Abstract
Atherosclerosis is a chronic inflammatory condition that is considered a major cause of death worldwide. Striking phenomena of atherosclerosis associated with systemic lupus erythematosus (SLE) is its high incidence in young patients. Macrophages are heterogeneous cells that differentiate from hematopoietic progenitors and reside in different tissues to preserve tissue integrity. Macrophages scavenge modified lipids and play a major role in the development of atherosclerosis. When activated, macrophages secret inflammatory cytokines. This activation triggers apoptosis of cells in the vicinity of macrophages. As such, macrophages play a significant role in tissue remodeling including atherosclerotic plaque formation and rupture. In spite of studies carried on identifying the role of macrophages in atherosclerosis, this role has not been studied thoroughly in SLE-associated atherosclerosis. In this review, we address factors released by macrophages as well as extrinsic factors that may control macrophage behavior and their effect on accelerated development of atherosclerosis in SLE.
Keywords: Macrophage, lupus, atherosclerosis, sphingolipids, lipoprotein immune complexes, autoantibodies
1. Introduction
This is a PDF ?le of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its ?nal citable form. Please note that during the production process errors may be discovered which could a?ect the content, and all legal disclaimers that apply to the journal pertain.
Atherosclerosis is a chronic inflammatory disease affecting the arterial intima (1), involving adaptive and innate immunological responses (2, 3). Noninvasive investigations have shown increases in intima-media thickness, carotid plaque build-up, and coronary artery calcifications in subjects with SLE (4). Atherosclerosis has been recognized as a major co-morbid condition in systemic lupus erythematosus (SLE) with a 5-10 fold increase in risk of developing cardiovascular disease (CVD) compared to age-matched controls (5). Women with SLE in the 35-44 year age group have an estimated 50-fold increased risk of myocardial infarction compared to age and sex-matched controls (6). Although patients with SLE are subject to the same traditional CVD risk factors (e.g., hypertension, dyslipidemia, diabetes, old age, tobacco use, and postmenopausal status) as the general population, several studies showed that these factors do not sufficiently account for the increased level of CVD in SLE patients (reviewed in 7-9). Controlling for traditional risk factors as defined by the Framingham studies, there is a substantial and statistically significant increase in CVD and stroke among SLE patients (10). It has been found, however, that 53% of patients with SLE had at least three traditional CVD risk factors (11). Some traditional risk factors may also interact with the management of SLE disease activity (e.g., smoking, diabetes and hyperlipidemia) (7). Systemic inflammation, anti-phospholipid antibodies, and low levels of the natural anti-phosphorylcholine antibodies are examples of non-traditional CVD risk factors in lupus (7-9). Anti-phospholipid antibodies (anticardiolipin antibody, anti-β2-glycoprotein I, and lupus anticoagulant), which are usually generated against phospholipid binding proteins, have been associated with myocardial infarction in the general population (12). However, the association of anti-phospholipid antibodies with CVD in patients with SLE has been inconsistent (7). The underlying mechanisms responsible for the increase in morbidity and mortality due to SLE-related CVD remain unclear.
Atherosclerosis is identified by a passive accumulation of lipids in the vessel wall forming an atherosclerotic plaque. However, inflammation plays a role not only in the development of the atherosclerotic lesion but also in acute plaque ruptures resulting in acute myocardial ischemic events (13, 14). Lupus is an autoimmune disease in which the immune system attacks its own cells and tissues causing inflammation. A hallmark of both atherosclerosis and SLE-related inflammation is increased modification of lipoproteins and increased production of autoantibodies (15-18). In addition, SLE patients have a tendency to develop pro-atherogenic lipids (19, 20), and/or a defect in endothelial cell function (21). More specifically, SLE patients show decreased levels of high-density lipoproteins (HDL) with increased triglycerides and oxidized low-density lipoproteins (oxLDL) levels compared to healthy controls (22-24), thus predisposing them to coronary artery disease. Moreover, modified lipoproteins and circulating antibodies act in concert, accelerating chronic inflammation and the development of atherosclerosis largely by the transformation of monocytes into foam cells (25). While it is established that foam cell accumulation is a critical event in atherogenesis, the underlying mechanisms responsible for CVD intensification in SLE are not yet fully understood. Numerous studies have improved our understanding of the diverse components of immune dysregulation in SLE; however, the pathophysiologic role(s) of monocytes/macrophages remains unclear.
The macrophage is a heterogeneous cell that differentiates from hematopoietic progenitors which can generate dendritic cells, osteoclasts, and monocytes as well as macrophages (26). Monocytes circulate in the blood, but when activated reside in tissues as macrophages. Macrophages are active in both innate and adaptive immune responses, in addition to their known role in reverse cholesterol transport (27). They are also involved in normal tissue homeostasis through the uptake and clearance of apoptotic cells, and in resorption of bone during normal bone remodeling (26). A classically activated macrophage requires both interferon gamma (IFN-γ) and either the induction of tumor necrosis factor alpha (TNF-α), or the engagement of toll-like receptors typically by a microbial product (28). In response, the macrophage secretes inflammatory cytokines such as interleukin-1β (IL-1β) and TNF-α, and chemokines that drive an immune reaction (29).
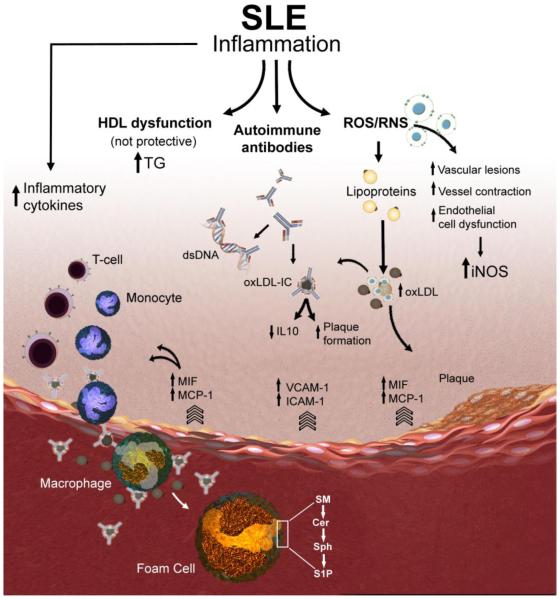
Theories formulated in the 1980s proposed that monocytes/macrophages in lupus display a defective phagocytic function, enabling the aberrant accumulation of apoptotic debris. This in turn can lead to a sequel of autoimmune events. However, studies exploring lupus nephritis suggested a more active role of monocytes/macrophages in mediating tissue inflammation and injury (30). The mechanisms by which SLE disease activity could be related to CVD have not been fully defined. The goal of this review is to focus on the role of monocytes/macrophages in developing accelerated vascular disease in SLE and describing the factors influencing the monocyte/macrophage function in this disease including inflammatory cytokines/chemokines, reactive oxygen and nitrogen intermediates, lipid profile changes, autoantibodies, and the potential role of bioactive sphingolipids in macrophage activation (Figure 1).
Figure 1. Schematic representation of putative macrophage-related mechanisms contributing to accelerated atherosclerosis in SLE.
Atherosclerotic plaques begin with the recruitment of inflammatory cells such as monocytes and T cells to the endothelial lining of the blood vessel. The endothelial cells release leukocyte adhesion molecules (e.g., VCAM-1, ICAM-1 and E-selectin) to facilitate sub-endothelial cell migration. MIF and MCP-1 levels are elevated in SLE patients and lead to increased pro-inflammatory cytokines. Increase in the production of reactive oxygen and nitrogen species (ROS and RNS) has been implicated in vascular lesion formation. LDL modified by the effect of ROS results in the formation of oxidized LDL (oxLDL). SLE and other autoimmune diseases produce autoantibodies which form complexes with either lipid metabolizing enzymes like lipoprotein lipase or with apolipoproteins and slow down lipoprotein catabolism, which may produce varieties of dyslipidemia. oxLDL complexed to IgG (oxLDL-IC) can be internalized by macrophages and results in the release of pro-inflammatory cytokines and promotes plaque formation. Pro-inflammatory HDL has been strongly associated with the development of carotid plaques and with intima media thickness in SLE patients. Targeting key metabolizing enzymes in the sphingolipid pathway (e.g., sphingomyelinases, ceramidases, and sphingosine kinases) that regulate the generation of the bioactive lipids sphingomyelin (SM), ceramide (Cer), sphingosine (Sph), and sphingosine 1- phosphate (S1P) in activated macrophages and foam cells might avert accelerated vascular disease in SLE.
2. Macrophage phagocytosis, activation and proliferation
Defective clearance of apoptotic bodies by monocytes may present one model in which SLE patients may develop Atherosclerosis (31). The build-up of unphagocytosed apoptotic bodies can at once both present an important source of autoantigens and also, through natural degradation, release proteins and enzymes that exacerbate local inflammation. While defective phagocytosis has been proposed to contribute to the build-up of immune-complexes in lupus nephritis, studies using lineage-specific transgenic reconstitution of Fc-γR in Fc-γR knockout animals have suggested that the build-up of immune complexes in the kidney activate monocyte/macrophages (32). Additionally, it has been suggested that monocytes from SLE patients have impaired IL-12p70 secretion (T cell stimulating factor) leading to high levels of IL-10 release (33). Interestingly, while B cell-derived IL-10 is anti-inflammatory, monocyte-derived IL-10 leads to increased estradiol-2-mediated anti-dsDNA antibody release (34).
Monocytes/macrophages play a key role in the early pathogenesis of atherosclerosis by ingesting modified lipoproteins, transforming into lipid-laden foam cells, and recruiting additional monocytes and macrophages to the vessel wall. Many clinical observations also acknowledge the role for monocyte/macrophage activation in the pathogenesis of atherosclerosis and its complications (35), and were concordant with histological studies in which monocytes/macrophages were prominent in atherosclerotic lesions (36). These findings suggest that high levels of macrophage activation and inflammatory chemokines mediate atherosclerosis development in SLE patients. In fact, macrophage activation syndrome (MAS), a life-threatening inflammatory emergency of children with rheumatic diseases, is increasingly recognized in pediatric systemic lupus erythematosus (37). Recently, it has been shown that concentrations of neopterin, a marker of macrophage activation and IFN-γ activity, are increased in large cohorts of patients with SLE or rheumatoid arthritis (RA), and have a more robust association with mediators of inflammation and homocysteine in SLE than in RA (38). More studies will be required to determine whether the increased concentrations of neopterin in patients with SLE could reflect a greater burden of unstable atheromatous plaque, or an increase in the processes by which stable plaque becomes unstable.
It has been recently suggested that expansion of macrophages necessary for pathogen control or wound repair can occur without recruitment of potentially tissue-destructive inflammatory cells. It has been found that proliferation of local macrophages (F4/80 negative) rather than recruitment from the blood (F4/80 positive) is fundamental in T helper-related inflammation (39). In aortas obtained from lupus mice lacking NOS2 or NOS3 genes encoding inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS), respectively, we have shown that F4/80-positive macrophages are mostly in the center of the nodule-like lesions found in the adventitia (40). Furthermore, sphingosine kinase (SK), the enzyme that generates sphingosine 1- phosphate (S1P), was found present in abundance around the F4/80-negative macrophages in the periphery of the lesion (40). It is now established that S1P promotes cell division and proliferation (41). It has been previously shown that the more advanced the atherosclerotic lesion, the greater the cell infiltration in the adventitia (42), predominantly the infiltration of B lymphocytes (42, 43). B-cells from RA patients are resistant to Fas-mediated apoptosis due in part to heightened SK activity and increased levels of S1P that can inhibit apoptosis and regulate lymphoid migration from the lymph node (44, 45). Hence, it is reasonable to suggest that increased SK levels in aortic lesions may be generating S1P, thus promoting B-cell infiltration and production of auto-antibodies that include anti-oxLDL IgG required to form oxLDL immune complexes (oxLDL-IC) (40). The oxLDL-IC-induced S1P generation that we have shown in human monocytic cells can lead to macrophage proliferation in the aorta and could expose more auto-antigens to be recognized by auto-antibodies generated locally (40). More studies to identify the exact role of macrophages in SLE are needed in order to mitigate the deleterious complications of this disease.
3. Inflammatory chemokines and cytokines in the pathogenesis of atherosclerosis in SLE
Atherosclerotic lesions begin with the recruitment of inflammatory cells such as monocytes and T cells to the endothelial wall. In response to this recruitment, the endothelial cells release leukocyte adhesion molecules like E-selectin, vascular cell adhesion molecule-1 (VCAM-1), and intercellular adhesion molecule-1 (ICAM-1) (46). The expression of adhesion molecules can be induced by pro-inflammatory cytokines such as TNF-α and IL-1, both of which up-regulate leukocyte adhesion molecules in an NF-κB-dependent process (46). A recent study by Gerry et al showed that a modest decrease in extracellular pH (pH of 7.0) can have marked effects on NF-κB activation and cytokine secretion in macrophages (47). Atherosclerotic lesions, as well as other sites of chronic inflammation, such as rheumatoid joints and tumors, have areas of low extracellular pH. It remains to be investigated whether this mechanism occurs in SLE patients.
VCAM-1 is induced when endothelial cells are exposed to lipopolysaccharides of gram negative bacteria, and oxidized phospholipids such as oxLDL (48, 49). Conversely, HDL inhibits the expression of adhesion molecules (50, 51). The soluble levels of VCAM-1 have been found elevated in humans with coronary artery disease (52, 53). However, in one cross sectional carotid ultrasound study of SLE patients neither levels of soluble VCAM nor ICAM were significantly associated with carotid plaque (54).
After the adherence of the leukocytes to the cell surface, they migrate through the endothelium into the intima (46). This transmigration occurs due to several chemotactic proteins including monocyte chemotactic protein-1 (MCP-1) produced by the smooth muscle layer and endothelial cells (55). MCP-1 expression in endothelial cells and smooth muscle cells can be up-regulated by cytokines such as TNF-α, and IL-1, as well as by oxLDL (55, 56). The elevated levels of MCP-1 in the circulation are positively correlated to increased carotid artery intima media thickness in humans (57). In addition, in LDLR−/− mice (a model for atherosclerosis), knockout of MCP-1 reduces the atherosclerosis induced by a high-fat diet (58). It has been shown that patients with SLE have increased concentrations of pro-inflammatory IL-6 and MCP-1 and that these cytokines correlate with some traditional coronary risk factors (59).
Macrophage migration inhibitory factor (MIF) is a pleiotropic cytokine with roles in several inflammatory diseases. MIF has emerged as a potential link between SLE and atherosclerosis development (60, 61). Elevated levels of serum MIF have been detected in SLE patients compared with healthy control individuals. MIF also induces the pro-inflammatory mediators TNF-α, IL-1, IL-6 and matrix metalloproteinases. It can activate T cells, promote angiogenesis and induce proliferation of cells, while inhibiting p53 expression and apoptosis of the same cells (61, 62). MIF can be induced by oxLDL, which is an initiating factor in atherogenesis, and so expression of MIF early on may enhance pro-inflammatory responses and lesion progression (62). The role of MCP-1 and MIF in atherosclerosis and SLE suggest that both are likely promising targets to treat the accelerated vascular disease in lupus.
In atherosclerosis, macrophages also play an important role in remodeling the plaque morphology. Macrophage activation leads to the secretion of pro-inflammatory cytokines and cytotoxic substances. The following cytokines secreted by activated macrophages are thought to play a role in the development of the atherosclerotic plaque
3.1. TNF-α
TNF-α constitutes an activating cytokine and a maturation factor of dendritic cells, which are essential in immune regulation and have also been implicated in autoimmunity in general, and in SLE in particular (63). In addition, the elevated circulating levels of TNF-α in SLE patient have been found to be associated with high triglyceride and low HDL levels (64). TNF-α and IL-1 enhance production of macrophage colony stimulating factor (M-CSF), granulocyte-monocyte colony stimulating factor, and granulocyte colony stimulating factor by smooth muscle cells, endothelial cells, and monocytes (65). These mediators activate monocytes and stimulate their transformation into macrophages and foam cells (66). It has been shown for example that inhibition of TNF-α decreased the progression of atherosclerosis in apolipoprotein E knockout mice (67). Whereas the association of TNF-α with human coronary atherosclerosis has been documented; its role in SLE-related atherosclerosis remains to be evaluated.
3.2. Interferon gamma (IFNγ)
IFNγ has also been detected in human plaques (46). IFNγ acts as a powerful growth inhibitor for smooth muscle cells, endothelial cells, and collagen production, thus promotes plaque instability (68). IFNγ has been shown to influence many features of atherosclerosis such as foam cell formation, the adaptive TH1-specific immune response and plaque development (69). IFNs, (IFNα, and IFNγ) are often profoundly dysregulated in SLE and induce B lymphocyte stimulator expression (70). IFNγ also improves the efficiency of antigen presentation, and leads to increased synthesis of TNF-α and IL-1 (71). Eid et al. reported a concomitant presence of IL-17 and IFNγ in patients and clinical specimens of coronary atherosclerosis (72). Both IL-17 and IFNγ increase T cells within coronary plaques, and a synergistic effect of IL-17 and IFNγ play an important role in the elicitation of pro-inflammatory cytokine and chemokine production by cultured human vascular smooth muscle cells (72). Significant evidence implicates IL-17 (produced by T cells) in the pathogenesis of SLE (73).
3.3. IL-1
The main role of IL-1 is to promote inflammation. In human atherosclerotic plaques, the expression of IL-1α and IL-1β seems to correlate with the progression of atherosclerotic plaques, with minimal expression in healthy coronary arteries, increased expression in simple atherosclerotic plaques, and high expression in complicated plaques (74). Experimental studies in atherosclerosis-prone animals have constantly revealed that genetic deletion or pharmacological inhibition of IL-1 signaling reduces the establishment and progression of atherosclerotic plaques, whereas increased IL-1 activity (by exogenous administration of IL-1β or down regulation of the naturally occurring receptor antagonist IL-1Ra) favors its progression (75, 76). Bone marrow chimeric studies recognized IL-1 signaling in the vessel wall, rather than in leukocytes, as a major determinant of atheroma formation (77, 78). IL-1 has been associated with lupus through correlation between lupus activity and IL-1 and IL-1Ra levels, as well as IL-1 genetic polymorphism (79).
3.4. IL-6
IL-6 is another cytokine, whose level is elevated in patients with SLE. IL-6 is produced by a wide range of cells including macrophages, lymphocytes and others. It acts in a paracrine, autocrine and endocrine fashion on target cells (80). IL-6 shares similar biological effect with IL-1. Elevated IL-6 levels have been considered as markers for atherosclerosis and inflammation in patients with SLE as well as for disease activity (81).
3.5. IL-12
Monocytes exposed to oxLDL result in the production of IL-12 (82). Moreover, it has been found that IL-12 levels were elevated in atherosclerotic plaques (83) . Inhibition of IL-12 using a vaccination technique that fully blocks the action of IL-12 led to decrease atherosclerosis in mice (83). STAT4, which is a key transcription factor induced in response to IL-12 in macrophages, is implicated in the pathogenesis of autoimmune diseases, such as SLE, RA, Type 1 diabetes and systemic sclerosis (84).
3.6. IL-10
IL-10 is an anti-inflammatory, regulatory cytokine that inhibits Th1 cytokine production and proliferation of CD4+ T cells via its indirect effects on antigen presenting cell function or through direct effects on T cells (85). IL-10 secretion disturbs the initial segment of inflammation by impeding the attachment of circulating immune cells to the endothelial wall by down-regulating adhesion molecules such as CD18, CD60L, and ICAM-1 (86). Yin et al showed that knocking out IL-10 in a lupus mouse model leads to develop severe lupus in these mice, and they had higher mortality than their IL-10 intact littermate controls (87). However, enhanced IFNγ production by both CD4 and CD8 cells and increased serum concentration of IgG2a anti-dsDNA autoantibodies were associated with the severity of the lupus in this model (87). Our recent findings showed that plasma level of IL-10 in the NOS2 and NOS3 knockout MRL/lpr mice significantly decreased compared to controls (40). Modulation of the level of IL-10 and more complete understanding of the interplay between IL-10, immune-complexes, and IFNα may identify a target that can be of potential therapeutic benefit for human lupus.
It is important therefore to find a link between chronic inflammation and the initiation and progression of vascular stiffening and atherosclerosis. Comprehensive characterization of the cytokines involved in the pathway of accelerated vascular complication in SLE is important to find the safest and most effective pharmacologic approaches to limit the flare up of this disease.
4. Reactive oxygen and nitrogen intermediates and accelerating vascular disease in SLE
There are two forms of nitric oxide synthase (NOS), inducible NOS (iNOS), and endothelial NOS (eNOS). SLE patients display a defect in eNOS functions that leads to reduced endothelium-dependent vasodilation (88). This results in increased vascular lesion formation, thus contributes to the severity of atherogenesis (89-91).
Nitric oxide (NO) has a dual effect on the cellular processes based on its concentration. NO can have a protective and proliferative effect at a concentration range 30-100nM (92). However, concentrations above 400nM can lead to cell cycle arrest and apoptosis, and more than 800nM of NO may cause nitrosative stress with the formation of N2O3 (93). Mitochondrial NO can combine with superoxide to form a peroxynitrate (ONOO-), which nitrates cytochrome c, leading to mitochondria-mediated apoptosis. On the other hand, NO in the cytoplasm has an anti-apoptotic effect (94). ONOO- also can oxidize lipids such as those found in LDL or arachidonic acid (95). Antibodies to the oxidized LDL and β2 glycoprotein I complexes were shown to be elevated in SLE. This antibody/antigen complex enhances oxLDL influx into foamy macrophages, providing a mechanism for accelerated atherosclerosis in SLE (96). In vascular tissue of murine models of lupus, prostocyclin synthase (97) and eNOS (98) are inactivated by ONOO-, a by-product of iNOS activity, leading to vasoconstriction. This suggests that iNOS activity has a pathogenic effect via deactivation of tissue protective enzyme.
Based on experimental evidence and clinical studies, oxidative and nitrosative stresses have been shown to be induced by atherosclerosis risk factors and to contribute to the onset and development of atherosclerotic vascular damage (99). There are several independent studies which have demonstrated a significant correlation between markers of systemic NO production and global lupus disease activity (100). Oates et al. demonstrated more increase in markers of NO production among African-Americans with lupus disease activity (101). It has been shown that excess production of reactive oxygen species and reactive nitrogen species has been implicated with vascular lesion formation and functional defects (102, 103), and are currently being pursued as possible new biomarkers for lupus (100). Recently we showed that human monocytic cells treated with free oxLDL had decreased mitochondrial membrane potential and significantly increased intracellular H2O2 and NO compared to cells treated with the vehicle alone (104).
In an attempt to study the effect of the iNOS inhibitor drug SD-3651 on prevention of SLE glomerulonephritis, it was shown that the drug prevented endothelial cell and podocyte pathology in MRL/lpr lupus mouse model lacking iNOS gene (105). However, the drug might have also acted via a non iNOS-mediated mechanism, indicating that other mechanisms can be involved in SLE. Our previously published findings suggest that advanced vascular disease in NOS2 and NOS3 knockout MRL/lpr mice may be caused by increased plasma triglycerides, ceramide and S1P; decreased plasma IL-10; and accumulation of oxLDL-IC in the vessel wall (40). This information provides a rational to develop therapeutic interventions that may modify the harmful effects of iNOS. Also, restoration of eNOS function may be another approach that can be used to treat SLE and prevent the flare up of its complications
5. Modified lipoproteins in SLE-associated atherosclerosis
One of the known mechanisms of atherosclerosis is that LDL particles are transported into artery walls, where they are trapped and bound to the extracellular matrix of the sub-endothelial space. These trapped LDL particles are then modified by reactive oxygen species, resulting in the formation of pro-inflammatory oxLDL (106). Cytokines such as MCP-1 and M-CSF are released when the endothelial cells are exposed to oxLDL, resulting in monocyte binding, chemotaxis, and differentiation into macrophage (107). It has been shown that SLE patients have higher levels of circulating oxLDL, especially in those with a history of CVD (17, 108). The higher levels of circulating oxLDL are strongly associated with coronary artery disease in the general population (109).
Several mechanisms by which oxLDL can be cleared from the sub-endothelial space have been previously described. One of these mechanisms is the macrophage engulfment of oxLDL by scavenger receptors (110, 111), then macrophages become foam cells around which atherosclerotic lesions are built (112). The other mechanism is enhanced reverse cholesterol transport mediated by lipoprotein transporters in HDL (113, 114). In addition, HDL removes reactive oxygen species from LDL, thus preventing the formation of oxLDL and subsequent recruitment of inflammatory mediators (107). Moreover, it has been shown that normal HDL inhibits the expression of MCP-1 (115).
It is now established that oxLDL complexed to IgG (oxLDL-IC) can be internalized by macrophages and this process is mediated primarily through cross-linking of FCγ receptors on the cell surface and results in the release of pro-inflammatory cytokines (116, 117). We found that oxLDL-IC can induce an immediate translocation and release of SK1 into the medium (116), which indicate that S1P may be generated extracellularly in response to oxLDL-IC and may therefore promote cell survival and prolong cytokine release by activated macrophages. We have also found that oxLDL-IC can induce the synthesis and release of heat shock protein 70B' (HSP70B'), and once stimulated, HSP70B' binds to the cell-associated and internalized lipid moiety of oxLDL-IC (118). The data implicated HSP70B' in the regulation of SK1 activity and release of IL-10, which influence macrophage activation and survival (118). The cross-linking of the FCγ receptors can also induce the secretion of acid sphingomyelinase, which can bind to oxLDL-IC and allows for the uptake and trafficking of the complex (119). We also showed that treatment of U937 macrophage with exogenous sphingomyelinase increases the uptake of oxLDL-IC (119). Interestingly, activated acid sphingomyelinase induced by oxLDL-IC also stimulates the release of exosomes containing IL-1β (119).
It has been shown that low levels of HDL are associated with increased risk of atherosclerosis (120). Also, during the acute phase response (occurring soon after the onset of infection, trauma, inflammatory processes, and some malignant conditions) HDL can be converted from anti-inflammatory state to pro-inflammatory and can usually cause increased oxidation of LDL (121). This acute phase response can become chronic, and may be a mechanism for HDL dysfunction in SLE (122). The pro-inflammatory HDL is significantly associated with carotid plaque and intima media thickness in patients with SLE (123). Moreover, in a study undertaken to determine the role of HDL in SLE, 45% of SLE patients were found to have abnormal HDL function, compared to 20% of RA patients and 4% of control patients, and had pro-inflammatory HDL that was not only unable to prevent oxidation of LDL but caused increased level of oxidation (24).
The interplay between HDL function, LDL oxidation, and macrophage activation might be a possible mechanism that can mediate vascular disease in SLE. Therefore, early detection of the levels of oxLDL and the pro-inflammatory HDL may identify the SLE patients at high risk of clinical atherosclerosis. Furthermore, HDL modification in SLE patients may protect from developing blood vessel complications.
6. Autoantibodies to modified lipoproteins in SLE
Elevated levels of antibodies against oxLDL in the general population and their role in the prediction of myocardial infarction and the progression of atherosclerosis have been previously described (124-126). Up to 80% of patient with SLE have elevated anti-oxLDL antibodies (16, 18, 109), and the titers of antibodies to oxLDL have also been associated with disease activity in SLE (15). Furthermore, SLE patients generate autoantibodies which form complexes with the lipid metabolizing enzyme lipoprotein lipase with a consequent dyslipoproteinemia characterized by elevated levels of very low-density lipoproteins and triglycerides and lower HDL levels. This pattern favors an enhanced LDL oxidation with a subsequent detrimental foam cell formation (127). Serum IgG anti-apoA1, anti-HDL, and anti C-reactive protein (CRP) were higher in patients with SLE compared to controls. In addition, the persistence of disease activity is associated with significant increase in IgG anti-apoA1 and anti-HDL but not anti-CRP (128).
Immune complexes have also been described as a risk factor for atherosclerosis in the general population. In a study of 257 healthy men, the levels of circulating immune complexes at age 50 correlated with the development of myocardial infarction (126). Several in vitro studies showed that lipoprotein-containing immune complexes may play a role in atherogenesis. For instance, macrophages that ingest these complexes become activated, and release TNF-α, IL-1, oxygen activated radicals, and matrix metalloproteinase-1 (129). In several studies of SLE subjects, lipoprotein-containing immune complexes have been examined, with different results. In one study of a pediatric SLE population, there was an increase in the level of anti-oxLDL IgG and IgG immune complexes with LDL in SLE subjects compared to healthy controls; however, there was no association with endothelial dysfunction (16). Another study showed that the levels of autoantibodies to oxLDL are elevated in SLE patients with CVD compared to SLE control patients with no CVD (17). On the other hand, Romero et al showed that anti-oxLDL IgG was not associated with arterial disease in SLE patients (130). Recently, we showed that in a lupus mouse model, oxLDL-IC can accumulate in the blood vessel wall but mainly in the adventitia (40). This controversy on the role of immune complexes in the contest of accelerated vascular disease in lupus warrants further studies.
7. Sphingolipids and vascular complications in SLE
Sphingolipids are ubiquitous constituents of bio-membranes and their metabolic products sphingomyelin, ceramide, sphingosine, and S1P are involved in the regulation of cell growth, differentiation, and apoptosis by acting as messengers in specific signal transduction pathways (131, 132). There is strong evidence that NO-activated pathways regulate many of the biological processes in which sphingolipids are involved (133, 134). Ceramides are one of the lipid components that make up sphingomyelin, which is one of the major lipids in the cell membrane lipid bilayer. Ceramide generation can also occur through breakdown of complex sphingolipids that are ultimately broken down into sphingosine. Sphingosine is a carbon amino alcohol with an unsaturated hydrocarbon chain, which forms primary structures of sphingolipids. Sphingosine can be phosphorylated in vivo via two kinases, SK1 and SK2 leading to the formation of the bioactive molecule S1P (135, 136).
S1P is a blood borne lipid mediator, in particular in association with lipoproteins such as HDL (137-139). High concentrations of S1P are present in body fluids and at lower levels in tissues (135). In a study designed to test the ability of serum sphingolipids to predict obstructive coronary artery disease, serum S1P was found to be a remarkably strong and robust predictor of both the occurrence and severity of coronary stenosis (140). We have previously reported that S1P is able to induce increases in released TNF-α, cyclooxygenase, and prostaglandin E2 in macrophages in vitro (141). Recently, we showed that levels of S1P in MRL/lpr mice lacking the eNOS and iNOS genes were significantly higher compared to their counterpart controls (40). Increased production of S1P has been linked to various pathological conditions suggesting that it may be a target for therapy for disorders such as cancer (142), atherosclerosis, and autoimmune diseases such as SLE and multiple sclerosis (143). A study by Snider et al. showed that inhibition of SK2 activity in MRL/lpr mice using the specific SK2 inhibitor ABC294640 can improve the glomerular pathology (144). Both S1P and dihydrosphingosine 1- phosphate (dh-S1P) levels in the circulation were significantly reduced with the SK2 inhibitor; however, kidney levels of dh-S1P were elevated. The authors suggested that inhibition of SK1 or perhaps both SK isoforms would better prevent elevations in circulating S1P and dh-S1P and potentially better protect against lupus nephritis (144).
It has been suggested that the sphingomyelin content of lipoprotein particles may influence lipid metabolism by affecting binding or activity of lecithin-cholesterol acyltransferase and lipoprotein lipase (145). However, sphingomyelin-rich lipoproteins can be converted to foam cell substrates by sphingomyelinase in the artery wall thereby promoting foam cell formation (146). Moreover, ceramide and related products of sphingomyelin synthesis and breakdown are potent regulators of cell proliferation, activation, and apoptosis and hence may affect plaque growth and stability (146).
Sphingomyelin in cellular membranes has a high affinity for cholesterol with which it associates in lipid rafts (147). T cells from SLE patients have been reported to contain larger lipid rafts compartments and that the lipid rafts formed are more robust when compared to T cells derived from normal individuals (148). The generation of these rafts was correlated with an increased response to CD3 cross-linking. Moreover, treatment of MRL/lpr mice with the immunosuppressor FTY720, an S1P receptor antagonist, induced apoptosis in double-negative splenic T cells and suppressed the development of SLE in these mice (149). It has been shown that natural killer (NK) T cell ablation decreases the total infiltration of macrophages in intra-abdominal adipose tissue (150). It has also been shown that in lupus patients NK cells up-regulates the secretion of anti-DNA antibodies(151). Morshed et al. showed that treatment with β-galctosylceramide, a glycolipid that reduces the cytokine secretion, down-regulated NK cell activity in NZB/W mice and ameliorated the symptoms of SLE (152). In contrast, repeated administration of α-galactosylceramide, a marine sponge-derived glycolipid that potently activates both mouse and human NK cells, led to the expansion of NK cells that could inhibit the development of inflammatory dermatitis in MRL/lpr mice (153) and pristane (hydrocarbon oils)-induced SLE in BALB/c mice. However, pristane could exacerbate SLE symptoms in SJL mice, known mouse model for immunological defects (154).
Recently Nowling et al. found early and significant dysfunction of the glycosphingolipid metabolic pathway in the kidneys of lupus mice and patients with lupus nephritis and suggested that molecules in this pathway may serve as early markers in lupus nephritis (155). Future studies can be directed towards finding therapeutic targets to modulate sphingolipid metabolic pathway which may have anti-atherogenic effects with eventual protection from accelerated vascular disease in the SLE patients. Furthermore, the development of methods to determine levels of circulating bioactive sphingolipids (156) in SLE patients and validation of these methods to be a routine clinical laboratory test could be a pioneering diagnostic and prognostic approach to SLE therapy.
8. Conclusions
In conclusion, SLE-associated atherosclerosis is a serious health problem for patients with SLE. More factors are involved in the development of this condition with SLE than with non-immune associated atherosclerosis. Many of these factors are cytokines and chemokines released by inflammatory cells, most significantly activated macrophages. Given the pivotal role of macrophages in the development, remodeling and thus stability of plaque in SLE, it is suggestive that macrophages play a similar role in the acceleration of development of atherosclerosis in SLE taking into consideration the amount of immune dysregulation associated with SLE. Future studies should be directed towards regulatory mechanisms of macrophage functions that can be targeted therapeutically to improve the outcome of SLE-associated atherosclerosis.
Highlights.
Accelerated atherosclerosis is a serious health problem for patients with SLE
Macrophages play a role in tissue remodeling including plaque formation and rupture
Macrophages release factors that accelerate development of atherosclerosis in SLE
Extrinsic factors can control macrophage behavior in SLE
Measuring and targeting sphingolipids in SLE can be a novel approach to therapy
9. Acknowledgments
S.M.H. was supported by NIH grant HL079274, NIH (ARRA) grant R01 HL079274 04S1, and the South Carolina COBRE in Lipidomics and Pathobiology (P20RR17677 from NCRR).
Abbreviations
- SLE
systemic lupus erythematosus
- CVD
cardiovascular disease
- oxLDL
oxidized low-density lipoprotein
- oxLDL-IC
oxidized low-density lipoprotein immune complex
- HDL
high-density lipoprotein
- dsDNA
double stranded DNA
- IFNγ
interferon gamma
- TNFα
tumor necrosis factor alpha
- IL-1α
interleukin 1 alpha
- IL-1β
interleukin 1 beta
- VCAM-1
vascular cell adhesion molecule-1
- ICAM-1
intercellular adhesion molecule-1
- MCP-1
monocyte chemotactic protein-1
- MIF
macrophage migration inhibitory factor
- CRP
C-reactive protein
- SK
sphingosine kinase
- S1P
sphingosine 1- phosphate
- dh-S1P
dihydrosphingosine 1- phosphate
- RA
rheumatoid arthritis
- NO
nitric oxide
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- M-CSF
macrophage colony stimulating factor
- ONOO-
peroxynitrate
- HSP70B'
heat shock protein 70B'
- NK cells
natural killer cells
References
- 1.Sherer Y, Shoenfeld Y. Mechanisms of disease: atherosclerosis in autoimmune diseases. Nature Reviews Rheumatology. 2006;2(2):99–106. doi: 10.1038/ncprheum0092. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson J, Hansson GK. Autoimmunity in atherosclerosis: a protective response losing control? Journal of Internal Medicine. 2008;263(5):464–78. doi: 10.1111/j.1365-2796.2008.01945.x. [DOI] [PubMed] [Google Scholar]
- 3.Pereira IA, Borba EF. The role of inflammation, humoral and cell mediated autoimmunity in the pathogenesis of atherosclerosis. Swiss Medical Weekly. 2008;138(37-38):534–9. doi: 10.4414/smw.2008.12287. [DOI] [PubMed] [Google Scholar]
- 4.Shoenfeld Y, Gerli R, Doria A, Matsuura E, Cerinic MM, Ronda N, et al. Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation. 2005;112(21):3337–47. doi: 10.1161/CIRCULATIONAHA.104.507996. [DOI] [PubMed] [Google Scholar]
- 5.Elliott JR, Manzi S, Edmundowicz D. The role of preventive cardiology in systemic lupus erythematosus. Current Rheumatology Reports. 2007;9(2):125–30. doi: 10.1007/s11926-007-0006-1. [DOI] [PubMed] [Google Scholar]
- 6.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jr., Jansen-McWilliams L, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. American Journal of Epidemiology. 1997;145(5):408–15. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 7.McMahon M, Skaggs B. Pathogenesis and treatment of atherosclerosis in lupus. Rheumatic Disease Clinics of North America. 2014;40(3):475–95. doi: 10.1016/j.rdc.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frostegård J. Prediction and management of cardiovascular outcomes in systemic lupus erythematosus. Expert Review of Clinical Immunology. 2014 Dec 17;:1–7. doi: 10.1586/1744666X.2015.993970. (in press) [DOI] [PubMed] [Google Scholar]
- 9.Sinicato NA, da Silva Cardoso PA, Appenzeller S. Risk factors in cardiovascular disease in systemic lupus erythematosus. Current Cardiology Reviews. 2013;9(1):15–9. doi: 10.2174/157340313805076304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis & Rheumatology. 2001;44(10):2331–7. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 11.Petri M, Perez-Gutthann S, Spence D, Hochberg MC. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. The American Journal of Medicine. 1992;93(5):513–9. doi: 10.1016/0002-9343(92)90578-y. [DOI] [PubMed] [Google Scholar]
- 12.Urbanus RT, Siegerink B, Roest M, Rosendaal FR, de Groot PG, Algra A. Antiphospholipid antibodies and risk of myocardial infarction and ischaemic stroke in young women in the RATIO study: a case-control study. The Lancet Neurology. 2009;8(11):998–1005. doi: 10.1016/S1474-4422(09)70239-X. [DOI] [PubMed] [Google Scholar]
- 13.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. The New England Journal of Medicine. 2005;352(16):1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 14.Ross R. Atherosclerosis--an inflammatory disease. The New England Journal of Medicine. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Zumaquero JM, Tinahones FJ, De Ramon E, Camps M, Garrido L, Soriguer FJ. Association of biological markers of activity of systemic lupus erythematosus with levels of anti- oxidized low-density lipoprotein antibodies. Rheumatology (Oxford) 2004;43(4):510–3. doi: 10.1093/rheumatology/keh109. [DOI] [PubMed] [Google Scholar]
- 16.Soep JB, Mietus-Snyder M, Malloy MJ, Witztum JL, von Scheven E. Assessment of atherosclerotic risk factors and endothelial function in children and young adults with pediatric-onset systemic lupus erythematosus. Arthritis & Rheumatology. 2004;51(3):451–7. doi: 10.1002/art.20392. [DOI] [PubMed] [Google Scholar]
- 17.Svenungsson E, Jensen-Urstad K, Heimburger M, Silveira A, Hamsten A, de Faire U, et al. Risk factors for cardiovascular disease in systemic lupus erythematosus. Circulation. 2001;104(16):1887–93. doi: 10.1161/hc4101.097518. [DOI] [PubMed] [Google Scholar]
- 18.Vaarala O, Alfthan G, Jauhiainen M, Leirisalo-Repo M, Aho K, Palosuo T. Crossreaction between antibodies to oxidised low-density lipoprotein and to cardiolipin in systemic lupus erythematosus. Lancet. 1993;341(8850):923–5. doi: 10.1016/0140-6736(93)91213-6. [DOI] [PubMed] [Google Scholar]
- 19.Borba EF, Bonfa E. Dyslipoproteinemias in systemic lupus erythematosus: influence of disease, activity, and anticardiolipin antibodies. Lupus. 1997;6(6):533–9. doi: 10.1177/096120339700600610. [DOI] [PubMed] [Google Scholar]
- 20.Nuttall SL, Heaton S, Piper MK, Martin U, Gordon C. Cardiovascular risk in systemic lupus erythematosus--evidence of increased oxidative stress and dyslipidaemia. Rheumatology (Oxford) 2003;42(6):758–62. doi: 10.1093/rheumatology/keg212. [DOI] [PubMed] [Google Scholar]
- 21.Bruce IN. 'Not only…but also': factors that contribute to accelerated atherosclerosis and premature coronary heart disease in systemic lupus erythematosus. Rheumatology (Oxford) 2005;44(12):1492–502. doi: 10.1093/rheumatology/kei142. [DOI] [PubMed] [Google Scholar]
- 22.Haque S, Mirjafari H, Bruce IN. Atherosclerosis in rheumatoid arthritis and systemic lupus erythematosus. Current Opinion in Lipidology. 2008;19(4):338–43. doi: 10.1097/MOL.0b013e328304b65f. [DOI] [PubMed] [Google Scholar]
- 23.Yassin LM, Londono J, Montoya G, De Sanctis JB, Rojas M, Ramirez LA, et al. Atherosclerosis development in SLE patients is not determined by monocytes ability to bind/endocytose Ox-LDL. Autoimmunity. 2011;44(3):201–10. doi: 10.3109/08916934.2010.530626. [DOI] [PubMed] [Google Scholar]
- 24.McMahon M, Grossman J, FitzGerald J, Dahlin-Lee E, Wallace DJ, Thong BY, et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis & Rheumatology. 2006;54(8):2541–9. doi: 10.1002/art.21976. [DOI] [PubMed] [Google Scholar]
- 25.Griffith RL, Virella GT, Stevenson HC, Lopes-Virella MF. Low density lipoprotein metabolism by human macrophages activated with low density lipoprotein immune complexes. A possible mechanism of foam cell formation. Journal of Experimental Medicine. 1988;168(3):1041–59. doi: 10.1084/jem.168.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nature Reviews Immunology. 2010;10(6):453–60. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcel YL, Ouimet M, Wang MD. Regulation of cholesterol efflux from macrophages. Current Opinion in Lipidology. 2008;19(5):455–61. doi: 10.1097/MOL.0b013e32830f4a1d. [DOI] [PubMed] [Google Scholar]
- 28.Schroder K, Sweet MJ, Hume DA. Signal integration between IFNgamma and TLR signalling pathways in macrophages. Immunobiology. 2006;211(6-8):511–24. doi: 10.1016/j.imbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Mosser DM. The many faces of macrophage activation. Journal of Leukocyte Biology. 2003;73(2):209–12. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 30.Kuroiwa T, Lee EG. Cellular interactions in the pathogenesis of lupus nephritis: the role of T cells and macrophages in the amplification of the inflammatory process in the kidney. Lupus. 1998;7(9):597–603. doi: 10.1191/096120398678920712. [DOI] [PubMed] [Google Scholar]
- 31.Gaipl US, Munoz LE, Grossmayer G, Lauber K, Franz S, Sarter K, et al. Clearance deficiency and systemic lupus erythematosus (SLE) Journal of Autoimmunity. 2007;28(2-3):114–21. doi: 10.1016/j.jaut.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Bergtold A, Gavhane A, D'Agati V, Madaio M, Clynes R. FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis. Journal of Immunology. 2006;177(10):7287–95. doi: 10.4049/jimmunol.177.10.7287. [DOI] [PubMed] [Google Scholar]
- 33.Liu TF, Jones BM. Impaired production of IL-12 in systemic lupus erythematosus. I. Excessive production of IL-10 suppresses production of IL-12 by monocytes. Cytokine. 1998;10(2):140–7. doi: 10.1006/cyto.1997.0268. [DOI] [PubMed] [Google Scholar]
- 34.Kanda N, Tsuchida T, Tamaki K. Estrogen enhancement of anti-double-stranded DNA antibody and immunoglobulin G production in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Arthritis & Rheumatology. 1999;42(2):328–37. doi: 10.1002/1529-0131(199902)42:2<328::AID-ANR16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 35.Weiss G, Willeit J, Kiechl S, Fuchs D, Jarosch E, Oberhollenzer F, et al. Increased concentrations of neopterin in carotid atherosclerosis. Atherosclerosis. 1994;106(2):263–71. doi: 10.1016/0021-9150(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 36.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 37.Gerstein M, Sukhdeo S, Levy DM, Feldman BM, Benseler SM, Ng LW, et al. A15: predicting macrophage activation syndrome in pediatric systemic lupus erythematosus patients at diagnosis. Arthritis & Rheumatology (Hoboken, N.J.) 2014;66(Suppl 11):S25. [Google Scholar]
- 38.Rho YH, Solus J, Raggi P, Oeser A, Gebretsadik T, Shintani A, et al. Macrophage activation and coronary atherosclerosis in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Care & Research (Hoboken) 2011;63(4):535–41. doi: 10.1002/acr.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 332;(6035):1284–8. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al Gadban MM, German J, Truman JP, Soodavar F, Riemer EC, Twal WO, et al. Lack of nitric oxide synthases increases lipoprotein immune complex deposition in the aorta and elevates plasma sphingolipid levels in lupus. Cellular Immunology. 2012;276(1-2):42–51. doi: 10.1016/j.cellimm.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nature Reviews Molecular Cell Biology. 2008;9(2):139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz CJ, Mitchell JR. Cellular infiltration of the human arterial adventitia associated with atheromatous plaques. Circulation. 1962;26:73–8. doi: 10.1161/01.cir.26.1.73. [DOI] [PubMed] [Google Scholar]
- 43.Ramshaw AL, Parums DV. Immunohistochemical characterization of inflammatory cells associated with advanced atherosclerosis. Histopathology. 1990;17(6):543–52. doi: 10.1111/j.1365-2559.1990.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 44.Lai WQ, Melendez AJ, Leung BP. Role of sphingosine kinase and sphingosine-1- phosphate in inflammatory arthritis. World Journal of Biological Chemistry. 2010;1(11):321–6. doi: 10.4331/wjbc.v1.i11.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pi X, Tan SY, Hayes M, Xiao L, Shayman JA, Ling S, et al. Sphingosine kinase 1- mediated inhibition of Fas death signaling in rheumatoid arthritis B lymphoblastoid cells. Arthritis and Rheumatism. 2006;54(3):754–64. doi: 10.1002/art.21635. [DOI] [PubMed] [Google Scholar]
- 46.Hansson GK. Immune mechanisms in atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21(12):1876–90. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- 47.Gerry AB, Leake DS. Effect of low extracellular pH on NF-kappaB activation in macrophages. Atherosclerosis. 2014;233(2):537–44. doi: 10.1016/j.atherosclerosis.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kume N, Cybulsky MI, Gimbrone MA., Jr. Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. The Journal of Clinical Investigation. 1992;90(3):1138–44. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watson AD, Leitinger N, Navab M, Faull KF, Horkko S, Witztum JL, et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. The Journal of Biological Chemistry. 1997;272(21):13597–607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 50.Cockerill GW, Rye KA, Gamble JR, Vadas MA, Barter PJ. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arteriosclerosis, Thrombosis, and Vascular Biology. 1995;15(11):1987–94. doi: 10.1161/01.atv.15.11.1987. [DOI] [PubMed] [Google Scholar]
- 51.Calabresi L, Franceschini G, Sirtori CR, De Palma A, Saresella M, Ferrante P, et al. Inhibition of VCAM-1 expression in endothelial cells by reconstituted high density lipoproteins. Biochemical and Biophysical Research Communications. 1997;238(1):61–5. doi: 10.1006/bbrc.1997.7236. [DOI] [PubMed] [Google Scholar]
- 52.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170(2):191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 53.Mehra VC, Ramgolam VS, Bender JR. Cytokines and cardiovascular disease. Journal of Leukocyte Biology. 2005;78(4):805–18. doi: 10.1189/jlb.0405182. [DOI] [PubMed] [Google Scholar]
- 54.Roman MJ, Shanker BA, Davis A, Lockshin MD, Sammaritano L, Simantov R, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. The New England Journal of Medicine. 2003;349(25):2399–406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 55.Wang JM, Sica A, Peri G, Walter S, Padura IM, Libby P, et al. Expression of monocyte chemotactic protein and interleukin-8 by cytokine-activated human vascular smooth muscle cells. Arteriosclerosis and Thrombosis. 1991;11(5):1166–74. doi: 10.1161/01.atv.11.5.1166. [DOI] [PubMed] [Google Scholar]
- 56.Torzewski J, Oldroyd R, Lachmann P, Fitzsimmons C, Proudfoot D, Bowyer D. Complement-induced release of monocyte chemotactic protein-1 from human smooth muscle cells. A possible initiating event in atherosclerotic lesion formation. Arteriosclerosis, Thrombosis, and Vascular Biology. 1996;16(5):673–7. doi: 10.1161/01.atv.16.5.673. [DOI] [PubMed] [Google Scholar]
- 57.Larsson PT, Hallerstam S, Rosfors S, Wallen NH. Circulating markers of inflammation are related to carotid artery atherosclerosis. International Angiology. 2005;24(1):43–51. [PubMed] [Google Scholar]
- 58.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Molecular Cell. 1998;2(2):275–81. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 59.Asanuma Y, Chung CP, Oeser A, Shintani A, Stanley E, Raggi P, et al. Increased concentration of proatherogenic inflammatory cytokines in systemic lupus erythematosus: relationship to cardiovascular risk factors. The Journal of Rheumatology. 2006;33(3):539–45. [PubMed] [Google Scholar]
- 60.Full LE, Ruisanchez C, Monaco C. The inextricable link between atherosclerosis and prototypical inflammatory diseases rheumatoid arthritis and systemic lupus erythematosus. Arthritis Research & Therapy. 2009;11(2):217. doi: 10.1186/ar2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morand EF, Leech M, Bernhagen J. MIF: a new cytokine link between rheumatoid arthritis and atherosclerosis. Nature Reviews Drug Discovery. 2006;5(5):399–410. doi: 10.1038/nrd2029. [DOI] [PubMed] [Google Scholar]
- 62.Zernecke A, Bernhagen J, Weber C. Macrophage migration inhibitory factor in cardiovascular disease. Circulation. 2008;117(12):1594–602. doi: 10.1161/CIRCULATIONAHA.107.729125. [DOI] [PubMed] [Google Scholar]
- 63.Palucka AK, Banchereau J, Blanco P, Pascual V. The interplay of dendritic cell subsets in systemic lupus erythematosus. Immunology and Cell Biology. 2002;80(5):484–8. doi: 10.1046/j.1440-1711.2002.01112.x. [DOI] [PubMed] [Google Scholar]
- 64.Svenungsson E, Fei GZ, Jensen-Urstad K, de Faire U, Hamsten A, Frostegard J. TNF-alpha: a link between hypertriglyceridaemia and inflammation in SLE patients with cardiovascular disease. Lupus. 2003;12(6):454–61. doi: 10.1191/0961203303lu412oa. [DOI] [PubMed] [Google Scholar]
- 65.Takahashi M, Kitagawa S, Masuyama JI, Ikeda U, Kasahara T, Takahashi YI, et al. Human monocyte-endothelial cell interaction induces synthesis of granulocyte-macrophage colony-stimulating factor. Circulation. 1996;93(6):1185–93. doi: 10.1161/01.cir.93.6.1185. [DOI] [PubMed] [Google Scholar]
- 66.Filonzi EL, Zoellner H, Stanton H, Hamilton JA. Cytokine regulation of granulocyte-macrophage colony stimulating factor and macrophage colony-stimulating factor production in human arterial smooth muscle cells. Atherosclerosis. 1993;99(2):241–52. doi: 10.1016/0021-9150(93)90026-q. [DOI] [PubMed] [Google Scholar]
- 67.Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(11):2137–42. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 68.Hansson GK, Jonasson L, Holm J, Clowes MM, Clowes AW. Gamma-interferon regulates vascular smooth muscle proliferation and Ia antigen expression in vivo and in vitro. Circulation Research. 1988;63(4):712–9. doi: 10.1161/01.res.63.4.712. [DOI] [PubMed] [Google Scholar]
- 69.McLaren JE, Ramji DP. Interferon gamma: a master regulator of atherosclerosis. Cytokine Growth Factor Reviews. 2009;20(2):125–35. doi: 10.1016/j.cytogfr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 70.Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. Journal of Experimental Medicine. 1999;189(11):1747–56. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annual Review of Immunology. 2003;21:713–58. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 72.Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, et al. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119(10):1424–32. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Apostolidis SA, Crispín JC, Tsokos GC. IL-17-producing T cells in lupus nephritis. Lupus. 2011;20(2):120–4. doi: 10.1177/0961203310389100. [DOI] [PubMed] [Google Scholar]
- 74.Dewberry R, Holden H, Crossman D, Francis S. Interleukin-1 receptor antagonist expression in human endothelial cells and atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20(11):2394–400. doi: 10.1161/01.atv.20.11.2394. [DOI] [PubMed] [Google Scholar]
- 75.Bashkaran K, Zunaina E, Bakiah S, Sulaiman SA, Sirajudeen K, Naik V. Anti-inflammatory and antioxidant effects of Tualang honey in alkali injury on the eyes of rabbits: experimental animal study. BMC Complementary and Alternative Medicine. 2011;11:90. doi: 10.1186/1472-6882-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Isoda K, Sawada S, Ishigami N, Matsuki T, Miyazaki K, Kusuhara M, et al. Lack of interleukin-1 receptor antagonist modulates plaque composition in apolipoprotein E-deficient mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(6):1068–73. doi: 10.1161/01.ATV.0000127025.48140.a3. [DOI] [PubMed] [Google Scholar]
- 77.Chamberlain J, Francis S, Brookes Z, Shaw G, Graham D, Alp NJ, et al. Interleukin-1 regulates multiple atherogenic mechanisms in response to fat feeding. PLoS One. 2009;4(4):e5073. doi: 10.1371/journal.pone.0005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Devlin CM, Kuriakose G, Hirsch E, Tabas I. Genetic alterations of IL-1 receptor antagonist in mice affect plasma cholesterol level and foam cell lesion size. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(9):6280–5. doi: 10.1073/pnas.092324399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tackey E, Lipsky PE, Illei GG. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus. 2004;13(5):339–43. doi: 10.1191/0961203304lu1023oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clark DN1, Markham JL, Sloan CS, Poole BD. Cytokine inhibition as a strategy for treating systemic lupus erythematosus. Clinical Immunology. 2013;148(3):335–43. doi: 10.1016/j.clim.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 81.Sabry AA, Elbasyouni SR, Kalil AM, Abdel-Rahim M, Mohsen T, Sleem A. Markers of inflammation and atherosclerosis in Egyptian patients with systemic lupus erythematosus. Nephrology (Carlton) 2006;11(4):329–35. doi: 10.1111/j.1440-1797.2006.00573.x. [DOI] [PubMed] [Google Scholar]
- 82.Chung SW, Kang BY, Kim SH, Pak YK, Cho D, Trinchieri G, et al. Oxidized low density lipoprotein inhibits interleukin-12 production in lipopolysaccharide-activated mouse macrophages via direct interactions between peroxisome proliferator-activated receptor-gamma and nuclear factor-kappa B. The Journal of Biological Chemistry. 2000;275(42):32681–7. doi: 10.1074/jbc.M002577200. [DOI] [PubMed] [Google Scholar]
- 83.Hauer AD, Uyttenhove C, de Vos P, Stroobant V, Renauld JC, van Berkel TJ, et al. Blockade of interleukin-12 function by protein vaccination attenuates atherosclerosis. Circulation. 2005;112(7):1054–62. doi: 10.1161/CIRCULATIONAHA.104.533463. [DOI] [PubMed] [Google Scholar]
- 84.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annual Review of Immunology. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 85.Song S, Ling-Hu H, Roebuck KA, Rabbi MF, Donnelly RP, Finnegan A. Interleukin-10 inhibits interferon-gamma-induced intercellular adhesion molecule-1 gene transcription in human monocytes. Blood. 1997;89(12):4461–9. [PubMed] [Google Scholar]
- 86.Liang Y, Pan HF, Ye DQ. Therapeutic potential of STAT4 in autoimmunity. Expert Opinion on Therapeutic Targets. 2014;18(8):945–60. doi: 10.1517/14728222.2014.920325. [DOI] [PubMed] [Google Scholar]
- 87.Yin Z, Bahtiyar G, Zhang N, Liu L, Zhu P, Robert ME, et al. IL-10 regulates murine lupus. The Journal of Immunology. 2002;169(4):2148–55. doi: 10.4049/jimmunol.169.4.2148. [DOI] [PubMed] [Google Scholar]
- 88.El-Magadmi M, Bodill H, Ahmad Y, Durrington PN, Mackness M, Walker M, et al. Systemic lupus erythematosus: an independent risk factor for endothelial dysfunction in women. Circulation. 2004;110(4):399–404. doi: 10.1161/01.CIR.0000136807.78534.50. [DOI] [PubMed] [Google Scholar]
- 89.Anderson TJ. Assessment and treatment of endothelial dysfunction in humans. Journal of the American College of Cardiology. 1999;34(3):631–8. doi: 10.1016/s0735-1097(99)00259-4. [DOI] [PubMed] [Google Scholar]
- 90.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23(2):168–75. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 91.Belmont HM, Levartovsky D, Goel A, Amin A, Giorno R, Rediske J, et al. Increased nitric oxide production accompanied by the up-regulation of inducible nitric oxide synthase in vascular endothelium from patients with systemic lupus erythematosus. Arthritis & Rheumatism. 1997;40(10):1810–6. doi: 10.1002/art.1780401013. [DOI] [PubMed] [Google Scholar]
- 92.Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radical Biology and Medicine. 2008;45(1):18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ridnour LA, Thomas DD, Mancardi D, Espey MG, Miranda KM, Paolocci N, et al. The chemistry of nitrosative stress induced by nitric oxide and reactive nitrogen oxide species. Putting perspective on stressful biological situations. Biological Chemistry. 2004;385(1):1–10. doi: 10.1515/BC.2004.001. [DOI] [PubMed] [Google Scholar]
- 94.Leon L, Jeannin JF, Bettaieb A. Post-translational modifications induced by nitric oxide (NO): implication in cancer cells apoptosis. Nitric Oxide. 2008;19(2):77–83. doi: 10.1016/j.niox.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 95.Moore KP, Darley-Usmar V, Morrow J, Roberts LJ., 2nd Formation of F2-isoprostanes during oxidation of human low-density lipoprotein and plasma by peroxynitrite. Circulation Research. 1995;77(2):335–41. doi: 10.1161/01.res.77.2.335. [DOI] [PubMed] [Google Scholar]
- 96.Lopez LR, Kobayashi K, Matsunami Y, Matsuura E. Immunogenic oxidized low-density lipoprotein/beta2-glycoprotein I complexes in the diagnostic management of atherosclerosis. Clinical Reviews in Allergy & Immunology. 2009;37(1):12–9. doi: 10.1007/s12016-008-8096-8. [DOI] [PubMed] [Google Scholar]
- 97.Zou MH, Cohen R, Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium. 2004;11(2):89–97. doi: 10.1080/10623320490482619. [DOI] [PubMed] [Google Scholar]
- 98.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. Journal of Clinical Investigation. 2002;109(6):817–26. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Puddu P, Puddu GM, Cravero E, De Pascalis S, Muscari A. The emerging role of cardiovascular risk factor-induced mitochondrial dysfunction in atherogenesis. Journal of Biomedical Science. 2009;16:112. doi: 10.1186/1423-0127-16-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oates JC, Gilkeson GS. The biology of nitric oxide and other reactive intermediates in systemic lupus erythematosus. Clinical Immunology. 2006;121(3):243–50. doi: 10.1016/j.clim.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oates JC, Christensen EF, Reilly CM, Self SE, Gilkeson GS. Prospective measure of serum 3-nitrotyrosine levels in systemic lupus erythematosus: correlation with disease activity. Proceedings of the Association of American Physicians. 1999;111(6):611–21. doi: 10.1046/j.1525-1381.1999.99110.x. [DOI] [PubMed] [Google Scholar]
- 102.Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radical Biology and Medicine. 1996;20(5):707–27. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 103.Freeman BA, White CR, Gutierrez H, Paler-Martinez A, Tarpey MM, Rubbo H. Oxygen radical-nitric oxide reactions in vascular diseases. Advances in Pharmacology. 1995;34:45–69. doi: 10.1016/s1054-3589(08)61080-7. [DOI] [PubMed] [Google Scholar]
- 104.Al Gadban MM, Smith KJ, Soodavar F, Piansay C, Chassereau C, Twal WO, et al. Differential trafficking of oxidized LDL and oxidized LDL immune complexes in macrophages: impact on oxidative stress. PLoS One. 5(9) doi: 10.1371/journal.pone.0012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Njoku C, Self SE, Ruiz P, Hofbauer AF, Gilkeson GS, Oates JC. Inducible nitric oxide synthase inhibitor SD-3651 reduces proteinuria in MRL/lpr mice deficient in the NOS2 gene. Journal of Investigative Medicine. 2008;56(7):911–9. doi: 10.231/JIM.0b013e3181889e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Camejo G, Olofsson SO, Lopez F, Carlsson P, Bondjers G. Identification of Apo B-100 segments mediating the interaction of low density lipoproteins with arterial proteoglycans. Arteriosclerosis. 1988;8(4):368–77. doi: 10.1161/01.atv.8.4.368. [DOI] [PubMed] [Google Scholar]
- 107.Navab M, Hama SY, Anantharamaiah GM, Hassan K, Hough GP, Watson AD, et al. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. Journal of Lipid Research. 2000;41(9):1495–508. [PubMed] [Google Scholar]
- 108.Frostegard J, Svenungsson E, Wu R, Gunnarsson I, Lundberg IE, Klareskog L, et al. Lipid peroxidation is enhanced in patients with systemic lupus erythematosus and is associated with arterial and renal disease manifestations. Arthritis & Rheumatism. 2005;52(1):192–200. doi: 10.1002/art.20780. [DOI] [PubMed] [Google Scholar]
- 109.Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. The New England Journal of Medicine. 2005;353(1):46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 110.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, et al. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. The Journal of Biological Chemistry. 2002;277(41):38503–16. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 111.Berliner J, Leitinger N, Watson A, Huber J, Fogelman A, Navab M. Oxidized lipids in atherogenesis: formation, destruction and action. Thrombosis and Haemostasis. 1997;78(1):195–9. [PubMed] [Google Scholar]
- 112.Navab M, Berliner JA, Watson AD, Hama SY, Territo MC, Lusis AJ, et al. The Yin and Yang of oxidation in the development of the fatty streak. A review based on the 1994 George Lyman Duff Memorial Lecture. Arteriosclerosis, Thrombosis, and Vascular Biology. 1996;16(7):831–42. doi: 10.1161/01.atv.16.7.831. [DOI] [PubMed] [Google Scholar]
- 113.Remaley AT, Thomas F, Stonik JA, Demosky SJ, Bark SE, Neufeld EB, et al. Synthetic amphipathic helical peptides promote lipid efflux from cells by an ABCA1-dependent and an ABCA1-independent pathway. Journal of Lipid Research. 2003;44(4):828–36. doi: 10.1194/jlr.M200475-JLR200. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Y, Zanotti I, Reilly MP, Glick JM, Rothblat GH, Rader DJ. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108(6):661–3. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- 115.Barter PJ, Baker PW, Rye KA. Effect of high-density lipoproteins on the expression of adhesion molecules in endothelial cells. Current Opinion in Lipidology. 2002;13(3):285–8. doi: 10.1097/00041433-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 116.Hammad SM, Taha TA, Nareika A, Johnson KR, Lopes-Virella MF, Obeid LM. Oxidized LDL immune complexes induce release of sphingosine kinase in human U937 monocytic cells. Prostaglandins & Other Lipid Mediators. 2006;79(1-2):126–40. doi: 10.1016/j.prostaglandins.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 117.Huang Y, Jaffa A, Koskinen S, Takei A, Lopes-Virella MF. Oxidized LDL-containing immune complexes induce Fc gamma receptor I-mediated mitogen-activated protein kinase activation in THP-1 macrophages. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19(7):1600–7. doi: 10.1161/01.atv.19.7.1600. [DOI] [PubMed] [Google Scholar]
- 118.Smith KJ, Twal WO, Soodavar F, Virella G, Lopes-Virella MF, Hammad SM. Heat shock protein 70B' (HSP70B') expression and release in response to human oxidized low density lipoprotein immune complexes in macrophages. The Journal of Biological Chemistry. 2010;285(21):15985–93. doi: 10.1074/jbc.M110.113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Truman JP, Al Gadban MM, Smith KJ, Jenkins RW, Mayroo N, Virella G, et al. Differential regulation of acid sphingomyelinase in macrophages stimulated with oxidized low-density lipoprotein (LDL) and oxidized LDL immune complexes: role in phagocytosis and cytokine release. Immunology. 2012;136(1):30–45. doi: 10.1111/j.1365-2567.2012.03552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circulation Research. 2004;95(8):764–72. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 121.Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, et al. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. Journal of Clinical Investigation. 1995;96(6):2758–67. doi: 10.1172/JCI118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. The New England Journal of Medicine. 1999;340(6):448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 123.McMahon M, Grossman J, Skaggs B, Fitzgerald J, Sahakian L, Ragavendra N, et al. Dysfunctional proinflammatory high-density lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. Arthritis & Rheumatism. 2009;60(8):2428–37. doi: 10.1002/art.24677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu R, Nityanand S, Berglund L, Lithell H, Holm G, Lefvert AK. Antibodies against cardiolipin and oxidatively modified LDL in 50-year-old men predict myocardial infarction. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;17(11):3159–63. doi: 10.1161/01.atv.17.11.3159. [DOI] [PubMed] [Google Scholar]
- 125.Salonen JT, Yla-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, et al. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992;339(8798):883–7. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 126.Mustafa A, Nityanand S, Berglund L, Lithell H, Lefvert AK. Circulating immune complexes in 50-year-old men as a strong and independent risk factor for myocardial infarction. Circulation. 2000;102(21):2576–81. doi: 10.1161/01.cir.102.21.2576. [DOI] [PubMed] [Google Scholar]
- 127.Borba EF, Carvalho JF, Bonfa E. Mechanisms of dyslipoproteinemias in systemic lupus erythematosus. Clinical & Developmental Immunology. 2006;13(2-4):203–8. doi: 10.1080/17402520600876945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.O'Neill SG, Giles I, Lambrianides A, Manson J, D'Cruz D, Schrieber L, et al. Antibodies to apolipoprotein A-I, high-density lipoprotein, and C-reactive protein are associated with disease activity in patients with systemic lupus erythematosus. Arthritis & Rheumatism. 2010;62(3):845–54. doi: 10.1002/art.27286. [DOI] [PubMed] [Google Scholar]
- 129.Virella G, Atchley D, Koskinen S, Zheng D, Lopes-Virella MF, Group DER. Proatherogenic and proinflammatory properties of immune complexes prepared with purified human oxLDL antibodies and human oxLDL. Clinical Immunology. 2002;105(1):81–92. doi: 10.1006/clim.2002.5269. [DOI] [PubMed] [Google Scholar]
- 130.Romero FI, Amengual O, Atsumi T, Khamashta MA, Tinahones FJ, Hughes GR. Arterial disease in lupus and secondary antiphospholipid syndrome: association with anti-beta2-glycoprotein I antibodies but not with antibodies against oxidized low-density lipoprotein. British Journal of Rheumatology. 1998;37(8):883–8. doi: 10.1093/rheumatology/37.8.883. [DOI] [PubMed] [Google Scholar]
- 131.Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274(5294):1855–9. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 132.Spiegel S, Merrill AH., Jr. Sphingolipid metabolism and cell growth regulation. The FASEB Journal. 1996;10(12):1388–97. doi: 10.1096/fasebj.10.12.8903509. [DOI] [PubMed] [Google Scholar]
- 133.Bulotta S, Barsacchi R, Rotiroti D, Borgese N, Clementi E. Activation of the endothelial nitric-oxide synthase by tumor necrosis factor-alpha. A novel feedback mechanism regulating cell death. The Journal of Biological Chemistry. 2001;276(9):6529–36. doi: 10.1074/jbc.M006535200. [DOI] [PubMed] [Google Scholar]
- 134.Sciorati C, Rovere P, Ferrarini M, Heltai S, Manfredi AA, Clementi E. Autocrine nitric oxide modulates CD95-induced apoptosis in gammadelta T lymphocytes. The Journal of Biological Chemistry. 1997;272(37):23211–5. doi: 10.1074/jbc.272.37.23211. [DOI] [PubMed] [Google Scholar]
- 135.Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO Reports. 2004;5(8):777–82. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zeidan YH, Hannun YA. Translational aspects of sphingolipid metabolism. Trends in Molecular Medicine. 2007;13(8):327–36. doi: 10.1016/j.molmed.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 137.Hammad SM, Al Gadban MM, Semler AJ, Klein RL. Sphingosine 1-phosphate distribution in human plasma: associations with lipid profiles. Journal of Lipids. 2012;2012:180705. doi: 10.1155/2012/180705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hammad SM, Pierce JS, Soodavar F, Smith KJ, Al Gadban MM, Rembiesa B, et al. Blood sphingolipidomics in healthy humans: impact of sample collection methodology. Journal of Lipid Research. 2010;51(10):3074–87. doi: 10.1194/jlr.D008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee MH, Hammad SM, Semler AJ, Luttrell LM, Lopes-Virella MF, Klein RL. HDL3, but not HDL2, stimulates plasminogen activator inhibitor-1 release from adipocytes: the role of sphingosine-1-phosphate. Journal of Lipid Research. 2010;51(9):2619–28. doi: 10.1194/jlr.M003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Deutschman DH, Carstens JS, Klepper RL, Smith WS, Page MT, Young TR, et al. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. American Heart Journal. 2003;146(1):62–8. doi: 10.1016/S0002-8703(03)00118-2. [DOI] [PubMed] [Google Scholar]
- 141.Hammad SM, Crellin HG, Wu BX, Melton J, Anelli V, Obeid LM. Dual and distinct roles for sphingosine kinase 1 and sphingosine 1 phosphate in the response to inflammatory stimuli in RAW macrophages. Prostaglandins & Other Lipid Mediators. 2008;85(3-4):107–14. doi: 10.1016/j.prostaglandins.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nature Reviews Cancer. 2010;10(7):489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 143.Groves A, Kihara Y, Chun J. Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. Journal of the Neurological Sciences. 2013;328(1-2):9–18. doi: 10.1016/j.jns.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Snider AJ, Ruiz P, Obeid LM, Oates JC. Inhibition of sphingosine kinase-2 in a murine model of lupus nephritis. PLoS One. 2013;8(1):e53521. doi: 10.1371/journal.pone.0053521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schissel SL, Keesler GA, Schuchman EH, Williams KJ, Tabas I. The cellular trafficking and zinc dependence of secretory and lysosomal sphingomyelinase, two products of the acid sphingomyelinase gene. The Journal of Biological Chemistry. 1998;273(29):18250–9. doi: 10.1074/jbc.273.29.18250. [DOI] [PubMed] [Google Scholar]
- 146.Park TS, Panek RL, Mueller SB, Hanselman JC, Rosebury WS, Robertson AW, et al. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2004;110(22):3465–71. doi: 10.1161/01.CIR.0000148370.60535.22. [DOI] [PubMed] [Google Scholar]
- 147.Brown DA, London E. Structure and function of sphingolipid-and cholesterol-rich membrane rafts. The Journal of Biological Chemistry. 2000;275(23):17221–4. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 148.Krishnan S, Nambiar MP, Warke VG, Fisher CU, Mitchell J, Delaney N, et al. Alterations in lipid raft composition and dynamics contribute to abnormal T cell responses in systemic lupus erythematosus. Journal of Immunology. 2004;172(12):7821–31. doi: 10.4049/jimmunol.172.12.7821. [DOI] [PubMed] [Google Scholar]
- 149.Okazaki H, Hirata D, Kamimura T, Sato H, Iwamoto M, Yoshio T, et al. Effects of FTY720 in MRL-lpr/lpr mice: therapeutic potential in systemic lupus erythematosus. The Journal of Rheumatology. 2002;29(4):707–16. [PubMed] [Google Scholar]
- 150.O'Rourke RW, Meyer KA, Neeley CK, Gaston GD, Sekhri P, Szumowski M, et al. Systemic NK cell ablation attenuates intra-abdominal adipose tissue macrophage infiltration in murine obesity. Obesity (Silver Spring) 2014 doi: 10.1002/oby.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hahn BH. Antibodies to DNA. The New England Journal of Medicine. 1998;338(19):1359–68. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- 152.Morshed SR, Takahashi T, Savage PB, Kambham N, Strober S. Beta-galactosylceramide alters invariant natural killer T cell function and is effective treatment for lupus. Clinical Immunology. 2009;132(3):321–33. doi: 10.1016/j.clim.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang JQ, Saxena V, Xu H, Van Kaer L, Wang CR, Singh RR. Repeated alpha-galactosylceramide administration results in expansion of NK T cells and alleviates inflammatory dermatitis in MRL-lpr/lpr mice. Journal of Immunology. 2003;171(8):4439–46. doi: 10.4049/jimmunol.171.8.4439. [DOI] [PubMed] [Google Scholar]
- 154.Singh AK, Yang JQ, Parekh VV, Wei J, Wang CR, Joyce S, et al. The natural killer T cell ligand alpha-galactosylceramide prevents or promotes pristane-induced lupus in mice. European Journal of Immunology. 2005;35(4):1143–54. doi: 10.1002/eji.200425861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nowling TK, Mather AR, Thiyagarajan T, Hernandez-Corbacho MJ, Powers TW, Jones EE, et al. Renal Glycosphingolipid Metabolism Is Dysfunctional in Lupus Nephritis. Journal of the American Society of Nephrology : JASN. 2014 doi: 10.1681/ASN.2014050508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hammad SM. Blood sphingolipids in homeostasis and pathobiology. Advances in Experimental Medicine and Biology. 2011;721:57–66. doi: 10.1007/978-1-4614-0650-1_4. [DOI] [PubMed] [Google Scholar]