Abstract
Clinical studies have established the important impact of atherosclerotic disease in Western societies. This disease is characterized by the accumulation of lipids and the migration of various cell types in the sub-endothelial space of blood vessels. As demonstrated by many studies, endothelial cells play an essential role in the development of this disease. The endothelium acts as a gatekeeper of blood vessel integrity and cardiovascular health status. For instance, the transfer of lipids via the transport of lipoproteins in the arterial intima is believed to be mediated by endothelial cells through a process termed transcytosis. In addition, lipoproteins that accumulate in the sub-endothelial space may also be modified, in a process that can direct the activation of endothelial cells. These steps are essential for the initiation of an atherosclerotic plaque and may be mediated, at least in part, by caveolae and their associated protein caveolin-1. In the present study, we evaluate the role of caveolin-1/caveolae in the regulation of these two steps in endothelial cells. Our data clearly demonstrate that caveolin-1 is involved in the regulation of lipoprotein transcytosis across endothelial cells and in the regulation of vascular inflammation.
Keywords: Caveolae, Caveolin, Lipoprotein, LDL, Cholesterol
Introduction
Atherosclerosis is a disease characterized by the accumulation of lipids and lipoproteins in the arterial vessel wall (Libby et al. 2011). The subendothelial space, where atheroma initiation and progression occur, plays an important role in the development of this disease. Endothelial cells are believed to be the initial barrier crossed by lipoproteins and immune cells such as monocytes and lymphocytes. Before their accumulation in the intima, lipoproteins must cross the endothelium barrier via a process termed transcytosis. The transcytosis of low-density lipoproteins (LDL) into the intima is followed by their modification (e.g., oxidation), which leads to the activation of endothelial cells and eventually the transmigration of monocytes. The latter process is believed to be mediated by various adhesion molecules that allow monocytes–endothelium interaction. These molecules includes selectins, intercellular adhesion molecule 1 (ICAM-1) and vascular adhesion molecule 1 (VCAM-1) (Ley et al. 2007). Therefore, the entry of LDL is associated with a cascade of events leading to the formation of an atheroma (Simionescu 2007). It is therefore believed to be an essential step associated with the development of atherosclerosis.
Caveolae are 50–100 nm cell surface plasma membrane invaginations observed at the surface of differentiated cells. The existence of these invaginations is regulated by the presence of lipids such as cholesterol and sphingolipids but also by members of the caveolin protein family (Parat 2009). In this family, caveolin-1 is the most ubiquitously expressed protein and is essential for caveolae formation in non-muscle cells. In particular, caveolin-1 is highly expressed in endothelial cells (Frank et al. 2003), which play an important role in the development of atherosclerosis (Bonetti et al. 2003). As a consequence, the number of caveolae in this cell type is remarkably elevated compared to other cell types (Frank et al. 2003). A role for these structures has been proposed by Palade’s early studies (Palade and Bruns 1968). It has been suggested that caveolae may allow the transport of macromolecules across endothelial cells via transcytosis. Increasing evidence has now suggested an important role for caveolin-1 and caveolae in the regulation of endothelial cell function (Frank et al. 2003). Accordingly, the absence of caveolin-1 in endothelial cells has been associated with a protective effect in the development of atherosclerosis (Fernandez-Hernando et al. 2009, Frank et al. 2004). One possible mechanism by which caveolin-1 could regulate atherosclerosis development may be by regulating the transcytosis of macromolecules across endothelial cells. In agreement with this hypothesis, in vivo studies have now clearly demonstrated the importance of caveolin-1/caveolae in the regulation of albumin transcytosis across endothelial cells (Pascariu et al. 2004, Schubert et al. 2001). As postulated by others and our laboratory (Frank and Lisanti 2004, Frank et al. 2009, Vasile et al. 1983), caveolae and caveolin-1 may also play an important role in the regulation of LDL transfer (i.e., transcytosis) in the vascular wall (intimal accumulation). Additionally, caveolin-1 has been shown to play an important role in the regulation of many cellular signaling pathways. In particular, caveolin-1 regulates several endothelial functions associated with vascular dysfunction (Sharma et al. 2010). Caveolin-1 has been shown to regulate pathways such as adhesion molecule functions and expression levels, angiogenesis and vascular permeability (Pavlides et al. 2012). Given the high levels of caveolin-1 protein in endothelial cells, it is likely that endothelial caveolin-1 plays many important roles in this cell type and in the development of atherosclerosis.
In the present study, we examine the role of caveolin-1 in the regulation of endothelial cell function. We specifically investigate the role of caveolin-1 in two endothelial functions that play a critical role in the development of atherosclerosis. They include the transcytosis of LDL across endothelial cells and the regulation of adhesion molecule expression.
Materials and methods
Materials
Antibodies and their sources were as follows: PECAM-1 (CD31) antibodies were obtained from BD Biosciences, Inc. (San Diego, CA, USA). VCAM-1 was obtained from Meridian Life Science, Inc. (Memphis, TN, USA). Beta-actin antibody was obtained from Sigma-Aldrich Corp. (St. Louis, MO, USA). Total and phospho-p65 and TNFR antibodies were obtained from Cell Signaling, Inc. (Danvers, MA, USA). Caveolin-1 and TLR4 mouse monoclonal antibody were obtained from Santa Cruz Biotechnology, Inc. (Palo Alto, CA, USA). Fluorescently-labeled LDL (BODIPY® FL LDL), transferrin (Alexa Fluor® 488 Conjugate) and Albumin (Alexa Fluor® 555 Conjugate) were obtained from Life Technologies (Grand Island, NY, USA). Oxidized LDL (oxLDL) preparations were obtained from Biomedical Technologies, Inc. (Stoughton, MA, USA).
Animals
All animals were housed and maintained in a barrier facility at the Kimmel Cancer Center at Thomas Jefferson University (Philadelphia, PA, USA). Animals used in these studies were backcrossed at least 6 times in the C57Bl/6J genetic background and were genotyped by PCR as previously described (Razani et al. 2001). Cav-1−/− mice were crossed with Apoe−/− mice to generate double knockout Cav-1−/−Apoe−/− mice, as described (Frank et al. 2004). Mice were kept on a 12-h light/dark cycle and on a normal chow diet or a Western-type diet (0.2 % cholesterol (w/w) (TestDiet, Richmond, IN). Animal protocols used in this study were approved by the Institutional Animal Care and Use Committee at Thomas Jefferson University.
Bone marrow transplantation
Six- to 7-week-old mice to be transplanted were submitted to 10 Gy irradiation. Bone marrow (BM) was collected from donor mice from hind leg femurs and tibias. Cells were washed and resuspended in RPMI 1640 containing 2 % FBS and 5 U/ml heparin. Four hours after irradiation, 107 bone marrow cells were intravenously injected into each mouse. One month after transplantation, blood was collected to control for the efficiency of the bone marrow transplant. Genotyping was performed by PCR as previously described (Frank et al. 2004). Transplanted mice were then fed a Western diet for 12 weeks.
HUVEC cell culture
Human umbilical vein endothelial cells (HUVEC) were obtained from AllCells (AllCells, LLC, Emeryville, CA, USA). HUVEC were cultured in M199 media (Life Technologies, Grand Island, NY, USA) containing 10 % FBS (Life Technologies, Grand Island, NY, USA) 50 µg/ml heparin (Sigma-Aldrich Corp., St. Louis, MO, USA) and 50 µg/ml Endothelial Cell Growth Supplement (Sigma-Aldrich Corp., St. Louis, MO, USA). They were treated for 24 h with control siRNA (Negative Control siRNA, Cat. #1022076; Qiagen, Inc., Valencia, CA, USA) or caveolin-1 siRNA (FlexiTube siRNA Hs_Cav1_9, Cat. #SI00299635; Qiagen, Inc.) and then incubated with fluorescently labeled albumin alone (Alexa Fluor 555 conjugated; Life Technologies, Grand Island, NY, USA), or in the presence of transferrin (Alexa Fluor 488 conjugated; Life Technologies) and in the presence of unlabeled albumin (competitive assay) for 0, 15, 30 and 60 min. In a separate study, fluorescently-labeled albumin was replaced with BODIPY® FL LDL to follow the uptake of LDL. Fluorescence confocal images were acquired on a Zeiss LSM 510 META confocal microscope.
For endothelial cell activation studies, HUVEC were subjected to siRNA treatment as described above. After 24 h of siRNA treatment, cells were incubated for 24 h under four conditions (TNFα, oxLDL, both TNFα and oxLDL, no treatment). Signaling pathways were examined in the cell lysates obtained after this incubation.
Western blot analysis
Protein concentrations were measured with the bicinchoninic acid protein assay (Thermo Fisher Scientific, Rockford, IL, USA) with bovine serum albumin as the protein standard. Equal amounts of protein for each sample were loaded and run on sodium dodecyl sulfate–polyacrylamide 12 % gels. After transfer to nitrocellulose, the expression levels of caveolin-1 and other proteins were examined by using specific antibodies.
Immunohistochemistry analysis
Frozen sections of aorta (5 µm) were prepared in OCT compound and kept at −80 °C until ready. Sections were fixed with 4%paraformaldehyde in PBS for 10 min at 4 °C and washed 3 times with PBS. After fixation, the sections were blocked with 10 % rabbit serum and incubated overnight at 4 °C with either rat monoclonal CD31 or VCAM-1 antibodies. The sections were then incubated with biotinylated rabbit anti-rat IgG (Vector Labs, Inc., Burlingame, CA, USA) and streptavidin-HRP (Dako, Inc., Carpinteria, CA, USA). Immunoreactivity was revealed with 3,3′ diaminobenzidine.
Statistics
Values were reported as the mean ± SE. Comparisons between samples were performed using the Student t-test or by ANOVA when appropriate.
Results
Albumin uptake is dependent on Cav-1 expression in HUVEC
The initial goal of this study was to examine the role of caveolin-1/caveolae in the regulation of transcytosis. The two most important macromolecules that are transcytosed via caveolae are albumin and LDL (Simionescu et al. 2009). However, the transfer of albumin in the sub-endothelial space can also occur via a paracellular pathway between endothelial cells (Schubert et al. 2002). Because of their larger diameter, LDL particles can only be transferred via transcytosis across endothelial cells. In the first experiment of this study, we decided to examine the role of caveolae/caveolin-1 in the regulation of albumin transcytosis by immunofluorescence microscopy. However, in this study and in the following ones, we only examined the initial uptake of albumin (or LDL) and not the release in a model sub-endothelial space. Therefore, we examined the uptake/endocytosis of albumin and LDL in these experiments.
To differentiate between clathrin-coated pits and caveolae-mediated endocytosis, fluorescent microscopy with various molecules was carried out. In this assay, the effect of acute downregulation of caveolin-1 in HUVECs on albumin (caveolae-mediated transfer pathway) and transferrin (clathrin-coated pits mediated pathway) uptake was analyzed. Previous studies have shown that transferrin exclusively utilizes the clathrin-coated pit pathway (Monks and Neville 2004), whereas albumin is predominantly transported through a caveolae-mediated pathway (Minshall et al. 2002, Schubert et al. 2001).
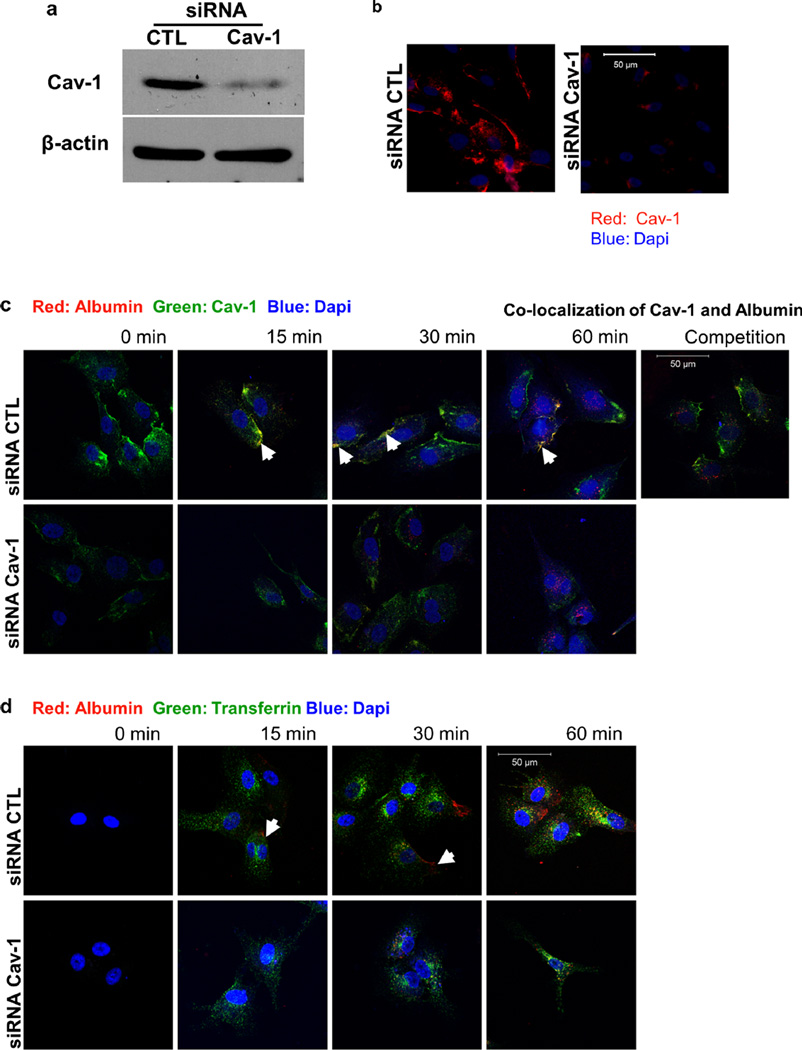
The function of caveolin-1 during endocytosis in endothelial cells was assessed using HUVECs treated with siRNA against the caveolin-1 mRNA transcript. At 24 h post-transfection with siRNA, caveolin-1 protein levels were decreased by over 90 % compared with control treated cells, as evaluated by Western blot analysis and immunofluorescence microscopy (Fig. 1a and b). After 24 h, transfected cells were incubated with fluorescently-labeled albumin (Alexa Fluor® 555 Conjugate, red) for specific time points (0, 15, 30, 60min) before fixation. Immunofluorescence microscopy using a caveolin-1 antibody confirmed that albumin follows a caveolae-mediated internalization pathway (Fig. 1c). Furthermore, internalization of fluorescently-labeled albumin was remarkably reduced in cells with reduced caveolin-1 protein levels or in the presence of a large excess of unlabelled albumin. In a similar experiment, cells were co-incubated with fluorescently labeled transferrin (Alexa Fluor® 488 Conjugate, green) and fluorescently labeled albumin (Alexa Fluor® 555 Conjugate, red) for various amounts of time (0, 15, 30, 60 min). Transferrin internalization followed a pathway different from that of albumin, based on their lack of sub-cellular colocalization (Fig. 1d). In addition, when cells were treated with siRNA against caveolin-1, while transferrin uptake appeared to be unaffected, transport of albumin was remarkably reduced (Fig. 1d). This experiment indicates that the uptake and endocytosis of molecules such as albumin are exclusively and specifically dependent on the presence of caveolin-1.
Fig. 1.
Caveolae-mediated uptake of albumin. a Western blot analysis of siRNA-treated HUVEC was performed to quantify caveolin-1 protein levels. Caveolin-1 siRNA-treated HUVEC displayed significantly decreased caveolin-1 protein levels when compared to CTL siRNA-treated cells. b Expression of caveolin-1 in HUVECs was also examined via immunofluorescence staining using an antibody specific to caveolin-1. c HUVECs were treated with control and caveolin-1 siRNA and then incubated with fluorescently labeled albumin (rhodamine-labeled, red) alone for 0, 15, 30 and 60 min or in the presence of unlabeled albumin (competition for 60 min). Colocalization (yellow) of albumin and caveolin-1 (FITC-labeled, green) was evident (white arrows) in cells treated with control siRNA. The uptake of albumin (red) by HUVEC was exclusively mediated by the caveolae pathway. Unlabeled albumin competed with labeled albumin internalization in the control siRNA treated cells (competition for 60 min). d siRNA-treated HUVEC were incubated with fluorescently labeled albumin (rhodamine, red) and fluorescently-labeled transferrin (FITC, green) for 0, 15, 30 and 60 min. Albumin was taken up only via caveolae. In the control siRNA-treated cells, transferrin and albumin did not colocalize, confirming the independent internalization of albumin (caveolae) and transferrin (clathrin-coated pits)
Cav-1 silencing in endothelial cells prevents the endocytosis of LDL
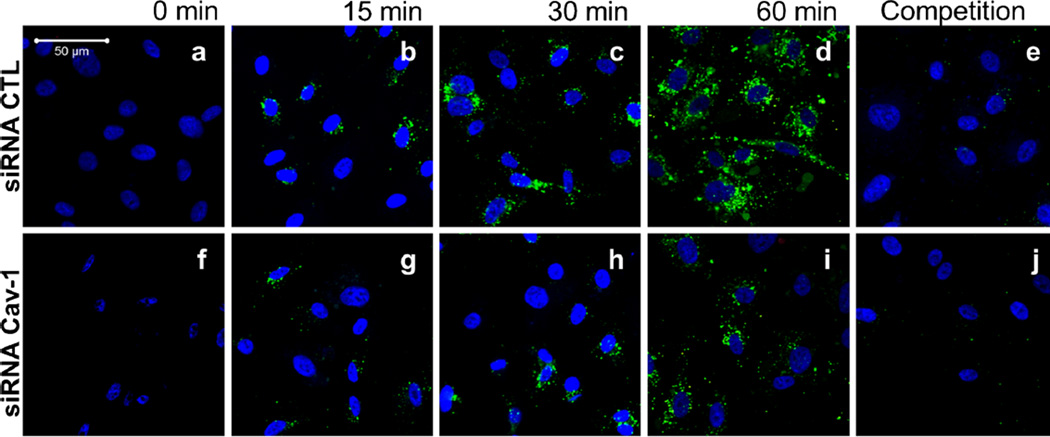
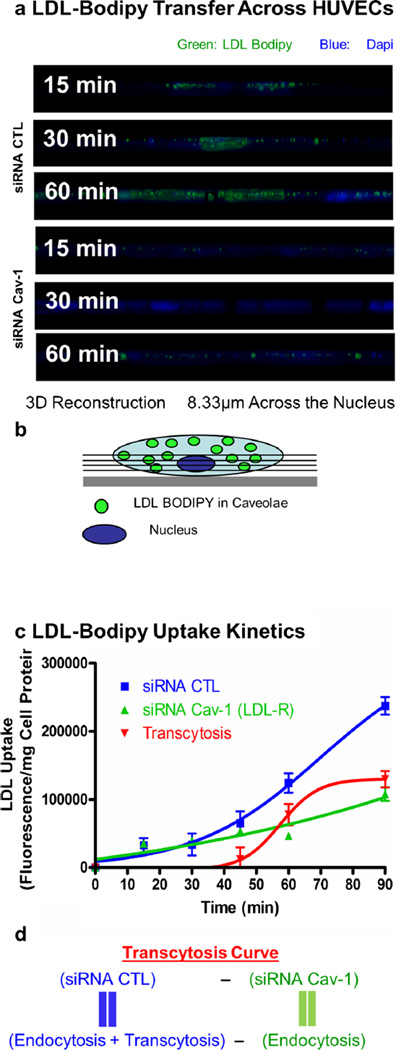
Having established an assay to study caveolae-mediated transport, we were further interested in examining the uptake of LDL in endothelial cells. The accumulation of LDL in the sub-endothelial space is believed to be the initial step leading to the development of an atheroma. This transfer of lipoprotein may be mediated by the transcytosis of LDL across endothelial cells (Frank et al. 2009, Vasile et al. 1983). To determine the role of caveolin-1 in the regulation of LDL endocytosis in endothelial cells, HUVEC cells treated with control and caveolin-1 siRNA were incubated with BODIPY-labeled LDL particles (BODIPY-LDL) for various amounts of time (15, 30, 45, 60 min). Confocal microscopy showed that depletion of caveolin-1 in HUVECs was sufficient to radically diminish the uptake of BODIPY-LDL (60 min), whereas in the siRNA control-treated cells, LDL particles were clearly endocytosed (Fig. 2a–j). To better illustrate these findings, a three-dimensional reconstruction from confocal scanning laser microscopy images was performed. Cross-section analysis demonstrated that BODIPY-LDL is transported only in the control siRNA-treated HUVECs as LDL particles move across endothelial cells over time from the apical to the basal side of the cells (Fig. 3a–b). Uptake and transport of BODIPY-LDL were also quantified by spectrofluorometry by measuring the cellular fluorescence due to BODIPY-LDL accumulation in HUVECs at different time points. LDL transport show different kinetics depending on the presence or absence of caveolin-1 (Fig. 3c). Accumulation of LDL in the cell may be due to endocytosis via other pathways, mainly clathrin-coated pits. Therefore, to differentiate between caveolae-dependent and -independent endocytosis, non-caveolae-dependent internalization of LDL (fluorescence obtained from caveolin-1 siRNA-treated cells) was subtracted from total LDL internalization (fluorescence obtained from control siRNA-treated cells). We hypothesized that HUVECs treated with control siRNA would be able to utilize both pathways (caveolae- and non-caveolae-mediated), whereas the caveolin-1 siRNA-treated-cells should only be able to utilize non-caveolae-associated pathways (e.g., clathrin-coated pits mediated endocytosis). Therefore, an estimation of the level of caveolae-mediated transport (possibly resulting in the transcytosis of LDL) in the presence of caveolin-1 (in control siRNA-treated cells) was derived as followed (Fig. 3d): the contribution of non-caveolae-mediated endocytosis (corresponding to the fluorescence in HUVEC treated with caveolin-1 siRNA) was subtracted from endocytosis and potential transcytosis (fluorescence in HUVECs treated with control siRNA). In this experiment, we assumed an almost complete inhibition of potential transcytosis via acute downregulation of caveolin-1, as suggested by the fluorescence microscopy experiment (Figs. 2 and 3a). Consequently, we were able to validate qualitatively and quantitatively the existence of a caveolae-mediated LDL endocytotic pathway for the first time. These findings suggest that caveolin-1 function is critical for the endocytosis of LDL and potentially transcytosis across endothelial cells and plays an important role in this initial step of lesion formation.
Fig. 2.
HUVEC treated with Cav-1 siRNA exhibit significant and selective blockage of labeled-LDL (BODIPY-LDL) uptake. The function of caveolin-1 during transcytosis in endothelial cells was assessed using HUVECs treated with siRNA against the caveolin-1 mRNA transcript, as described in the previous figure. Cells were incubated with BODIPY-labeled LDL (LDL BODIPY) alone for different times (0, 15, 30, 45, 60 min; a–d, f–i) or with a 20× excess of unlabeled LDL (competition for 60 min; e, j). After fixation, cells were visualized using a confocal microscope
Fig. 3.
The absence of Cav-1 impairs LDL transcytosis across endothelial cells. a Visualization of cells incubated with labeled LDL from z-stacks side views (8.33 µm) across the nucleus (used as a cellular marker). b Schematic representation of z-stack side view across the nucleus as observed in a. c Quantification of LDL uptake kinetics via cellular fluorescence quantification was performed to obtain the level of transcytosis. For this experiment, cells were incubated for different amounts of time and solubilized with DMSO. Fluorescence content of each extract was measured using a fluorescence spectrophotometer. A significant reduction (57 %) of LDL internalization at 60 min was observed when cells were treated with caveolin-1 siRNA compared with the CTL siRNA-treated cells. The level of transcytosis was estimated as described in d
Endothelial cell activation is reduced when Cav-1 is downregulated
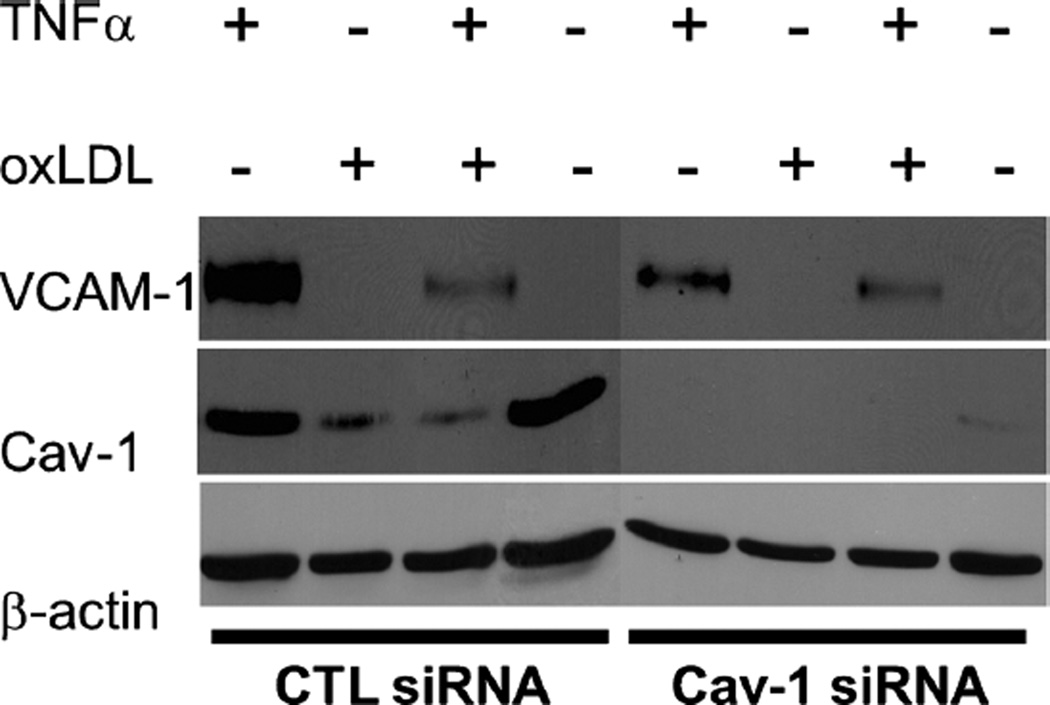
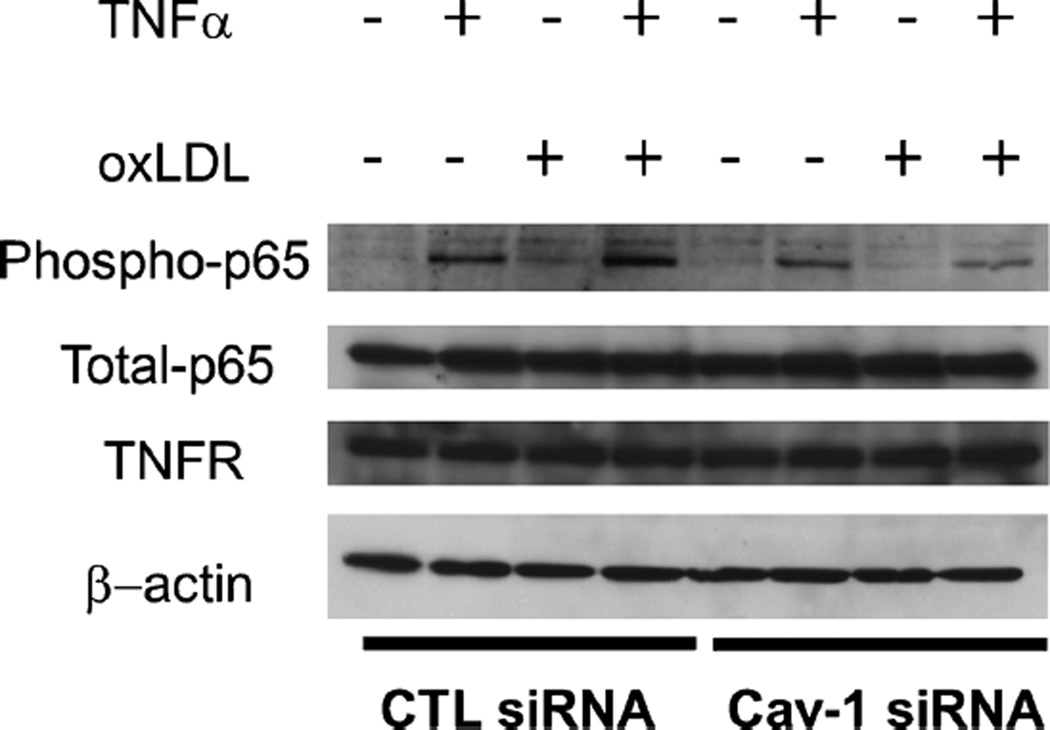
Activation of endothelial cells is a major step during atherosclerosis development and follows LDL transcytosis and modification in the sub-endothelial space. It leads to the expression of adhesion molecules that can facilitate the adherence of monocytes to the endothelium and their subsequent migration into the arterial intima (Lusis 2000). To determine the role of caveolin-1 in the regulation of endothelial cell inflammation, HUVECs were subjected to siRNA treatment for 24 h to downregulate caveolin-1 and exposed to conditions that mimic an in-vivo inflammatory environment. Control siRNA and caveolin-1 siRNA-treated cells were incubated under four conditions to stimulate VCAM-1 production (TNF-α, oxLDL, both TNF-α and oxLDL and control untreated) and then subjected to Western blot analysis. Caveolin-1 siRNA-treated HUVECs incubated with TNF-α alone, or in combination with TNF-α and oxLDL exhibited reduced activation (i.e., expression of VCAM-1), compared with HUVEC treated with CTL siRNA (Fig. 4). Interestingly, control cells treated with oxLDL also displayed reduced caveolin-1 expression, which may have altered the outcome of the experiment. As a consequence, activation of oxLDL-treated cells (as measured by VCAM-1 expression levels) was not as robust as in the case of control cells treated with TNFα alone.
Fig. 4.
Endothelial cells activation is decreased when Cav-1 is downregulated. HUVEC were subjected to siRNA treatment. After 24 h of siRNA treatment, cells were incubated for 24 h under four conditions (TNFα, oxLDL, both TNFα and oxLDL, no treatment). Caveolin-1 siRNA-treated HUVEC incubated with TNFα alone, or a combination of TNFα and oxLDL exhibited reduced activation (i.e., expression ofVCAM-1), in comparison with HUVEC treated with CTL siRNA. β-actinwas used as a loading control
Caveolin-1 depletion alters signaling via the NF-κB inflammatory pathway
To further examine the mechanism regulating endothelial cell activation by caveolin-1, a more detailed analysis of the signaling pathway involved was undertaken. To induce activation in endothelial cells, TNFα binds to its cognate receptor (TNFR) and induces a signaling cascade involving phosphorylation of IκB and the NF-κB subunit p65. Under basal conditions, the NF-κB complex p65/p50 is maintained by IκBα in an inactive state in the cytoplasm. Upon stimulation, IκBα and p65 are phosphorylated and the NF-κB dimer can translocate into the nucleus to activate transcription of target genes (de Winther et al. 2005). To examine the activation of this pathway, phospho-p65 and total p65 protein levels were quantified in cells activated under varying conditions. In control cells, treatment with oxLDL resulted in reduced p65 phosphorylation compared to cells treated with TNFα. However, a combination of TNFα and oxLDL could still activate NF-κB. This finding is in contrast with the result observed with the stimulation of VCAM-1 expression and suggests that the oxLDL may have a direct effect on VCAM-1. Our results also show that defective signaling takes place in cells with reduced caveolin-1 protein levels (Fig. 5). In that case, reduced p65 phosphorylation was observed with both TNFα and oxLDL treatment. Importantly, TNFα receptor levels were not affected by caveolin-1 protein levels (Fig. 5). In control cells, while phospho-p65 levels were increased in cells treated with TNFα and oxLDL compared to cells treated with TNFα alone or oxLDL alone, VCAM-1 expression was reduced. These data suggest that in the conditions used in the present study, VCAM-1 expression is dependent on the presence of caveolin-1 (Fig. 4).
Fig. 5.
Endothelial cell activation is decreased when Cav-1 is downregulated. HUVEC were subjected to siRNA treatment. After 24 h of siRNA treatment, cells were incubated for 24 h under four conditions (TNFα, oxLDL, both TNFα and oxLDL, no treatment). Caveolin-1 siRNA-treated HUVEC incubated with TNF-α alone, or a combination of TNFα and oxLDL exhibited reduced activation (i.e., expression of phosphop65), in comparison with HUVEC treated with CTL siRNA. β-actin was used as a loading control
Endothelial cell activation is increased in the presence of Cav-1 in aortic sections
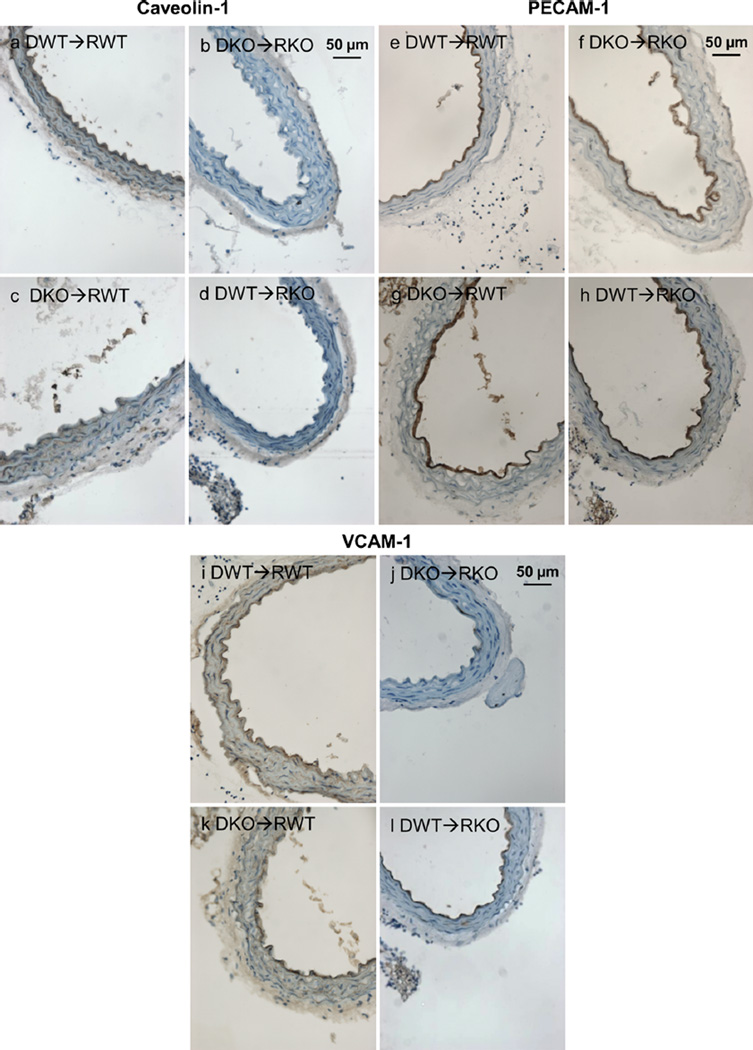
To determine the physiological relevance of the previous findings during atherosclerosis development, aorta sections obtained from ApoE-deficient (Apoe−/−) mice were analyzed after bone marrow transplantation with bone-marrow-derived cells obtained from Cav-1−/− or Cav-1+/+ mice. The different bone-marrow transplantation groups were analyzed to confirm the in-vitro data showing that activation of endothelial cells is dependent on caveolin-1 expression in the context of atherogenesis. Cross-sections of the descending thoracic aorta were immune-stained for caveolin-1 (Fig. 6a–d), PECAM-1 (CD31) (Fig. 6e–h) and for the adhesion molecule VCAM-1 (Fig. 6i–l). Our results show that sections obtained from mice on the Cav-1+/+ background (caveolin-1 expressed in endothelial cells) displayed increased endothelial VCAM-1 expression, especially in mice transplanted with bone marrow lacking caveolin-1 (Fig. 6k). This is in agreement with the aorta lesion quantification results that demonstrate a positive correlation between endothelial cell activation (VCAM-1 expression) and increased fatty streak lesion formation (Data not shown).
Fig. 6.
Endothelial cell activation is decreased in the Cav-1−/− background. Representative immunohistochemistry of aortic sections from the four transplanted groups: a–d As expected, caveolin-1 was not expressed in Cav-1−/− mice (right panels: DKO➔RKO, DWT➔RKO). However, caveolin-1 expression was observed in Cav-1+/+ recipient mice (left panels: DKO➔RWT, DWT➔RWT) specifically on endothelial cells and arterial intima. e–h PECAM is expressed homogeneously in endothelial cells of all groups, which indicates an intact endothelial cell layer forming a distinct inner ring of the aortic cross-section (arrowheads). i–l VCAM-1 expression (arrowheads) was increased in Cav-1+/+ mice transplanted with bone marrow cells obtained fromeither Cav-1−/− or Cav-1+/+ mice (left panels: DKO➔RWT, DWT➔RWT), in comparison to VCAM-1 expression in mice on the Cav-1−/− background (right panels: DKO➔RKO, DWT➔RKO). Donors (D) and recipients (R) are all on the Apoe−/− atherosclerosis susceptible background. D: donor, R: recipient, KO: Cav-1−/−, WT: Cav-1+/+
Discussion
Several studies have now demonstrated a clear and important role for caveolin-1 in the development of atherosclerosis (Fernandez-Hernando et al. 2010, Fernandez-Hernando et al. 2009, Frank et al. 2004). However, studies suggest that this role may depend on the cell type examined, as we previously hypothesized (Frank and Lisanti 2004). For example, while smooth muscle caveolin-1 may reduce neointima formation (Hassan et al. 2004), conclusive evidence that demonstrates a negative role for endothelial caveolin-1 in the development of atherosclerosis has been obtained (Fernandez-Hernando et al. 2010, 2009). In the present study, we decided to focus on two critical aspects regulating the atherogenic function of endothelial cells. More specifically, we show that caveolin-1 regulates the endocytosis of albumin and LDL in endothelial cells. Furthermore, we show that caveolae-mediated endocytosis of LDL occurs via a specific pathway (i.e., mediated by caveolae) that remains to be completely characterized. In addition, we also show that caveolin-1 regulates endothelial cell activation via the regulation of NF-κB activation mediated by TNFα and oxidized LDL. Taken together, these data show that caveolin-1 promotes the transfer of atherogenic LDL into the sub-endothelial space of arteries and promotes endothelial cell activation and, eventually, inflammation.
Caveolin-1 and the endothelial transcytosis
Previous studies including those from our laboratory have suggested an important role for endothelial caveolin-1 in the regulation of atherosclerosis development (Fernandez-Hernando et al. 2009, Frank et al. 2004). To further determine the role of caveolin-1 in the regulation of endothelial cell function, we examined the role of caveolin-1/caveolae in the regulation of albumin/LDL endocytosis and endothelial activation. In the present study, we show that the uptake/transcytosis of fluorescently-labeled albumin and LDL is significantly decreased when caveolin-1 is downregulated. We also demonstrate that the downregulation of caveolin-1 in endothelial cells is sufficient to inhibit the endocytosis of albumin and LDL across endothelial cells in vitro. Our report is the first to directly demonstrate a direct role for caveolin-1 in the regulation of LDL uptake/transcytosis. Previous studies have demonstrated a role for caveolin-1/caveolae in the regulation of albumin transfer across endothelial cells (Schnitzer and Oh 1994, Schubert et al. 2001) and it may be mediated by gp60 (Schnitzer and Oh 1994, Tiruppathi et al. 1997). Gp60 is associated with caveolin-1 in endothelial cells and has been shown to play an important role in the regulation of vascular permeability (Vogel et al. 2001). Our study demonstrates that caveolae-1/caveolae mediate the endocytosis of LDL and albumin in a specific manner. While the receptor for albumin has previously been characterized, the mechanisms regulating LDL endocytosis/transcytosis remain to be established. Simionescu and collaborators were the first to examine the existence of an LDL transcytosis pathway in endothelial cells (Vasile et al. 1983). When incubated with endothelial cells, LDL particles can follow two pathways, either endocytosis to respond to cellular cholesterol requirements or transcytosis across endothelial cells. The latter pathway appears to be predominant in this system (Vasile et al. 1983). Our data demonstrate the existence of a caveolae-dependent pathway that may mediate the endocytosis/transcytosis of LDL in endothelial cells. These findings are important, as they suggest an important role for the transfer or flux of LDL into the arterial wall. Accordingly, the regulation of caveolin-1 expression and caveolae formation in endothelial cells may play an important role in early atheroma formation. These data are also consistent with those obtained by Fernández-Hernando et al. (Fernández-Hernando et al. 2010), who showed that endothelial caveolin-1 can regulate LDL entry into the arterial wall. Moreover, they also support our previous studies showing that the permeability of the aorta to 125I-LDL is reduced in caveolin-1-deficient mice (Frank et al. 2008).
Caveolin-1 and the regulation of endothelial cell activation
A consequence of LDL transcytosis is intimal LDL particle accumulation and their modification. Modified LDL particles that are taken up by infiltrated monocyte-derived macrophages induce an inflammatory response characterized by the secretion of cytokines such as TNFα. As suggested by Frostegard et al., monocytes or macrophages exposed to oxLDL may secrete cytokines responsible for the increased adhesiveness of monocytes to endothelial cells (Frostegard et al. 1993). Signaling pathways activated by TNFα induce the expression of adhesion molecules at the surface of endothelial cells (Collins and Cybulsky 2001). These molecules include VCAM-1, ICAM-1, P-selectin and E-selectin (Galkina and Ley 2007). Increased VCAM-1 expression at the surface of endothelial cells has been associated with increased monocyte recruitment and increased atherosclerotic lesions formation in Apoe−/− mice (Dansky et al. 2001, Nakashima et al. 1998). Since LDL transfer and accumulation in the arterial wall is reduced in Cav-1−/−Apoe−/− compared to Apoe−/−, we can propose that, as an indirect consequence, endothelial cell activation is also reduced. This simple finding may explain at least in part, the reduced endothelium activation observed in Cav-1−/−Apoe−/− mice (Frank et al. 2004). Our study also demonstrates that caveolin-1 can regulate the TNFα signaling pathway in endothelial cells, thereby regulating the activation of NF-κB. Moreover, we show that oxLDL also regulates this pathway, although to a lesser extent than TNFα alone. An explanation for this finding may be that incubation of endothelial cell with oxLDL is also associated with reduced caveolin-1 protein levels. The latter finding is consistent with a role of oxLDL components (e.g., lysophosphatidylcholine) on plasma membrane cholesterol content and/or distribution. In agreement with this hypothesis, previous studies by Blair et al. (Blair et al. 1999) have shown that oxLDL can deplete caveolar cholesterol and induce the transfer of caveolin-1 to intracellular compartments. In that case, modification of plasma membrane cholesterol by oxidized lipids may be associated with a modification of cholesterol molecules and, eventually, caveolin-1 internalization. As a consequence, caveolin-1 protein half-life may be reduced. Since incubation of oxLDL with endothelial cells is associated with reduced caveolin-1 expression, which leads to reduced TNFα activation, our studies provide an explanation for the lack of effect of oxLDL on VCAM-1 expression in endothelial cells that we and others have observed (Khan et al. 1995).
When examining the development of atherosclerosis, it is believed that the transcytosis of LDL is an essential step leading to the formation of an atheroma. When LDL are transferred to the sub-endothelial space, they are transformed into modified LDL, which are believed to become trapped in the intima (Williams and Tabas 1995). These modified LDL could reduce caveolin-1 protein levels on the basal side of endothelial cells. Reduced caveolin-1 levels on the basal side could further enhance the movement and transcytosis of caveolae/caveolin-1 coming from the apical side of endothelial cells and worsen atherosclerotic lesion formation. Finally, oxLDL may not only affect caveolin-1 function but may also alter the plasma membrane localization of other proteins that play key roles in the development of atherosclerosis. These proteins include eNOS or SR-BI (Shaul 2003).
Conclusions
Our findings are in agreement with our previous results showing an increased VCAM-1 expression in the presence of caveolin-1 in the Apoe−/− aorta (Frank et al. 2004) compared to Cav-1−/−Apoe−/−. This data could explain the proatherogenic role of endothelial caveolin-1 during the development of atherosclerosis. It is likely that macrophage caveolin plays a role at a step downstream from the transcytosis of LDL. Therefore, in the Cav-1−/− background, the effect of macrophage caveolin-1 is masked by the inability of these mice to initiate lesion formation. Taken together, these data show that endothelial caveolin-1 is involved in the earliest stages of atherosclerosis development through LDL transcytosis and endothelial cell activation. Both of these processes promote atherogenesis through the accumulation of LDL in the arterial intima and recruitment of monocytes at the site of lesion.
Acknowledgments
The authors would like to thank Dr. Iset Medina Vera for her technical support. PGF was supported by grants from the Jane Barsumian/Mary Lyons Trust and theW.W. Smith Trust Fund.MPL was supported by grants from the National Institutes of Health and the American Heart Association. The Bioimaging Shared Resource of the Kimmel Cancer Center (NCI 5 P30 CA-56036) was used in this study.
Contributor Information
Stephanos Pavlides, Manchester Breast Centre & Breakthrough Breast Cancer Research Unit; Paterson Institute for Cancer Research; Institute of Cancer Sciences; Manchester Academic Health Science Centre, University of Manchester, Manchester, UK.
Jorge L. Gutierrez-Pajares, Department of Stem Cell Biology & Regenerative Medicine, Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA, USA
Jeannette Iturrieta, Department of Stem Cell Biology & Regenerative Medicine, Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA, USA.
Michael P. Lisanti, Manchester Breast Centre & Breakthrough Breast Cancer Research Unit; Paterson Institute for Cancer Research; Institute of Cancer Sciences; Manchester Academic Health Science Centre, University of Manchester, Manchester, UK
Philippe G. Frank, Email: philippe.frank@jefferson.edu, Department of Stem Cell Biology & Regenerative Medicine, Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA, USA; Department of Biochemistry and Molecular Biology, Kimmel Cancer Center, Thomas Jefferson University, 233 South 10th Street, Philadelphia, PA 19107, USA.
References
- Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem. 1999;274:32512–32519. doi: 10.1074/jbc.274.45.32512. [DOI] [PubMed] [Google Scholar]
- Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- Collins T, Cybulsky MI. NF-kappaB: pivotal mediator or innocent bystander in atherogenesis? J Clin Invest. 2001;107:255–264. doi: 10.1172/JCI10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansky HM, Barlow CB, Lominska C, Sikes JL, Kao C, Weinsaft J, Cybulsky MI, Smith JD. Adhesion of monocytes to arterial endothelium and initiation of atherosclerosis are critically dependent on vascular cell adhesion molecule-1 gene dosage. Arterioscler Thromb Vasc Biol. 2001;21:1662–1667. doi: 10.1161/hq1001.096625. [DOI] [PubMed] [Google Scholar]
- de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:904–914. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- Fernandez-Hernando C, Yu J, Davalos A, Prendergast J, Sessa WC. Endothelial-specific overexpression of caveolin-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2010;177:998–1003. doi: 10.2353/ajpath.2010.091287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Hernando C, Yu J, Suarez Y, Rahner C, Davalos A, Lasuncion MA, Sessa WC. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab. 2009;10:48–54. doi: 10.1016/j.cmet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- Frank PG, Lisanti MP. Caveolin-1 and caveolae in atherosclerosis: differential roles in fatty streak formation and neointimal hyperplasia. Curr Opin Lipidol. 2004;15:523–529. doi: 10.1097/00041433-200410000-00005. [DOI] [PubMed] [Google Scholar]
- Frank PG, Pavlides S, Cheung MW, Daumer K, Lisanti MP. Role of caveolin-1 in the regulation of lipoprotein metabolism. Am J Physiol Cell Physiol. 2008;295:C242–C248. doi: 10.1152/ajpcell.00185.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank PG, Pavlides S, Lisanti MP. Caveolae and transcytosis in endothelial cells: role in atherosclerosis. Cell Tissue Res. 2009;335:41–47. doi: 10.1007/s00441-008-0659-8. [DOI] [PubMed] [Google Scholar]
- Frank PG, Woodman SE, Park DS, Lisanti MP. Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol. 2003;23:1161–1168. doi: 10.1161/01.ATV.0000070546.16946.3A. [DOI] [PubMed] [Google Scholar]
- Frostegard J, Wu R, Haegerstrand A, Patarroyo M, Lefvert AK, Nilsson J. Mononuclear leukocytes exposed to oxidized low density lipoprotein secrete a factor that stimulates endothelial cells to express adhesion molecules. Atherosclerosis. 1993;103:213–219. doi: 10.1016/0021-9150(93)90264-u. [DOI] [PubMed] [Google Scholar]
- Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- Hassan GS, Jasmin JF, Schubert W, Frank PG, Lisanti MP. Caveolin-1 deficiency stimulates neointima formation during vascular injury. Biochemistry. 2004;43:8312–8321. doi: 10.1021/bi049609t. [DOI] [PubMed] [Google Scholar]
- Khan BV, Parthasarathy SS, Alexander RW, Medford RM. Modified low density lipoprotein and its constituents augment cytokine-activated vascular cell adhesion molecule-1 gene expression in human vascular endothelial cells. J Clin Invest. 1995;95:1262–1270. doi: 10.1172/JCI117776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshall RD, Tiruppathi C, Vogel SM, Malik AB. Vesicle formation and trafficking in endothelial cells and regulation of endothelial barrier function. Histochem Cell Biol. 2002;117:105–112. doi: 10.1007/s00418-001-0367-x. [DOI] [PubMed] [Google Scholar]
- Monks J, Neville MC. Albumin transcytosis across the epithelium of the lactating mouse mammary gland. J Physiol. 2004;560:267–280. doi: 10.1113/jphysiol.2004.068403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:842–851. doi: 10.1161/01.atv.18.5.842. [DOI] [PubMed] [Google Scholar]
- Palade GE, Bruns RR. Structural modification of plasmalemma vesicles. J Cell Biol. 1968;37:633–649. doi: 10.1083/jcb.37.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parat MO. The biology of caveolae: achievements and perspectives. Int Rev Cell Mol Biol. 2009;273:117–162. doi: 10.1016/S1937-6448(08)01804-2. [DOI] [PubMed] [Google Scholar]
- Pascariu M, Bendayan M, Ghitescu L. Correlated endothelial caveolin overexpression and increased transcytosis in experimental diabetes. J Histochem Cytochem. 2004;52:65–76. doi: 10.1177/002215540405200107. [DOI] [PubMed] [Google Scholar]
- Pavlides S, Gutierrez-Pajares JL, Danilo C, Lisanti MP, Frank PG. Atherosclerosis, caveolae and caveolin-1. Adv Exp Med Biol. 2012;729:127–144. doi: 10.1007/978-1-4614-1222-9_9. [DOI] [PubMed] [Google Scholar]
- Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P. Albondin-mediated capillary permeability to albumin. Differential role of receptors in endothelial transcytosis and endocytosis of native and modified albumins. J Biol Chem. 1994;269:6072–6082. [PubMed] [Google Scholar]
- Schubert W, Frank PG, Razani B, Park DS, Chow CW, Lisanti MP. Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J Biol Chem. 2001;276:48619–48622. doi: 10.1074/jbc.C100613200. [DOI] [PubMed] [Google Scholar]
- Schubert W, Frank PG, Woodman SE, Hyogo H, Cohen DE, Chow CW, Lisanti MP. Microvascular hyperpermeability in caveolin-1 (−/−) knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, L-name, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem. 2002;277:40091–40098. doi: 10.1074/jbc.M205948200. [DOI] [PubMed] [Google Scholar]
- Sharma A, Yu C, Bernatchez PN. New insights into caveolae, caveolins and endothelial function. Can J Cardiol. 2010;26(Suppl A):5A–8A. doi: 10.1016/s0828-282x(10)71053-9. [DOI] [PubMed] [Google Scholar]
- Shaul PW. Endothelial nitric oxide synthase, caveolae and the development of atherosclerosis. J Physiol. 2003;547:21–33. doi: 10.1113/jphysiol.2002.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu M. Implications of early structural-functional changes in the endothelium for vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:266–274. doi: 10.1161/01.ATV.0000253884.13901.e4. [DOI] [PubMed] [Google Scholar]
- Simionescu M, Popov D, Sima A. Endothelial transcytosis in health and disease. Cell Tissue Res. 2009;335:27–40. doi: 10.1007/s00441-008-0688-3. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem. 1997;272:25968–25975. doi: 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- Vasile E, Simionescu M, Simionescu N. Visualization of the binding, endocytosis, and transcytosis of low density lipoprotein in the arterial endothelium in situ. J Cell Biol. 1983;96:1677–1689. doi: 10.1083/jcb.96.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel SM, Easington CR, Minshall RD, Niles WD, Tiruppathi C, Hollenberg SM, Parrillo JE, Malik AB. Evidence of transcellular permeability pathway in microvessels. Microvasc Res. 2001;61:87–101. doi: 10.1006/mvre.2000.2274. [DOI] [PubMed] [Google Scholar]
- Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]