Abstract
Background
Photon involved-field radiation therapy (IFRT), the standard for locally advanced non-small cell lung cancer (LA-NSCLC), results in favorable outcomes without increased isolated nodal failures, perhaps from scattered dose to elective nodal stations. Given the high conformality of intensity-modulated proton therapy (IMPT), proton IFRT could increase nodal failures. We investigated the feasibility of IMPT for elective nodal irradiation (ENI) in LA-NSCLC.
Materials and Methods
IMPT IFRT plans were generated to the same total dose of 66.6–72 Gy received by 20 LA-NSCLC patients treated with photon IFRT. IMPT ENI plans were generated to 46 CGE to elective nodal (EN) planning treatment volumes (PTV) plus 24 CGE to involved field (IF)-PTVs.
Results
Proton IFRT and ENI both improved D95 involved field (IF)-PTV coverage by 4% (p<0.01) compared to photon IFRT. All evaluated dosimetric parameters improved significantly with both proton plans. Lung V20 and mean lung dose decreased 18% (p<0.01) and 36% (p<0.01), respectively, with proton IFRT and 11% (p=0.03) and 26% (p<0.01) with ENI. Mean esophagus dose decreased 16% with IFRT and 12% with ENI; heart V25 decreased 63% with both (all p<0.01).
Conclusions
This study demonstrates the feasibility of IMPT for LA-NSCLC ENI. Potential decreased toxicity indicates IMPT could allow ENI while maintaining a favorable therapeutic ratio compared to photon IFRT.
Keywords: Non-small cell lung cancer, elective nodal irradiation, proton therapy, dosimetric, involved-field radiation therapy
INTRODUCTION
The overall survival for locally advanced (LA) non-small cell lung cancer (NSCLC) patients remains poor, with 5-year survival estimated at 15–25%.1 High local failure rates led to approaches emphasizing local control, including trimodality therapy and dose escalation to the primary tumor and mediastinum.2–4 Both approaches, however, have been limited by increased toxicity.5–10 Involved-field radiation therapy (IFRT) remains the current standard given that elective nodal irradiation (ENI) has demonstrated higher rates of toxicity with limited elective nodal failures.11,12
The improved dose localization permitted by proton therapy provides a potential avenue for lowering the toxicity of radiotherapy. Proton therapy has been successfully used to decrease normal tissue toxicity in multiple types of cancer.13–15 For NSCLC, several early-phase proton trials showed efficacy and decreased pneumonitis and esophagitis, and the first successful clinical implementation of intensity-modulated proton therapy (IMPT) for thoracic cancers has been recently published.16–22
Previous dosimetric analyses of 5-patient cohorts showed the feasibility of covering high-risk lymph nodes (LN) with double-scatter proton therapy.23,24 In this study, we compared dosimetric parameters from 20 LA-NSCLC patients’ actual photon IFRT plans with those from IMPT IFRT and ENI plans to investigate whether IMPT can allow for treatment of elective nodal stations (ENS) without reducing coverage of gross disease or increasing the dose to organs at risk (OAR).
MATERIALS AND METHODS
In this institutional review board-approved study, we identified a cohort of 20 consecutive patients with biopsy-proven inoperable LA-NSCLC who had a positron emission tomography (PET)/computed tomography (CT) scan as part of their staging workup and were treated with definitive photon IFRT at the University of Pennsylvania Department of Radiation Oncology.
For clinical photon treatment, patients were immobilized in the supine position with arms overhead and underwent CT-based simulation with four-dimensional (4D)-CT to account for respiratory motion. Each 4D-CT image set was comprised of 8 images distributed equally across a single respiratory cycle. Gross tumor volumes (GTV) were contoured to include all sites of known disease; LN were included if enlarged on CT scan (>1 cm on short axis), had increased standard uptake value over background on PET, or biopsy-positive. Utilizing the 4D-CT scan, the GTV was expanded to incorporate motion to create an internal GTV (IGTV). The primary IGTV was expanded by 8 mm and the nodal IGTV by 3 mm to encompass microscopic extension of disease, creating the clinical target volume (CTV). The planning target volume (PTV) was a 5 mm uniform expansion of the CTV. OAR, including lungs, esophagus, and heart, were contoured. These volumes and expansions represent the standard volumes and expansions utilized at the University of Pennsylvania at the time the patients on this study were definitively treated. All photon treatment plans were generated using the Eclipse planning system (Varian Medical Systems, Palo Alto, CA). Three-dimensional conformal radiation therapy (3D-CRT) or intensity-modulated radiation therapy (IMRT) plans were generated to deliver 66.6–72 Gy while respecting normal tissue constraints. Plans were first dosimetrically optimized with 3D-CRT; an IMRT plan was delivered only if dose constraints could not be met with a 3D-CRT plan.
For each case, ENS were separately contoured to create an elective nodal (EN) CTV (CTV). The International Association for the Study of Lung Cancer CT-based lymph node atlas was used to define LN levels.25 The ipsilateral hilum (levels 10–11), subcarina (level 7), and bilateral mediastinum (levels 2–4) were included for all cases. The ipsilateral supraclavicular LN (level 1) were included for upper lobe lesions. The contralateral hilum (levels 10–11) was included when involved. The aortopulmonary window (levels 5–6) was included for left upper lobe primaries. Inferior mediastinal LN below the subcarina (levels 8–9) and the peripheral zone (levels 12–14) were not specifically targeted. The elective nodal PTV (EN-PTV) was a 5 mm uniform volumetric expansion of the EN-CTV. The same involved-field (IF) IGTV, CTV, and PTV from the actual treatment were used in the proton planning process.
A modulated-scanning technique with multi-field optimization was utilized for IMPT planning with default beams from IBA (Louvain-la-Neuve, Belgium). The spot size was 5 mm and target margins were 1 cm laterally and 0 cm proximally and distally. Two (n=1), 3 (n=18), or 4 (n=1) fields were used, with 3-field plans typically employing AP, lateral, and oblique fields. The EN-PTV was planned to 46 CGE, followed by a 24 CGE boost to the original IF-PTV for a total dose of 70 CGE. The optimization objectives were to maximize PTV coverage and minimize spinal cord and esophageal dose; no constraints were entered for the heart and lungs.
Data analysis was performed using the paired t-test for parametric data (esophagus V60, V55, and V50 and heart V25) and the Wilcoxon’s rank-sum test for non-parametric data (IF-PTV D95, lung V20 and V5, and mean lung and esophagus doses). All tests were two-tailed, with α for significance set at p≤0.05.
RESULTS
Patient Characteristics
Patient characteristics are summarized in Table 1. Patients had T1 (n=2), T2 (n=2), T3 (n=5), or T4 (n=11) primary NSCLC with predominantly node-positive (n=16) and right-sided (n=17) disease. Thoracic stage grouping was IIIA in 14 and IIIB in 6 patients. Most patients (n=17) received concurrent chemotherapy with a platinum-based doublet. Patients were treated to a median of 40 fractions (range 36–40) in 1.8–2.0 Gy daily fractions to a mean of 72 Gy (range 66.6–72 Gy) with 3D-CRT (n=16) or IMRT (n=4).
Table 1.
Patient, tumor, and treatment regimen characteristics
| Patient | T Stage | N Stage | Thoracic Stage Grouping | Primary Location* | Concurrent Chemo-radiation | Chemotherapy | # of Fractions | Dose per Fraction (Gy) | Total Dose (Gy) | 3DCRT† or IMRT |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | T2 | N2 | IIIA | RML | Yes | Carboplatin, Taxol | 40 | 1.8 | 72.0 | 3DCRT (AP, PA, LAO, RPO) |
| 2 | T4 | N2 | IIIB | RLL | Yes | Cisplatin, Etoposide | 40 | 1.8 | 72.0 | 3DCRT (AP, PA, LAO, RPO) |
| 3 | T4 | N0 | IIIA | LUL | Yes | Cisplatin, Etoposide | 40 | 1.8 | 72.0 | IMRT (6 fields) |
| 4 | T3 | N1 | IIIA | RUL | No | None | 36 | 2.0 | 72.0 | 3DCRT (LSAO, LPO, RPO) |
| 5 | T3 | N2 | IIIA | RUL | Yes | Carboplatin, Taxotere | 40 | 1.8 | 72.0 | 3DCRT (AP, PA, LAO, RPO) |
| 6 | T1 | N2 | IIIA | RUL | Yes | Carboplatin, Taxol | 40 | 1.8 | 72.0 | 3DCRT (AP, PA, LAO, RPO) |
| 7 | T4 | N2 | IIIB | RUL | No | Carboplatin, Gemcitabine | 40 | 1.8 | 72.0 | 3DCRT (AP, PA, LAO, RPO) |
| 8 | T4 | N0 | IIIA | RUL | Yes | Carboplatin, Taxol | 40 | 1.8 | 72.0 | 3DCRT (AP, PA, LAO, RPO) |
| 9 | T4 | N2 | IIIB | RUL | Yes | Carboplatin, Taxol | 40 | 1.8 | 72.0 | 3DCRT (AP, PA, LAO, RPO) |
| 10 | T4 | N2 | IIIB | RUL | Yes | Cisplatin, Pemetrexed | 40 | 1.8 | 72.0 | 3DCRT (AP, PA, LAO, RPO) |
| 11 | T3 | N2 | IIIA | RUL | Yes | Carboplatin, Taxol | 40 | 1.8 | 72.0 | 3DCRT (AP, PA, LAO, RPO) |
| 12 | T3 | N2 | IIIA | RUL | Yes | Cisplatin, Etoposide | 37 | 1.8 | 66.6 | IMRT (6 fields) |
| 13 | T4 | N1 | IIIA | LUL/LLL | Yes | Cisplatin, Etoposide | 37 | 1.8 | 66.6 | 3DCRT (AP, PA, LPO, RAO) |
| 14 | T1 | N3 | IIIB | RUL | Yes | Carboplatin, Taxol | 37 | 1.8 | 66.6 | IMRT (5 fields) |
| 15 | T4 | N2 | IIIB | RUL/RML | Yes | Cisplatin, Etoposide | 37 | 1.8 | 66.6 | 3DCRT (AP, PA, LAO, RPO) |
| 16 | T4 | N0 | IIIA | LUL | Yes | Cisplatin, Etoposide | 40 | 1.8 | 72.0 | 3DCRT (AP, PA, LPO, RAO) |
| 17 | T4 | N1 | IIIA | RML/RLL | Yes | Carboplatin, Taxol | 40 | 1.8 | 72.0 | 3DCRT (AP, PA, LAO, RPO) |
| 18 | T2 | N2 | IIIA | RLL | No | None | 40 | 1.8 | 72.0 | 3DCRT (AP, PA, LAO, RPO) |
| 19 | T4 | N0 | IIIA | RUL | Yes | Cisplatin, Etoposide | 40 | 1.8 | 72.0 | IMRT (5 field) |
| 20 | T3 | N2 | IIIA | RUL | Yes | Cisplatin, Etoposide | 36 | 2.0 | 72.0 | 3DCRT (AP, PA, LAO, RPO) |
Abbreviations: RUL = right upper lobe, RML = right middle lobe, RLL = right lower lobe, LUL = left upper lobe, LLL = left lower lobe
Abbreviations: AP = anterior-anterior, PA = posterior-anterior, LAO = left anterior oblique, LPO = left posterior oblique, LSAO = left superior anterior oblique, RAO = right anterior oblique, RPO = right posterior oblique
Photon IFRT vs. Proton IFRT
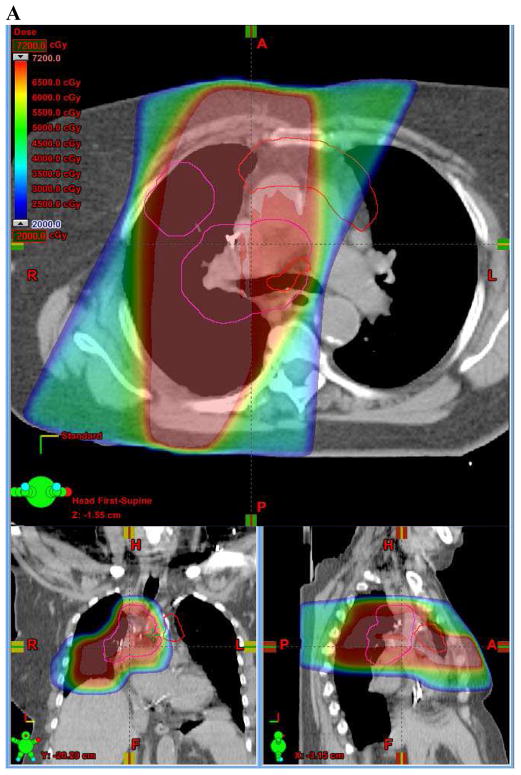
Dose-volume parameters from proton IFRT plans were compared with those from the originally delivered photon IFRT plans (Figures 1A, 1B). All dose-volume parameters were significantly improved by proton IFRT (Figure 1C, Table 2). Comparison of mean D95 of the IF-PTV indicated that proton IFRT improved coverage by 4% relative to photon IFRT (98.0 vs. 94.4%, p<0.01). Proton IFRT also reduced the mean lung dose by 36% (11.0 vs. 17.2 CGE/Gy, p<0.01), lung V20 by 18% (22.9 vs. 27.9%, p<0.01), and lung V5 by 30% (31.2 vs. 44.2%, p<0.01). Similarly, mean esophagus dose was reduced by 16% with proton IFRT (31.6 vs. 37.7 CGE/Gy, p<0.01) and esophagus V60, V55, and V50 were reduced by 21% (32.5 vs. 41.1%, p<0.01), 19% (35.8 vs. 44.2%, p<0.01), and 18% (38.4 vs. 46.7%, p<0.01), respectively. Average heart V25 was 63% lower (10.7 vs. 29.3%, <0.01) with proton IFRT compared to photon IFRT.
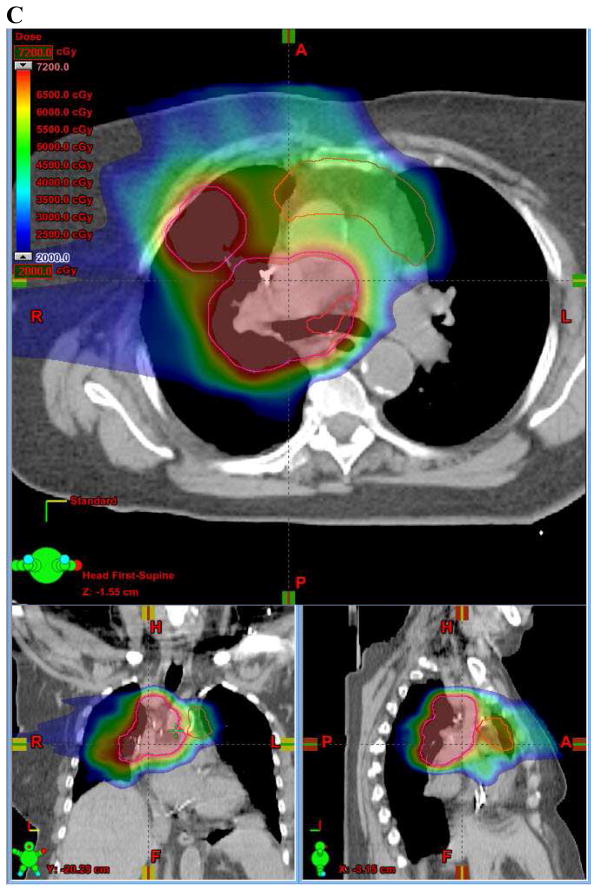
Figure 1.
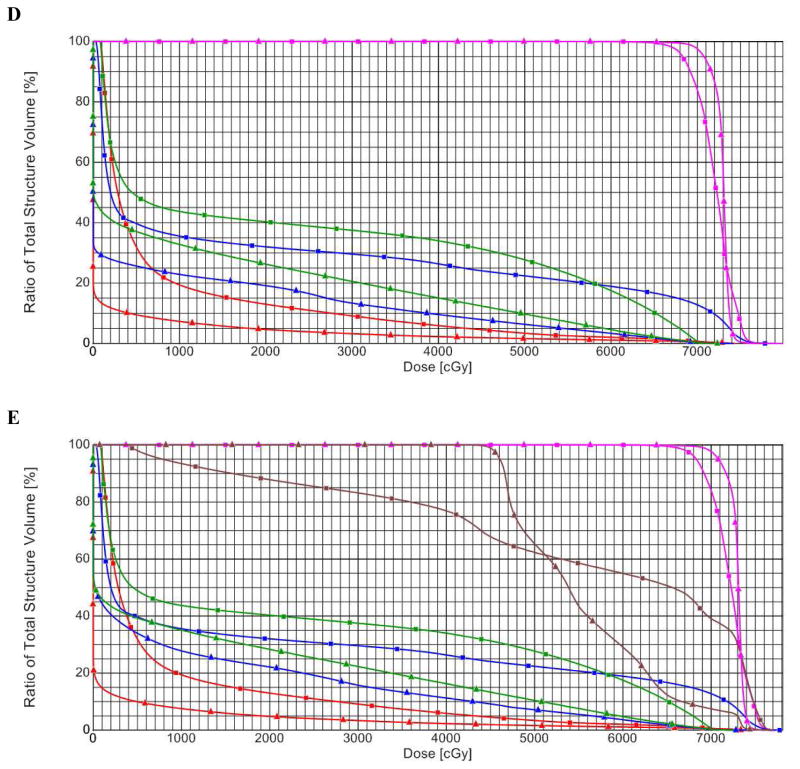
(A) Axial, sagittal, and coronal (ASC) views of photon involved-field radiation therapy (IFRT) plan. (B) ASC views of proton IFRT plan. (C) ASC views of proton elective nodal irradiation (ENI) plan. In (A), (B), and (C), the involved-field PTV is outlined in pink and the ENI PTV is outlined in red. (D) Dose-volume histogram (DVH) comparing photon IFRT (squares) with proton IFRT (triangles). (E) DVH comparing photon IFRT (squares) with proton ENI (triangles). In (D) and (E), structures and organs at risk are: Involved-field PTV (pink), ENI PTV (brown), esophagus (green), lung (blue), and heart (red).
Table 2.
Dosimetric comparison of photon involved-field, proton involved-field, and proton elective nodal irradiation plans
| Photon IFRT | Proton IFRT | Proton ENI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Structure or Organ | Mean ± SE | Mean ± SE | % Change* | p* | Mean ± SE | % Change* | p* | % Change† | p† |
| Involved-field PTV | |||||||||
| D95 (%) | 94.4 ± 0.9 | 98.0 ± 0.9 | ↑4% | 0.002 | 98.1 ± 0.9 | ↑4% | 0.002 | 0% | 0.70 |
| Lung | |||||||||
| Mean dose (Gy/CGE) | 17.2 ± 0.9 | 11.0 ± 0.8 | ↓36% | <0.001 | 12.5 ± 0.7 | ↓26% | <0.001 | ↑14% | <0.001 |
| V20 (%) | 27.9 ± 1.6 | 22.9 ± 1.5 | ↓18% | 0.002 | 24.8 ± 1.4 | ↓11% | 0.03 | ↑8% | 0.009 |
| V5 (%) | 44.2 ± 2.7 | 31.2 ± 1.9 | ↓30% | <0.001 | 37.4 ± 1.7 | ↓15% | 0.02 | ↑20% | <0.001 |
| Esophagus | |||||||||
| Mean dose (Gy/CGE) | 37.7 ± 3.3s | 31.6 ± 3.2 | ↓16% | <0.001 | 33.0 ± 3.0 | ↓12% | <0.001 | ↑4% | 0.006 |
| V60 (%) | 41.1 ± 5.4 | 32.5 ± 4.8 | ↓21% | <0.001 | 32.5 ± 4.8 | ↓21% | <0.001 | ↑0% | 0.95 |
| V55 (%) | 44.2 ± 5.2 | 35.8 ± 4.8 | ↓19% | <0.001 | 36.0 ± 4.8 | ↓19% | <0.001 | ↑1% | 0.67 |
| V50 (%) | 46.7 ± 5.1 | 38.4 ± 4.7 | ↓18% | <0.001 | 38.9 ± 4.8 | ↓17% | <0.001 | ↑1% | 0.37 |
| Heart | |||||||||
| V25 (%) | 29.3 ± 4.0 | 10.7 ± 1.9 | ↓63% | <0.001 | 10.8 ± 2.0 | ↓63% | <0.001 | ↑1% | 0.97 |
Abbreviations: IFRT = involved-field radiation therapy, ENI = elective nodal irradiation, SE = standard error, PTV = planning treatment volume,
V5, V20, V25, V55, V60 = percentage of volume receiving 5, 20, 25, 55, 60 Gy (photon) or CGE (proton), respectively.
Compared with photon IFRT plans.
Compared with proton IFRT plans.
Photon IFRT vs. Proton ENI
Dose-volume parameters were also compared between photon IFRT and proton ENI plans (Figures 1A, 1C, 1E). As with proton IFRT, proton ENI significantly improved every parameter compared to the delivered photon IFRT plans (Figure 1E, Table 2). Mean D95 was improved by 4% (98.1 vs. 94.4%, p<0.01) with proton ENI. Proton ENI reduced mean lung dose by 26% (12.5 vs. 17.2 CGE/Gy, p<0.01) and mean esophageal dose by 12% (33.0 vs. 37.7 CGE/Gy, p<0.01). Proton ENI decreased lung V20 by 11% (24.8 vs. 27.9%, p=0.03) and lung V5 by 15% (37.4 vs. 44.2%, p<0.01). Similarly, proton ENI reduced esophagus V60 by 21% (32.5 vs. 41.1%, p<0.01), esophagus V55 by 19% (36.0 vs. 44.2%, p<0.01), esophagus V50 by 17% (38.9% vs. 38.4%, p<0.01), and heart V25 by 63% (10.8 vs. 29.3%, p<0.01).
Proton IFRT vs. Proton ENI
Next, dose-volume parameters between proton ENI and proton IFRT were compared (Figure 1E, Table 2). Mean D95 values were nearly identical (98.1 vs. 98.0%, p=0.42), as were esophagus V60 (32.5 vs. 32.5%, p=0.95), esophagus V55 (36.0 vs. 35.8%, p=0.67), esophagus V50 (38.9 vs. 38.4%, p=0.37), and heart V25 (10.8 vs. 10.7%, p=0.97). Relative to proton IFRT, proton ENI increased lung V20 by 8% (24.8 vs. 22.9%, p<0.01), V5 by 11% (37.4% vs. 31.2%, p<0.01), mean lung dose by 14% (12.5 vs. 11.0 CGE, p<0.01), and mean esophagus dose by 4% (33.0 vs. 31.6 CGE, p<0.01).
Location of Primary Tumor
Dose-volume parameters were next compared with regard to the location of the primary tumor, specifically laterality and cranial-caudal position (upper vs. middle or lower lobe). The data were asymmetric in this regard: 17/20 tumors were right-sided and 17/20 tumors had an upper lobe primary. The significance of all proton vs. photon comparisons in right-sided tumors remained unchanged. In upper-lobe tumors, the one difference was that the Lung V20 only trended toward an improvement with proton ENI compared to photon IFRT (24.8% vs 27.8%, p=0.07), while proton IFRT still significantly improved the Lung V20 compared to photon IFRT (23.8% vs 27.8%, p=0.03).
The 3/20 left-sided and middle/lower lobe primary tumors were also analyzed, although small sample sizes resulted in less robust statistical analysis. All three left-sided tumors were located in the left upper lobe. For left-sided tumors, comparison of the mean D95 of the IF-PTV showed that proton IFRT significantly improved PTV coverage (99.2% vs 95.7%, p=0.005). Although both proton IFRT and proton ENI decreased the mean lung dose (8.1 Gy and 9.1 Gy, respectively) compared to photon IFRT (12.8 Gy), the improvement was not statistically significant (p=0.28 and p=0.51, respectively). Similarly, comparing the dosimetric parameters from photon IFRT vs. proton IFRT vs. proton ENI for all other organs resulted in decreases that were not statistically significant, such as esophagus V60 (31.8% vs. 25.2% vs. 23.1%; p=0.34 for proton IFRT vs. photon IFRT and p=0.24 for proton ENI vs. photon IFRT) and heart V25 (29.8% vs. 13.2% vs. 12.6%; p=0.35 for proton IFRT vs. photon IFRT and p=0.32 for proton ENI vs. photon IFRT). A parallel analysis of the 3/20 middle/lower lobe primary tumors, all of which were right-sided, revealed similar results, although proton IFRT did not significantly improve PTV coverage compared to photon IFRT (98.6% vs 97.1%, p=0.13). Esophagus V60 decreased from 46.7% with photon IFRT to 33.5% with proton IFRT and 32.6% with proton ENI, but neither was significant (p=0.13 and p=0.12, respectively), as was the case with heart V25 (37.0% vs. 11.8% vs. 11.4%; p=0.21 for proton IFRT vs. photon IFRT and p=0.20 for proton ENI vs. photon IFRT).
Intensity-Modulated Radiation Therapy
Finally, dose-volume parameters were compared with regard to the type of photon plan delivered, specifically the 4/20 patients treated with IMRT. Three of the four were right-sided and all included an upper lobe primary tumor. Compared to photon IMRT plans only, proton IFRT improved PTV coverage from 90.5% to 95.2% and proton ENI to 95.2%, although these improvements were not significant (p=0.22 and p=0.23, respectively). As with the analysis of the small number of left-sided and middle/lower lobe primary tumors, IMRT improved dosimetric parameters, but not significantly. For example, esophagus V60 decreased from 21.9% with photon IFRT to 19.1% with proton IFRT and 17.9% with proton ENI, but neither was significant (p=0.40 and p=0.19, respectively), while heart V25 decreased from 11.0% with photon IFRT to 5.1% with proton IFRT and 5.2% with proton ENI (p=0.47 for both proton IFRT vs. photon IFRT and proton ENI vs. photon IFRT).
DISCUSSION
This study demonstrates the potential of IMPT to enhance the therapeutic window in LA-NSCLC by reducing dose to OAR while covering gross disease and at-risk ENS. This analysis is important because of the possibility of increased nodal failures with proton IFRT compared to photon IFRT as a result of decreased scatter to adjacent nodal regions. With photon IFRT, incidental dose to uninvolved nodal stations can reach as high as 40 Gy for node-positive patients and 13 Gy for hilar stations in node-negative patients,26–28 doses likely to contribute to control of microscopic disease.
The radiotherapy standard of care for LA-NSCLC continues to evolve. While two-dimensional radiation therapy plans covered ENS, the field moved away from ENI as it progressed into the three-dimensional era. Toxicity remains the primary argument against the use of photon ENI. In the only prospective trial comparing IFRT and ENI, patients in the IFRT arm had higher response rates, improved local control, longer overall survival, and lower rates of pneumonitis, although this study has been criticized for a lower dose of radiation (60–64 Gy vs. 68–74 Gy) and higher lung V20 in the ENI arm.29 A single-institution retrospective analysis confirmed that IFRT had a favorable therapeutic ratio compared to ENI based on reduced acute toxicity rather than improved disease control 11. No patient in the present study had a proton IFRT or ENI plan with either poorer IF-PTV coverage or higher dose to any OAR compared to the photon IFRT plan they actually received as measured by any dosimetric parameter assessed.
A second major argument against ENI is the relatively low rate of isolated ENS failure.11,12,30,31 Memorial-Sloan Kettering reported 2-year ENS control of 92.4% when targeting only initially-involved LN. Although few ENS failures were reported, preventing them could be quite significant in the LA-NSCLC population, which has limited expected survival. Furthermore, the true rate of ENS failures is unknown and likely underreported, given the difficulty of detecting mediastinal disease and because patients often undergo re-imaging only at the time of suspected systemic disease. These patients are then categorized as having simultaneous local and systemic failures, even though exclusive mediastinal disease may have been present prior to extrathoracic metastases.
Improved imaging techniques are another argument against ENI. While PET has unquestionably improved both initial staging and target delineation, thereby decreasing the likelihood of unintentional exclusion of involved nodal stations, the limit of PET detection, is estimated to be in the range of 105–106 malignant cells.32–34 In one randomized trial comparing pre-operative PET/CT with conventional staging, 24% of patients in the PET/CT arm underwent thoracotomy that was futile because of N2 disease detected in the surgical specimen; another similarly-structured trial revealed a 15% false-negative rate in the mediastinum in patients randomized to PET/CT staging.35,36 One estimate of the sensitivity of PET is as low as 50% after incorporation of verification bias.37 It is precisely those patients with microscopic disease below the limit of PET detection that may benefit the most from ENI.
We recognize the present study is limited by both its retrospective design and dosimetric endpoints. Specific limitations of the design included the inability to select patients with limited tumor motion nor re-plan the IMPT courses adaptively, which may be necessary in select patients; the recently published implementation of IMPT in thoracic cancers both selected patients for limited motion as well as adaptively planned with repeated 4D-CT simulation during treatment.22,38 With regard to organ motion, previous studies have quantified the amount of expected motion for mediastinal lymph nodes. A study utilizing 4D-CT simulation showed the mean motion for radiologically positive lymph nodes to be 2.6 mm mediolateral, 2.5 mm anterior-posterior, and 5.2 mm superior-inferior, with greater motion in the subcarinal lymph nodes.39 In our planning, we accounted for both primary tumor and mediastinal nodal motion with 4D-CT simulation followed by a further expansion of the 4D-CT-based iGTV. We recognize that the 3 mm expansion on nodal iGTV is tighter than is often utilized: in the recently opened RTOG 1308, a prospective randomized trial of proton versus photon radiotherapy in LA-NSCLC, the iGTV is expanded by 8 mm. This expansion reflects the University of Pennsylvania standard at the time the patients on this study were treated; histologic analysis has demonstrated that the extent of extracapsular extension is less than or equal to 3 mm in 95% of mediastinal lymph nodes.40 We were again constrained by the retrospective design of this study, as these expansions and volumes represent the actual treated volumes in these cases. Furthermore, the tighter photon IFRT volumes might have biased the dosimetric parameters in this analysis in favor of IFRT, thereby strengthening the conclusion that IMPT-based ENI may be able to overcome the toxicity limitations that have restricted photon-based ENI.
Results of this study should also be assessed in the context of the relatively high percentage of our cohort treated with 3D-CRT and/or to 72 Gy with photons. Patients in this study were treated with 3D-CRT unless dosimetric parameters could not be met, in which case they were treated with IMRT, a real-world strategy which accurately reflects that decisions are often based on insurance approvals rather than physician preference. Recent evidence suggests that this is not a localized issue: data from RTOG 0617 presented in the Plenary Session of the 2013 ASTRO Annual Meeting demonstrated that IMRT was used in only 41% and 44% of the QOL patients on the 60 Gy and 74 Gy arms, respectively.41 RTOG 1308 is also allowing 3D-CRT on the photon arm.
Although similar to a previously published analysis, this study included a larger number of patients, helping to address a concern from those authors that there may be cases in which adding ENI may lead to unacceptable OAR dose, which we did not find in any of our patients.24 While the previous study examined double-scatter proton plans, we were able to analyze IMPT with multi-field optimization, which the same institution has recently demonstrated, albeit in a pediatric clinical scenario, to have superior conformity and target coverage.42
CONCLUSIONS
In spite of these limitations, this study shows that ENI with IMPT is an approach worth considering in LA-NSCLC. Improved local control has previously translated to improved overall survival, but only at the expense of increased toxicity.43,44 While photon dose escalation showed promise in phase II studies, the high-dose arm of Radiation Therapy Oncology Group (RTOG) trial 0617 was closed early due to overall survival decrement and was also associated with worse quality of life.45,46 Previous trials, including a phase II trial with concurrent chemotherapy, suggest that proton therapy can more safely allow for dose escalation;47 RTOG 1308is treating to 70 Gy/CGE. IMPT-based ENI may be able to overcome the toxicity limitations that have restricted both photon-based dose escalation and ENI and translate clear dosimetric advantages into desperately-needed improved clinical outcomes.
CLINICAL PRACTICE POINTS.
Proton radiotherapy is being increasingly used as definitive treatment for non-small cell lung cancer for its potential to decrease radiation doses to organs at risk.
It is currently unknown if proton involved-field radiotherapy results in increased rates of isolated nodal failures compared to standard photon involved-field radiotherapy secondary to its high conformality and subsequent decreased scattered dose to elective nodal stations.
In this study, we show that the dosimetric advantages of intensity-modulated proton therapy allow for treatment of full elective nodal and primary target volumes while still reducing radiation doses to all evaluated organs at risk compared to photon involved-field radiotherapy.
Acknowledgments
This research was supported in part by the Intramural Research Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health.
ABBREVIATIONS
- 3D-CRT
three-dimensional conformal radiation therapy
- 4D
four-dimensional
- CTV
clinical target volume
- EN
elective nodal
- ENI
elective nodal irradiation
- ENS
elective nodal stations
- GTV
gross tumor volume
- IF
involved-field
- IFRT
involved-field radiation therapy
- IGTV
internal gross tumor volume
- IMPT
intensity-modulated proton therapy
- IMRT
intensity-modulated radiation therapy
- LA
locally advanced
- LN
lymph nodes
- OAR
organs at risk
- PTV
planning treatment volume
- RTOG
Radiation Therapy Oncology Group
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edge SB. AJCC cancer staging manual. 7. New York: Springer; 2010. American Joint Committee on Cancer. [DOI] [PubMed] [Google Scholar]
- 2.Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999 Sep;17(9):2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 3.Curran WJ., Jr Evolving chemoradiation treatment strategies for locally advanced non-small-cell lung cancer. Oncology (Williston Park) 2003 Dec;17(12 Suppl 13):7–14. [PubMed] [Google Scholar]
- 4.Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study. J Clin Oncol. 2005 Sep 1;23(25):5910–5917. doi: 10.1200/JCO.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 5.Rengan R, Rosenzweig KE, Venkatraman E, et al. Improved local control with higher doses of radiation in large-volume stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004 Nov 1;60(3):741–747. doi: 10.1016/j.ijrobp.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Belderbos JS, Heemsbergen WD, De Jaeger K, Baas P, Lebesque JV. Final results of a Phase I/II dose escalation trial in non-small-cell lung cancer using three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2006 Sep 1;66(1):126–134. doi: 10.1016/j.ijrobp.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 7.Kong FM, Ten Haken RK, Schipper MJ, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys. 2005 Oct 1;63(2):324–333. doi: 10.1016/j.ijrobp.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Zhao L, West BT, Hayman JA, Lyons S, Cease K, Kong FM. High radiation dose may reduce the negative effect of large gross tumor volume in patients with medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2007 May 1;68(1):103–110. doi: 10.1016/j.ijrobp.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 9.Uy KL, Darling G, Xu W, et al. Improved results of induction chemoradiation before surgical intervention for selected patients with stage IIIA-N2 non-small cell lung cancer. J Thorac Cardiovasc Surg. 2007 Jul;134(1):188–193. doi: 10.1016/j.jtcvs.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 10.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009 Aug 1;374(9687):379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandes AT, Shen J, Finlay J, et al. Elective nodal irradiation (ENI) vs. involved field radiotherapy (IFRT) for locally advanced non-small cell lung cancer (NSCLC): A comparative analysis of toxicities and clinical outcomes. Radiother Oncol. 2010 May;95(2):178–184. doi: 10.1016/j.radonc.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Rosenzweig KE, Sura S, Jackson A, Yorke E. Involved-field radiation therapy for inoperable non small-cell lung cancer. J Clin Oncol. 2007 Dec 10;25(35):5557–5561. doi: 10.1200/JCO.2007.13.2191. [DOI] [PubMed] [Google Scholar]
- 13.Kozak KR, Adams J, Krejcarek SJ, Tarbell NJ, Yock TI. A dosimetric comparison of proton and intensity-modulated photon radiotherapy for pediatric parameningeal rhabdomyosarcomas. Int J Radiat Oncol Biol Phys. 2009 May 1;74(1):179–186. doi: 10.1016/j.ijrobp.2008.06.1942. [DOI] [PubMed] [Google Scholar]
- 14.Taheri-Kadkhoda Z, Bjork-Eriksson T, Nill S, et al. Intensity-modulated radiotherapy of nasopharyngeal carcinoma: a comparative treatment planning study of photons and protons. Radiat Oncol. 2008;3:4. doi: 10.1186/1748-717X-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simone CB, 2nd, Ly D, Dan TD, et al. Comparison of intensity-modulated radiotherapy, adaptive radiotherapy, proton radiotherapy, and adaptive proton radiotherapy for treatment of locally advanced head and neck cancer. Radiother Oncol. 2011 Dec;101(3):376–382. doi: 10.1016/j.radonc.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang JY, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in Stage I or Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006 Jul 15;65(4):1087–1096. doi: 10.1016/j.ijrobp.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 17.Chang JY, Dong L, Liu H, et al. Image-guided radiation therapy for non-small cell lung cancer. J Thorac Oncol. 2008 Feb;3(2):177–186. doi: 10.1097/JTO.0b013e3181622bdd. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama H, Satoh H, Sugahara S, et al. Proton beam therapy of Stage II and III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011 Nov 15;81(4):979–984. doi: 10.1016/j.ijrobp.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Li Y, Pan X, et al. Intensity-modulated proton therapy reduces the dose to normal tissue compared with intensity-modulated radiation therapy or passive scattering proton therapy and enables individualized radical radiotherapy for extensive stage IIIB non-small-cell lung cancer: a virtual clinical study. Int J Radiat Oncol Biol Phys. 2010 Jun 1;77(2):357–366. doi: 10.1016/j.ijrobp.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang JY, Komaki R, Wen HY, et al. Toxicity and patterns of failure of adaptive/ablative proton therapy for early-stage, medically inoperable non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011 Aug 1;80(5):1350–1357. doi: 10.1016/j.ijrobp.2010.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sejpal S, Komaki R, Tsao A, et al. Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer. 2011 Jul 1;117(13):3004–3013. doi: 10.1002/cncr.25848. [DOI] [PubMed] [Google Scholar]
- 22.Chang JY, Li H, Zhu XR, et al. Clinical Implementation of Intensity Modulated Proton Therapy for Thoracic Malignancies. Int J Radiat Oncol Biol Phys. 2014 Sep 24; doi: 10.1016/j.ijrobp.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nichols RC, Huh SH, Hoppe BS, et al. Protons safely allow coverage of high-risk nodes for patients with regionally advanced non-small-cell lung cancer. Technol Cancer Res Treat. 2011 Aug;10(4):317–322. doi: 10.7785/tcrt.2012.500208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichols RC, Jr, Huh SH, Henderson RH, et al. Selective nodal irradiation of regionally advanced non-small-cell lung cancer with proton therapy and IMRT: A dosimetric comparison. Thoracic Cancer. 2012;3:169–174. doi: 10.1111/j.1759-7714.2011.00100.x. [DOI] [PubMed] [Google Scholar]
- 25.Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009 May;4(5):568–577. doi: 10.1097/JTO.0b013e3181a0d82e. [DOI] [PubMed] [Google Scholar]
- 26.Kepka L, Maciejewski B, Withers RH. Does incidental irradiation with doses below 50 gy effectively reduce isolated nodal failures in non-small-cell lung cancer: dose-response relationship. Int J Radiat Oncol Biol Phys. 2009 Apr 1;73(5):1391–1396. doi: 10.1016/j.ijrobp.2008.07.070. [DOI] [PubMed] [Google Scholar]
- 27.Kimura T, Togami T, Nishiyama Y, Ohkawa M, Takashima H. Impact of incidental irradiation on clinically uninvolved nodal regions in patients with advanced non-small-cell lung cancer treated with involved-field radiation therapy: does incidental irradiation contribute to the low incidence of elective nodal failure? Int J Radiat Oncol Biol Phys. 2010 Jun 1;77(2):337–343. doi: 10.1016/j.ijrobp.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 28.Zhao L, Chen M, Ten Haken R, et al. Three-dimensional conformal radiation may deliver considerable dose of incidental nodal irradiation in patients with early stage node-negative non-small cell lung cancer when the tumor is large and centrally located. Radiother Oncol. 2007 Feb;82(2):153–159. doi: 10.1016/j.radonc.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Yuan S, Sun X, Li M, et al. A randomized study of involved-field irradiation versus elective nodal irradiation in combination with concurrent chemotherapy for inoperable stage III nonsmall cell lung cancer. Am J Clin Oncol. 2007 Jun;30(3):239–244. doi: 10.1097/01.coc.0000256691.27796.24. [DOI] [PubMed] [Google Scholar]
- 30.Senan S, Burgers S, Samson MJ, et al. Can elective nodal irradiation be omitted in stage III non-small-cell lung cancer? Analysis of recurrences in a phase II study of induction chemotherapy and involved-field radiotherapy. Int J Radiat Oncol Biol Phys. 2002 Nov 15;54(4):999–1006. doi: 10.1016/s0360-3016(02)03028-6. [DOI] [PubMed] [Google Scholar]
- 31.Kepka L, Bujko K, Zolciak-Siwinska A. Risk of isolated nodal failure for non-small cell lung cancer (NSCLC) treated with the elective nodal irradiation (ENI) using 3D-conformal radiotherapy (3D-CRT) techniques--a retrospective analysis. Acta Oncol. 2008;47(1):95–103. doi: 10.1080/02841860701441855. [DOI] [PubMed] [Google Scholar]
- 32.Bradley J, Bae K, Choi N, et al. A phase II comparative study of gross tumor volume definition with or without PET/CT fusion in dosimetric planning for non-small-cell lung cancer (NSCLC): primary analysis of Radiation Therapy Oncology Group (RTOG) 0515. Int J Radiat Oncol Biol Phys. 2012 Jan 1;82(1):435–441. e431. doi: 10.1016/j.ijrobp.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geiger GA, Kim MB, Xanthopoulos EP, et al. Stage migration in planning PET/CT scans in patients due to receive radiotherapy for non-small-cell lung cancer. Clin Lung Cancer. 2014 Jan;15(1):79–85. doi: 10.1016/j.cllc.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Fischer BM, Olsen MW, Ley CD, et al. How few cancer cells can be detected by positron emission tomography? A frequent question addressed by an in vitro study. Eur J Nucl Med Mol Imaging. 2006 Jun;33(6):697–702. doi: 10.1007/s00259-005-0038-6. [DOI] [PubMed] [Google Scholar]
- 35.Fischer B, Lassen U, Mortensen J, et al. Preoperative staging of lung cancer with combined PET-CT. N Engl J Med. 2009 Jul 2;361(1):32–39. doi: 10.1056/NEJMoa0900043. [DOI] [PubMed] [Google Scholar]
- 36.Fischer BM, Mortensen J, Hansen H, et al. Multimodality approach to mediastinal staging in non-small cell lung cancer. Faults and benefits of PET-CT: a randomised trial. Thorax. 2011 Apr;66(4):294–300. doi: 10.1136/thx.2010.154476. [DOI] [PubMed] [Google Scholar]
- 37.Videtic GM, Rice TW, Murthy S, et al. Utility of positron emission tomography compared with mediastinoscopy for delineating involved lymph nodes in stage III lung cancer: insights for radiotherapy planning from a surgical cohort. Int J Radiat Oncol Biol Phys. 2008 Nov 1;72(3):702–706. doi: 10.1016/j.ijrobp.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 38.Hui Z, Zhang X, Starkschall G, et al. Effects of interfractional motion and anatomic changes on proton therapy dose distribution in lung cancer. Int J Radiat Oncol Biol Phys. 2008 Dec 1;72(5):1385–1395. doi: 10.1016/j.ijrobp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donnelly ED, Parikh PJ, Lu W, et al. Assessment of intrafraction mediastinal and hilar lymph node movement and comparison to lung tumor motion using four-dimensional CT. Int J Radiat Oncol Biol Phys. 2007 Oct 1;69(2):580–588. doi: 10.1016/j.ijrobp.2007.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan S, Meng X, Yu J, et al. Determining optimal clinical target volume margins on the basis of microscopic extracapsular extension of metastatic nodes in patients with non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2007 Mar 1;67(3):727–734. doi: 10.1016/j.ijrobp.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 41.Movsas B, Hu C, Sloan J, et al. Quality of Life (QOL) Analysis of the Randomized Radiation (RT) Dose-Escalation NSCLC Trial (RTOG 0617): The Rest of the Story. Int J Radiat Oncol. 2013 Oct 1;87(2):S1–S2. [Google Scholar]
- 42.Yeung D, McKenzie C, Indelicato DJ. A dosimetric comparison of intensity-modulated proton therapy optimization techniques for pediatric craniopharyngiomas: a clinical case study. Pediatric blood & cancer. 2014 Jan;61(1):89–94. doi: 10.1002/pbc.24593. [DOI] [PubMed] [Google Scholar]
- 43.Schaake-Koning C, Maat B, van Houtte P, et al. Radiotherapy combined with low-dose cis-diammine dichloroplatinum (II) (CDDP) in inoperable nonmetastatic non-small cell lung cancer (NSCLC): a randomized three arm phase II study of the EORTC Lung Cancer and Radiotherapy Cooperative Groups. Int J Radiat Oncol Biol Phys. 1990 Oct;19(4):967–972. doi: 10.1016/0360-3016(90)90020-k. [DOI] [PubMed] [Google Scholar]
- 44.Schaake-Koning C, van den Bogaert W, Dalesio O, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med. 1992 Feb 20;326(8):524–530. doi: 10.1056/NEJM199202203260805. [DOI] [PubMed] [Google Scholar]
- 45.Bradley JD, Paulus R, Komaki R, et al. A randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy with or without cetuximab for stage III non-small cell lung cancer: Results on radiation dose in RTOG 0617. J Clin Oncol. 2013;31(15_suppl):7501. [Google Scholar]
- 46.Movsas B, Hu C, Sloan J, et al. Quality of Life (QOL) Analysis of the Randomized Radiation (RT) Dose-Escalation NSCLC Trial (RTOG 0617): The Rest of the Story. Int J Radiat Oncol Biol Phys. 2013;87(2):S1–2. [Google Scholar]
- 47.Chang JY, Komaki R, Lu C, et al. Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer. 2011 Oct 15;117(20):4707–4713. doi: 10.1002/cncr.26080. [DOI] [PMC free article] [PubMed] [Google Scholar]