Abstract
Bortezomib (BZ) is the first clinically approved proteasome inhibitor that has shown remarkable anticancer activity in patients with hematological malignancies. However, many patients relapse and develop resistance; yet, the molecular mechanisms of BZ resistance are not fully understood. We have recently shown that in solid tumors, BZ unexpectedly increases expression of the pro-inflammatory and pro-angiogenic chemokine interleukin-8 (IL-8), while it inhibits expression of other NFκB-regulated genes. Since monocytes and macrophages are major producers of IL-8, the goal of this study was to test the hypothesis that BZ increases the IL-8 expression in human monocytes and macrophages. Here, we show that BZ dramatically increases the IL-8 expression in lipopolysaccharide (LPS)-stimulated U937 macrophages as well as in unstimulated U937 monocytes and peripheral blood mononuclear cells, while it inhibits expression of IL-6, IL-1 and tumor necrosis factor-α. In addition, our results show that the underlying mechanisms involve p38 mitogen-activated protein kinase, which is required for the BZ-induced IL-8 expression. Together, these data suggest that the BZ-increased IL-8 expression in monocytes and macrophages may represent one of the mechanisms responsible for the BZ resistance and indicate that targeting the p38-mediated IL-8 expression could enhance the BZ effectiveness in cancer treatment.
Keywords: Bortezomib, interleukin-8, macrophages, monocytes, NFκB, p38 MAPK
Introduction
Bortezomib (BZ, Velcade, PS-341) is the first Food and Drug Administration-approved proteasome inhibitor that has been used as a frontline therapy in refractory multiple myeloma (MM). BZ has shown promising results also in mantle cell lymphoma, cutaneous T cell lymphoma, and other hematological malignancies. However, many patients do not respond to BZ and those who do respond almost always relapse and develop resistance [1, 2]. The molecular mechanisms of BZ resistance are poorly understood; thus, a better understanding of the responsible mechanisms should lead to the development of more effective therapies.
The original rationale behind BZ development and use was the inhibition of inducible proteasomal degradation of IκBα, resulting in the inhibition of NFκB activity and transcription of NFκB-dependent anti-apoptotic and pro-inflammatory genes in cancer cells [3–5]. However, in addition to inhibiting the degradation of IκBα, proteasome inhibition likely stabilizes other proteins as well. Indeed, we have recently shown that proteasome inhibition by BZ increases the nuclear levels of IκB kinase (IKK) in prostate and ovarian cancer cells, resulting in increased expression of the pro-inflammatory chemokine interleukin-8 (IL-8), while the expression of other NFκB-regulated genes is suppressed, or is not affected [6, 7].
IL-8 was originally discovered as the neutrophil chemo-attractant and inducer of leukocyte-mediated inflammation [8–10]. However, in cancer cells, IL-8 contributes to cancer progression through its induction of tumor cell proliferation, survival, migration, and angiogenesis [11–14]. In addition, IL-8 induces neutrophil recruitment and activates neutrophils and the tumor-associated macrophages to release more IL-8, which further amplifies the pro-survival, angiogenic and metastatic effect [11–14]. Even though IL-8 is released by many cell types, monocytes and macrophages are the major producers [15, 16].
In this study, we tested the hypothesis that proteasome inhibition by BZ increases the IL-8 expression in human monocytes and macrophages. We show that BZ dramatically increases the expression of IL-8 in LPS-stimulated U937 macrophages as well as in unstimulated U937 monocytic cells and peripheral blood mononuclear cells (PBMC), while it inhibits the expression of IL-6, IL-1 and tumor necrosis factor-α (TNF). In addition, our results indicate that the underlying mechanisms involve stabilization of p38 mitogen-activated protein kinase (MAPK), which is required for the BZ-induced IL-8 expression. Together, these data suggest that the BZ-increased IL-8 expression in monocytes and macrophages may represent one of the mechanisms responsible for the BZ resistance and indicate that targeting the p38-mediated IL-8 expression could enhance the BZ effectiveness in cancer treatment.
Materials and Methods
Antibodies and reagents
Purified polyclonal antibodies against human IKKα (sc-7218), IKKβ (sc-8014), p38 MAPK (sc-7149), and lamin B (sc-6216) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Purified polyclonal antibody against lactate dehydrogenase (LDH; 20-LG22) was from Fitzgerald Industries International (Concord, MA, USA), and actin antibody was from Sigma (St Louis, MO, USA). Horseradish peroxidase (HRP)-conjugated anti-rabbit, anti-mouse and anti-goat secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Bortezomib was obtained from ChemieTek (Indianapolis, IN, USA). Lipopolysaccharide (LPS), PS1145, and SB203580 were purchased from Sigma (St Louis, MO, USA). All other reagents were molecular biology grade and were from Sigma (St Louis, MO, USA).
Cell culture
U937 human promyeloid cells were obtained from American Type Culture Collection (ATCC; Rockville, MD, USA). Peripheral blood mononuclear cells (PBMC) from healthy human volunteers were purchased from All Cells (PB003F; Alameda, CA, USA). The cells were grown in RPMI 1640 medium (Invitrogen, Grand Island, NY, USA) supplemented with 10% heat inactivated fetal bovine serum (FBS; Invitrogen, Grand Island, NY, USA), 2 mM L-glutamine, and antibiotics at 37°C in a 5% CO2 humidified atmosphere as described [17]. U937 cells were differentiated into macrophage-like cells by incubation with 10 ng/ml phorbol-12-myristate-13 acetate (PMA; Calbiochem, San Diego, CA, USA) for 24 hours as described [17].
Transfections
Prior to transfections, cells were seeded (5×105 cells/ml) into a 6-well plate and incubated in a humidified 5% CO2 atmosphere at 37°C in antibiotic-free RPMI medium supplemented with 10% FBS for 24 hours. For siRNA transfections, 50 nmol (final concentration) of control siRNA-A (sc-37007; Santa Cruz Biotechnology, CA, USA), IKKα (sc-29365), IKKβ (sc-35644), or p38 siRNA (sc-29433) were used. Cells were transfected with TransIT-siQUEST transfection reagent (Mirus Bio, Madison, WI, USA) according to manufacturer’s instructions as described [18]. After transfection, fresh RPMI medium supplemented with FBS and antibiotics was added and cells were incubated for 24 hours.
Real time RT-PCR
Total RNA was isolated by using RNeasy mini-kit (Qiagen, Valencia, CA, USA). The iScript one-step RT-PCR kit with SYBR Green (BioRad, Hercules, CA, USA) was used as a supermix and 20 ng of RNA was used as template on a Bio-Rad MyIQ Single Color Real-Time PCR Detection System (BioRad). The primers used for quantification of IL-1, IL-6, IL-8, TNF, and actin mRNA were purchased from SA Biosciences (Frederick, MD, USA).
Western analysis of cytoplasmic and nuclear extracts
Cytoplasmic (CE) and nuclear extracts (NE) were prepared and separated on 12% SDS gels as described previously [18, 19]. Contamination of nuclear and cytoplasmic fractions by cytoplasmic and nuclear proteins, respectively, was determined by western analysis using LDH and lamin B as specific markers as described [19]. To determine equal protein loading, membranes were stripped and re-probed with anti-actin antibody as described [19].
ELISA
IL-1, IL-6, IL-8 and TNF release was measured in cell culture supernatants by commercially available ELISA kits (R&D, Minneapolis, MN, USA) as previously described [6, 7].
Statistical analysis
The results represent at least three independent experiments. Numerical results are presented as means ± S.E. Data were analyzed by using an InStat software package (GraphPAD, San Diego, CA). Statistical significance was evaluated by using Mann-Whitney U test with Bonferroni correction for multiple comparisons; p<0.05 was considered significant, and is denoted by an asterisk.
Results and Discussion
Proteasome inhibition by BZ specifically induces IL-8 expression in LPS-stimulated U937 macrophages
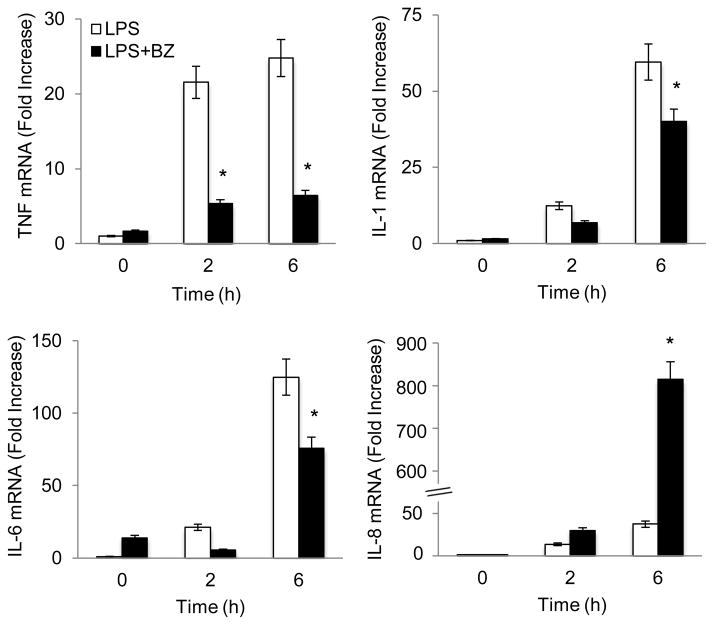
To investigate whether BZ induces IL-8 expression in stimulated human macrophages, we first used PMA differentiated U937 cells [19]. Differentiated U937 macrophages were stimulated with LPS (1 μg/ml) in the presence or absence of 100 nM BZ for 0, 2 and 6 hours, and mRNA levels of pro-inflammatory cytokines were analyzed by quantitative RT-PCR. As shown in Fig. 1, LPS stimulation increased expression of TNF, IL-1, IL-6, and IL-8. However, while the expression of TNF, IL-1, and IL-6 was inhibited by 100 nM BZ, which approximately corresponds to the clinically used BZ concentrations in cancer patients [20], the IL-8 expression was dramatically increased. Compared to cells stimulated 6 hours with LPS, BZ increased the IL-8 mRNA levels approximately 28-fold (Fig. 1).
Figure 1. Bortezomib specifically induces IL-8 mRNA expression in LPS-stimulated U937 macrophages.
U937 cells were differentiated with 10 ng/ml PMA for 24 hours, and stimulated with LPS (1 μg/ml) in the absence or presence of 100 nM BZ. mRNA levels were measured by quantitative RT-PCR using β-actin as a control. The values represent the mean +/− SE of four experiments. Asterisks denote a statistically significant (p<0.05) change compared to LPS alone.
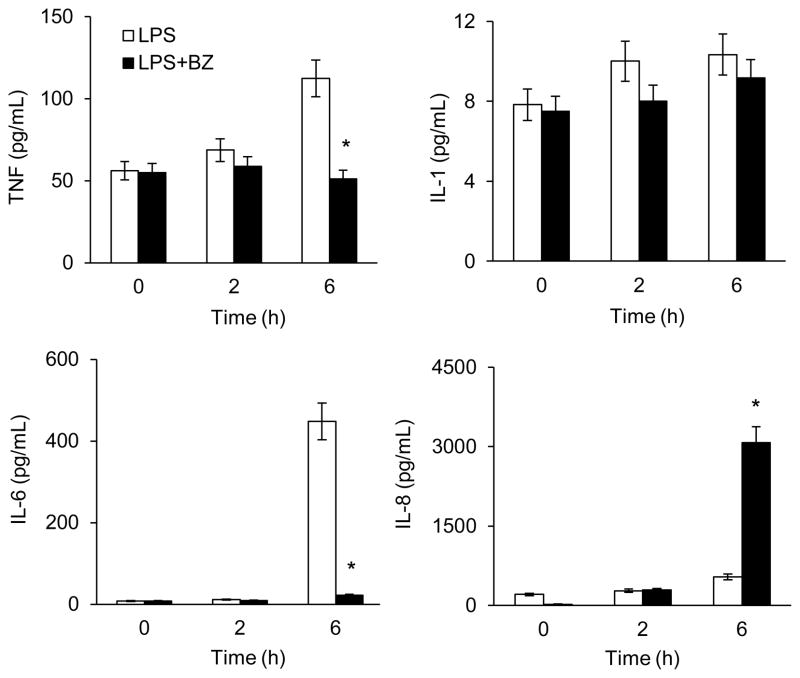
To determine whether BZ also increases the IL-8 release, we analyzed the release of TNF, IL-1, IL-6, and IL-8 in tissue culture supernatants of LPS-stimulated PMA-differentiated U937 macrophages by ELISA. Similarly, BZ significantly increased the IL-8 release from LPS-stimulated U937 macrophages, while it inhibited TNF, IL-1 and IL-6 release (Fig. 2). Compared to cells stimulated 6 hours with LPS, BZ increased the IL-8 release approximately 6-fold (Fig. 2), correlating with the mRNA data (Fig. 1).
Figure 2. Bortezomib specifically induces IL-8 release from LPS-stimulated U937 macrophages.
U937 cells were differentiated with 10 ng/ml PMA for 24 hours, and stimulated with LPS (1 μg/ml) in the absence or presence of 100 nM BZ. Cytokine release was measured in cell culture supernatants by ELISA. The values represent the mean +/− SE of four experiments. Asterisks denote a statistically significant (p<0.05) change compared to LPS alone.
BZ increases p38 MAPK nuclear accumulation in LPS-stimulated U937 macrophages
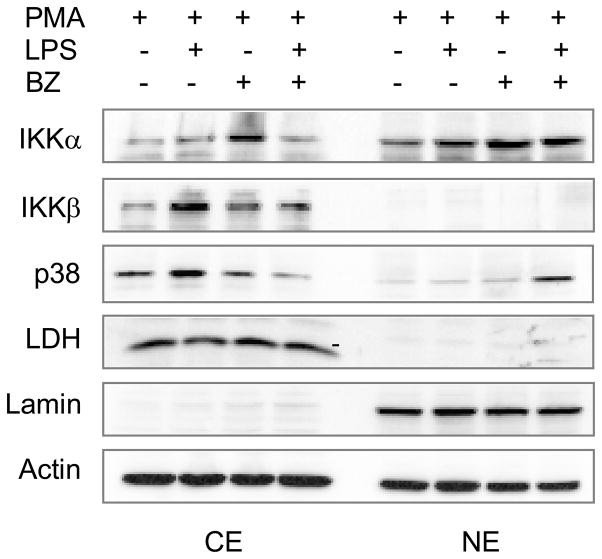
To investigate the mechanism responsible for the BZ-increased IL-8 expression in LPS-stimulated U937 macrophages, we analyzed the cellular levels of IκB kinases and p38 MAPK, since both IKK and p38 MAPK were shown to regulate the IL-8 expression [21–24]. To this end, we used western blotting to evaluate the cytoplasmic and nuclear levels of IKKα, IKKβ and p38 in PMA-differentiated U937 macrophages stimulated 6 hours with LPS in the absence and presence of 100 nM BZ. As a control of the purity of cytoplasmic and nuclear extract fractions, we used lactate dehydrogenase (LDH) and lamin B as specific cytoplasmic and nuclear markers, respectively. As shown in Fig. 3, IKKα was localized both in the cytoplasm and in the nucleus, while IKKβ and p38 MAPK were predominantly in the cytoplasm in unstimulated cells. Interestingly, BZ induced the nuclear accumulation of p38 MAPK in LPS-stimulated cells (Fig. 3), indicating that proteasome inhibition increases the stability of p38 MAPK, and suggesting that p38 MAPK might be involved in the BZ-increased IL-8 expression.
Figure 3. BZ increases p38 nuclear accumulation in LPS-stimulated U937 macrophages.
Western blotting of cytoplasmic (CE) and nuclear extracts (NE) prepared from U937 cells differentiated with 10 ng/ml PMA for 24 hours, and stimulated with LPS (1 μg/ml) in the absence or presence of 100 nM BZ. The western blots were analyzed by using IKKα, IKKβ, and p38 MAPK specific antibodies. The presence of cytoplasmic proteins in nuclear fraction was evaluated by re-probing the membrane with lactate dehydrogenase (LDH) antibody. Nuclear contamination in the cytoplasmic fraction was assessed by using lamin B specific antibody. To confirm equal protein loading, the membrane was stripped and re-probed with actin antibody.
The BZ-increased IL-8 expression in human monocytes is mediated by p38 MAPK
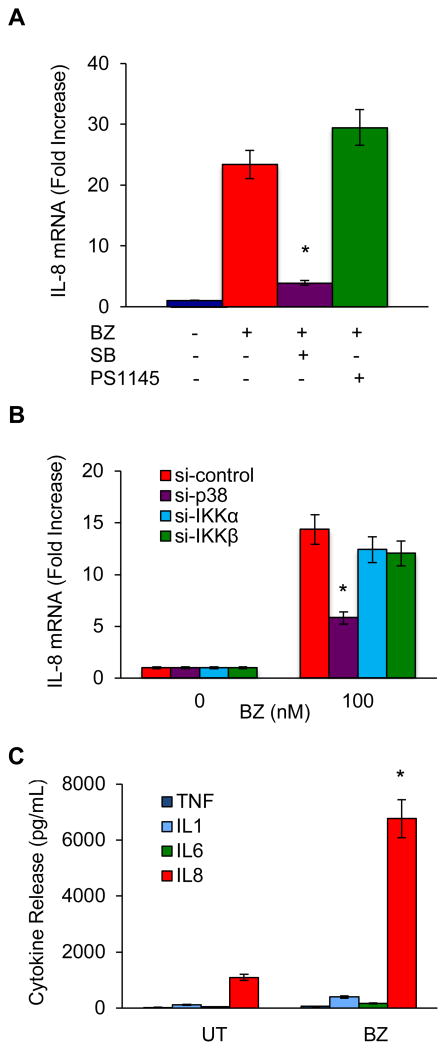
To investigate whether p38 MAPK mediates the BZ-increased IL-8 expression, and to determine whether BZ induces IL-8 expression also in unstimulated monocytic cells, we measured IL-8 mRNA levels in BZ-treated (100 nM, 6 hours) undifferentiated U937 cells in the presence of the p38 MAPK inhibitor SB203580 (10 μM), as well as the IKK inhibitor PS1145 (20 μM). As shown in Fig. 4A, BZ induced the IL-8 expression also in undifferentiated monocytic cells. Compared to untreated cells, BZ increased the IL-8 mRNA expression approximately 23-fold (Fig. 4A). Importantly, the p38 MAPK inhibitor SB203580 significantly decreased the BZ-induced IL-8 mRNA, while the IKK inhibitor PS1145 did not have any significant effect on the BZ-induced IL-8 mRNA expression (Fig. 4A), indicating that the BZ-induced IL-8 expression in monocytic cells is mediated by p38 MAPK.
Figure 4. The BZ-increased IL-8 in human monocytes is mediated by p38 MAPK.
(A) Real time RT-PCR analysis of IL-8 mRNA levels in U937 cells pre-treated 2 hours with p38 inhibitor SB203580 (10 μM) or IKK inhibitor PS1145 (20 μM), before 6-hour incubation with 100 nM BZ. The values represent the mean +/− SE of three experiments; the asterisk denotes a statistically significant (p<0.05) inhibition compared to cells treated with BZ only. (B) Real time RT-PCR analysis of IL-8 mRNA levels in U937 cells transfected with control, p38, IKKα, or IKK siRNA and then incubated 6 hours with 100 nM BZ. The values represent the mean +/− SE of three experiments; the asterisk denotes a statistically significant (p<0.05) inhibition compared to cells transfected with control siRNA. (C) Cytokine release measured by ELISA in cell culture supernatants of PBMC (1×106/ml) incubated 24 hours with 100 nM BZ. The values represent the mean +/− SE of three experiments. The asterisk denotes a statistically significant (p<0.05) change compared to untreated (UT) cells.
To confirm the p38 involvement in the BZ-induced IL-8 expression, we analyzed IL-8 mRNA levels in U937 cells transfected with control, p38, IKKα, and IKKβ siRNA, before 6-hour incubation with 100 nM BZ. As shown in Fig. 4B, suppression of p38 MAPK with siRNA significantly decreased the BZ-induced IL-8 expression, while suppression of IKKα or IKKβ did not have any significant effect on the IL-8 expression. These data demonstrate that the BZ-induced IL-8 expression in monocytic cells is not mediated by IKK, but by p38 MAPK.
BZ induces IL-8 release in primary human PBMC
To determine whether BZ induces the IL-8 release also in primary human monocytic cells, we analyzed the cytokine release in primary human peripheral blood mononuclear cells (PBMC) incubated 24 hours in the presence or absence of 100 nM BZ. Similarly as in U937 monocytic cells, BZ significantly induced the IL-8 release in human PBMC (Fig. 4C). In PBMC incubated with 100 nM BZ, the IL-8 release was induced about 7-fold compared to untreated PBMC, and reached approximately 7000 pg/mL. In contrast, BZ did not have any significant effect on the release of TNF, IL-6, or IL-1 in PBMC.
IL-8 contributes to cancer progression through its induction of tumor cell proliferation, survival, and angiogenesis. In this study, we show that BZ increases the IL-8 expression in human monocytes through a mechanism that involves the p38 MAPK activity. Thus, it seems that proteasome inhibition has two opposite effects. One mechanism of action consists of the inhibition of NFκB activity, resulting in the suppression of NFκB-dependent cytokines TNF, IL-1, and IL-6 (Fig. 1). The other, opposite, mechanism consists of the increased expression of IL-8, which is mediated by p38 MAPK (Fig. 4).
Interestingly, several studies have indicated that inhibition of p38 MAPK enhances the cytotoxicity of bortezomib, and may improve the outcome in MM patients [25–29]. The specific mechanisms by which BZ increases p38 activity and IL-8 expression in human monocytes are currently under investigation. Since monocytes and macrophages are major producers of IL-8, these data suggest that the BZ-increased IL-8 expression in monocytes and macrophages may represent one of the underlying mechanisms responsible for the BZ resistance and indicate that targeting the p38-mediated IL-8 expression could enhance the BZ effectiveness in cancer treatment.
Supplementary Material
Highlights.
Bortezomib (BZ) has anti-cancer activity but many patients develop resistance.
IL-8, produced by monocytes and macrophages, promotes angiogenesis and tumorigenesis.
We show that BZ specifically induces IL-8 in monocytes and macrophages.
The BZ-induced IL-8 expression is mediated by p38 MAPK.
Targeting the p38-mediated IL-8 could enhance BZ effectiveness in cancer treatment.
Acknowledgments
This work was supported by St. John’s University, and by the National Institutes of Health research grant CA173452 to I. Vancurova. S. Sanacora was supported by Clare Boothe Luce fellowship. J. Urdinez was sponsored by Fulbright scholarship.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shah JJ, Orlowski RZ. Proteasome inhibitors in the treatment of multiple myeloma. Leukemia. 2009;23:1964–1979. doi: 10.1038/leu.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lonial S, Anderson KC. Association of response endpoints with survival outcomes in multiple myeloma. Leukemia. 2014;28:258–268. doi: 10.1038/leu.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 4.Hideshima T, Mitsiades C, Akiyama M, et al. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood. 2003;101:1530–1534. doi: 10.1182/blood-2002-08-2543. [DOI] [PubMed] [Google Scholar]
- 5.Richardson PG, Mitsiades C, Hideshima T, Anderson KC. Proteasome inhibition in the treatment of cancer. Cell Cycle. 2005;4:290–296. [PubMed] [Google Scholar]
- 6.Manna S, Singha B, Phyo SA, Gatla HR, Chang TP, Sanacora S, Ramaswami S, Vancurova I. Proteasome Inhibition by Bortezomib Increases IL-8 Expression in Androgen-Independent Prostate Cancer Cells: The Role of IKKα. J Immunol. 2013;191:2837–2846. doi: 10.4049/jimmunol.1300895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singha B, Gatla HR, Manna S, Chang TP, Sanacora S, Poltoratsky V, Vancura A, Vancurova I. Proteasome inhibition increases recruitment of I B kinase β (IKKβ), S536P-p65, and transcription factor EGR1 to interleukin-8 (IL-8) promoter, resulting in increased IL-8 production in ovarian cancer cells. J Biol Chem. 2014;289:2687–2700. doi: 10.1074/jbc.M113.502641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunkel SL, Strieter RM, Chensue SW, Basha M, Standiford T, Ham J, Remick DG. Tumor necrosis factor-alpha, interleukin-8 and chemotactic cytokines. Prog Clin Biol Res. 1990;349:433–444. [PubMed] [Google Scholar]
- 10.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 11.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 12.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 13.Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010;16:133–144. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baggiolini M, Loetscher P, Moser B. Interleukin-8 and the chemokine family. Int J Immunopharmacol. 1995;17:103–138. doi: 10.1016/0192-0561(94)00088-6. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal R, Ghobrial IM, Roodman GD. Chemokines in multiple myeloma. Exp Hematol. 2006;34:1289–1295. doi: 10.1016/j.exphem.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, Hauschildt S. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011;31:379–446. doi: 10.1615/critrevimmunol.v31.i5.20. [DOI] [PubMed] [Google Scholar]
- 17.Sanacora S, Chang TP, Vancurova I. Chromatin immunoprecipitation analysis of bortezomib-mediated inhibition of NF B recruitment to IL-1β and TNFa gene promoters in human macrophages. Methods Mol Biol. 2014;1172:315–327. doi: 10.1007/978-1-4939-0928-5_29. [DOI] [PubMed] [Google Scholar]
- 18.Chang TP, Vancurova I. Bcl3 regulates pro-survival and pro-inflammatory gene expression in cutaneous T-cell lymphoma. Biochim Biophys Acta. 2014;1843:2620–2630. doi: 10.1016/j.bbamcr.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh CC, Ramaswami S, Juvekar A, Vu HY, Galdieri L, Davidson D, Vancurova I. Gene-specific repression of proinflammatory cytokines in stimulated human macrophages by nuclear IκBα. J Immunol. 2010;185:3685–3693. doi: 10.4049/jimmunol.0902230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levêque D, Carvalho MC, Maloisel F. Clinical pharmacokinetics of bortezomib. In Vivo. 2007;21:273–278. [PubMed] [Google Scholar]
- 21.Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- 22.Li H, Nord EP. CD40 ligation stimulates MCP-1 and IL-8 production, TRAF6 recruitment, and MAPK activation in proximal tubule cells. Am J Physiol Renal Physiol. 2002;282:1020–1033. doi: 10.1152/ajprenal.00291.2001. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes AF, Bian Q, Jiang JK, Thomas CJ, Taylor A, Pereira P, Shang F. Proteasome inactivation promotes p38 mitogen-activated protein kinase-dependent phosphatidylinositol 3-kinase activation and increases interleukin-8 production in retinal pigment epithelial cells. Mol Biol Cell. 2009;20:3690–3699. doi: 10.1091/mbc.E08-10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell LM, Maxwell PJ, Waugh DJ. Rationale and Means to Target Pro-Inflammatory Interleukin-8 (CXCL8) Signaling in Cancer. Pharmaceuticals (Basel) 2013;6:929–959. doi: 10.3390/ph6080929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hideshima T, Podar K, Chauhan D, et al. p38 MAPK inhibition enhances PS-341 (bortezomib)-induced cytotoxicity against multiple myeloma cells. Oncogene. 2004;23:8766–8776. doi: 10.1038/sj.onc.1208118. [DOI] [PubMed] [Google Scholar]
- 26.Yu C, Rahmani M, Dent P, Grant S. The hierarchical relationship between MAPK signaling and ROS generation in human leukemia cells undergoing apoptosis in response to the proteasome inhibitor Bortezomib. Exp Cell Res. 2004;295:555–566. doi: 10.1016/j.yexcr.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Navas TA, Nguyen AN, Hideshima T, et al. Inhibition of p38alpha MAPK enhances proteasome inhibitor-induced apoptosis of myeloma cells by modulating Hsp27, Bcl-X(L), Mcl-1 and p53 levels in vitro and inhibits tumor growth in vivo. Leukemia. 2006;20:1017–1027. doi: 10.1038/sj.leu.2404200. [DOI] [PubMed] [Google Scholar]
- 28.Yasui H, Hideshima T, Ikeda H, et al. BIRB 796 enhances cytotoxicity triggered by bortezomib, heat shock protein (Hsp) 90 inhibitor, and dexamethasone via inhibition of p38 mitogen-activated protein kinase/Hsp27 pathway in multiple myeloma cell lines and inhibits paracrine tumor growth. Br J Haematol. 2007;136:414–423. doi: 10.1111/j.1365-2141.2006.06443.x. [DOI] [PubMed] [Google Scholar]
- 29.Ishitsuka K, Hideshima T, Neri P, et al. p38 mitogen-activated protein kinase inhibitor LY2228820 enhances bortezomib-induced cytotoxicity and inhibits osteoclastogenesis in multiple myeloma; therapeutic implications. Br J Haematol. 2008;141:598–606. doi: 10.1111/j.1365-2141.2008.07044.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.