | |
| 78 | 0 | N/A | 0 | 0 | 9.0 | 2.2 | 89 | – |
PTD = posttransplant day; Hgb = hemoglobin; WBC = white blood cell; Plt = platelets; y = years; F = female; N/A = not applicable; PP = plasmapheresis. stain intensity: 0 = negative; +1 = low; +2 = moderate; +3 = high.
Stains performed retrospectively after diagnosis of acute AMR.
Biopsy showed early ductopenia; LFTs and DSA were elevated.
Patient 1
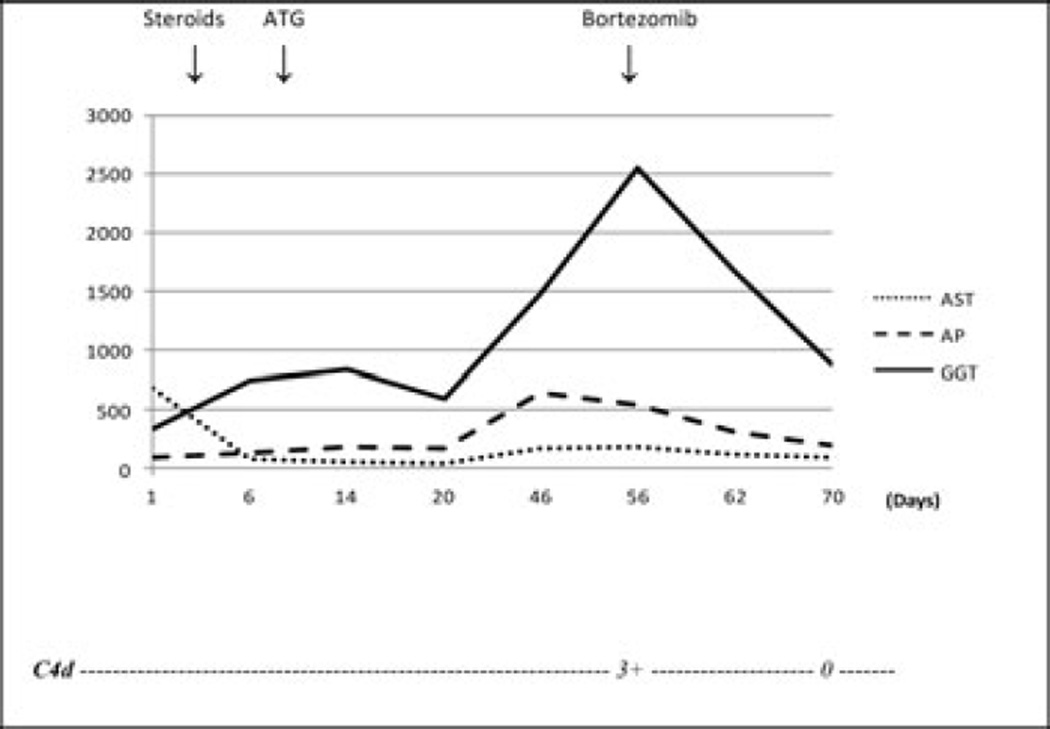
A 62-year-old Caucasian woman with end-stage liver disease because of primary sclerosing cholangitis and hepatocellular carcinoma underwent orthotopic liver transplant in October 2010 in the presence of a negative crossmatch. Posttransplant immunosuppression included tacrolimus, sirolimus and corticosteroids. One week after transplant, her alkaline phosphatase level quadrupled, her bilirubin level increased from 0.9 mg/dL to 2.2 mg/dL and her gamma-glutamyl transpeptidase (GGT) level reached 13 times the upper limit of normal (Figure 1). The Doppler ultrasound of her liver was normal and the biopsy revealed lymphocytic portal infiltrates with bile duct damage and endothelialitis compatible with Banff grade II (RAI 5) ACR. The patient received a steroid cycle (methylprednisolone 1 g/day for two doses followed by a taper for a week). After completion of the steroid cycle, her LFTs remained elevated and a repeat liver biopsy showed persistent rejection resulting in OKT-3 initiation (5 mg/day). After eight doses of OKT-3, the patient presented with a perforated duodenal ulcer and underwent emergent laparotomy with a Graham patch repair.
Figure 1. Posttransplant course of patient 1.
Aspartate aminotransferase (AST, U/L), alkaline phosphatase (AP, U/L), gamma glutamyl transferase (GGT, U/L) and C4d immunostain. Ster, steroids; ATG, antithymocyte globulin.
After resolution of her acute abdominal process, her liver biopsy showed persistent rejection, prompting treatment with Thymoglobulin (rabbit antithymocyte globulin, 1.5 mg/kg, 10 doses). The follow-up liver biopsy showed focal residual rejection that was treated with steroids (methylprednisolone 1 g IV every other day for six doses).
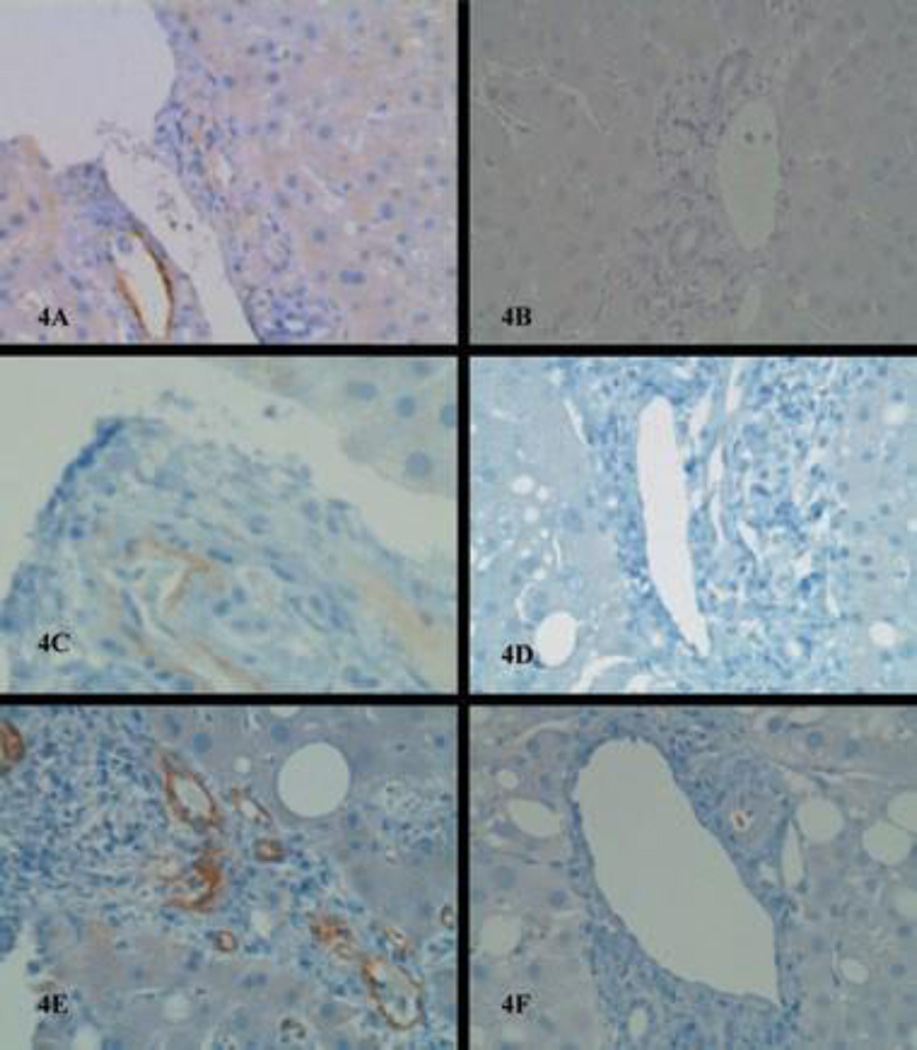
Despite this intense immunosuppression, the patient’s LFTs increased and repeat liver histology showed worsening rejection. At that time, diffuse C4d deposits were noted in the walls of the portal venules (Figure 4A), with large numbers of plasma cells in the portal tracts. Her DSAs were positive for several HLA class II antibodies with high MFI (Table 3). She was then treated with bortezomib (1.3 mg/m2 IV, weekly for four doses) and her LFTs declined after completion of treatment, normalizing in 7 weeks. The posttreatment biopsy showed resolution of rejection with negative C4d (Figure 4B) and her DSAs became negative (Table 3).
Figure 4. Liver biopsy before treatment and after treatment with bortezomib.
C4d immunostain shows strong perivenular deposition in patient 1, 2 and 3 before treatment with bortezomib (A, C, E) and resolution of C4d deposition after treatment in the same patients (B, D, F).
Table 3.
HLA donor-specific antibody MFI before and after treatment with bortezomib
| Patient 1 | DR13 | DR15 | DR51 | DR52 |
| Time | ||||
| 7 days before bortezomib first dose | 20708 | 1618 | 2151 | 3881 |
| 146 days after bortezomib last dose | 892 | 0 | 21 | 214 |
| Patient 2 | DQ2 | DQ6 | – | – |
| Time | ||||
| 7 days before bortezomib first dose | 2658 | 5877 | – | – |
| 110 days after bortezomib last dose | 1062 | 3658 | – | – |
| 7 days after last bortezomib + PP | 312 | 1416 | – | – |
| Patient 3 | B51 | Cw2 | DQ7 | – |
| Time | ||||
| 7 days before bortezomib first dose | 1121 | 1963 | 5574 | – |
| 7 days after bortezomib last dose | 293 | 939 | 5996 | – |
PP = plasmapheresis.
Five months after completion of bortezomib, the patient was admitted for cytomegalovirus (CMV) pneumonia and pericarditis, which were treated and resolved with ganciclovir and anti-CMV immunoglobulin (Cytogam). At 1-year follow-up, the patient was doing well with normal graft function. Her annual follow-up liver biopsy did not show any plasma cell infiltrate or C4d deposition.
Patient 2
A 28-year-old Caucasian woman underwent orthotopic liver transplant in February 2009 for fulminant hepatic failure in the presence of a negative crossmatch. The patient’s immediate postoperative course was uneventful. She received induction with OKT-3 (5 mg/day, five doses) and her immunosuppression included tacrolimus, mycophenolate mofetil and steroids.
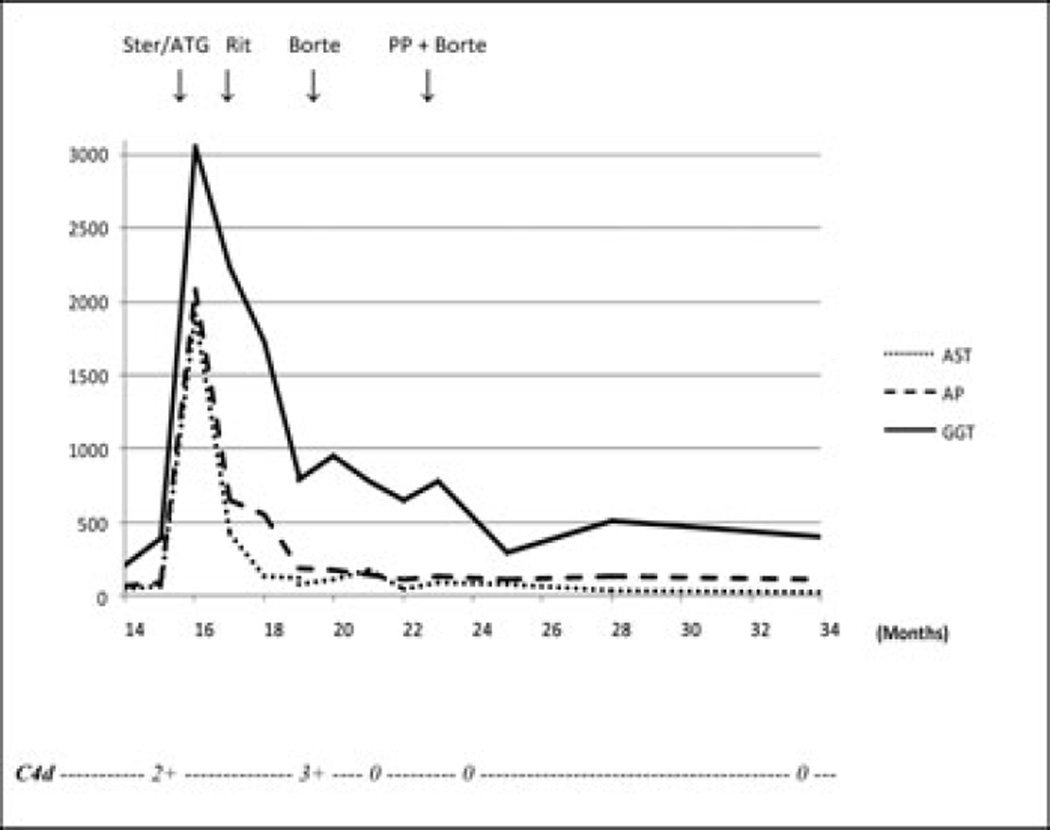
One year after transplant, the patient presented with elevated LFTs (Figure 2) in the presence of a normal Doppler ultrasound. The liver biopsy showed lymphocytic portal infiltrates with severe bile duct injury and endothelialitis compatible with Banff grade III (RAI 8) ACR. After completion of a steroid cycle, her LFTs remained elevated and her liver biopsy demonstrated persistent rejection. Despite 10 days of Thymoglobulin (1.5 mg/kg/day), her LFTs remained abnormal and a repeat liver biopsy showed significant plasma cell infiltrate, persistent ductal injury and C4d deposition. Her DSA testing was positive for two HLA class II antibodies (Table 3), AMR was suspected and rituximab was administered (375 mg/m2 weekly for four doses). The postrituximab biopsy showed increased lymphoplasmacytic infiltrates with strong and diffuse C4d staining in the portal venules (Figure 4C). Bortezomib (1.3 mg/m2 IV, twice weekly for four doses) was initiated, her decreased inflammatory infiltrates on the follow-up liver biopsy.
Figure 2. Posttransplant course of patient 2.
Aspartate amino-transferase (AST, U/L), alkaline phosphatase (AP, U/L), gamma glutamyl transferase (GGT, U/L) and C4d immunostain. Ster, steroids; ATG, antithymocyte globulin; Rit, rituximab; Borte, bortezomib; PP, plasmapheresis.
Four weeks later, the patient represented with markedly abnormal LFTs. Her liver biopsy showed lymphoplasmacytic portal infiltrates, damaged bile ducts and C4d deposition in the venules of portal tracts (Figure 4E). HLA DSAs were also elevated (see Table 3). She was treated with bortezomib (1.3 mg/m2 IV weekly for four doses). After completion of her treatment, her LFTs improved and her DSA MFI decreased. The posttreatment liver biopsy showed resolution of the plasma cell infiltrates and negative C4d immunostain (Figure 4F).
Three months later, the patient’s LFTs increased again and a liver biopsy showed early ductopenia in the presence of persistently elevated DSA levels (Table 3). Four cycles of weekly plasmapheresis and bortezomib were administered and resulted in improved LFTs and decreased DSA MFI. At 9-month follow-up, the patient was doing well and her LFTs were normal except for a mild GGT elevation. Her annual follow-up liver biopsy showed no inflammatory infiltrates and persistent mild ductopenia.
Patient 3
A 53-year-old Hispanic woman underwent orthotopic liver transplant in October 2011 for hepatitis C-related cirrhosis and hepatocellular carcinoma. Both the T cell and B cell flow cytometric crossmatches were positive. Posttransplant immunosuppression included tacrolimus, sirolimus and corticosteroids. On day 6, her bilirubin increased from 1.4 mg/dL to 4.9 mg/dL and her GGT increased to 13 times the upper limit of normal (Figure 3). A liver biopsy showed lymphocytic portal infiltrates, bile duct damage and endothelialitis compatible with Banff grade II ACR. A steroid cycle was administered without significant improvement. A course of Thymoglobulin (1.5 mg/kg/day for 7 days) was administered with improvement in her LFTs and decreased inflammatory infiltrates on the follow-up liver biopsy.
Figure 3. Posttransplant course of patient 3.
Aspartate aminotransferase (AST, U/L), alkaline phosphatase (AP, U/L), gamma glutamyl transferase (GGT, U/L) and C4d immunostain. Ster, steroids; ATG, antithymocyte globulin.
Four weeks later, the patient represented with markedly abnormal LFTs. Her liver biopsy showed lymphoplasmacytic portal infiltrates, damaged bile ducts and C4d deposition in the venules of portal tracts (Figure 4E). HLA DSAs were also elevated (see Table 3). She was treated with bortezomib (1.3 mg/m2 IV weekly for four doses). After completion of her treatment, her LFTs improved and her DSA MFI decreased. The posttreatment liver biopsy showed resolution of the plasma cell infiltrates and negative C4d immunostain (Figure 4F).
Discussion
The incidence of acute AMR in ABO-compatible liver transplant recipients is unknown because of the lack of specific diagnostic criteria and practitioner awareness. According to the National Institutes of Health criteria for kidney transplant, the diagnosis of acute AMR is based on the combination of clinical, pathologic and immunologic findings. The diagnosis requires graft dysfunction, histologic evidence of acute tissue injury, C4d deposition in the vascular walls and elevated DSA MFI (1). These criteria have been fulfilled in the most recent reports of AMR in liver transplants (Table 3) (3–5,13,20). C4d is a component of complement cascade. It is considered a marker of complement regulation. Complement factors, which are produced in the liver, can be activated for a variety of reasons including ischemic/reperfusion injury, inflammation and alternative pathway activation. C4d deposits in the arteriolar elastic lamina were found in about 30% of liver biopsies from “normal livers”, while strong linear deposition along the sinusoid endothelium was associated with rejection or recurrent hepatitis (5). However, even a diffuse endothelial and sinusoidal C4d staining alone cannot be considered specific for the diagnosis of AMR. Diffuse endothelial and sinusoidal C4d staining has been found in AMR and other common allograft disorders such as ACR, chronic rejection, biliary obstruction and recurrent viral or autoimmune hepatitis (19,20). A substantial plasma cell infiltrate (CD-138 positive) was also seen in all three patients. The isolated finding of HLA DSA is not specific for AMR because it has been found in 60% of liver transplant recipients without rejection (7). Because of the lack of specific markers for acute AMR, the diagnosis is based on the combination of multiple clinical, pathologic, immunohistochemical and serologic findings. A high index of suspicion is necessary to diagnose acute AMR, which should be suspected in patients with steroid-resistant rejection that does not improve after treatment with antibody agents (e.g. Thymoglobulin)
In such patients, clinicians may want to consider the diagnosis of acute AMR by testing for DSA and evaluating the liver biopsy for plasma cell infiltrates and C4d deposition. The diagnosis and treatment of these complex acute rejections can be more complicated due to the presence of both cellular and humoral components. In our patients both components were present, the cellular rejection was treated first with steroids and thymoglobulin while the humoral component was treated with bortezomib.
The treatment of acute AMR in ABO-compatible liver transplants is not clearly determined because of the limited number of cases. Most of the evidence in this field derives from studies in kidney transplantation. Traditional treatments for AMR in kidney transplant include intravenous immunoglobulins, plasmapheresis, rituximab and Thymoglobulin. Most of these treatments are effective in depleting immature B cells, but are not effective on plasma cells that produce the offending antibodies. Therefore, the administration of a drug such as bortezomib, which has been shown to induce apoptosis in mature plasma cells, could be more effective. Flechner et al. (14) reported a series of 20 kidney transplant patients with AMR that were treated with plasmapheresis and bortezomib with a graft survival of 85% at 10-month follow-up. Previous reports on AMR in ABO-compatible liver transplants describe conflicting results after treatment with plasmapheresis, rituximab, Thymoglobulin or steroids (Table 4), leaving an opportunity for improvement in therapy (3–5,13). Although all of our patients demonstrated excellent clinical and pathologic response to bortezomib, the rate of response was different in each patient. It is still unclear if there is a target DSA MFI in the treatment of AMR. A previous study on kidney transplant recipients with AMR treated with plasmapheresis and bortezomib showed that the mean decrease in DSA MFI was 55% and only 10% of patients achieved undetectable DSA (14). It is reasonable to monitor DSA levels after treatment, but it is necessary to correlate them to clinical and histologic findings.
Table 4.
Previous reports of acute antibody-mediated rejection in ABO-compatible liver transplants
| Author, year | N | C4d | DSA | Onset (PTD) | Treatment | Patient outcome |
|---|---|---|---|---|---|---|
| Kamar, 2009 (3) | 2 | + | + | 6 | PP, rituximab | 1 survived, 1 died |
| Wilson, 2006 (13) | 1 | + | + | 1460 | PP, Thymoglobulin, rituximab | Survived |
| Watson, 2006 (4) | 1 | + | + | 1 | PP, rituximab, steroids | Died |
| Rostron, 2005 (11) | 1 | +/− | + | 6 | PP, steroids, IVIG, mycophenolate | Survived |
| Kozlowski, 2011 (5) | 3 | + | + | 1 | PP, rituximab, steroids, IVIG, Thymoglobulin, OKT-3 | 2 died, 1 retransplant |
N = number of patients; DSA = donor-specific antibodies; PTD = posttransplant day; PP = plasmapheresis; IVIG = intravenous immunoglobulins.
The treatment with bortezomib was generally well tolerated, but we did note some side effects. Fatigue was present in all three patients. One patient received bortezomib on a biweekly dosing and reported nausea and diffuse musculo-skeletal pain. These side effects resolved after switching to a weekly dosing. The other two patients received bortezomib on a weekly dosing and did not experience these effects. Fortunately, no major toxicity was observed. Patient 1 developed severe CMV infection five months after bortezomib treatment. It is unclear if this opportunistic infection was prompted by bortezomib, OKT- 3 or Thymoglobulin. Regardless, the patient was at high risk for CMV infection because she was CMV negative before transplant and her donor was CMV positive. Nevertheless, CMV infection was treated and resolved with ganciclovir and anti-CMV immunoglobulin. No opportunistic infections or neoplasms have been associated with bortezomib treatment in the renal transplant literature (14,15).
The results of this study are preliminary and limited because of the small number of patients reported. However, these outcomes are intriguing from a clinical and biologic perspective, offering an alternative treatment option for patients who have historically been refractory to other treatments. More studies are necessary to identify specific markers of acute AMR in liver transplantation and to test the efficacy of bortezomib. In addition, there are still unanswered questions on the relationship with chronic rejection.
In conclusion, acute AMR is a type of acute rejection that can rarely complicate ABO-compatible liver transplants, resulting in graft injury and potentially loss. Clinicians should suspect this diagnosis in patients with steroid-resistant rejection refractory to treatment with antibody agents. In selected cases with documented abnormal C4d deposition, plasma cell infiltrates and elevated DSA titers, treatment with bortezomib could be considered.
Abbreviations
- ACR
acute cellular rejection
- AMR
antibody-mediated rejection
- CMV
cytomegalovirus
- DSA
donor-specific antibodies
- GGT
gamma- glutamyl transpeptidase
- HLA
human leukocyte antigen
- LFT
liver function test
- MFI
mean fluorescence intensity
- RAI
Rejection Activity Index.
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclosure as described by the American Journal of Transplantation.
References
- 1.Takemoto SK, Zeevi A, Feng S, et al. National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant. 2004;4:1033–1041. doi: 10.1111/j.1600-6143.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 2.Chan KM, Lee CS, Wu TJ, Lee CF, Chen TC, Lee WC. Clinical perspective of acute humoral rejection after blood type-compatible liver transplantation. Transplantation. 2011;91:e29–e30. doi: 10.1097/TP.0b013e318208138c. [DOI] [PubMed] [Google Scholar]
- 3.Kamar N, Lavayssié re L, Muscari F, et al. Early plasmapheresis and rituximab for acute humoral rejection after ABO-compatible liver transplantation. World J Gastroenterol. 2009;15:3426–3430. doi: 10.3748/wjg.15.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watson R, Kozlowski T, Nickeleit V, et al. Isolated donor specific alloantibody-mediated rejection after ABO compatible liver transplantation. Am J Transplant. 2006;6:3022–3029. doi: 10.1111/j.1600-6143.2006.01554.x. [DOI] [PubMed] [Google Scholar]
- 5.Kozlowski T, Rubinas T, Nickeleit V, et al. Liver allograft antibody-mediated rejection with demonstration of sinusoidal C4d staining and circulating donor-specific antibodies. Liver Transpl. 2011;17:357–368. doi: 10.1002/lt.22233. [DOI] [PubMed] [Google Scholar]
- 6.Musat AI, Agni RM, Wai PY, et al. The significance of donor-specific HLA antibodies in rejection and ductopenia development in ABO compatible liver transplantation. Am J Transplant. 2011;11:500–510. doi: 10.1111/j.1600-6143.2010.03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Leary JG, Kaneku H, Susskind BM, et al. High mean fluorescence intensity donor-specific anti-HLA antibodies associated with chronic rejection postliver transplant. Am J Transplant. 2011;11:1868–1876. doi: 10.1111/j.1600-6143.2011.03593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morioka D, Togo S, Kumamoto T, et al. Six consecutive cases of successful adult ABO-incompatible living donor liver transplantation: A proposal for grading the severity of antibody-mediated rejection. Transplantation. 2008;85:171–178. doi: 10.1097/TP.0b013e31815e9672. [DOI] [PubMed] [Google Scholar]
- 9.Raut V, Uemoto S. Management of ABO-incompatible living-donor liver transplantation: past and present trends. Surg Today. 2011;41:317–322. doi: 10.1007/s00595-010-4437-3. [DOI] [PubMed] [Google Scholar]
- 10.Colvin RB. C4d in liver allografts: A sign of antibody-mediated rejection? Am J Transplant. 2006;6:447–448. doi: 10.1111/j.1600-6143.2006.01245.x. [DOI] [PubMed] [Google Scholar]
- 11.Rostron A, Carter V, Mutunga M, et al. A case of acute humoral rejection in liver transplantation: Successful treatment with plasma- pheresis and mycophenolate mofetil. Transpl Int. 2005;18:1298–1301. doi: 10.1111/j.1432-2277.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 12.Ratner LE, Phelan D, Brunt EM, Mohanakumar T, Hanto DW. Probable antibody-mediated failure of two sequential ABO-compatible hepatic allografts in a single recipient. Transplantation. 1993;55:814–819. doi: 10.1097/00007890-199304000-00025. [DOI] [PubMed] [Google Scholar]
- 13.Wilson CH, Agarwal K, Carter V, et al. Late humoral rejection in a compliant ABO-compatible liver transplant recipient. Transplantation. 2006;82:988–989. doi: 10.1097/01.tp.0000229939.85412.27. [DOI] [PubMed] [Google Scholar]
- 14.Flechner SM, Fatica R, Askar M, et al. The role of proteasome inhibition with bortezomib in the treatment of antibody-mediated rejection after kidney-only or kidney-combined organ transplantation. Transplantation. 2010;90:1486–1492. doi: 10.1097/TP.0b013e3181fdd9b0. [DOI] [PubMed] [Google Scholar]
- 15.Walsh RC, Everly JJ, Brailey P, et al. Proteasome inhibitor-based primary therapy for antibody-mediated renal allograft rejection. Transplantation. 2010;89:277–284. doi: 10.1097/TP.0b013e3181c6ff8d. [DOI] [PubMed] [Google Scholar]
- 16.Everly MJ, Everly JJ, Susskind B, et al. Bortezomib provides effective therapy for antibody-and cell-mediated acute rejection. Transplantation. 2008;86:1754–1761. doi: 10.1097/TP.0b013e318190af83. [DOI] [PubMed] [Google Scholar]
- 17.Banff schema for grading liver allograft rejection: An international consensus document. Hepatology. 1997;25:658–663. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 18.Sakashita H, Haga H, Ashihara E, et al. Significance of C4d staining in ABO-identical/compatible liver transplantation. Mod Pathol. 2007;20:676–684. doi: 10.1038/modpathol.3800784. [DOI] [PubMed] [Google Scholar]
- 19.Lunz J, Ruppert KM, Cajaiba MM, et al. Re-examination of the lymphocytotoxic crossmatch in liver transplantation: Can C4d stains help in monitoring? Am J Transplant. 2012;12:171–182. doi: 10.1111/j.1600-6143.2011.03786.x. [DOI] [PubMed] [Google Scholar]
- 20.Ali S, Ormsby A, Shah V, et al. Significance of complement split product C4d in ABO-compatible liver allograft: Diagnosing utility in acute antibody mediated rejection. Transpl Immunol. 2012;26:62–69. doi: 10.1016/j.trim.2011.08.005. [DOI] [PubMed] [Google Scholar]