Summary
Programmed cell death eliminates unneeded and dangerous cells in a timely and effective manner during development. In this review, we examine the role cell death plays during development in worms, flies and mammals. We discuss signaling pathways that regulate developmental cell death, and describe how they communicate with the core cell death pathways. In most organisms the majority of developmental cell death is seen in the nervous system. Therefore we focus on what is known about the regulation of developmental cell death in this tissue. Understanding how the cell death is regulated during development may provide insight into how this process can be manipulated in the treatment of disease.
Keywords: cell death, apoptosis, Drosophila, development, Hox, Notch, Ras
1. Introduction
Cells die throughout the lifespan of multicellular organisms, and this physiologic cell death is critical for developmental plasticity and for organismal health [1]. In this review we describe the general functions of developmental cell death, focusing on nervous system development. The core cell death pathways that contribute to the majority of developmental cell death will be introduced, and the upstream regulation of these pathways in the context of the developing organism will be discussed.
2. The canonical pathway of cell death
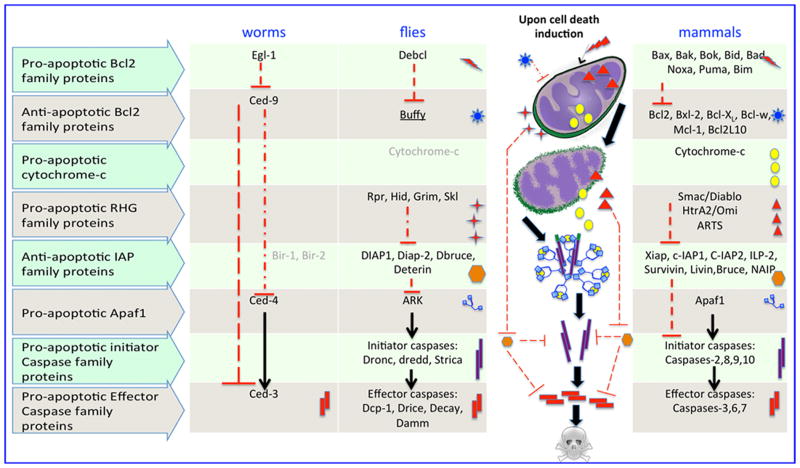
Genes important for apoptosis are highly conserved from worms to man, and include the caspases, and their regulators (Fig. 1). The control of caspase activity is central to the regulation of developmental death. Caspase activity can be controlled by regulating both activation and inhibition. The relative importance of these two apoptosis control strategies varies between species and also between cell types, as well as in response to different apoptotic stimuli.
Figure 1. Core cell death machinery in C. elegans, Drosophila and mammals.
The core cell death pathway is evolutionarily conserved. Homologues across each row are represented with symbols. Their interactions are shown graphically. Bir1,2 in worms and Cytochrome c in flies have not been shown to play a major role in programmed cell death (gray). Buffy (underlined) can be either pro-[155] or anti- apoptotic [156, 157]. Details of individual genes in the pathway can be found in [17, 26, 158]
In C. elegans, the activation of the ced3 caspase by the ced4 adapter is inhibited by the bcl2-like ced9 protein [2]. The egl1 protein, a BH3 only family protein, is transcribed in cells fated to die [1]. In the presence of egl1, ced9 is inhibited and ced 3 is activated.
In mammalian systems the role of the bcl2 family proteins in regulating caspase activation is more indirect. Upstream signals influence the balance of pro-and anti-apoptotic bcl2 family members, impacting Bax and Bak on the mitochondrial membrane [2]. Bax and Bak induce changes in the mitochondrial membrane, resulting in the release of mitochondrial proteins including Cytochrome-c. Cytochrome-c binds to Apaf-1, forming an apoptosome complex with procaspase-9. Caspase-9 is activated at the apoptosome. Subsequent activation of effector caspases results in cell death.
In flies, a caspase inhibitor, DIAP1, restrains caspase activity in most cells, and cell death is activated when this inhibition is removed [2]. DIAP1 is a member of the Inhibitor of Apoptosis Protein (IAP) family, which can act as direct caspase inhibitors. The RHG proteins, reaper, hid, grim and sickle, bind to DIAP1 and inhibit its anti-apoptotic activity resulting in cell death. The 4 RHG genes are transcribed in various combinations in cells fated to die [3–7]. Interestingly, the process of cell death in flies is very rapid; cells are eliminated within hours of RHG protein expression [3–5]. The bias in the Drosophila system towards this more poised apoptotic state may reflect the need for rapid apoptosis activation during development.
IAP proteins can also regulate cell death in mammals. There are 8 IAP family members in humans [8] (see Fig. 1). In the nervous system, there is a role for IAPs in inhibiting caspase activity in apoptosis and in axonal and dendrite pruning [9–11]. SMAC/DIABLO and OMI/HTRA2 are functional homologs of the fly RHG family. These proteins bind to and negatively regulate IAPs and can kill cells under certain conditions [8, 12].
3. Functions of cell death in development
Cell death is prevalent during the development of multicellular organisms. The majority of developmental cell death appears to be apoptotic [13], although alternative death pathways such as autophagy and necrosis may also contribute to the elimination of cells. The amount of cell death occurring during development can be underestimated, as phagocytes often eliminate dying cells within an hour of the initiation of death [14–16].
Examination of the distribution of dying cells and genetic disruption of cell death pathways has revealed important functions of cell death during development. These include the removal of unneeded tissues and cells and amelioration of developmental errors [17]. In certain situations isolated cells die, while in other cases, whole tissues are eliminated.
3.1. Removal of unneeded tissues
Entire tissues can be removed by programmed cell death as part of the initial shaping of the developing organism, or after the tissue has served its function [18, 19]. For example, during the development of the mammalian reproductive system Mullerian ducts are differentially eliminated from males and Wolffian ducts from females, in response to hormonal signaling [13, 18] The Drosophila salivary glands are also removed in response to a steroid hormone pulse after they have served their function [20, 21]. Interestingly, the elimination of the salivary gland requires both apoptotic and autophagic pathways [22–24]. Digit separation in vertebrates involves the elimination of inter-digital mesenchymal cells in response to developmental signaling pathways [25, 26]. Analysis of mutants in the apoptotic machinery suggests that there are backup non-apoptotic pathways that can also contribute to the elimination of these cells [27]. Other examples of tissue elimination during development include the removal of the pronephric kidney in mammals and loss of the tadpole tail and intestine [17].
3.2. Removal of unneeded cells
The death of isolated cells is seen in many developing tissues, but is best described in the developing nervous system. Many more cells are generated during nervous system development than are present in the fully developed tissue. Roughly 10 % of cells in the C. elegans nervous system are removed by apoptosis [28, 29]. In Drosophila and mammals the number of cells eliminated rises to more than 50% [3, 28–31]. Programmed cell death in the nervous system is likely to play an important role in developmental plasticity. Production of excess cells and their later elimination facilitates the matching of neurons to their targets [32].
The phenotype of cell death mutants illustrates the importance of apoptosis in nervous system development. Deletion of pro-death genes can result in severely malformed nervous systems in mammals and flies. Mice deficient for Caspase-3, caspase-9 and Apaf-1 show persistent neural precursors and exhibit nervous system patterning defects such as multiple indentations of the cerebrum and periventricular masses [33–38]. Interestingly, these phenotypes may not be caused by the survival of large numbers of cells, but rather may be secondary to the inappropriate survival of an FGF8 signaling center, resulting in defects in neural tube closure and insufficient brain ventricle expansion [39].
Bcl-2 family proteins also play a significant role in developmental cell death. Mice null for Bcl-2 family genes show defects in early nervous system development. For example, mice deficient for the anti-apoptotic Bcl-XL and Mcl-1 proteins die early in development due to massive apoptosis of immature neurons and hematopoietic system [40, 41]. Mice null for Bax, a pro-apoptotic member of the Bcl-2 family, have increased numbers of neurons, which are resistant to apoptosis induced by nerve growth factor deprivation [42]. Pro-apoptotic BH3-only proteins have redundant functions in developmental apoptosis. Individual knockouts of Bid, Bim and Puma result in only minor delays in developmental death of neurons. However, simultaneous deletion of all the three genes results in a strong resistance to stress-induced death in cerebellar granule neurons [43].
In the fly, apoptosis also plays an important role in shaping the developing central nervous system. Apoptosis is required to eliminate both dividing progenitors and differentiated neurons in Drosophila development [3, 44–47]. For example, neural stem cells are eliminated in the abdominal segments of the developing ventral nerve cord at the end of embryogenesis [3, 48]. This alters the morphology of the central nervous system to match the transition from a crawling larva to an adult fly. In the absence of apoptosis, the ventral nerve cord is massively expanded [44, 47]. Neural stem cells in the abdominal ventral nerve cord are eliminated in response to developmental pathways including Hox genes and Notch signaling [49]. Interestingly, individual neuronal lineages are also shaped by selective cell death in response to Notch signaling, as described below [50].
Apoptosis is required for the precise organization of the ommatidial units in the developing Drosophila eye. Waves of apoptosis sculpt the developing eye, from the earliest specification of photoreceptor and accessory cells to the final tightening of the ommatidial clusters, removing around 2000 excess cells [51]. The regulation of these deaths involve complex interactions between several developmentally important pathways, including the Epithelial Growth Factor Receptor and Notch pathways, as described below [52, 53].
3.3. Developmental correction
Apoptosis can be activated to normalize the developmental defects caused by mutations. This suggests that cell death may also be important for error correction in normal development. For example, in Drosophila embryos, misexpression of the bicoid morphogen results in mis-specification of the anterior posterior axis of the fly. Surprisingly, this can be corrected by apoptosis, resulting in a normal animal [54–56]. Mis-expression of the cell cycle regulators cycE or E2F with DP results in a massive increase in cell number due to proliferation. This hypertrophy is also eventually reversed by induced apoptosis, resulting in nearly normal adults [54, 57, 58]. There are numerous other examples where genetic manipulations result in the formation of ectopic cells but the defect is finally corrected by apoptosis [59–62]. Defects caused by the genetic manipulation of Dpp and Wg signaling is corrected by JNK induced apoptosis in the developing Drosophila wing [63]. The role of apoptosis in correcting developmental errors is less well studied in mammals, but increased apoptosis is noted in mis-specified cells in mutant mice [64, 65].
The precise patterning of sensory bristles on the fly thorax provides an elegant example of the role of apoptosis in developmental error correction. During normal development, an excess number of cells become specified as sensory organ precursors. To maintain precise spacing between sensory cells, these aberrant cells are eliminated by Notch induced apoptosis [66].
The examples above provide a glimpse into how apoptosis is an essential aspect of the developmental program. Below we discuss some of the signaling pathways that regulate developmental apoptosis.
4. Regulation of developmental cell death
As described above, the central components of the apoptotic pathway have been extensively characterized. Less is known about how these components are activated in the correct cells at the correct time in development. Many pathways implicated in other developmental processes also regulate developmental cell death (summarized in table 1).
Table 1.
Signaling pathways that regulate developmental cell death
| Gene | Effect on cell death | Model | References |
|---|---|---|---|
| AbdA | Positive | Drosophila | [45, 49, 129] |
| Deformed | Positive | Drosophila | [125] |
| AbdB | Positive and negative | Drosophila | [125–127] |
| hoxa1 | Positive | Mammals | [121, 122] |
| LIN-39 | Negative | C. elegans | [123] |
| PcG proteins (Polycomb, Sex combs extra & Enhancer of zeste) | Negative | Drosophila | [128, 131] |
| Notch, Notch 1, Notch 3, Su(H) | Positive and negative | Drosophila, Mammals | [46, 49, 50, 52, 53, 66–74, 90] |
| Ras signaling | Negative | Drosophila | [75, 79, 80, 85] |
| MAPK/ERK Mek1 and Mek2, signaling | Negative | Drosophila, mammals | [76, 77, 86] |
| Epidermal Growth Factor Receptor (EGFR) | Negative | Drosophila | [78, 81–84] |
| Argos | Positive | Drosophila | [83] |
| Retinoblastoma (Rb) | Negative | Drosophila, mammals | [87, 88] |
| miR998 | Negative | Drosophila | [87] |
| Cbl | Negative | Drosophila, mammals | [87] |
| AKT | Positive and negative | Drosophila, mammals | [91–93] |
| PI3K pathway | Negative | Drosophila, mammals | [75, 91, 92, 94] |
| Steroid hormone signaling | Positive and negative | Drosophila Mammals | [95–107, 159] |
| Hippo pathway | Positive | Drosophila mammals | [108–116] |
| Yorkie/YAP | Negative | Drosophila, mammals | [116–120] |
| Wnt/Wg pathway | Positive and negative | Drosophila mammals | [132–140] |
| BMP/Dpp family | Positive | Drosophila, mammals | [140–147] |
4.1. Notch signaling
Notch signaling is involved in numerous binary cell fate decisions in development, including the life or death decision. In both flies and mammals, Notch is found to bias the choice between survival and death, acting in both directions. Notch influences the survival of neural precursors and differentiating neurons in the mammalian brain. Conditional knockdown of Notch1 and Notch3 in vivo results in increased apoptosis of large number of neural progenitors and differentiating neurons [67]. Notch can also promote apoptosis in the mammalian nervous system. Notch knockouts have been reported to show reduced apoptosis of early neural progenitors [68].
A beautiful example of the two-sided nature of Notch in regulating cell death is provided by the study of hemilineages in the developing Drosophila ventral nerve cord. During the postembryonic development of this tissue, neural stem cell divisions can give rise to two distinct hemilineages. In some cases, one of these hemilineages undergoes apoptosis. Notch signaling determines which of these lineages survives [46, 69]. Interestingly, Notch “on” dictates death in 50% of the dying lineages, while Notch “off” promotes death in the remainder. The lack of initiator caspases Dronc results in ectopic survival of doomed hemi-lineages, implying that Notch can regulate the canonical apoptotic pathway to eliminate neuronal lineages [46]. In an elegant recent study of Drosophila optic lobe neurons, temporal patterning by specific transcription factors was shown to differentially control Notch dependent death. Interestingly, the upstream regulators of most of the Drosophila apoptosis, rpr grim and hid genes in RHG cluster, were found to be differentially responsible for the fate of Notch “on” and Notch “off” lineages. A hid specific genetic deletion rescues Notch “off” lineages while a genetic deletion that removes rpr and a regulatory region for grim rescues Notch “on” lineages. Thus Notch “on” neurons die in a reaper and grim dependent manner, while the death of Notch “off” neurons requires hid [47, 70].
In other Drosophila tissues, Notch has been reported to induce apoptosis. For example during eye development, superfluous inter-ommatidial cells are eliminated in a Notch-dependent manner to maintain the correct spacing between ommatidia [71–73]. Recent studies have demonstrated the Notch target Su(H) can directly regulate transcription of the RHG gene hid [74]. Given the extensive involvement of Notch in many developmental processes, it is likely that Notch contributes widely to life or death decisions in development.
4.2. RAS/MAPK signaling
Ras signaling is activated downstream of receptor tyrosine kinases, many of which promote cell survival [75]. Ras activates both the Raf/MAPK (ERK) and the PI3K pathways, and is often hyperactivated in tumors. Alongside its well-known role in cancer development, Ras signaling regulates cell death in development. For example, deletion of the Ras/ERK pathway components Mek1 and Mek2 in mouse skin results in significant apoptosis in both the embryo and the adult [76]. Deletion of ERK 1 and ERK2 in the developing mouse central nervous system leads to significant apoptosis in a variety of cell types [77].
In the developing Drosophila eye, the Epidermal Growth Factor Receptor (EGFR), acting through the Raf/MAPK pathway, provides a precise short-range signal for cell survival [78–81]. Loss of EGFR signaling or ectopic expression of EGFR inhibitor, Argos, results in massive apoptosis in the Drosophila eye, while ectopic expression of EGFR signaling results in persistent cell survival and tissue hypertrophy [71, 78, 82, 83]. Loss of RAS and MAPK signaling also results in tissue loss [84, 85]. EGFR/Ras/MAPK signaling suppresses apoptosis in the fly by suppressing both the expression and the activity of the pro-apoptotic RHG gene hid [79, 80]. Loss of Ras activity results in increased hid transcription [79]. MAPK also negatively regulates hid activity through phosphorylation [80]. Similarly, in mammalian systems, ERK may promote survival by phosphorylating glycogen synthase kinase-3 (GSK-3) to desensitize mitochondria to apoptotic permeabilization [86].
In the fly, cross talk between the EGFR pathway and other regulatory pathways has been shown to regulate cell death. The Rb tumor suppressor can regulate cell death acting through the EGFR pathway [87]. Rb inhibits apoptosis by keeping E2F1 dependent apoptosis in check [88]. It was shown recently that miR998 acts downstream of Rb and inhibits dCbl, a negative regulator of EGFR pathway, to suppress cell death in flies [87]. Furthermore, cell death inhibitory interactions between miR998/miR29 RNA and Cbl is conserved in human cells [87]. Crosstalk between the EGFR and Notch signaling pathways can also be important in precisely regulating developmental cell death. In the developing Drosophila eye, Notch signaling induces apoptosis in the inter-ommatidial cells whereas EGFR/RAS pathway counteracts this signal [89, 90].
4.3 PI3K signaling
The conserved PI3K kinase pathway is important for the regulation of cell growth, proliferation and survival. PI3K signaling is activated downstream of many growth factor receptors, either by direct receptor binding or downstream of Ras activation (see above) [75]. PI3K signaling can promote cell survival through AKT activation. AKT phosphorylates and inactivates a number of targets, including the pro-apoptotic Bcl2 family member Bad and Caspase 9, and regulates gene expression through the Foxo transcription factor [91]. Knockouts of other PI3K pathway components display pleiotropic phenotypes that are consistent with a role for this pathway in promoting survival during development [92]. Paradoxically, when multiple AKT isoforms are deleted in the mouse, there is increased survival of some cell types during development [93].
In the Drosophila brain, the elimination of mushroom body stem cells is regulated by a cross talk between insulin/PI3K signaling and the RHG-mediated apoptotic pathway [94]. Normally, mushroom body stem cells are eliminated around mid pupal life through RHG mediated apoptosis [45, 94]. Reduced insulin/PI3 kinase signaling precedes this death, and inhibition of both RHG activity and PI3K signaling prolongs the survival of these cells.
4.4. Hormone regulation
Nuclear receptor signaling pathways are highly conserved between flies and mammals. In both, flies and vertebrates, the survival or death of many cells or entire tissues is influence by steroid hormones [95, 96]. Steroid hormones can be either pro-or anti-apoptotic, mediating the expression of targets such as Bcl2 to inhibit death, or pro-apoptotic Bcl2 family members to activate death.
In Drosophila, apoptotic and autophagic pathway components are transcriptionally regulated in response to the steroid hormone ecdysone [23, 97–99]. The pro-apoptotic genes reaper, and hid, the caspases Dronc and Drice, and the adaptor dark are transcriptionally up regulated in response to ecdysone signaling, while the antiapoptotic proteins DIAP1 and DIAP2 are down regulated [98, 100–104]. Recently, it has been shown that the ecdysone receptor complex cooperates with chromatin-modifying cofactors to regulate the transcription of several cell death genes [105–107]#
4.5. Hippo signaling
The Fat/Hippo signaling pathway also regulates cell death. Yorkie (Yki), the most downstream target of the hippo pathway, promotes cell survival in Drosophila by negatively regulating the pro-death gene hid and activating pro-survival factors such as bantam and DIAP1 [108]–[109]. Upon activation, hippo signaling exerts its pro-death effect by negatively regulating Yki through phosphorylation. In hippo mutants, retina cells show exuberant proliferation and persistent survival due to inhibition of apoptosis [110–115]. Similarly, the human homologue of Yki, YAP, is elevated in cancer and contributes to cell survival in development and cancer [116–120].
4.6. Hox and Polycomb
Hox genes play a conserved role in controlling anterior-posterior patterning in many species. The regulation of apoptosis is an important element of this patterning in worms, mammals and flies. In mammals, several Hox genes control targets that regulate apoptosis [121]. For example, in the mammalian brain hoxa1 regulates the pruning of neurons in the hindbrain. When hoxa1 is deleted, ectopic surviving neurons make functional circuits in the postnatal brain [122]. In C. elegans, the Hox gene LIN-39 regulates the survival of VC neurons by suppressing the transcription of the egl1 pro-apoptotic regulator [123].
Hox gene function in Drosophila development has been extensively characterized [124]. Many of the Hox genes regulate apoptosis. The Deformed gene, which is involved in head formation, initiates apoptosis to maintain a boundary between the maxillary and mandibular segments of the head [125]. Similarly, AbdB, which regulates the formation of posterior structures, activates apoptosis to generate a precise boundary between abdominal segments A6/A7 and A7/A8 [125]. In both cases, the Hox genes regulate the expression of the proapoptotic reaper gene directly. AbdB also regulates the survival and death of neuropeptide-expressing neurons in the embryonic nervous system [126, 127]. The AbdA Hox gene regulates neural stem cell death in the Drosophila embryonic and post-embryonic nervous system, acting upstream of RHG gene expression [49, 128]. Hox-dependent apoptosis is also required in several neuroblast lineages for the proper elimination of unneeded neurons [129]. Interestingly, Hox proteins can suppress autophagy by repressing the expression of autophagy genes [130]. This suggests that Hox proteins can coordinate death and survival pathways to control developmental cell death.
The Polycomb-group protein (PcG) family of chromatin remodelers play a global role in epigenetic gene silencing. The Hox genes are important targets of PcG activity; PcG keeps the activation of Hox genes in check during normal development. Loss of PcG genes like Polycomb, Sex combs extra, and Enhancer of zeste result in the apoptotic removal of neural stem cells, due to aberrant activation of Hox genes [128]. PcG genes may also have a more direct effect on the expression of cell death genes. ChIP analysis indicates that the RHG gene region is tightly suppressed by the activity of PcG proteins [131]. Interestingly this suppression is regulated temporally to alter the sensitivity of RHG genes to be activated at different developmental stages [131].
4.7. Other developmental pathways
Given the complex and dynamic patterns of apoptosis seen in development, it is clear that additional developmental pathways must influence the core death pathways. These may include intrinsic regulators that dictate the identity of a cell at a specific time and place in development, as well as additional extrinsic signaling pathways such as Wnts and BMPs.
The Wnt/Wingless (Wg) pathway is required to regulate numerous developmental processes including apoptosis [132]. Depending on the cellular context it influences apoptosis positively as well as negatively [133–135]. Activation of Wnt induces apoptosis in hematopoietic progenitor cells[136]. On the other hand, its suppression induces apoptosis in mouse cranial neural crest and Drosophila neurons [137, 138]. On the other hand Wnt/Wg induces RHG mediated apoptosis to precisely remove incomplete ommatidial units at periphery of developing eye in Drosophila [139]. In human disease, Wnt/Wg signaling is implicated in photoreceptor apoptosis in retinal degeneration and neural apoptosis in Alzheimer’s disease [140].
The BMP/Dpp family of secreted ligands are members of the TGF-beta super-family. BMP signaling is important for the removal of the interdigital web in mammals [141]. Moreover, BMP signaling is known to regulate apoptosis in the context of forebrain development, where it triggers apoptosis in prospective neural crest cells [142, 143], and during morphogenesis of the chick eye [144]. Ectopic induction of BMP/Dpp signaling can cause apoptosis in isolated mouse neural stem cells [145]. In Drosophila, BMP/Dpp can regulate apoptosis through JNK signaling [63, 146, 147].
Intrinsic temporal and cell identity pathways clearly play a role in regulating cell death in the mammalian and Drosophila nervous system. A recent study demonstrates that cortical interneuron death in mice is independent of external signals. Cultured or transplanted interneurons die at the same time and at the same rate as their counterparts in vivo [148]. Drosophila neural stem fate is autonomously regulated by the temporal series of transcription factors that dictates neural stem cell identity [149]. These transcription factors also decide the fate of neural stem cells by precisely regulating the timing of their death [45].
5. Future directions
Apoptosis is a prevalent cell fate in during development. Pathways that regulate other developmental events can also regulate cell death. It is interesting to note that some regulatory pathways can activate or suppress cell death, depending on the developmental context. The temporal and tissue specific context cues that alter the cell death response will be an interesting area for future research. Until now, these studies have been difficult in vivo, given the rapidity of the apoptotic process from initiation to clearance. New technical advances should facilitate such studies, including new methods for isolating and analyzing gene expression and chromatin changes in small numbers of cells [150–154].
Perturbations in cell death contribute to a variety of human diseases such as cancer and neuro-degeneration. We expect that a greater understanding of the context specific activation of cell death during development could provide insight into how cell death can be accurately manipulated in the treatment of human disease. By examining how developmental pathways intersect with the conserved core apoptotic machinery, we may learn how cancer cells become resistant to cell death or how to prevent cell death in neurodegenerative diseases such as Parkinsonism. In vivo studies are essential to fully appreciate the complexity of cell death regulation.
Highlights.
Apoptosis is a common developmental cell fate
Core apoptotic signaling is highly conserved
Several developmentally important signaling pathways influence the regulation of apoptosis.
Pathways regulating developmental cell death may contribute to disease.
Acknowledgments
We would like to acknowledge funding from the MGH Fund for Medical Discovery (RA) and R01GM55568 and R01GM110477v(KW). Tatevik Sarkissian and Ritu Tomar provided important comments on the manuscript.
Gene abbreviations
- AbdA
Abdominal A
- AbdB
Abdominal B
- apaf-1
Apoptotic protease-activating factor 1
- BMP
Bone morphogenetic protein
- ced3
cell death protein-3
- Ced4
Cell death protein 4
- Ced9
cell death protein-9
- cycE
Cyclin E
- Cyt-c
Cytochrome-c
- Dark
Apaf-1 related killer DARK
- DIABLO
direct IAP binding protein with low pI
- DIAP1
Drosophila inhibitor apoptosis protein 1
- DP
Dimerizing partner protein
- Dpp
Decapentaplegic
- E2F
Transcription factor E2f
- EGFR
Epidermal growth factor receptor
- Egl1
Egg-laying defective protein 1
- ERK 1
extracellular-signal-regulated kinases1
- ERK2
extracellular-signal-regulated kinases2
- FGF8
fibroblast growth factor 8
- Foxo
Forkhead box O
- GSK-3
glycogen synthase kinase-3
- Hox
Homeobox genes
- IAPs
Inhibitor of Apoptosis
- JNK
c-Jun N-terminal kinases
- LIN-39
abnormal cell LINeage-39
- MAPK
mitogen-activated protein kinase 1
- Mek1
mitogen-activated protein kinase kinase 1
- Mek2
mitogen-activated protein kinase kinase 2
- OMI/HTRA2
HtrA serine peptidase 2
- PcG
Polycomb-group protein
- PI3K
Phosphoinositide 3-kinase
- Rb
Retinoblastoma
- RHG
Reaper, hid, grim
- sce
Sex combs extra
- SMAC
Second mitochondria-derived activator of caspase
- Su(H)
Suppressor of Hairless
- Wg
Wingless
- YAP
Yes-associated protein
- Yki
Yorkie
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Conradt B, Horvitz HR. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell. 1998;93(4):519–29. doi: 10.1016/s0092-8674(00)81182-4. [DOI] [PubMed] [Google Scholar]
- 2.Kornbluth S, White K. Apoptosis in Drosophila: neither fish nor fowl (nor man, nor worm) J Cell Sci. 2005;118(Pt 9):1779–87. doi: 10.1242/jcs.02377. [DOI] [PubMed] [Google Scholar]
- 3.White K, et al. Genetic control of programmed cell death in Drosophila. Science. 1994;264(5159):677–83. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- 4.Chen P, et al. grim, a novel cell death gene in Drosophila. Genes Dev. 1996;10(14):1773–82. doi: 10.1101/gad.10.14.1773. [DOI] [PubMed] [Google Scholar]
- 5.Grether ME, et al. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev. 1995;9(14):1694–708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- 6.Christich A, et al. The damage-responsive Drosophila gene sickle encodes a novel IAP binding protein similar to but distinct from reaper, grim, and hid. Curr Biol. 2002;12(2):137–40. doi: 10.1016/s0960-9822(01)00658-3. [DOI] [PubMed] [Google Scholar]
- 7.Wing JP, Karres JS, Ogdahl JL, Zhou L, Schwartz LM, Nambu JR. Drosophila sickle is a novel grim-reaper cell death activator. Curr Biol. 2002;12:131–5. doi: 10.1016/s0960-9822(01)00664-9. [DOI] [PubMed] [Google Scholar]
- 8.Berthelet J, Dubrez L. Regulation of Apoptosis by Inhibitors of Apoptosis (IAPs) Cells. 2013;2(1):163–87. doi: 10.3390/cells2010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potts PR, et al. Critical function of endogenous XIAP in regulating caspase activation during sympathetic neuronal apoptosis. J Cell Biol. 2003;163(4):789–99. doi: 10.1083/jcb.200307130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cusack CL, et al. Distinct pathways mediate axon degeneration during apoptosis and axon-specific pruning. Nat Commun. 2013;4:1876. doi: 10.1038/ncomms2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unsain N, et al. XIAP regulates caspase activity in degenerating axons. Cell Rep. 2013;4(4):751–63. doi: 10.1016/j.celrep.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Wang K, Lin B. Inhibitor of apoptosis proteins (IAPs) as regulatory factors of hepatic apoptosis. Cell Signal. 2013;25(10):1970–80. doi: 10.1016/j.cellsig.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Glucksmann A. Cell deaths in normal vertebrate ontogeny. Biological Reviews. 1951;26:59–86. doi: 10.1111/j.1469-185x.1951.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 14.Savill J. Recognition and phagocytosis of cells undergoing apoptosis. Br Med Bull. 1997;53:491–508. doi: 10.1093/oxfordjournals.bmb.a011626. [DOI] [PubMed] [Google Scholar]
- 15.Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol. 2013;5(1):a008748. doi: 10.1101/cshperspect.a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barres BA, et al. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70(1):31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- 17.Baehrecke EH. How death shapes life during development. Nat Rev Mol Cell Biol. 2002;3(10):779–87. doi: 10.1038/nrm931. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88(3):347–54. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 19.Milligan CE, Schwartz LM. Programmed cell death during animal development. Br Med Bull. 1997;53(3):570–590. doi: 10.1093/oxfordjournals.bmb.a011631. [DOI] [PubMed] [Google Scholar]
- 20.Robertson CW. The metamorphosis of Drosophila melanogaster, including an accurately timed account of the principal morphological changes. Journal of Morphology. 1936;59(2):351–399. [Google Scholar]
- 21.Bodenstein D. The postembryonic development of Drosophila. In: Demerec M, editor. Biology of Drosophila. New York: Hafner Publishing Co; 1965. [Google Scholar]
- 22.Berry DL, Baehrecke EH. Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell. 2007;131(6):1137–48. doi: 10.1016/j.cell.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CY, Baehrecke EH. Steroid regulation of autophagic programmed cell death during development. Development. 2001;128(8):1443–55. doi: 10.1242/dev.128.8.1443. [DOI] [PubMed] [Google Scholar]
- 24.Martin DN, Baehrecke EH. Caspases function in autophagic programmed cell death in Drosophila. Development. 2004;131(2):275–84. doi: 10.1242/dev.00933. [DOI] [PubMed] [Google Scholar]
- 25.Zuzarte-Luis V, Hurle JM. Programmed cell death in the developing limb. Int J Dev Biol. 2002;46(7):871–6. [PubMed] [Google Scholar]
- 26.Conradt B. Genetic control of programmed cell death during animal development. Annu Rev Genet. 2009;43:493–523. doi: 10.1146/annurev.genet.42.110807.091533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan J, Kroemer G. Alternative cell death mechanisms in development and beyond. Genes Dev. 2010;24(23):2592–602. doi: 10.1101/gad.1984410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56(1):110–56. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- 29.Sulston JE, et al. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100(1):64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 30.Rogulja-Ortmann A, et al. Programmed cell death in the embryonic central nervous system of Drosophila melanogaster. Development. 2007;134(1):105–16. doi: 10.1242/dev.02707. [DOI] [PubMed] [Google Scholar]
- 31.Buss RR, Sun W, Oppenheim RW. Adaptive roles of programmed cell death during nervous system development. Annu Rev Neurosci. 2006;29:1–35. doi: 10.1146/annurev.neuro.29.051605.112800. [DOI] [PubMed] [Google Scholar]
- 32.Hamburger V. Cell death in the development of the lateral motor column of the chick embryo. J Comp Neurol. 1975;160(4):535–46. doi: 10.1002/cne.901600408. [DOI] [PubMed] [Google Scholar]
- 33.Cecconi F, et al. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94(6):727–37. doi: 10.1016/s0092-8674(00)81732-8. [DOI] [PubMed] [Google Scholar]
- 34.Hakem R, et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94(3):339–52. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 35.Honarpour N, et al. Adult Apaf-1-deficient mice exhibit male infertility. Dev Biol. 2000;218(2):248–58. doi: 10.1006/dbio.1999.9585. [DOI] [PubMed] [Google Scholar]
- 36.Kuida K, et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94(3):325–37. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida H, et al. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 1998;94(6):739–50. doi: 10.1016/s0092-8674(00)81733-x. [DOI] [PubMed] [Google Scholar]
- 38.Blaschke AJ, Staley K, Chun J. Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development. 1996;122(4):1165–74. doi: 10.1242/dev.122.4.1165. [DOI] [PubMed] [Google Scholar]
- 39.Nonomura K, et al. Local apoptosis modulates early mammalian brain development through the elimination of morphogen-producing cells. Dev Cell. 2013;27(6):621–34. doi: 10.1016/j.devcel.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Motoyama N, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267(5203):1506–10. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 41.Arbour N, et al. Mcl-1 is a key regulator of apoptosis during CNS development and after DNA damage. J Neurosci. 2008;28(24):6068–78. doi: 10.1523/JNEUROSCI.4940-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hyman BT, Yuan J. Apoptotic and non-apoptotic roles of caspases in neuronal physiology and pathophysiology. Nat Rev Neurosci. 2012;13(6):395–406. doi: 10.1038/nrn3228. [DOI] [PubMed] [Google Scholar]
- 43.Ren D, et al. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330(6009):1390–3. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peterson C, et al. reaper is required for neuroblast apoptosis during Drosophila development. Development. 2002;129(6):1467–76. doi: 10.1242/dev.129.6.1467. [DOI] [PubMed] [Google Scholar]
- 45.Maurange C, Cheng L, Gould AP. Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila. Cell. 2008;133(5):891–902. doi: 10.1016/j.cell.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 46.Truman JW, et al. Role of Notch signaling in establishing the hemilineages of secondary neurons in Drosophila melanogaster. Development. 2010;137(1):53–61. doi: 10.1242/dev.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan Y, et al. Coordinated expression of cell death genes regulates neuroblast apoptosis. Development. 2011;138(11):2197–206. doi: 10.1242/dev.058826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Truman JW, Bate M. Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev Biol. 1988;125(1):145–57. doi: 10.1016/0012-1606(88)90067-x. [DOI] [PubMed] [Google Scholar]
- 49.Arya R, et al. Neural stem cell progeny regulate stem cell death in a Notch and Hox dependent manner. Cell Death and Differentiation. 2015 doi: 10.1038/cdd.2014.235. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Truman JW. Metamorphosis of the central nervous system of Drosophila. J Neurobiol. 1990;21(7):1072–84. doi: 10.1002/neu.480210711. [DOI] [PubMed] [Google Scholar]
- 51.Bonini NM, Fortini ME. Surviving Drosophila eye development: integrating cell death with differentiation during formation of a neural structure. Bioessays. 1999;21(12):991–1003. doi: 10.1002/(SICI)1521-1878(199912)22:1<991::AID-BIES3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 52.Rusconi JC, Hays R, Cagan RL. Programmed cell death and patterning in Drosophila. Cell Death Differ. 2000;7(11):1063–70. doi: 10.1038/sj.cdd.4400767. [DOI] [PubMed] [Google Scholar]
- 53.Wolff T, Ready DF. Cell death in normal and rough eye mutants of Drosophila. Development. 1991;113(3):825–39. doi: 10.1242/dev.113.3.825. [DOI] [PubMed] [Google Scholar]
- 54.Namba R, et al. Drosophila embryonic pattern repair: how embryos respond to bicoid dosage alteration. Development. 1997;124(7):1393–403. doi: 10.1242/dev.124.7.1393. [DOI] [PubMed] [Google Scholar]
- 55.Bangs P, White K. Regulation and execution of apoptosis during Drosophila development. Dev Dyn. 2000;218(1):68–79. doi: 10.1002/(SICI)1097-0177(200005)218:1<68::AID-DVDY6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 56.Werz C, et al. Mis-specified cells die by an active gene-directed process, and inhibition of this death results in cell fate transformation in Drosophila. Development. 2005;132(24):5343–52. doi: 10.1242/dev.02150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du W, Xie JE, Dyson N. Ectopic expression of dE2F and dDP induces cell proliferation and death in the Drosophila eye. EMBO J. 1996;15(14):3684–92. [PMC free article] [PubMed] [Google Scholar]
- 58.Li QJ, Pazdera TM, Minden JS. Drosophila embryonic pattern repair: how embryos respond to cyclin E-induced ectopic division. Development. 1999;126(10):2299–307. doi: 10.1242/dev.126.10.2299. [DOI] [PubMed] [Google Scholar]
- 59.Herz HM, et al. vps25 mosaics display non-autonomous cell survival and overgrowth, and autonomous apoptosis. Development. 2006;133(10):1871–80. doi: 10.1242/dev.02356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brumby AM, Richardson HE. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 2003;22(21):5769–79. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Igaki T, Pagliarini RA, Xu T. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr Biol. 2006;16(11):1139–46. doi: 10.1016/j.cub.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 62.Namba R, et al. Drosophila embryonic pattern repair: how embryos respond to bicoid dosage alteration. Development. 1997;124:1393–403. doi: 10.1242/dev.124.7.1393. [DOI] [PubMed] [Google Scholar]
- 63.Adachi-Yamada T, et al. De novo synthesis of sphingolipids is required for cell survival by down-regulating c-Jun N-terminal kinase in Drosophila imaginal discs. Mol Cell Biol. 1999;19(10):7276–86. doi: 10.1128/mcb.19.10.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rossel M, Capecchi MR. Mice mutant for both Hoxa1 and Hoxb1 show extensive remodeling of the hindbrain and defects in craniofacial development. Development. 1999;126(22):5027–40. doi: 10.1242/dev.126.22.5027. [DOI] [PubMed] [Google Scholar]
- 65.Millen KJ, et al. Transformation of the cerebellum into more ventral brainstem fates causes cerebellar agenesis in the absence of Ptf1a function. Proc Natl Acad Sci U S A. 2014;111(17):E1777–86. doi: 10.1073/pnas.1315024111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koto A, Kuranaga E, Miura M. Apoptosis ensures spacing pattern formation of Drosophila sensory organs. Curr Biol. 2011;21(4):278–87. doi: 10.1016/j.cub.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 67.Mason HA, et al. Loss of notch activity in the developing central nervous system leads to increased cell death. Dev Neurosci. 2006;28(1–2):49–57. doi: 10.1159/000090752. [DOI] [PubMed] [Google Scholar]
- 68.Yang X, et al. Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev Biol. 2004;269(1):81–94. doi: 10.1016/j.ydbio.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 69.Lin S, et al. Lineage-specific effects of Notch/Numb signaling in post-embryonic development of the Drosophila brain. Development. 2010;137(1):43–51. doi: 10.1242/dev.041699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bertet C, et al. Temporal Patterning of Neuroblasts Controls Notch-Mediated Cell Survival through Regulation of Hid or Reaper. Cell. 2014;158(5):1173–86. doi: 10.1016/j.cell.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller DT, Cagan RL. Local induction of patterning and programmed cell death in the developing Drosophila retina. Development. 1998;125(12):2327–35. doi: 10.1242/dev.125.12.2327. [DOI] [PubMed] [Google Scholar]
- 72.Hay BA, Wolff T, Rubin GM. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120(8):2121–9. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- 73.Cagan RL, Ready DF. Notch is required for successive cell decisions in the developing Drosophila retina. Genes Dev. 1989;3(8):1099–112. doi: 10.1101/gad.3.8.1099. [DOI] [PubMed] [Google Scholar]
- 74.Housden BE, et al. Transcriptional dynamics elicited by a short pulse of notch activation involves feed-forward regulation by E(spl)/Hes genes. PLoS Genet. 2013;9(1):e1003162. doi: 10.1371/journal.pgen.1003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramjaun AR, Downward J. Ras and phosphoinositide 3-kinase: partners in development and tumorigenesis. Cell Cycle. 2007;6(23):2902–5. doi: 10.4161/cc.6.23.4996. [DOI] [PubMed] [Google Scholar]
- 76.Scholl FA, et al. Mek1/2 MAPK kinases are essential for Mammalian development, homeostasis, and Raf-induced hyperplasia. Dev Cell. 2007;12(4):615–29. doi: 10.1016/j.devcel.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 77.Satoh Y, et al. Deletion of ERK1 and ERK2 in the CNS causes cortical abnormalities and neonatal lethality: Erk1 deficiency enhances the impairment of neurogenesis in Erk2-deficient mice. J Neurosci. 2011;31(3):1149–55. doi: 10.1523/JNEUROSCI.2243-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87(4):651–60. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- 79.Kurada P, White K. Ras promotes cell survival in Drosophila by downregulating hid expression. Cell. 1998;95(3):319–29. doi: 10.1016/s0092-8674(00)81764-x. [DOI] [PubMed] [Google Scholar]
- 80.Bergmann A, et al. The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell. 1998;95(3):331–41. doi: 10.1016/s0092-8674(00)81765-1. [DOI] [PubMed] [Google Scholar]
- 81.Fan Y, Bergmann A. Multiple Mechanisms Modulate Distinct Cellular Susceptibilities toward Apoptosis in the Developing Drosophila Eye. Dev Cell. 2014;30(1):48–60. doi: 10.1016/j.devcel.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sawamoto K, et al. Argos induces programmed cell death in the developing Drosophila eye by inhibition of the Ras pathway. Cell Death Differ. 1998;5(6):548. doi: 10.1038/sj.cdd.4400398. [DOI] [PubMed] [Google Scholar]
- 83.Baker NE, Rubin GM. Effect on eye development of dominant mutations in Drosophila homologue of the EGF receptor. Nature. 1989;340(6229):150–3. doi: 10.1038/340150a0. [DOI] [PubMed] [Google Scholar]
- 84.Diaz-Benjumea FJ, Hafen E. The sevenless signalling cassette mediates Drosophila EGF receptor function during epidermal development. Development. 1994;120(3):569–78. doi: 10.1242/dev.120.3.569. [DOI] [PubMed] [Google Scholar]
- 85.Simon MA, et al. Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell. 1991;67(4):701–716. doi: 10.1016/0092-8674(91)90065-7. [DOI] [PubMed] [Google Scholar]
- 86.Rasola A, et al. Activation of mitochondrial ERK protects cancer cells from death through inhibition of the permeability transition. Proc Natl Acad Sci U S A. 2010;107(2):726–31. doi: 10.1073/pnas.0912742107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Truscott M, et al. An intronic microRNA links Rb/E2F and EGFR signaling. PLoS Genet. 2014;10(7):e1004493. doi: 10.1371/journal.pgen.1004493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moon NS, Di Stefano L, Dyson N. A gradient of epidermal growth factor receptor signaling determines the sensitivity of rbf1 mutant cells to E2F-dependent apoptosis. Mol Cell Biol. 2006;26(20):7601–15. doi: 10.1128/MCB.00836-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gorski SM, et al. Delta and notch promote correct localization of irreC-rst. Cell Death Differ. 2000;7(10):1011–3. doi: 10.1038/sj.cdd.4400742. [DOI] [PubMed] [Google Scholar]
- 90.Kooh PJ, Fehon RG, Muskavitch MA. Implications of dynamic patterns of Delta and Notch expression for cellular interactions during Drosophila development. Development. 1993;117(2):493–507. doi: 10.1242/dev.117.2.493. [DOI] [PubMed] [Google Scholar]
- 91.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13(22):2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 92.Renner O, Blanco-Aparicio C, Carnero A. Genetic modelling of the PTEN/AKT pathway in cancer research. Clin Transl Oncol. 2008;10(10):618–27. doi: 10.1007/s12094-008-0262-1. [DOI] [PubMed] [Google Scholar]
- 93.Yang ZZ, et al. Dosage-dependent effects of Akt1/protein kinase Balpha (PKBalpha) and Akt3/PKBgamma on thymus, skin, and cardiovascular and nervous system development in mice. Mol Cell Biol. 2005;25(23):10407–18. doi: 10.1128/MCB.25.23.10407-10418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Siegrist SE, et al. Inactivation of both Foxo and reaper promotes long-term adult neurogenesis in Drosophila. Curr Biol. 2010;20(7):643–8. doi: 10.1016/j.cub.2010.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.King-Jones K, Thummel CS. Nuclear receptors--a perspective from Drosophila. Nat Rev Genet. 2005;6(4):311–23. doi: 10.1038/nrg1581. [DOI] [PubMed] [Google Scholar]
- 96.Medh RD, Thompson EB. Hormonal regulation of physiological cell turnover and apoptosis. Cell Tissue Res. 2000;301(1):101–24. doi: 10.1007/s004419900159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Robinow S, et al. Programmed cell death in the Drosophila CNS is ecdysone-regulated and coupled with a specific ecdysone receptor isoform. Development. 1993;119(4):1251–9. doi: 10.1242/dev.119.4.1251. [DOI] [PubMed] [Google Scholar]
- 98.Jiang C, Baehrecke EH, Thummel CS. Steroid regulated programmed cell death during Drosophila metamorphosis. Development. 1997;124(22):4673–83. doi: 10.1242/dev.124.22.4673. [DOI] [PubMed] [Google Scholar]
- 99.Neufeld TP, Baehrecke EH. Eating on the fly: function and regulation of autophagy during cell growth, survival and death in Drosophila. Autophagy. 2008;4(5):557–62. doi: 10.4161/auto.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dorstyn L, et al. DRONC, an ecdysone-inducible Drosophila caspase. Proc Natl Acad Sci U S A. 1999;96(8):4307–12. doi: 10.1073/pnas.96.8.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Robinow S, Draizen TA, Truman JW. Genes that induce apoptosis: transcriptional regulation in identified, doomed neurons of the Drosophila CNS. Dev Biol. 1997;190(2):206–13. doi: 10.1006/dbio.1997.8696. [DOI] [PubMed] [Google Scholar]
- 102.Cakouros D, et al. Ecdysone-induced expression of the caspase DRONC during hormone-dependent programmed cell death in Drosophila is regulated by Broad-Complex. J Cell Biol. 2002;157(6):985–95. doi: 10.1083/jcb.200201034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kilpatrick ZE, Cakouros D, Kumar S. Ecdysone-mediated up-regulation of the effector caspase DRICE is required for hormone-dependent apoptosis in Drosophila cells. J Biol Chem. 2005;280(12):11981–6. doi: 10.1074/jbc.M413971200. [DOI] [PubMed] [Google Scholar]
- 104.Kang Y, Bashirullah A. A steroid-controlled global switch in sensitivity to apoptosis during Drosophila development. Dev Biol. 2014;386(1):34–41. doi: 10.1016/j.ydbio.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cakouros D, Daish TJ, Kumar S. Ecdysone receptor directly binds the promoter of the Drosophila caspase dronc, regulating its expression in specific tissues. J Cell Biol. 2004;165(5):631–40. doi: 10.1083/jcb.200311057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cakouros D, et al. dLKR/SDH regulates hormone-mediated histone arginine methylation and transcription of cell death genes. J Cell Biol. 2008;182(3):481–95. doi: 10.1083/jcb.200712169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Denton D, et al. UTX coordinates steroid hormone-mediated autophagy and cell death. Nat Commun. 2013;4:2916. doi: 10.1038/ncomms3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang J, et al. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122(3):421–34. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 109.Nolo R, et al. The bantam microRNA is a target of the hippo tumor-suppressor pathway. Curr Biol. 2006;16(19):1895–904. doi: 10.1016/j.cub.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 110.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138(1):9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tapon N, et al. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110(4):467–78. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 112.Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114(4):457–67. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- 113.Jia J, et al. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 2003;17(20):2514–9. doi: 10.1101/gad.1134003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Udan RS, et al. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;5(10):914–20. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- 115.Wu S, et al. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114(4):445–56. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- 116.Zender L, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125(7):1253–67. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sawada A, et al. Redundant roles of Tead1 and Tead2 in notochord development and the regulation of cell proliferation and survival. Mol Cell Biol. 2008;28(10):3177–89. doi: 10.1128/MCB.01759-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dong J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130(6):1120–33. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao B, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21(21):2747–61. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morin-Kensicki EM, et al. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol. 2006;26(1):77–87. doi: 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Svingen T, Tonissen KF. Hox transcription factors and their elusive mammalian gene targets. Heredity (Edinb) 2006;97(2):88–96. doi: 10.1038/sj.hdy.6800847. [DOI] [PubMed] [Google Scholar]
- 122.del Toro ED, et al. Generation of a novel functional neuronal circuit in Hoxa1 mutant mice. J Neurosci. 2001;21(15):5637–42. doi: 10.1523/JNEUROSCI.21-15-05637.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Potts MB, Wang DP, Cameron S. Trithorax, Hox, and TALE-class homeodomain proteins ensure cell survival through repression of the BH3-only gene egl-1. Dev Biol. 2009;329(2):374–85. doi: 10.1016/j.ydbio.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 124.Miller DF, et al. Cross-regulation of Hox genes in the Drosophila melanogaster embryo. Mech Dev. 2001;102(1–2):3–16. doi: 10.1016/s0925-4773(01)00301-x. [DOI] [PubMed] [Google Scholar]
- 125.Lohmann I, et al. The Drosophila Hox gene deformed sculpts head morphology via direct regulation of the apoptosis activator reaper. Cell. 2002;110(4):457–66. doi: 10.1016/s0092-8674(02)00871-1. [DOI] [PubMed] [Google Scholar]
- 126.Miguel-Aliaga I, Thor S. Segment-specific prevention of pioneer neuron apoptosis by cell-autonomous, postmitotic Hox gene activity. Development. 2004;131(24):6093–105. doi: 10.1242/dev.01521. [DOI] [PubMed] [Google Scholar]
- 127.Suska A, Miguel-Aliaga I, Thor S. Segment-specific generation of Drosophila Capability neuropeptide neurons by multi-faceted Hox cues. Dev Biol. 2011;353(1):72–80. doi: 10.1016/j.ydbio.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bello B, Holbro N, Reichert H. Polycomb group genes are required for neural stem cell survival in postembryonic neurogenesis of Drosophila. Development. 2007;134(6):1091–9. doi: 10.1242/dev.02793. [DOI] [PubMed] [Google Scholar]
- 129.Kuert PA, et al. Neuroblast lineage identification and lineage-specific Hox gene action during postembryonic development of the subesophageal ganglion in the Drosophila central brain. Dev Biol. 2014;390(2):102–15. doi: 10.1016/j.ydbio.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 130.Banreti A, et al. Hox proteins mediate developmental and environmental control of autophagy. Dev Cell. 2014;28(1):56–69. doi: 10.1016/j.devcel.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 131.Zhang Y, et al. Epigenetic blocking of an enhancer region controls irradiation-induced proapoptotic gene expression in Drosophila embryos. Dev Cell. 2008;14(4):481–93. doi: 10.1016/j.devcel.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pecina-Slaus N. Wnt signal transduction pathway and apoptosis: a review. Cancer Cell Int. 2010;10:22. doi: 10.1186/1475-2867-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yeo W, Gautier J. Early neural cell death: dying to become neurons. Dev Biol. 2004;274(2):233–44. doi: 10.1016/j.ydbio.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 134.Brocardo M, Henderson BR. APC shuttling to the membrane, nucleus and beyond. Trends Cell Biol. 2008;18(12):587–96. doi: 10.1016/j.tcb.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 135.Ahmed Y, et al. Regulation of armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell. 1998;93(7):1171–82. doi: 10.1016/s0092-8674(00)81461-0. [DOI] [PubMed] [Google Scholar]
- 136.Ming M, et al. Activation of Wnt/beta-catenin protein signaling induces mitochondria-mediated apoptosis in hematopoietic progenitor cells. J Biol Chem. 2012;287(27):22683–90. doi: 10.1074/jbc.M112.342089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mirkovic I, et al. Drosophila nemo is an essential gene involved in the regulation of programmed cell death. Mech Dev. 2002;119(1):9–20. doi: 10.1016/s0925-4773(02)00289-7. [DOI] [PubMed] [Google Scholar]
- 138.Brault V, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128(8):1253–64. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 139.Lin HV, Rogulja A, Cadigan KM. Wingless eliminates ommatidia from the edge of the developing eye through activation of apoptosis. Development. 2004;131(10):2409–18. doi: 10.1242/dev.01104. [DOI] [PubMed] [Google Scholar]
- 140.Hackam AS. The Wnt signaling pathway in retinal degenerations. IUBMB Life. 2005;57(6):381–8. doi: 10.1080/15216540500137586. [DOI] [PubMed] [Google Scholar]
- 141.Zou H, Niswander L. Requirement for BMP signaling in interdigital apoptosis and scale formation. Science. 1996;272(5262):738–41. doi: 10.1126/science.272.5262.738. [DOI] [PubMed] [Google Scholar]
- 142.Graham A, et al. The signalling molecule BMP4 mediates apoptosis in the rhombencephalic neural crest. Nature. 1994;372(6507):684–6. doi: 10.1038/372684a0. [DOI] [PubMed] [Google Scholar]
- 143.Furuta Y, Piston DW, Hogan BL. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124(11):2203–12. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- 144.Trousse F, Esteve P, Bovolenta P. Bmp4 mediates apoptotic cell death in the developing chick eye. J Neurosci. 2001;21(4):1292–301. doi: 10.1523/JNEUROSCI.21-04-01292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gambaro K, et al. BMP-4 induces a Smad-dependent apoptotic cell death of mouse embryonic stem cell-derived neural precursors. Cell Death Differ. 2006;13(7):1075–87. doi: 10.1038/sj.cdd.4401799. [DOI] [PubMed] [Google Scholar]
- 146.Moreno E, Basler K, Morata G. Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature. 2002;416(6882):755–9. doi: 10.1038/416755a. [DOI] [PubMed] [Google Scholar]
- 147.Yang SA, Su MT. Excessive Dpp signaling induces cardial apoptosis through dTAK1 and dJNK during late embryogenesis of Drosophila. J Biomed Sci. 2011;18:85. doi: 10.1186/1423-0127-18-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Southwell DG, et al. Intrinsically determined cell death of developing cortical interneurons. Nature. 2012;491(7422):109–13. doi: 10.1038/nature11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Doe CQ. Molecular markers for identified neuroblasts and ganglion mother cells in the Drosophila central nervous system. Development. 1992;116(4):855–63. doi: 10.1242/dev.116.4.855. [DOI] [PubMed] [Google Scholar]
- 150.Bonn S, et al. Cell type-specific chromatin immunoprecipitation from multicellular complex samples using BiTS-ChIP. Nat Protoc. 2012;7(5):978–94. doi: 10.1038/nprot.2012.049. [DOI] [PubMed] [Google Scholar]
- 151.Schauer T, et al. CAST-ChIP maps cell-type-specific chromatin states in the Drosophila central nervous system. Cell Rep. 2013;5(1):271–82. doi: 10.1016/j.celrep.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Henry GL, et al. Cell type-specific genomics of Drosophila neurons. Nucleic Acids Res. 2012;40(19):9691–704. doi: 10.1093/nar/gks671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bowman SK, et al. Multiplexed Illumina sequencing libraries from picogram quantities of DNA. BMC Genomics. 2013;14:466. doi: 10.1186/1471-2164-14-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Patel AP, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wu JN, et al. grim promotes programmed cell death of Drosophila microchaete glial cells. Mech Dev. 2010;127(9–12):407–17. doi: 10.1016/j.mod.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Quinn L, et al. Buffy, a Drosophila Bcl-2 protein, has anti-apoptotic and cell cycle inhibitory functions. Embo J. 2003;22(14):3568–79. doi: 10.1093/emboj/cdg355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sevrioukov EA, et al. Drosophila Bcl-2 proteins participate in stress-induced apoptosis, but are not required for normal development. Genesis. 2007;45(4):184–93. doi: 10.1002/dvg.20279. [DOI] [PubMed] [Google Scholar]
- 158.Kumar S. Caspase function in programmed cell death. Cell Death Differ. 2007;14(1):32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- 159.Yin VP, Thummel CS, Bashirullah A. Down-regulation of inhibitor of apoptosis levels provides competence for steroid-triggered cell death. J Cell Biol. 2007;178(1):85–92. doi: 10.1083/jcb.200703206. [DOI] [PMC free article] [PubMed] [Google Scholar]