Abstract
Functional neuroimaging has become an increasingly common tool for studying drug craving. Furthermore, functional neuroimaging studies, which have addressed an incredibly diverse array of questions regarding the nature and treatment of craving, have had a substantial impact on theoretical models of addiction. Here, we offer three points related to this sizeable and influential body of research. First, we assert that the craving most investigators seek to study represents not just a desire but a strong desire to use drugs, consistent with prominent theoretical and clinical descriptions of craving. Second, we highlight that, despite the clear conceptual and clinical emphasis on craving as an intense desire, brain imaging studies often have been explicitly designed in a way that reduces the ability to generate powerful cravings. We illustrate this point by reviewing the peak urge levels endorsed by participants in functional magnetic resonance imaging (fMRI) studies of cigarette craving in nicotine-deprived versus nondeprived smokers. Third, we suggest that brain responses measured during mild states of desire (such as following satiety) differ in fundamental ways from those measured during states of overpowering desire (i.e., craving) to use drugs. We support this position by way of a meta-analysis revealing that fMRI cue exposure studies using nicotine-deprived smokers have produced different patterns of brain activation than those using nondeprived smokers. Regarding brain imaging studies of craving, intensity of the urges matter, and more explicit attention to urge intensity in future work has the potential to yield valuable information about the nature of craving.
Functional neuroimaging has become one of the most widely used tools for studying drug addiction (1). In particular, an enormous amount of functional neuroimaging research has focused on drug craving, a construct that has been central to the study of addiction for more than half a century (2). Huge sums of money are spent each year on functional neuroimaging experiments that relate to craving, as evidenced by the proliferation of such studies since our review of the literature a decade ago (3). This now sizeable (and still rapidly growing) literature includes basic investigations designed to understand the neural architecture of craving (4), translational studies testing a range of pharmacological and psychological treatments designed to curb cravings (5), and expensive protocols aimed at evaluating genetic moderators of craving (6). These examples, which provide only a narrow sampling of this work, reveal both the clinical and theoretical importance of functional neuroimaging research on craving (1, 2).
Here, we offer three points related to this expansive and influential body of research. First, we suggest the these studies as a whole have been aimed at shedding light on craving that is best conceptualized as an intense and overwhelming desire – and not simply any desire – to use drugs (2, 7, 8). Second, we point out that, despite the clear focus on craving as a particularly strong desire from both scientific and clinical perspectives, many studies have been designed in such a way that precludes the ability to provoke strong desires. Specifically, using functional magnetic resonance imaging (fMRI) investigations examining cue-elicited desire to smoke as a prevalent and representative subset of the literature, we find that studies often have produced a relatively modest desire state because they create satiety by requiring participants to smoke a cigarette just prior to conducting their tests. Third, we contend that mild desire differs in fundamental ways from the strong desire state that characterizes conceptually and clinically meaningful craving (2), much like a mild fear is distinct from a full blown panic attack. In support of this view, we conduct a quantitative meta-analysis of fMRI smoking cue exposure studies to reveal that they have produced different results according to the degree of deprivation required of participants at the outset. Stated differently, urge intensity matters. We propose that a greater focus on urge intensity has the potential to provide insight into some of the most significant questions currently being debated in the experimental drug craving field. Our overarching goal is to stimulate discussion regarding the need to be more mindful of how craving is manipulated and measured in functional brain imaging research.
Craving is Widely Viewed as an Overpowering State of Desire
A review of the language used in functional neuroimaging studies incorporating cue-reactivity methods (i.e., exposing addicted individuals to drug-related stimuli) makes clear that investigating the nature of a subjective craving experience has been an important – and often primary – goal of this research. For example, with few exceptions, studies have highlighted the clinical significance of craving as a rationale for investigating brain activity linked to drug cue exposure (with many articles containing the word craving in the title or as a keyword). Similarly, studies have routinely interpreted and discussed the implications of their findings in relation to craving. Thus, we believe it is reasonable to assert that neuroimaging cue-reactivity studies have generally sought to induce clinically-relevant states of craving. But what exactly constitutes clinically-relevant craving?
Our first point is that craving at its core represents not just a desire to use drugs but rather a strong desire to do so (2, 7, 8). To find support for this idea, one need look no further than the descriptions of craving offered by internationally recognized leaders in the field of addiction research. Consider the following quote from a recent review by Volkow and colleagues (8):
Some of the most pernicious features of drug addiction are the overwhelming craving to take drugs that can reemerge even after years of abstinence, and the severely compromised ability of addicted individuals to inhibit drug seeking once the craving erupts in spite of well-known negative consequences. (p. 753)
This portrayal of craving as an overpowering experience is consistent with the notion that the desire to use drugs takes on particular clinical significance when it reaches an intense level (2, 9). The following depiction provided by George and Koob (7) similarly underscores the idea that craving is a state of strong desire:
Craving is what makes addiction to drugs so difficult to overcome. The intense craving that follows a cue that has been previously associated with the drug, combined with a stressful state or a dysphoric state, represents an unstoppable force that leads to drug intake and relapse for most addicted individuals. (p. 4165)
The emphasis on the penetrating nature of craving is also reflected in the latest edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM–5; 10). Craving, which was added as a key symptom of addictive disorders, is defined in the DSM-5 as an “intense desire or urge,” with the manual noting that craving should be assessed by “asking [individuals] if there has ever been a time when they had such strong urges to use the drug that they could not think of anything else” (p. 483). As noted elsewhere (11): for most individuals addicted to drugs, “a ‘weak craving’ is an oxymoron” (p. 11). Clearly, then, from both conceptual and clinical perspectives, the focus is on craving as a robust state and not a period of weak to moderate desire.
Success at Provoking Craving has Varied Widely across Studies
We believe that many addiction researchers would agree with our first point. It is therefore noteworthy that many neuroimaging cue-reactivity studies have been explicitly designed in a way that minimizes the ability to provoke powerful desires (cravings). To illustrate this point, we conducted a review of fMRI studies in which cigarette cues were presented in an attempt to elicit a desire to smoke in adults. We identified studies by searching the Medline/Pubmed database using a combination of keywords related to smoking/craving (cigarette, craving, cue, desire, smoker, smoking, or urge) and fMRI (blood-oxygen-level dependent, BOLD, brain imaging, fMRI, imaging, MRI, or neuroimaging). Of the identified studies, we included those for which peak self-reported urge, expressed as a percentage of the maximum scale value, could be discerned (those reporting only baseline urge levels, only changes in urge, or failing to report scale endpoints were excluded), yielding a total of 32 (sub)samples across 24 studies (several studies included multiple subgroups and/or conditions; see Table 1).
Table 1.
Means (SD) for Select Sample Characteristics
| First Author | Year | Subgroup and/or Condition | Cue(s) | Urge Measure | % of Scale |
|---|---|---|---|---|---|
| Nicotine-Deprived | |||||
| Canterberry | 2013 | Prior to neurofeedback | Pic | 1–10 scale | 64 |
| David | 2005 | n/a | Pic | SJCS | 50 |
| David | 2007 | First abstinent session | Pic | SJCS | 57 |
| David | 2007 | Second abstinent session | Pic | SJCS | 76 |
| Due | 2002 | n/a | Pic | 0–6 scale | 84 |
| Goudriaan | 2010 | Heavy smokers | Pic | QSU (partialc) | 59 |
| Kobera | 2010 | Focusing on immediate effects of smoking | Pic | 1–5 scale | 85 |
| Li | 2013 | Not resisting urge | Pic; Handle | 1–5 scale | 73 |
| McBridea | 2006 | Expectant group; abstinent session | Vid | QSU (partialb) | 70 |
| McBridea | 2006 | Nonexpectant group; abstinent session | Pic | QSU (partialb) | 75 |
| McClernona | 2005 | Abstinent session | Pic | SJCS | 69 |
| Stippekohl | 2010 | Abstinent group; images of 2nd smoking stage | Pic | 1–9 scale | 74 |
| Westbrook | 2011 | Responding naturally | Pic | 1–5 scale | 56 |
| Wilson | 2005 | Expectant group | Handle | 0–100 scale | 72 |
| Wilson | 2005 | Nonexpectant group | Handle | 0–100 scale | 77 |
| Wilson | 2012 | Quitting-unmotivated; expectant group | Handle | 0–100 scale | 75 |
| Wilson | 2012 | Quitting-unmotivated; nonexpectant group | Handle | 0–100 scale | 73 |
| Wilson | 2012 | Quitting-motivated; expectant group | Handle | 0–100 scale | 77 |
| Wilson | 2012 | Quitting-motivated; nonexpectant group | Handle | 0–100 scale | 70 |
| Xu | 2012 | Weighted mean of high and low urge groups | Pic | 0–100 scale | 50 |
| M = 69.3 SD = 10.1 | |||||
| Nondeprived | |||||
| Bourque | 2013 | n/a | Pic | 0–100 scale | 47 |
| Brody | 2007 | Not resisting urge | Vid | 1–5 scale | 58 |
| Culbertson | 2011 | Placebo group; pretreatment; not resisting urge | Vid | 1–5 scale | 61 |
| David | 2007 | First nonabstinent session | Pic | SJCS | 31 |
| David | 2007 | Second nonabstinent session | Pic | SJCS | 36 |
| Franklin | 2007 | n/a | Vid; Handle | 1–7 scale | 69 |
| McBridea | 2006 | Expectant group; nonabstinent session | Vid | QSU (partialb) | 50 |
| McBridea | 2006 | Nonexpectant group; nonabstinent session | Vid | QSU (partialb) | 62 |
| McClernona | 2005 | Nonabstinent session | Pic | SJCS | 50 |
| Smolka | 2006 | n/a | Pic | 0–100 scale | 49 |
| Stippekohl | 2010 | Nonabstinent group; images of 2nd smoking stage | Pic | 1–9 scale | 52 |
| Vollstädt-Klein | 2011 | n/a | Pic | 0–100 scale | 41 |
| M = 50.5 SD = 11.0 | |||||
Note. Handle = holding and viewing an unlit cigarette; QSU = Questionnaire on Smoking Urges; Pic = viewing smoking-related pictures; SJCS = Shiffman-Jarvik Craving Scale; Vid = viewing smoking-related videos.
Urge scores were estimated from a figure.
Participants completed a subset of 7 items selected from the QSU.
Participants completed subset of 10 items selected from the QSU (it is unclear whether this was the 10-item version of the QSU referred to as the QSU-brief).
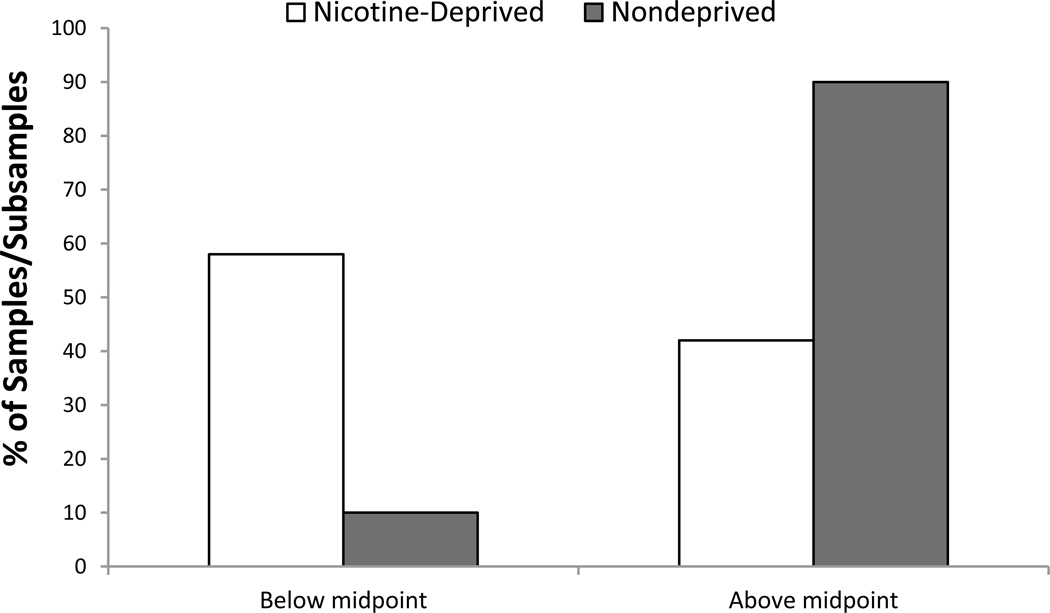
As presented in Table 1, 12 of the 32 samples instructed participants to smoke ad libitum before the experiment (12–21), while the remaining 20 required participants to abstain from smoking for a period of time (ranging from 2–16 hours) prior to the onset of the experimental visit(s) (15, 17,18,20,22–31). As expected, mean ratings of the desire to smoke were noticeably larger in nicotine-deprived smokers (69.3% of scale maximum) relative to nondeprived smokers (50.5% of scale maximum). The difference in average smoking desire is even more striking when considering the proportion of nicotine-deprived versus nondeprived samples for which mean ratings fell above or below the midpoint of the scale. As shown in Figure 1, mean ratings fell in the upper half of the scale for almost all (90%) of nicotine-deprived samples but only a minority (42%) of the nondeprived samples.
Figure 1.
The percentage of samples of nicotine-deprived (white bars) and nondeprived (gray bars) smokers for which mean self-reported urge ratings fell above or below the midpoint of the urge rating scale.
In some cases (15, 17, 18), studies measured responses in smokers under both nondeprived and deprived conditions with the goal of directly examining potential differences in brain activation between these states. Nevertheless, we argue that the field generally has not paid sufficient attention to the fact that many previous studies have used procedures that dampen cravings. For instance, results obtained from addicted individuals in nondeprived and deprived states are often lumped together with relatively little consideration of the very different conditions under which such results were obtained (but see 4). Indeed, we have done so ourselves in prior work (3). This overlooks what we believe is a crucial point. Namely, for some studies – especially those requiring periods of nicotine deprivation – brain responses to cigarette cues have been measured in the context of a strong desire/craving to smoke [akin to what was described by Drs. Volkow, Koob, and their colleagues (7, 8), and that is featured in DSM-5 (10)]. For many other studies, however, cue-elicited brain activity has been assessed in smokers experiencing a more modest desire to smoke. Moreover, we suspect that this remains an ongoing issue for the field (i.e., that additional brain imaging studies using suboptimal procedures for provoking craving are being conducted).
Studying Mild Desire may not be the same as Studying Craving
The patterns presented in Figure 1 are of little consequence if there are only quantitative differences between the responses measured during mild versus strong desires/cravings. To the contrary, there are reasons to challenge this notion on both conceptual and empirical grounds. From a conceptual perspective, there would seem to be a clear distinction between how drug cues are processed by addicts during a modest desire to consume drugs – such as immediately following substance use – relative to those in the midst of an intense desire that is fueled in part by acute abstinence (15–18). The idea that there is a qualitative difference between desires of high and low intensity is compatible with basic theory and research regarding the nature of emotional experiences (32). To the extent that affect is an important component of the desire to use drugs (32), such desire states may be expected to change qualitatively or nonlinearly and take on unique properties as they become particularly robust (see 9).
In line with this view, Sayette and colleagues found that disparate measures of cigarette craving converge on a single common factor only at high levels of desire created through the combination of nicotine deprivation and smoking cue exposure; craving measures did not covary at comparatively weak levels of desire (i.e., in nondeprived smokers exposed to smoking cues) (33). Similarly, Gwaltney et al. observed that quitting smokers exhibited a significant drop in their confidence to remain abstinent from smoking only during maximal urge states (34). Abstinence self-efficacy and craving were not associated when smoking desire was not at its peak, suggesting that cravings may be a categorically different experience from less potent states of desire. Collectively, such research indicates that conclusions may be limited in studies that measure cue-elicited brain activity in those whose desire to smoke has recently been satisfied. For instance, unlike nicotine-deprived smokers in a state of craving, nondeprived smokers with low levels of desire may not be especially useful for characterizing the appetitive motivational responses that contribute to relapse in those trying to quit smoking (32).
Pertinent to this issue, Engelmann and colleagues (4) recently compared the responses of four studies of nicotine-deprived smokers to eight studies of nondeprived smokers. Although craving was not a focus of their review and the number of studies was small, the authors nevertheless found that cue exposure was more reliably associated with increases in activation of the inferior occipital cortex and superior frontal gyrus in nicotine-derived than nondeprived smokers. Results also suggested that smoking cues were associated with the activation of a larger extent of the prefrontal cortex (PFC) in nicotine-deprived relative to nondeprived smokers, although this pattern was not significant in a formal subtraction analysis. These findings reinforce the idea that smoking satiety influences brain responses to cigarette cues, perhaps because of differences in the level of smoking desire experienced by nicotine-deprived and nondeprived smokers. The small number of smoking studies reviewed limited the ability to draw conclusions, however, especially in light of relevant studies that were omitted. [Because this review focused on cue-specific reactivity rather than craving, per se, it excluded research in which participants reported some of the most robust cravings (29).]
Given these constraints, we conducted a meta-analysis of fMRI studies of smoking cue-elicited craving using activation likelihood estimation (ALE) (35), as implemented with GingerALE version 2.3 (http://www.brainmap.org). This provided an opportunity to more than double the number of studies reviewed by Engelmann and colleagues (4). Studies were identified using the same search strategy described above. We included all available fMRI studies that assessed cue-reactivity in adult smokers, conducted whole-brain analyses, and reported coordinates in Montreal Neurological Institute (MNI) or Talairach space (with Talairach coordinates converted to MNI) for regions exhibiting significant cue-related increases in activation. Like Engelmann et al. (4), we excluded studies in which participants were instructed to inhibit or cope with craving or were taking smoking-cessation medications at the time of scanning (unless the study also reported results for a pre-treatment scan or a condition in which participants were instructed not to resist craving), and those in which smoking cues were presented in the background or periphery while participants performed a separate task.
We identified 26 (sub)samples meeting these criteria (see Table 2): 12 from studies that required participants to abstain from smoking for a period of time before the experiment (15, 23,25,27–30, 36–40) and 14 from studies that instructed participants to smoke ad libitum prior to the scan session (12, 13,15,17,21,41–48). [Note that David et al. (15) included both nicotine-deprived and nondeprived samples and that this set of studies only partially overlaps with those included in the analysis of peak craving described above. In addition, our classification of one study differed from that of Engelmann and colleagues (4). Specifically, we included the report by Hartwell et al. (37) among the studies of deprived smokers, as participants were instructed to abstain from smoking for two hours prior to the experiment.]
Table 2.
Studies Included in Activation Likelihood Estimation Meta-Analysis
| First Author | Year | Contrast | Cue(s) | n | Foci |
|---|---|---|---|---|---|
| Nicotine-Deprived | |||||
| Claus | 2013 | Cig > Food-related | Vid | 116 | 11 |
| David | 2005 | Cig > Neu | Pic | 9 | 3 |
| David | 2007 | Cig > Neu (abstinent session) | Pic | 8 | 2 |
| Goudriaan | 2010 | Cig - Neu (high FTND smokers > nonsmokers) | Pic | 10 | 7 |
| Hartwell | 2011 | Cig > Neu (not resisting urge) | Pic | 31 | 12 |
| Kang | 2012 | Cig > Neu | Pic | 25 | 17 |
| Li | 2013 | Cig > Rest (not resisting urge) | Pic; Handle | 10 | 10 |
| McClernon | 2009 | Cig > Neu (abstinent session) | Pic | 18 | 19 |
| Westbrook | 2011 | Cig > Neu (responding naturally) | Pic | 47 | 2 |
| Wilson | 2005 | Cig > Neu | Handle | 20 | 9 |
| Wilson | 2012 | Cig > Neu | Handle | 90 | 12 |
| Zhang | 2011 | Cig - Neu (smokers > nonsmokers) | Pic | 22 | 6 |
| Nondeprived | |||||
| Bourque | 2013 | Cig > Neu | Pic | 31 | 5 |
| Brody | 2007 | Cig > Neu (not resisting urge) | Vid | 42 | 17 |
| Dagher | 2009 | Cig > Neu (no stress condition) | Vid | 15 | 3 |
| David | 2007 | Cig > Neu (nonabstinent session) | Pic | 8 | 2 |
| Diggs | 2013 | Cig > Neu | Pic | 9 | 1 |
| Franklin | 2009 | Cig > Neu (9 repeats) | Vid; Handle | 10 | 9 |
| Franklin | 2009 | Cig > Neu (10/10 repeats) | Vid; Handle | 9 | 13 |
| Franklin | 2011 | Cig > Neu | Vid | 26 | 1 |
| Janes | 2009 | Cig > Neu (pre-treatment scan) | Pic | 13 | 25 |
| Janes | 2012 | Cig > Neu | Pic | 24 | 11 |
| McBride | 2006 | Cig > Neu (nonabstinent session) | Vid | 19 | 5 |
| Versace | 2011 | Cig > Neu | Pic | 35 | 13 |
| Vollstädt-Klein | 2010 | Cig > Neu | Pic | 22 | 13 |
| Yalachkov | 2009 | Cig - Neu (smokers > nonsmokers) | Pic | 15 | 12 |
Note. Cig = cigarette-related cues; FTND = Fagerström Test for Nicotine Dependence; Handle = holding and viewing an unlit cigarette; Neu = neutral cues; Pic = viewing smoking-related pictures; Vid = viewing smoking-related videos.
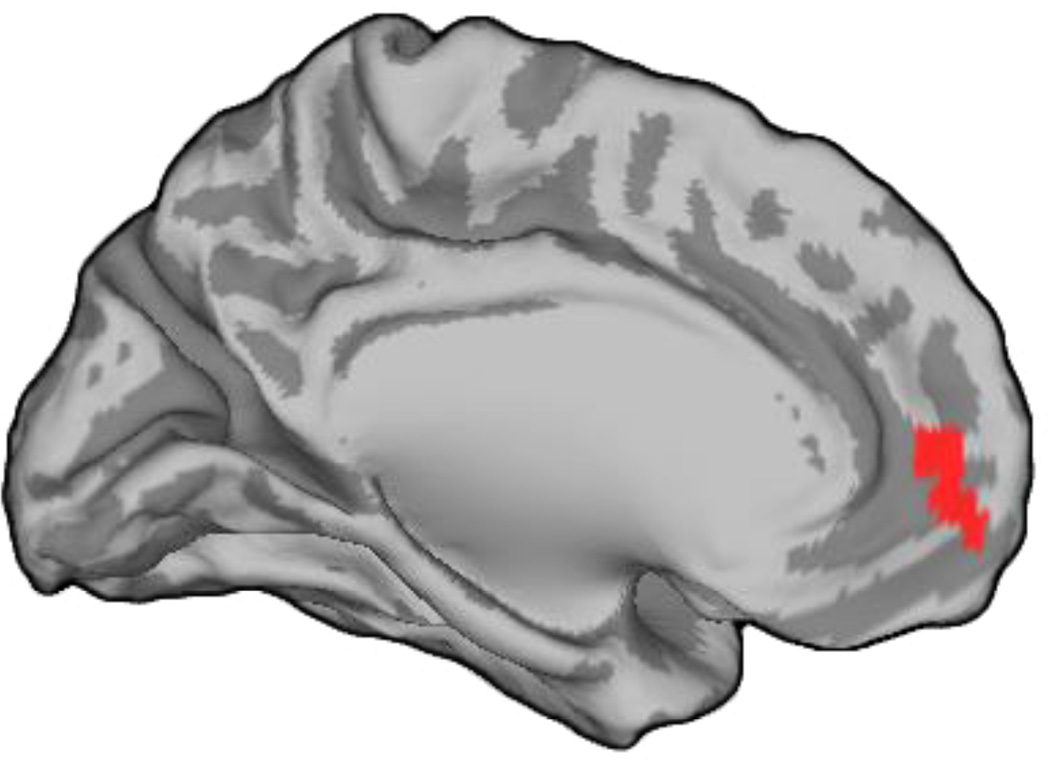
Our meta-analysis revealed that smoking cues have been associated with activation of a larger portion of the rostral anterior cingulate cortex (rACC) in nicotine-deprived smokers (4360 mm3) relative to nondeprived smokers (1184 mm3) when each was considered separately. In order to more directly evaluate the significance of this pattern, we conducted a subtraction analysis (35) contrasting the ALE maps generated for studies of nicotine-deprived versus nondeprived smokers. Results confirmed that a region encompassing the rACC and adjacent medial/ventromedial PFC (MNI coordinates x=−9, y=49, z=−8; size=960 mm3, Brodmann's areas 32 and 10) was more likely to exhibit increased activation during cigarette cue exposure in the nicotine-deprived samples (p<0.05 false discovery rate corrected, with a minimum cluster size of 200 mm3; see Figure 2). This difference is notable in light of emerging research highlighting the importance of the rACC in relation to the treatment of craving and the regulation of affect, more generally (1, 8,13,14,22,27, 37). There were no regions more reliably activated by cigarette cues in nondeprived relative to deprived smokers.
Figure 2.
Region of the rostral ACC and adjacent medial/ventromedial PFC (depicted in red) more reliably activated by cigarette cues in nicotine-deprived than nondeprived smokers, as indicated by ALE subtraction analysis.
We hypothesize that this differential engagement of the rACC was driven at least in part by differences in the degree to which nicotine-deprived versus nondeprived smokers experienced the desire to smoke. Although it was impossible to test this idea directly because peak ratings of smoking desire were not available for most studies, indirect support comes from research examining the neurobiological effects associated with interventions designed to reduce craving. In one line of research, Brody and colleagues have demonstrated that treatment with bupropion hydrochloride – a medication that reduces both background and cue-provoked craving – attenuates activation of the rACC by cigarette cues in smokers (e.g., 14). In a second line of work, Brady, George and colleagues have shown that smokers can be taught to decrease cue-elicited activation of the rACC using real-time fMRI neurofeedback and that doing so is associated with reductions in urge (22, 27). Thus, manipulations that weaken the desire to use drugs appear to reduce activation of the rACC during cue exposure.
Results from our meta-analysis are thus consistent with the idea that nondeprived smokers exhibit less reliable activation of the rACC because the desire to smoke is relatively modest when cues are paired with cigarette satiety, whereas the combination of deprivation and salient cigarette cues produce particularly robust desire/craving. More generally, we propose that, as with behavioral studies (8), it is possible – perhaps even likely – that neuroimaging studies assessing smokers in a modest state of desire (such as immediately following smoking) and those assessing smokers in the midst of a relatively powerful craving episode are to some degree measuring distinct concepts.
Furthermore, the differential activation of the rACC identified in our meta-analysis may represent only the tip of the iceberg regarding how neurobiological responses to smoking cues are shaped by urge intensity. As has been noted (30), it is the spatiotemporal relationships (i.e., connectivity) between brain regions, and not differences in the mean activation level within brain regions in isolation, that may best elucidate the nature of cue-elicited neural responses. Of particular relevance, our prior work suggests that connectivity between the rACC and other areas of the brain is especially sensitive to the motivational context associated with smoking cue exposure (e.g., smoking expectancy) (30). Hence, while the potential link between the strength of cravings and cue-elicited activation of the rACC itself has salient conceptual and clinical implications, we anticipate that the importance of craving intensity will become even clearer as the assessment of brain connectivity during urge states becomes more widespread.
Greater Attention to Urge Intensity Would Help to Advance the Field
We believe that more explicit attention to urge intensity in addiction neuroimaging research has the potential to yield valuable information about craving. Among many fruitful avenues for future research, the application of imaging to tightly manipulated levels of craving may be particularly effective for clarifying whether craving manifests as a linear or nonlinear phenomenon – a fundamental issue that remains unresolved within the field (9, 49, 50). Neuroimaging could be used to assess changes in the strength of the desire to use drugs from low to very high (e.g., as produced by manipulating the duration of nicotine deprivation and intensity of cue exposure) in relation to both the magnitude of activation within localized brain regions and patterns of connectivity among brain areas. Such research may reveal, for example, that changes in drug use desire are associated with corresponding adjustments in the magnitude of activation and/or connectivity within some relatively fixed set of brain areas (a “craving network”), suggesting that craving is best conceived of as linear. Alternatively, increases in desire above some threshold may result in the emergence of new brain areas/connections (e.g., brain responses linked to motivation and action preparation appearing only after desire is very high), suggesting that craving may be nonlinear. Neuroimaging methods are uniquely well-suited for distinguishing between these and other possibilities (e.g., some combination of linearity and nonlinearity) regarding the nature of craving. Relatedly, research is needed to directly examine the extent to which urge intensity moderates the association between cue-elicited brain responses and clinical relevant outcomes such as relapse. Though in some instances nicotine-deprived smokers’ baseline urges and their “peak” urges following smoking cue exposure are similarly linked to subsequent relapse (see 9), it may be that the neural responses during intense desire associated with nicotine deprived states ultimately prove to be more strongly associated with clinical outcomes than are responses during nondeprived states. This possibility requires experimental verification, as some work suggests that lighter states of desire may offer a sensitive predictor of relapse (see 50).
More generally, we believe that a greater focus on the intensity of substance use desires in neuroimaging research would benefit the field even if such work ultimately reveals that craving intensity has only modest effects on neurobiological responses to drug cues, as it would challenge the widespread argument that there is something unique about strong desires. Models of addiction and craving would require critical revisions in order to accommodate such an unexpected result. Regardless of the outcomes of future studies, however, our point of emphasis is that advancing knowledge regarding the neurobiology of craving requires deeper consideration of just how we manipulate and assess craving.
Summary and Conclusions
Functional neuroimaging research on craving for cigarettes and other drugs has come a long way in a remarkably short period of time. The field has generally moved beyond a focus on “mapping” brain responses to drug-related cues and is becoming dominated by studies attempting to address increasingly nuanced questions about urges. Collectively, this research has led to some of the most exciting advances in the study of addiction (1, 8). As a field, however, we have not paid sufficient attention to the very phenomenon under study. Namely, in many studies purporting to examine craving, participants endorse only a weak desire to use drugs, while in others participants endorse exceptionally strong desires. It is notable that the actual amount of craving reported by participants is not given much consideration in many imaging smoking cue reactivity studies. Indeed, one wonders what it means to talk about the “neural correlates of craving” in smokers reporting mild urges that seemingly fall far short of the powerful state that is believed to be important from both theoretical (2, 7, 8) and clinical/diagnostic (10) perspectives.
We believe that it is important to pay close attention to the intensity of the cravings that one is studying regardless of the research tools that are utilized and types of responses that are measured. We have emphasized functional brain imaging because it has become an especially prevalent and influential approach to studying craving – and because neuroimaging methods present distinct challenges when it comes to manipulating and measuring craving (e.g., the fMRI environment is noisy and cramped and generally precludes use of certain highly potent drug cues, such as a burning cigarette) – but we believe that our claims apply whether one is assessing brain activity or other response modalities (for additional discussion, see 9). Regarding functional brain imaging methods, specifically, it is useful to keep in mind that by themselves the data they provide are merely indicators of changes in electrophysiological (e.g., as measured using electroencephalography) or hemodynamic (e.g., as measured using fMRI) activity in the brain. In order to derive meaningful insights from such data, they must be considered in the context of the study manipulations and other measures (see 49). Urge intensity represents one such variable.
We have highlighted acute nicotine deprivation as a factor that affects the desire to smoke, and thus as a useful factor for parsing studies, but our more fundamental point is that craving intensity itself warrants greater attention. We recognize that there are several other relevant factors worth considering [e.g., robustness of the cues that are used to elicit craving (9), perceived opportunity to smoke during the study (30)]. Additionally, we did not address the possibility that drug withdrawal has effects on responses (including those measured in the brain) that are separate from those it has on craving, as we viewed this issue to be beyond the scope of our argument. Nonetheless, research exploring whether the effects of drug deprivation and craving can be disentangled would be useful (e.g., see 16). In reviewing peak urge ratings reported in previous studies, we necessarily collapsed across studies using different craving scales. The assumption is that the different end points of these scales do not affect how respondents use them, which may or may not be accurate. Finally, while we have concentrated on craving for cigarettes, we believe that the intensity of subjective experience also may be critical when studying craving for other drugs.
Notwithstanding these caveats, we believe that the points made herein have significant implications for research in which tools from neuroscience are used with the goal of examining drug craving. Simply put, studying brain responses during mild desires may not be the same as studying brain responses during overpowering urges. Therefore, inconsistency in urge intensity should be taken into account as a potentially important source of heterogeneity across prior studies. In addition, by focusing more on urge intensity in future work, addiction researchers have the potential to provide novel insight into some of the most pressing questions concerning the construct of craving itself.
Acknowledgments
Dr. Wilson’s research is supported by the National Institute on Drug Abuse (R03DA035929) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under BIRCWH award number K12HD055882, “Career Development Program in Women’s Health Research at Penn State.” Dr. Sayette’s research is supported by the National Cancer Institute (R01CA10605 and R01CA184779). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Contributor Information
Stephen J. Wilson, The Pennsylvania State University and the Center for Brain, Behavior, and Cognition
Michael A. Sayette, University of Pittsburgh and the Center for the Neural Basis of Cognition
References
- 1.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinha R. The clinical neurobiology of drug craving. Curr Opin Neurobiol. 2013;23:649–654. doi: 10.1016/j.conb.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelmann JM, Versace F, Robinson JD, et al. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69:695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karoly HC, Harlaar N, Hutchison KE. Substance use disorders: a theory-driven approach to the integration of genetics and neuroimaging. Annals of the New York Academy of Sciences. 2013;1282:71–91. doi: 10.1111/nyas.12074. [DOI] [PubMed] [Google Scholar]
- 7.George O, Koob GF. Control of craving by the prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4165–4166. doi: 10.1073/pnas.1301245110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkow ND, Wang GJ, Fowler JS, et al. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sayette MA, Tiffany ST. Peak provoked craving: an alternative to smoking cue-reactivity. Addiction. 2013;108:1019–1025. doi: 10.1111/j.1360-0443.2012.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 11.West R, Brown J. Theory of addiction. Hoboken, New Jersey: John Wiley and Sons Ltd; 2013. [Google Scholar]
- 12.Bourque J, Mendrek A, Dinh-Williams L, Potvin S. Neural circuitry of impulsivity in a cigarette craving paradigm. Front Psychiatry. 2013;4:67. doi: 10.3389/fpsyt.2013.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brody AL, Mandelkern MA, Olmstead RE, et al. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Culbertson CS, Bramen J, Cohen MS, et al. Effect of bupropion treatment on brain activation induced by cigarette-related cues in smokers. Arch Gen Psychiatry. 2011;68:505–515. doi: 10.1001/archgenpsychiatry.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.David SP, Munafo MR, Johansen-Berg H, et al. Effects of Acute Nicotine Abstinence on Cue-elicited Ventral Striatum/Nucleus Accumbens Activation in Female Cigarette Smokers: A Functional Magnetic Resonance Imaging Study. Brain Imaging Behav. 2007;1:43–57. doi: 10.1007/s11682-007-9004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franklin TR, Wang Z, Wang J, et al. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- 17.Mcbride D, Barrett SP, Kelly JT, AW A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- 18.McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smolka MN, Buhler M, Klein S, et al. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl) 2006;184:577–588. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- 20.Stippekohl B, Winkler M, Mucha RF, et al. Neural responses to BEGIN- and END-stimuli of the smoking ritual in nonsmokers, nondeprived smokers, and deprived smokers. Neuropsychopharmacology. 2010;35:1209–1225. doi: 10.1038/npp.2009.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vollstädt-Klein S, Kobiella A, Buhler M, et al. Severity of dependence modulates smokers' neuronal cue reactivity and cigarette craving elicited by tobacco advertisement. Addict Biol. 2011;16:166–175. doi: 10.1111/j.1369-1600.2010.00207.x. [DOI] [PubMed] [Google Scholar]
- 22.Canterberry M, Hanlon CA, Hartwell KJ, et al. Sustained Reduction of Nicotine Craving With Real-Time Neurofeedback: Exploring the Role of Severity of Dependence. Nicotine Tob Res. 2013 doi: 10.1093/ntr/ntt122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.David SP, Munafo MR, Johansen-Berg H, et al. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- 25.Goudriaan AE, de Ruiter MB, van den Brink W, Oosterlaan J, Veltman DJ. Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: an fMRI study. Addict Biol. 2010;15:491–503. doi: 10.1111/j.1369-1600.2010.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kober H, Mende-Siedlecki P, Kross EF, et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Hartwell KJ, Borckardt J, et al. Volitional reduction of anterior cingulate cortex activity produces decreased cue craving in smoking cessation: a preliminary real-time fMRI study. Addict Biol. 2013;18:739–748. doi: 10.1111/j.1369-1600.2012.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westbrook C, Creswell JD, Tabibnia G, et al. Mindful attention reduces neural and self-reported cue-induced craving in smokers. Soc Cogn Affect Neurosci. 2011 doi: 10.1093/scan/nsr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: a preliminary study. Nicotine Tob Res. 2005;7:637–645. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson SJ, Sayette MA, Fiez JA. Quitting-unmotivated and quitting-motivated cigarette smokers exhibit different patterns of cue-elicited brain activation when anticipating an opportunity to smoke. J Abnorm Psychol. 2012;121:198–211. doi: 10.1037/a0025112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Wang J, Aron A, et al. Intense passionate love attenuates cigarette cue-reactivity in nicotine-deprived smokers: an FMRI study. PLoS One. 2012;7:e42235. doi: 10.1371/journal.pone.0042235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker TB, Morse E, Sherman JE. The motivation to use drugs: a psychobiological analysis of urges. Nebr Symp Motiv. 1987;34:257–323. [PubMed] [Google Scholar]
- 33.Sayette MA, Martin CS, Hull JG, Wertz JM, Perrott MA. Effects of nicotine deprivation on craving response covariation in smokers. J Abnorm Psychol. 2003;112:110–118. [PMC free article] [PubMed] [Google Scholar]
- 34.Gwaltney CJ, Shiffman S, Sayette MA. Situational correlates of abstinence self-efficacy. Journal of Abnormal Psychology. 2005;114:649–660. doi: 10.1037/0021-843X.114.4.649. [DOI] [PubMed] [Google Scholar]
- 35.Laird AR, Fox PM, Price CJ, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Claus ED, Blaine SK, Filbey FM, Mayer AR, Hutchison KE. Association Between Nicotine Dependence Severity, BOLD Response to Smoking Cues, and Functional Connectivity. Neuropsychopharmacology. 2013;38:2363–2372. doi: 10.1038/npp.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartwell KJ, Johnson KA, Li X, et al. Neural correlates of craving and resisting craving for tobacco in nicotine dependent smokers. Addict Biol. 2011;16:654–666. doi: 10.1111/j.1369-1600.2011.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang OS, Chang DS, Jahng GH, et al. Individual differences in smoking-related cue reactivity in smokers: an eye-tracking and fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:285–293. doi: 10.1016/j.pnpbp.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 39.McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl) 2009;204:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X, Salmeron BJ, Ross TJ, et al. Anatomical differences and network characteristics underlying smoking cue reactivity. Neuroimage. 2011;54:131–141. doi: 10.1016/j.neuroimage.2010.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dagher A, Tannenbaum B, Hayashi T, Pruessner JC, McBride D. An acute psychosocial stress enhances the neural response to smoking cues. Brain Res. 2009;1293:40–48. doi: 10.1016/j.brainres.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diggs HA, Froeliger B, Carlson JM, Gilbert DG. Smoker-nonsmoker differences in neural response to smoking-related and affective cues: an fMRI investigation. Psychiatry Res. 2013;211:85–87. doi: 10.1016/j.pscychresns.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Franklin TR, Lohoff FW, Wang Z, et al. DAT genotype modulates brain and behavioral responses elicited by cigarette cues. Neuropsychopharmacology. 2009;34:717–728. doi: 10.1038/npp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franklin TR, Wang Z, Li Y, et al. Dopamine transporter genotype modulation of neural responses to smoking cues: confirmation in a new cohort. Addict Biol. 2011;16:308–322. doi: 10.1111/j.1369-1600.2010.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janes AC, Frederick B, Richardt S, et al. Brain fMRI reactivity to smoking-related images before and during extended smoking abstinence. Exp Clin Psychopharmacol. 2009;17:365–373. doi: 10.1037/a0017797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janes AC, Smoller JW, David SP, et al. Association between CHRNA5 genetic variation at rs16969968 and brain reactivity to smoking images in nicotine dependent women. Drug Alcohol Depend. 2012;120:7–13. doi: 10.1016/j.drugalcdep.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Versace F, Engelmann JM, Jackson EF, et al. Do brain responses to emotional images and cigarette cues differ? An fMRI study in smokers. Eur J Neurosci. 2011;34:2054–2063. doi: 10.1111/j.1460-9568.2011.07915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yalachkov Y, Kaiser J, Naumer MJ. Brain regions related to tool use and action knowledge reflect nicotine dependence. J Neurosci. 2009;29:4922–4929. doi: 10.1523/JNEUROSCI.4891-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abrams DB. Transdisciplinary concepts and measures of craving: commentary and future directions. Addiction. 2000;95(Suppl 2):S237–S246. doi: 10.1080/09652140050111807. [DOI] [PubMed] [Google Scholar]
- 50.Shiffman S. Parsing peak provoked craving. Addiction. 2013;108:1026–1027. doi: 10.1111/j.1360-0443.2012.04062.x. [DOI] [PubMed] [Google Scholar]