Abstract
Background
Anti-Müllerian hormone (AMH) is secreted by the granulosa cells of growing follicles during the primary to large antral follicle stages. Abnormal levels of AMH and follicle stimulating hormone (FSH) may indicate a woman’s diminished ability or inability to conceive. Our aim is to investigate the changes in serum AMH and FSH concentrations at different age groups and its correlation with ovarian reserves in infertile women.
Materials and Methods
This cross-sectional study analyzed serum AMH and FSH levels from 197 infertile women and 176 healthy controls, whose mean ages were 19-47 years. Sample collection was performed by random sampling and analyzed with SPSS version 16 software.
Results
There were significantly lower mean serum AMH levels among infertile women compared to the control group. The mean AMH serum levels from different ages of infertile and control group (fertile women) decreased with increasing age. However, this reduction was greater in the infertile group. The mean FSH serum levels of infertile women were significantly higher than the control group. Mean serum FSH levels consistently increased with increasing age in infertile women; however mean luteinizing hormone (LH) levels were not consistent.
Conclusion
We have observed increased FSH levels and decreased AMH levels with increasing age in women from 19 to 47 years of age. Assessments of AMH and FSH levels in combination with female age can help in predicting ovarian reserve in infertile women.
Keywords: Anti-Müllerian Hormone, Follicle Stimulating Hormone, Infertility, Women, Age
Introduction
One of the main causes for infertility is decreased ovarian reserve. Ovarian reserve is the number of good quality oocytes that remain within the ovaries. As a woman’s age increases, her ovarian reserves decline (1,5). This decline may also result from autoimmune, genetic and iatrogenic conditions (6). In addition, autoimmune endocrinopathies, radiation therapy, or pelvic surgery may lead to decreased ovarian reserve (7,9). There are different tests to diagnose reductions in ovarian reserve such as elevated serum follicle stimulating hormone ( FSH ) levels on days 2 or 3 of the menstrual cycle FSH is mostly used in routine laboratories), low ovarian volume, an antral follicle count of <5 per ovary, low inhibin B levels, and <5 oocytes retrieved during an assisted reproductive technology ( ART ) cycle. A new test for the assessment of decreased ovarian reserve analyzes anti-Müllerian hormone ( AMH ) levels (10,14). AMH, a member of the transforming growth factor superfamily, is secreted in the human ovary by granulosa cells of primary growing follicles until the early antral stage (15,19). In females AMH regulates the growth of primary follicles by inhibiting further recruitment of other follicles during folliculogenesis (20). Serum AMH levels decline with increasing age in women and the levels are undetectable after menopause (21). Serum levels of AMH decrease prior to any increase in baseline FSH. FSH mostly indicates follicular maturation during the previous two weeks when gonadotropin follicles become sensitive. However AMH levels indicate pre-antral follicles to the post-primary pool that pass through stages before folliculogenesis (22,24).
Few studies have assessed the levels of FSH and AMH at various ages in infertile women, therefore our aim was to investigate the changes in serum concentrations of AMH and FSH at different ages and its correlation with ovarian reserves in infertile women.
Materials and Methods
This cross-sectional study was performed at the Research Center of Infertility, Shahid Sadoughi University, Yazd, Iran between May 2010 and September 2012. We assessed serum AMH and FSH levels on days 2 or 3 of the menstrual cycles of 197 infertile women with problem decreasing ovarian reserve and 176 healthy controls, without decreasing ovarian reserve and age 19-47 years, who were admitted to infertility clinic to investigate infertility. Inclusion criteria were: no history of gynecological surgical procedures, presence of a regular menstrual cycle, no signs of hyper-androgenemia, and normal sonographic appearance of the ovaries. Infertile women were excluded if they were using fertility drugs or had any autoimmune, genetic, or iatrogenic conditions, autoimmune endocrinopathies, radiation therapy or pelvic surgery, or polycystic ovary syndrome as these factors have been shown to alter serum AMH levels (6,10).
Patients were stratified into the following age categories: <25, 25-29.9, 30-34.9, 35-39.9, 40-45 and ≥45 years. Ethical approval for the study was received from the Women’s and Medical Ethics Committee.
This approval allowed for measurement of serum AMH levels in stored routine clinical samples without the need for the patient’s written permission in order to produce an age-related normal range data for AMH levels. All 197 patients and 176 healthy controls were asked to provide their consent in order to link their AMH results with IVF outcome.
Serum AMH levels were assessed in serum by the enzyme linked immunosorbent assay ( ELISA ) method ( Beckman Coulter, USA ). The sensitivity of the assay is 0.08 ng/ml with a reference range of 12.6 ng/ml. Interand intra-assay coefficients of variation ( CV ) are <7.7% and <5.8%, respectively. Luteinizing hormone ( LH ) and FSH levels were assessed in serum by the electrochemiluminescent immunoassay ( ECLIA ) method ( Cobas, England ). The assay sensitivity for FSH is <0.1 mIU/ml with interand intraassay CVs of <7.7% and <5.8%, respectively. Sample collection was performed by random sampling at days 3-5 of a spontaneous menstrual cycle. The serum was separated one hour after sampling and frozen at -20˚C until assayed.
Data analysis
The data were presented as mean±standard deviation as calculated in each group by SPSS software version 16 ( SPSS Inc., Chicago, IL, USA ). The student’s t test was used to assess differences between mean values of AMH, FSH and LH in the infertility and control groups with a confidence level of 95% and p value <0.05.
Results
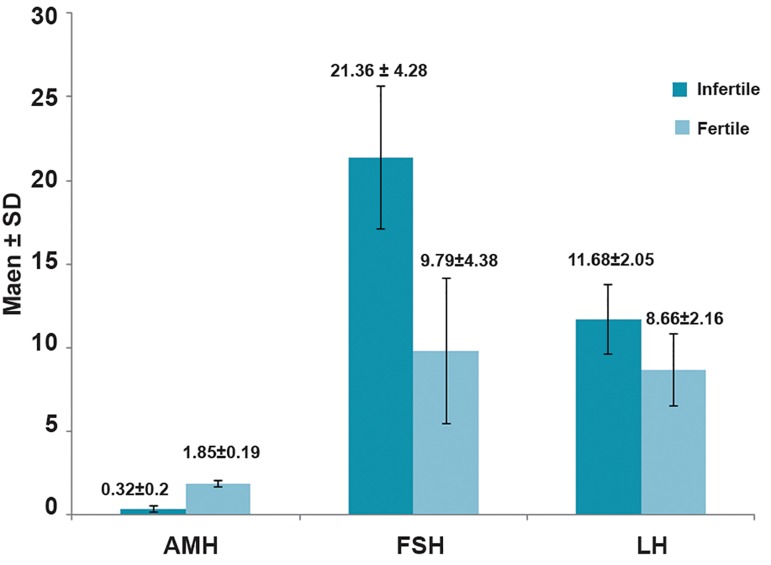
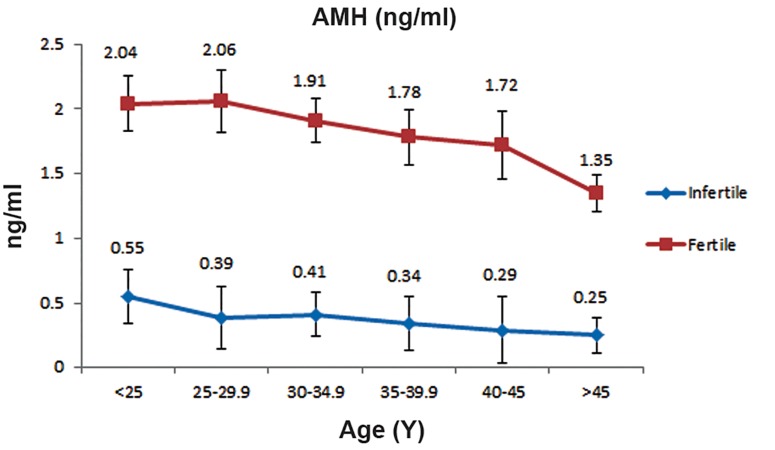
Table 1 shows the mean AMH, FSH and LH levels according to the six age categories. Overall there was a significantly lower mean AMH serum level in infertile women compared to the control group (Fig.1,p=0.002). Although the mean serum AMH levels consistently decreased with increasing age in both the infertile and control groups, the reduction seen in the infertile group was more (Fig.2,p<0.05). Overall, the mean FSH serum level in infertile women was significantly higher than the control group (Fig.1,p=0.03).
Fig.1.
Mean±standard deviation in anti-Müllerian hormone (AMH), follicle stimulating hormone (FSH) and luteinizing hormone (LH) in both fertile and infertile women.
Fig.2.
Mean±standard deviation values for anti-Müllerian hormone (AMH) over the reproductive age range in control group and infertile women.
Mean serum FSH levels consistently increased with increasing age in both the infertile and control groups.
However, this increase was higher in the infertile group (Fig.3,p<0.05). The mean LH serum level in infertile women was higher than the control group (Fig.1,p=0.32). The mean LH serum level in both groups with increasing age was not consistent (Fig.4).
Fig.3.
Mean±standard follicle stimulating hormone (FSH) values for the reproductive age range in control and infertile group women.
Fig.4.
Mean±standard deviation values for follicle luteinizing stimulating hormone (LH) over the reproductive age range in fertile and infertile women.
Table 1.
Changes in anti-Mullerian hormone (AMH), follicle stimulating hormone (FSH) and luteinizing hormone (LH) serum concentrations for different ages of women in the infertile and control groups
| Age (Y) | Infertile Mean±SD | Fertile Mean±SD | P value | |
|---|---|---|---|---|
| n=197 | n=176 | |||
| 19-47 Total sample | BMI (kg/m2) | 27±2 | 27±6 | |
| AMH (ng/ml) | 0.32±0.2 | 1.85±0.19 | 0.002 | |
| FSH (mIU/ml) | 21.36±4.28 | 9.79±4.38 | 0.03 | |
| LH (mIU/ml) | 11.68±2.05 | 8.66±2.16 | 0.32 | |
| n=17 | n=15 | |||
| <25 | BMI (kg/m2) | 28±3 | 27±5 | |
| AMH (ng/ml) | 0.55±0.16 | 2.04±0.21 | 0.000 | |
| FSH (mIU/ml) | 17.27±2.36 | 5.02±4.39 | 0.002 | |
| LH (mIU/ml) | 9.2±1.25 | 5.54±1.23 | 0.2 | |
| n=29 | n=27 | |||
| 25-29.9 | BMI (kg/m2) | 26±2 | 27±4 | |
| AMH (ng/ml) | 0.39±0.23 | 2.06±0.24 | 0.001 | |
| FSH (mIU/ml) | 20.41±4.49 | 6.7±3.35 | 0.04 | |
| LH (mIU/ml) | 12.32±2.54 | 8.98±1.35 | 0.27 | |
| n=38 | n=32 | |||
| 30-34.9 | BMI (kg/m2) | 28±1 | 26±7 | |
| AMH (ng/ml) | 0.41±0.21 | 1.91±0.17 | 0.000 | |
| FSH (mIU/ml) | 29.3±3.24 | 13.21±5.26 | 0.02 | |
| LH (mIU/ml) | 14.76±1.81 | 9.39±2.49 | 0.35 | |
| n=22 | n=57 | |||
| 35-39.9 | BMI (kg/m2) | 27±5 | 28±1 | |
| AMH (ng/ml) | 0.34±0.18 | 1.78±0.21 | 0.000 | |
| FSH (mIU/ml) | 27.48±5.62 | 7.02±1.66 | 0.004 | |
| LH (mIU/ml) | 12.38±2.53 | 6.89±3.39 | 0.55 | |
| n=61 | n=31 | |||
| 40-44.9 | BMI (kg/m2) | 28±6 | 26±3 | |
| AMH (ng/ml) | 0.29±0.25 | 1.72±0.26 | 0.032 | |
| FSH (mIU/ml) | 29.32±3.59 | 13.23±6.34 | 0.023 | |
| LH (mIU/ml) | 7.22±2.3 | 6.32±2.12 | 0.34 | |
| n=30 | n=14 | |||
| ≥45 | BMI (kg/m2) | 27±2 | 28±4 | |
| AMH (ng/ml) | 0.25±0.22 | 1.35±0.14 | 0.012 | |
| FSH (mIU/ml) | 32.54±6.42 | 17±5.32 | 0.043 | |
| LH (mIU/ml) | 10.61±1.87 | 8.34±2.43 | 0.21 | |
BMI; Body mass index.
Discussion
In this study infertile women had higher FSH levels and lower AMH levels than fertile women. The range of AMH observed in infertile women was <1 whereas in the control group it was approximately 1 to 3. Mean serum AMH levels steadily decreased with increasing age in the age range of 19 to 47 years. In addition, mean FSH level approximately increased with increasing age in this range ( 19 to 47 years ) and was attributed to reduced ovarian reserve. Since AMH are produced by preantral and antral follicles (15,18,24,25), hence with increasing age, levels of pre-antral follicles decrease, causing a reduction in the amount of AMH. Thus, lower levels of AMH and higher FSH show declining ovarian reserve. Increased levels of FSH and decreased AMH can be considered as a marker for reduced fertility potential. This fluctuation on the third day FSH levels makes it difficult to predict ovarian reserve. Hence, the most appropriate factor for the assessment of ovarian reserve is an evaluation of AMH levels, which are independent of the cycle.
We observed good correlation between serum AMH and FSH levels with ovarian reserve. These results supported those of previous studies in terms of the connections between low AMH serum levels and poor ovarian response (26,27). Several studies examined decreased AMH with age. Seifer et al. (25) reported that mean AMH levels decreased steadily with increasing age, in the range from 24 to 50 years, which was similar to the results of the current study.
La Marca et al. (28), in a study on 277 healthy women ( aged 18-50 years ) reported that serum AMH levels progressively declined with increasing age. Mulders et al. evaluated serum AMH levels in 98 infertile and 48 healthy control women. They reported that serum AMH levels decreased over time in both infertile and healthy control women, which was similar to the results of our study (29).
Conclusion
With increasing age AMH levels decrease due to reduced ovarian reserves. Hence AMH can be used as a marker for the assessment of ovarian reserves in the follicular and luteal phases.
Acknowledgments
The authors wish to express their gratitude to the staff of the Research and Clinical Infertility Center in Yazd, Iran. There is no financial support for this article. The authors declare that they have no conflict of interests.
References
- 1.Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7(10):1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 2.Klein NA, Battaglia DE, Fujimoto VY, Davis GS, Bremner WJ, Soules MR. Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J Clin Endocrinol Metab. 1996;81(3):1038–1045. doi: 10.1210/jcem.81.3.8772573. [DOI] [PubMed] [Google Scholar]
- 3.Gougeon A. Ovarian follicular growth in humans: ovarian ageing and population of growing follicles. Maturitas. 1998;30(2):137–142. doi: 10.1016/s0378-5122(98)00069-3. [DOI] [PubMed] [Google Scholar]
- 4.Gougeon A, Ecochard R, Thalabard JC. Age-related changes of the population of human ovarian follicles: increase in the disappearance rate of non-growing and earlygrowing follicles in aging women. Biol Reprod. 1994;50(3):653–663. doi: 10.1095/biolreprod50.3.653. [DOI] [PubMed] [Google Scholar]
- 5.te Velde ER, Scheffer GJ, Dorland M, Broekmans FJ, Fauser BC. Developmental and endocrine aspects of normal ovarian aging. Mol Cell Endocrinol. 1998;145(1-2):67–73. doi: 10.1016/s0303-7207(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, et al. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20(2):402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- 7.Bath LE, Wallace WH, Shaw MP, Fitzpatrick C, Anderson RA. Depletion of ovarian reserve in young women after treatment for cancer in childhood: detection by anti-Mullerian hormone, inhibin B and ovarian ultrasound. Hum Reprod. 2003;18(11):2368–2374. doi: 10.1093/humrep/deg473. [DOI] [PubMed] [Google Scholar]
- 8.Iwase A, Hirokawa W, Goto M, Takikawa S, Nagatomo Y, Nakahara T, et al. Serum anti-Mullerian hormone level is a useful marker for evaluating the impact of laparoscopic cystectomy on ovarian reserve. Fertil Steril. 2010;94(7):2846–2849. doi: 10.1016/j.fertnstert.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Papaleo E, De Santis L, Fusi F, Doldi N, Brigante C, Marelli G, et al. Natural cycle as first approach in aged patients with elevated follicle-stimulating hormone undergoing intracytoplasmic sperm injection: a pilot study. Gynecol Endocrinol. 2006;22(7):351–354. doi: 10.1080/09513590600818992. [DOI] [PubMed] [Google Scholar]
- 10.Coccia ME, Rizzello F. Ovarian reserve. Ann N Y Acad Sci. 2008;1127:27–30. doi: 10.1196/annals.1434.011. [DOI] [PubMed] [Google Scholar]
- 11.Bukman A, Heineman MJ. Ovarian reserve testing and the use of prognostic models in patients with subfertility. Hum Reprod Update. 2001;7(6):581–590. doi: 10.1093/humupd/7.6.581. [DOI] [PubMed] [Google Scholar]
- 12.Dennis NA, Houghton LA, Jones GT, van Rij AM, Morgan K, McLennan IS. The level of serum anti-Mullerian hormone correlates with vitamin D status in men and women but not in boys. J Clin Endocrinol Metab. 2012;97(7):2450–2455. doi: 10.1210/jc.2012-1213. [DOI] [PubMed] [Google Scholar]
- 13.Maheshwari A, Fowler P, Bhattacharya S. Assessment of ovarian reserve--should we perform tests of ovarian reserve routinely? Hum Reprod. 2006;21(11):2729–2735. doi: 10.1093/humrep/del188. [DOI] [PubMed] [Google Scholar]
- 14.van Rooij IA, Broekmans FJ, Hunault CC, Scheffer GJ, Eijkemans MJ, de Jong FH, et al. Use of ovarian reserve tests for the prediction of ongoing pregnancy in couples with unexplained or mild male infertility. Reprod Biomed Online. 2006;12(2):182–190. doi: 10.1016/s1472-6483(10)60859-0. [DOI] [PubMed] [Google Scholar]
- 15.Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 16.Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction. 2002;124(5):601–609. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- 17.Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97(5):1673–1680. doi: 10.1210/jc.2011-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Josso N. Anti-mullerian hormone. Clin Endocrinol (Oxf) 1986;25(3):331–345. doi: 10.1111/j.1365-2265.1986.tb01699.x. [DOI] [PubMed] [Google Scholar]
- 19.Li HW, Ng EH, Wong BP, Anderson RA, Ho PC, Yeung WS. Correlation between three assay systems for antiMullerian hormone (AMH) determination. J Assist Reprod Genet. 2012;29(12):1443–1446. doi: 10.1007/s10815-012-9880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Visser JA, de Jong FH, Laven JS, Themmen AP. AntiMullerian hormone: a new marker for ovarian function. Reproduction. 2006;131(1):1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- 21.de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77(2):357–362. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 22.Feyereisen E, Mendez Lozano DH, Taieb J, Hesters L, Frydman R, Fanchin R. Anti-Mullerian hormone: clinical insights into a promising biomarker of ovarian follicular status. Reprod Biomed Online. 2006;12(6):695–703. doi: 10.1016/s1472-6483(10)61081-4. [DOI] [PubMed] [Google Scholar]
- 23.La Marca A, Volpe A. Anti-Mullerian hormone (AMH) in female reproduction: is measurement of circulating AMH a useful tool? Clin Endocrinol (Oxf) 2006;64(6):603–610. doi: 10.1111/j.1365-2265.2006.02533.x. [DOI] [PubMed] [Google Scholar]
- 24.Fanchin R, Schonauer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. Serum anti-Mullerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18(2):323–327. doi: 10.1093/humrep/deg042. [DOI] [PubMed] [Google Scholar]
- 25.Seifer DB, Baker VL, Leader B. Age-specific serum antiMullerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril. 2011;95(2):747–750. doi: 10.1016/j.fertnstert.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum mullerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77(3):468–471. doi: 10.1016/s0015-0282(01)03201-0. [DOI] [PubMed] [Google Scholar]
- 27.van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, et al. Serum anti-Mullerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17(12):3065–3071. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 28.La Marca A, Sighinolfi G, Giulini S, Traglia M, Argento C, Sala C, et al. Normal serum concentrations of anti-Mullerian hormone in women with regular menstrual cycles. Reprod Biomed Online. 2010;21(4):463–469. doi: 10.1016/j.rbmo.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Mulders AG, Laven JS, Eijkemans MJ, de Jong FH, Themmen AP, Fauser BC. Changes in anti-Mullerian hormone serum concentrations over time suggest delayed ovarian ageing in normogonadotrophic anovulatory infertility. Hum Reprod. 2004;19(9):2036–2042. doi: 10.1093/humrep/deh373. [DOI] [PubMed] [Google Scholar]