Abstract
In utero bisphenol A (BPA) exposure affects reproductive function in the first generation (F1) of mice; however, not many studies have examined the reproductive effects of BPA exposure on subsequent generations. In this study, pregnant mice (F0) were orally dosed with vehicle, BPA (0.5, 20, and 50 µg/kg/day) or diethylstilbestrol (DES; 0.05 µg/kg/day) daily from gestation day 11 until birth. F1 females were used to generate the F2 generation, and F2 females were used to generate the F3 generation. Breeding studies at the ages of 3, 6, and 9 months were conducted to evaluate reproductive capacity over time. Further, studies were conducted to evaluate pubertal onset, litter size, and percentage of dead pups; and to calculate pregnancy rate, and mating, fertility, and gestational indices. The results indicate that BPA exposure (0.5 and 50 μg/kg/day) significantly delayed the age at vaginal opening in the F3 generation compared to vehicle control. Both DES (0.05 μg/kg/day) and BPA (50 μg/kg/day) significantly delayed the age at first estrus in the F3 generation compared to vehicle control. BPA exposure reduced gestational index in the F1 and F2 generations compared to control. Further, BPA exposure (0.5 μg/kg/day) compromised the fertility index in the F3 generation compared to control. Finally, in utero BPA exposure reduced the ability of female mice to maintain pregnancies as they aged. Collectively, these data suggest that BPA exposure affects reproductive function in female mice and that some effects may be transgenerational in nature.
Keywords: in utero exposure, bisphenol A (BPA), fertility, transgenerational, diethylstilbestrol (DES)
Introduction
Bisphenol A (BPA) is a chemical commonly used in polycarbonate plastics and epoxy resins. As a result, BPA is incorporated in many products including food and drink containers, toys, thermal receipt paper, and dental sealants. Previous studies indicate that BPA monomers can leach from products into food and drink consumed by humans (Michalowicz, 2014). Moreover, studies show that BPA is present in more than 92% of tested human urine samples, suggesting that humans are constantly exposed to BPA (Calafat et al., 2008). Further, unconjugated BPA has been measured in human ovarian follicular fluid (2.4 ± 0.8 ng/ml) (Ikezuki et al., 2002), placental tissue (1.0-104.9 ng/gr) (Schonfelder et al., 2002), and fetal plasma (0.2-9.2 ng/ml) (Schonfelder et al., 2002). The presence of BPA in maternal reproductive tissues and fluids is concerning because it suggests that exposure to BPA can occur at all developmental stages, including the embryonic stage, and thus, it has potential to affect subsequent generations through the germline.
A few recent studies have examined the effects of embryonic BPA exposure on reproductive outcomes. These studies show that embryonic BPA exposure (2, 20, and 200 μg/kg/day) followed by lactational exposure does not affect the timing of vaginal opening, fertility, or body weight gain in the first generation (F1) of Long Evans rats (Ryan et al., 2010). Additionally, embryonic BPA exposure (5 μg/kg) followed by lactational and lifelong BPA exposure affected maternal behavior in the F1 generation of Wistar rats, but not in the non-directly exposed second generation (Boudalia et al., 2014). Similarly, low dose (0.2-200 µg/kg/day) continuous BPA exposure did not affect estrous cyclicity, fertility index, litter size, pup weight or viability in either the F1 or F2 generations of IGS rats (Ema et al., 2001). In contrast, perinatal exposure to BPA (25 ng - 25 µg/kg/day) reduced pregnancies and the cumulative number of pups over time in the F1 generation in a forced breeding study in CD-1 mice (Cabaton et al., 2011). Further, embryonic exposure of BPA (0.5, 20, and 50 µg/kg/day) from gestation day (GD) 11 until birth caused significant effects in the F1 generation of friend leukemia virus B strain (FVB) mice, including advanced puberty onset, altered estrous cyclicity, and increased body weight (Wang et al., 2014). Also, in the same study, in utero BPA (0.5 µg/kg/day) exposure significantly reduced fertility in the F1 generation as the females aged when compared to control (Wang et al., 2014).
Although previous studies indicate that BPA exposure (depending on dose, timing of exposure, and species) may affect some, but not all reproductive outcomes, they did not examine in detail whether prenatal BPA exposure has transgenerational effects on female reproduction. Therefore, the current study was designed to evaluate the potential effects of dosing of pregnant dams (F0) from GD 11 until birth on the F2 and F3 generations of mice. Specifically, this study tested the hypothesis that in utero BPA exposure affects pubertal onset and reproductive capacity (mating, ability to conceive, and maintain pregnancy to term) of female mice over several generations.
Materials and Methods
Chemicals
Powder BPA (99%; obtained from the National Institutes of Environmental Health Sciences; USA) and diethylstilbestrol (DES; Sigma Chemical Co., USA) were dissolved in 100% ethanol. The solutions were further diluted in tocopherol-stripped corn oil (vehicle) to reach the planned animal exposures, while maintaining a final concentration of 0.0375% of ethanol (non-fetotoxic concentration) for the final oral dosing solutions. Diethylstilbestrol (DES; 0.05 µg/kg/day) was chosen as a positive control to ensure the animals were responsive to estrogenic compounds. BPA concentrations were chosen based on previous studies and their environmental relevance. Specifically, BPA 0.5 µg/kg/day was used in the study by Wang et al. (Wang et al., 2014) and is similar to the in utero BPA exposure level that was used in a study by Markey et al., and resulted in abnormalities in reproductive organs that appeared as the F1 female mice (CD-1) aged (Markey et al., 2005). BPA 20 µg/kg/day was selected because it disrupts proper oocyte maturation and function in later reproductive life in mice (Susiarjo et al., 2007). BPA 50 µg/kg/day was selected because it is the United States Environmental Protection Agency published reference safe dose (Rubin, 2011).
Animals
FVB female mice and FVB male mice (Charles River; USA) were housed at 25°C in conventional polysulfone cages on a 12L: 12D cycle. The mice were given Teklad Rodent Diet 8604 (Harlan) and highly purified water (reverse osmosis filtered) provided in glass water bottles ad libitum. All animal procedures were approved by the University of Illinois Institutional Animal Care and Use Committee.
Study design
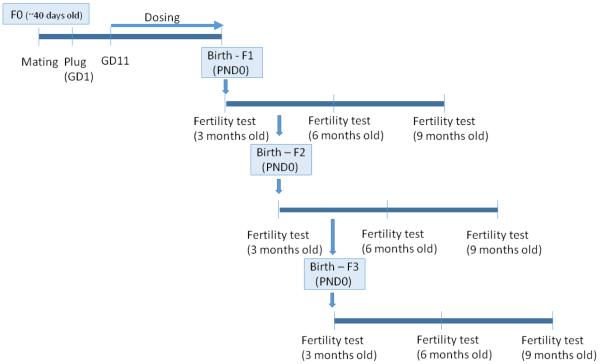
A schematic description of the study design is presented in Figure 1. Briefly, female mice (F0, 6-7 weeks of age) were weighed and then mated with proven male breeders. Mating was confirmed by the presence of a vaginal plug and the day the vaginal plug was observed was considered to be GD 1. On GD 1, plugged females were removed from the male, weighed, and individually caged. Subsequently, daily weights were recorded as an indication of a successful mating/pregnancy. On GD 11 until the birth of the pups, dams were orally dosed with either tocopherol stripped corn oil (vehicle control), DES (0.05 µg/kg/day) or BPA (0.5, 20, and 50 µg/kg/day). This dosing period encompasses the time frame in which primordial germ cells have migrated to the genital ridge to colonize the fetal ovaries and initial establishment of ovarian reserve begins (Pepling, 2006). This time period for treatment also corresponds to a major period of demethylation/methylation in the female germ cells that can potentially result with epigenetic changes if altered (Trasler, 2005). The doses were administered in a volume of 28-32 µl based on their daily body weight. The dams were allowed to deliver naturally and the birth date of the progeny was considered as postnatal day (PND) 0. After weaning on PND 21, at least one female from each litter per generation (and at least n = 3 per treatment group) was monitored daily for vaginal opening. Once vaginal opening was observed, daily vaginal smears were taken to detect the first estrus.
Figure 1.
Study design. Mature female mice mated with proven male breeders. On gestation day 11 until delivery, pregnant dams were dosed with either tocopherol stripped corn oil (control), diethylstilbestrol (DES; 0.05 µg/kg/day) or bisphenol A (BPA; 0.5, 20, 50 µg/kg/day) to examine the effects of in utero BPA exposure on fertility of subsequent generations at 3, 6, and 9 months of age.
At least one female per treatment group per litter from the F1, F2, and F3 generations was selected to examine fertility at the age of 3, 6, and 9 months. In each fertility test, the selected females were mated with proven male breeders until a vaginal plug was observed. Once the plug was observed, females were individually caged, monitored, and weighed twice per week until term. The number of pregnant dams and births were recorded. Once the females gave birth (mostly on GD 19), the litter sizes and the number of dead pups per litter were recorded.
Pregnancy Rate and Fertility, Mating and Gestational Indices
To examine the effect of in utero BPA exposure on fertility, we calculated the following indices: Pregnancy rate: number of pregnant females/ number of breeding pairs × 100;
Mating index: number of females who plugged/ number of breeding pairs × 100;
Fertility index: number of pregnant females/ number of females who plugged × 100; and Gestational index: number of females who delivered/ number of pregnant females × 100. Mating, fertility, and gestational indices were calculated based on the formulas suggested by Tyl et al. (Tyl et al., 2008), with a minor modification in the gestational index in which we included all females who delivered, including ones in which all pups were found dead on PND 0 (n = 9).
Statistical analyses
Data were expressed as the mean ± SEM from at least three separate biological replicates. Differences between vehicle control and the other treatment groups were statistically analyzed using SPSS software (SPSS Inc., Chicago, IL). For all comparisons, statistical significance was assigned at p ≤ 0.05. For continuous data, when normally distributed and homogeneity of variance assumption were met, we used one-way analysis of variance followed by a Dunnett’s post hoc test. When continuous data were not normally distributed and/or when the homogeneity of variance assumption was not met, we used a Kruskal-Wallis non-parametric test, followed by a Mann-Whitney test.
Results
Effect of in utero BPA exposure on timing of vaginal opening and first estrus
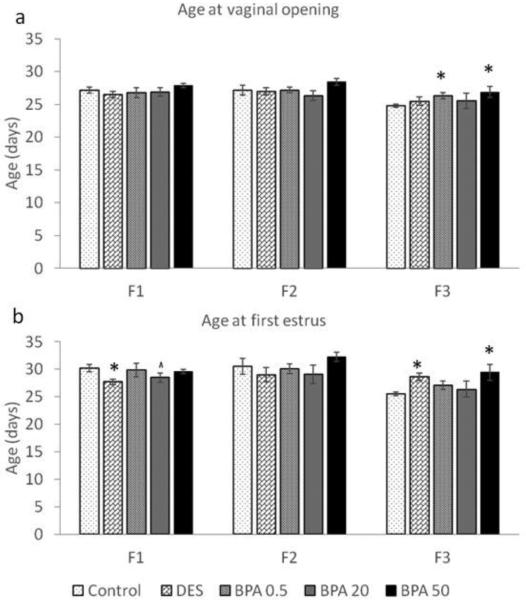
Starting at PND21, female mice were examined daily for vaginal opening. Then, estrous cyclicity was monitored after vaginal opening to detect the timing of the first estrus, as a hallmark of pubertal onset. In both the F1 and F2 generations, the average ages at vaginal opening in the BPA treatment groups were similar to the average ages of the control groups (Figure 2a). In contrast, the age at vaginal opening in F3 generation was significantly delayed with 0.5 μg/kg/day BPA exposure (n = 9) and 50 μg/kg/day in utero BPA exposure (n = 9) when compared to the control group (Figure 2a; n = 14; p ≤ 0.05).
Figure 2.
The effects of in utero BPA exposure on timing of vaginal opening (a) and first estrus (b) in the F1-F3 generations. Graphs represent mean ± SEM from 9-15 dams per treatment group. Asterisks (*) represent a significant difference from the control group (p ≤ 0.05); ^ represent a borderline difference from the control group (p = 0.1)
As previously published by Wang et al. (Wang et al., 2014), the age at first estrus was significantly earlier in the DES treatment group (Figure 2b; p ≤ 0.05; n = 11) and trended towards significance in the BPA 20 μg/kg/day treatment group (p = 0.1; n = 10) when compared to the control group (n = 15). When we expanded these findings by examining the age at first estrus in the F2 and F3 generations, we found that in the F2 generation, the age at first estrus was similar between all treatment groups (Figure 2b). However, in the F3 generation, the average age at first estrus was significantly delayed in the DES (n = 8) and BPA (50 μg/kg/day) treatment groups (n = 9) when compared to the control group (n = 14; p ≤ 0.05).
Effect of BPA on female weights at 3, 6, and 9 months of age
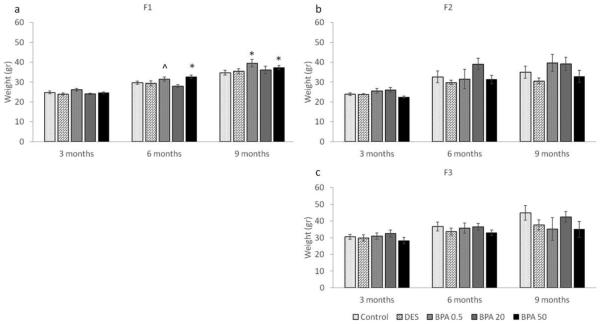
At the beginning of each breeding cycle, females were weighed and the average weights per treatment group per generation were calculated, and presented in Figure 3 (a-c). As previously shown by Wang et al., in utero BPA exposure significantly increased body weight at 6 months (Figure 3a) (Wang et al., 2014). When we expanded these findings by examining body weight in the F2 and F3 generations, we found that BPA-induced weight differences observed in the F1 generation were not maintained in the F2 and F3 generations (Figure 3b-c). Specifically, at 3, 6 and 9 months of age, the average weights per treatment group per generation were comparable (p > 0.05).
Figure 3.
The effects of in utero BPA exposure on body weight (grams) at 3, 6, and 9 months of age on the F1 (a), F2 (b), and F3 (c) generations. Graphs represent mean ± SEM from 4-15 dams per treatment group. Asterisks (*) represent a significant difference from the control group (p ≤ 0.05); ^ represents a borderline difference from the control group (0.05 < p ≤ 0.1)
Effect of in utero BPA exposure on pregnancy rate
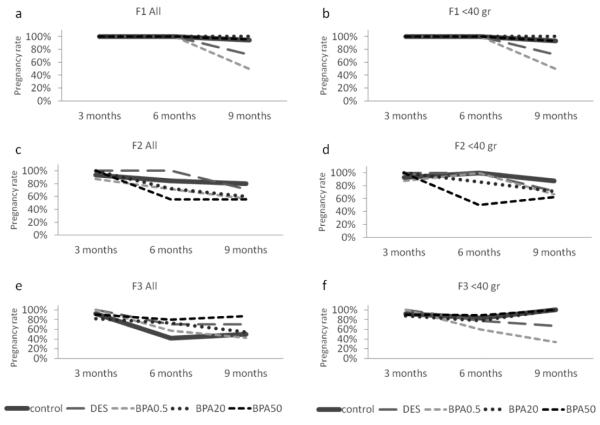
To examine the effects of in utero BPA exposure on the ability to get pregnant, we calculated a pregnancy rate as described in the methods section. Because we observed relatively high variability in weights within the groups as the dams aged, we elected to present the data both for the entire cohort (Figure 4a, c, e) and for dams who weighed less than 40 grams when each mating cycle was initiated (Figure 4b, d, f). The cut-off weight of 40 grams was chosen based on the results from the study by Wang et al. (Wang et al., 2014) and is typical for reproductive-aged mice (i.e., less than 40 grams). Similar to the data published by Wang et al. (Wang et al., 2014), in utero BPA exposure significantly reduced pregnancy rates in the F1 generation, with a lower pregnancy rate occurring in the BPA 0.5 μg /kg/day treatment group, and being the lowest (50%) at 9 months (Figure 4a and b).
Figure 4.
The effects of in utero BPA exposure on the percentage of pregnant dams of the total breeding pairs at 3, 6, and 9 months of age per generation (F1, F2, and F3). Graphs represent the percentage of pregnant dams from 3-15 dams per treatment group. Panels a, c, and e represent data for the entire cohort and panels b, d, and f represent data from females who weighed less than 40 grams prior to mating at each breeding cycle.
When we expanded these findings to examine fertility in the F2 and F3 generations, in utero BPA exposure decreased pregnancy rates in the entire F2 cohort, but the most notable BPA-induced decrease in pregnancy rate started at 6 months instead of 9 months in the 50 μg/kg/day BPA treatment group (56%), whereas the control and the other BPA treatment groups had higher pregnancy rates (71% and above; Figure 4c). At 9 months of age, all BPA treatment groups still had a reduced pregnancy rate (60% and less) compared to controls (80%; Figure 4c). When dams with a weight less than 40 grams prior to mating were examined at 6 months of age, only BPA 50 μg/kg/day still caused a severely reduced pregnancy rate (50%) compared to the control group (100%), whereas the other BPA treatment groups had pregnancy rates similar to the control group (86% and above). At 9 months of age, BPA 0.5 μg/kg/day and 50 μg/kg/day still reduced pregnancy rate (67% and 63% respectively) compared to controls, whereas the other treatment groups had pregnancy rates of 71% and above (Figure 4d).
In the F3 generation, in utero BPA exposure did not affect pregnancy rate as females aged compared to controls in the whole cohort at 3 months (Figure 4e). As the females aged, the control group had a relatively low pregnancy rate (42% and 50% at 6 and 9 months respectively; Figure 4e). However, when dams with a weight less than 40 grams prior to mating were examined, in utero BPA exposure (0.5 μg/kg/day) reduced pregnancy rate (60%) compared to controls (83%) at 6 months (Figure 4f). Similarly, in utero BPA exposure (0.5 μg/kg/day) reduced pregnancy rate (33%) compared to controls (100%) at 9 months (Figure 4f).
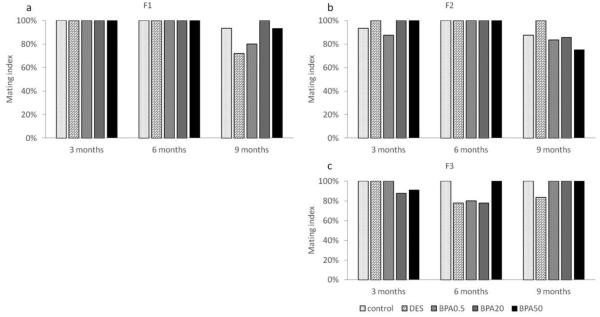
Effect of in utero BPA exposure on mating index
In the F1 generation, the mating index was 100% in all treatment groups at 3 and 6 months (Figure 5a). However, both the DES and BPA (0.5 μg/kg/day) treatment groups had a slightly lower mating index (72% and 80%, respectively) compared to the control group (100%) at 9 months (Figure 5a). In the F2 generation, the mating index was similar (> 80%) in all treatment groups at 3 and 6 months of age, but slightly lower in the 50 μg/kg/day BPA treatment group (75%) compared to controls at 9 months (Figure 5b). In the F3 generation, all treatment groups had a mating rate of 83% and higher at 3, 6, and 9 months (Figure 5c).
Figure 5.
The effects of in utero BPA exposure on mating indices at 3, 6, and 9 months of age in F1 (a), F2 (b), and F3 (c) generations. Data generated from 3-15 dams per treatment group. Data include only females who weighed less than 40 grams prior to mating at each breeding cycle.
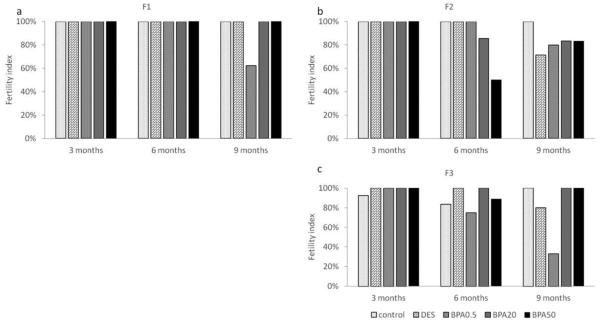
Effect of in utero BPA exposure on fertility index
In the F1 generation, all females who plugged also got pregnant at 3 and 6 months of age (Figure 6a). However, only 63% of the females got pregnant in the 0.5 μg/kg/day BPA treatment group at 9 months (Figure 6a). In the F2 generation, all females got pregnant at 3 months of age. Females in the 50 μg/kg/day treatment group only had a 50% fertility index compared to 86% and above in the other treatment groups at 6 months of age (Figure 6b). Females in the DES treatment group had a fertility index of 71% compared to 80% and higher in the other treatment groups at 9 months (Figure 6b).
Figure 6.
The effects of in utero BPA exposure on fertility indices at 3, 6, and 9 months of age in F1 (a), F2 (b), and F3 (c) generations. Data generated from 1-14 dams per treatment group. Data include only females who weighed less than 40 grams prior to mating at each breeding cycle.
In the F3 generation, all treatment groups had a fertility index greater than 92% at 3 months of age (Figure 6c). Females in the control, DES, and BPA (20 and 50 μg/kg/day) treatment groups had a relatively high fertility index (>83%), whereas the 0.5 μg/kg/day BPA treatment group had a fertility index of 75% at 6 months of age (Figure 6c). Lastly, the fertility indices of all treatment groups were similar to the ones calculated at 6 months of age (i.e. >80%), except in the 0.5 μg /kg/day BPA treatment group, which had a much lower index (33%) at 9 months of age (Figure 6c).
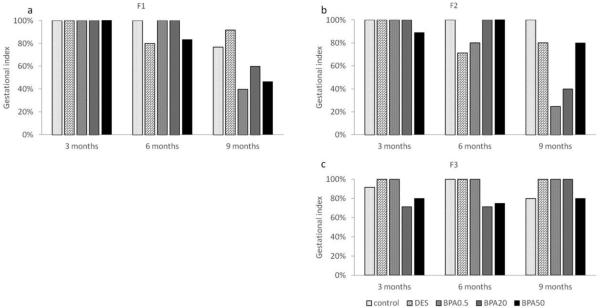
Effect of in utero BPA exposure on gestational index
In the F1 generation, all groups had 100% success in maintaining their pregnancy at 3 months of age (Figure 7a). All treatment groups had a relatively high gestational index of 80% and above at 6 months. All BPA treatment groups had a relatively low gestational index compared to controls, with the lowest index in the 0.5 μg/kg/day (40%) and 50 μg/kg/day (46%) BPA treatments at 9 months (Figure 7a).
Figure 7.
The effects of in utero BPA exposure on gestational indices at 3, 6, and 9 months of age in F1 (a), F2 (b), and F3 (c) generations. Data generated from 1-14 dams per treatment group. Data include only females who weighed less than 40 grams prior to mating at each breeding cycle.
In the F2 generation, all treatment groups had 89% and higher gestational indices at 3 months of age (Figure 7b). All treatment groups had 80% and above except in the DES treatment group (71%) at 6 months. However, only the control, DES and 50 μg/kg/day BPA treatment groups maintained a relatively high gestation index (>80%), whereas the BPA 0.5 μg/kg/day and BPA 20 μg/kg/day treatment groups had a sharp decrease in the gestation index (25% and 40% respectively) at 9 months (Figure 7b).
In the F3 generation, most treatment groups exhibited a high gestational index (> 80%), except in 20 μg/kg/day (71%) BPA treatment group at 3 months (Figure 7c). Similarly, a lower percentage of females was able to maintain their pregnancies in the 20 μg/kg/day (71%) and 50 μg/kg/day (75%) BPA treatment groups compared to the other treatment groups (100%) at 6 months (Figure 7c). In contrast, all treatment groups have a gestation index of 80% and above at 9 months (Figure 7c).
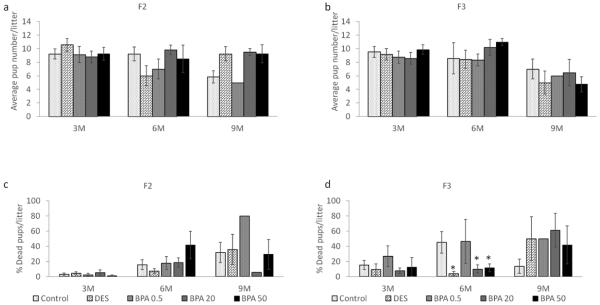
Effect of BPA on average litter size and percentage of dead pups
Previously Wang et al. (Wang et al., 2014) showed that in utero BPA exposure affects average litter size (0.5 and 50 μg/kg/day) and increases the percentage of dead pups (0.5 μg/kg/day) in the F1 generation. We expanded these findings by monitoring the number of live and dead pups in the F2 and F3 generations. For consistency purposes, we present the data from the dams that weighed less than 40 grams at the time of mating though there were no major differences when examining the entire cohort. We found that in the F2 generation, BPA and DES treatments did not affect litter size compared to control at any time points (Figure 8a). However, similar to the F1 results, only one of the seven dams in the 0.5 μg/kg/day BPA treatment group gave birth at 9 months, and only one pup out of the 5 that were born remained alive in the F2 generation. Also, the 20 μg/kg/day BPA treatment group exhibited a very low percentage of dead pups compared to control at 9 months, however, our sample size was too low for statistical analysis (n = 2; Figure 8c).
Figure 8.
The effects of in utero BPA exposure on average litter size (F2 generation in panel a and F3 generation in panel b) and percentage of dead pups per litter at 3, 6, and 9 months of age (F2 generation in panel c and F3 generation in panel d). Data generated from 1-14 dams per treatment group. Data include only females who weighed less than 40 grams prior to mating at each breeding cycle. Asterisks (*) represent a significant difference from the control group (p ≤ 0.05)
In the F3 generation, BPA and DES did not affect the average litter size compared to control at any time points (Figure 8b). However, the DES, 20 μg/kg/day BPA, and 50 μg/kg/day BPA treatment groups significantly lowered the percentage of dead pups compared to the control group at 6 months (p ≤ 0.05), whereas a similar percentage of dead pups to controls was observed in 0.5 μg/kg/day BPA group (p > 0.05).
Discussion
Previously, Wang et al. examined the effects of in utero BPA exposure on some reproductive outcomes in the F1 generation and reported that BPA significantly advances the age of first estrus, causes various fertility problems, and increases the percentage of dead pups compared to controls (Wang et al., 2014). In the current study, we present additional data on the effects of BPA on female reproductive parameters in the F1 generation as well as in the subsequent generations (F2 and F3 generations). Our data indicate that BPA exposure delays the age at vaginal opening and first estrus in the F3 generation, reduces the fertility of the dams as they age in the F2 and F3 generations, and reduces the percentage of dead pups in the F3 generation when compared to controls.
Our data indicate that the age at vaginal opening is similar between all treatment groups in the F1 generation, but it was delayed in 0.5 μg/kg/day and 50 μg/kg/day BPA treatment groups in the F3 generation. The findings in the F1 generation are in agreement with Ryan et al., who showed that in utero exposure to BPA (2, 20, and 200 μg/kg/day) from GD 7 until PND 18 did not affect age at vaginal opening in the F1 generation of rats (Ryan et al., 2010). Our data, however, differ from Honma et al., who showed that in utero BPA exposure (20 μg/kg/day) and DES exposure (0.02-2 μg/kg/day) decreased the age at vaginal opening in ICR/Jcl mice (Honma et al., 2002). The reasons for differences in our data versus those of Honma et al. are unclear, but could be due to strain differences, time of exposure, or the difference in doses of BPA and DES in the studies.
As far as our results on vaginal opening in the F2 and F3 generations, we could not find any published studies to which we could compare our results. However, Manikkam et al., found that exposure to a mixture of BPA and other plastics delayed pubertal onset in F1 females and caused early pubertal onset in F3 females (Manikkam et al., 2013). The mechanism by which BPA alters onset of puberty is unknown. It is possible that a potential target for BPA toxicity may be the hypothalamic-pituitary-gonadal (HPG) axis (Fernandez et al., 2009). Hence, we can only speculate that once the onset of puberty is affected in the F1 generation, it may take couple of generations to completely restore the alterations in the reproductive axis to the levels of the control animals. Future studies should elucidate if in utero BPA exposure of the F1 generation results in organizational or epigenetic changes that have long-term effects over several generations. It is possible that in later generations, the observed effects of BPA on pubertal onset will be restored completely. Similarly, BPA (50 μg/kg/day) delayed the age at first estrus in the F3 generation. Unfortunately, we could not examine hormone levels or conduct histological evaluation to better understand this outcome. Nevertheless, it is consistent with the observations regarding the delayed age at vaginal opening in the F3. Future studies are needed to explore these findings further.
Although in utero BPA exposure (0.5, 20, and 50 μg/kg/day) has been shown to increase body weight in the F1 generation (Wang et al., 2014), it does not appear to affect body weights or selected organ weights (liver, ovary, and uterus) in the F2 or F3 generations at selected time points (data not shown). These data are in contrast to those of Hiyama et al. who showed that BPA treatments (200, 500 and 1,000 mg/kg) significantly increased body weights compared to vehicle control group in the F2 generation (Hiyama et al., 2011). Our data also differ from Miyawaki et al., who showed that perinatal and postnatal exposure to BPA (1 or 10 µg/ml) administered via the drinking water increased F1 weight gain (Miyawaki et al., 2007). The reason for differences among studies is likely due to differences in the doses of BPA used in each study. A report by The National Toxicology Program – The Center for the Evaluation of Risks to Human Reproduction concluded that the effects of BPA on obesity/weight gain are inconsistent, but that in general, studies that utilized low doses of BPA (5 mg/kg bw/day) reported either no effect or reduced body weight compared to controls (Shelby, 2008). Given that we did not observe an effect of BPA on weight in the F2 and F3 generations, our data suggest that any effects of BPA observed in F1 generations are not likely to be carried over multiple generations. It also may be that the effects of BPA on body weight gain involve complicated mechanisms that should be explored in future studies.
Though there were no differences in body weight among treatment groups in the F2 or F3 generations, we did observe large variability in body weight within the same treatment groups (intra-animal variability). Thus, we analyzed reproductive outcomes using the whole cohort as well as just animals with weights that are typical for reproductive-aged mice (i.e. less than 40 grams) and were comparable to the data presented previously by Wang et al. on the F1 generation from the same study (Wang et al., 2014). Interestingly, 0.5 μg/kg/day BPA treatment caused the most profound effects on fertility across all generations as the mice aged. Specifically, in the F1 generation, the lowest pregnancy rate was observed at BPA 0.5 μg/kg/day as the females reached 9 months of age. In the F2 generation, while we observed a sharp decline in pregnancy rate in 50 μg/kg/day BPA treatment group at 6 months, both 0.5 μg/kg/day and 50 μg/kg/day BPA treatment decreased pregnancy rate at 9 months. In the F3 generation, 0.5 μg/kg/day BPA treatment caused the lowest pregnancy rate. While the mechanism by which BPA affects pregnancy in mice is unknown, Manikkam et al., showed that exposure to a plastics mixture decreased ovarian reserve in the F1 and F3 generation (Manikkam et al., 2013). Hence, it is possible that exposure to BPA induces ovotoxicity that is carried over several generations. It is also possible that in utero BPA exposure of the developing F1 pups affected the germ cells that later generated the F2 generation. These may have been long lasting effects that included epigenetic modifications such as DNA methylation that carried over to the F3 generation. In support of this hypothesis are several studies that provide some evidence for epigenetic modifications following exposure to endocrine disrupting chemicals, including BPA. For example, studies by Dolinoy et al. (Dolinoy et al., 2007) and Anderson et al. (Anderson et al., 2012) have shown that maternal dietary BPA intake causes epigenetic modifications in the offspring of agouti mice. Manikkam et al. have reported sperm epimutations in the F3 generation following in utero exposure to a plastic mixture that included BPA (Manikkam et al., 2013). Other endocrine disrupting chemicals such as methoxyclor (Anway et al., 2005) and vinclozolin (Anway et al., 2006) have also been shown to cause transgenerational effects (F1 to F4) on male infertility in rats via epigenetic reprogramming; however, further studies are needed to determine if that is the case in our study.
To better understand the differences in pregnancy rates among treatment groups, we evaluated which steps in the pathway to a successful pregnancy were most impacted by BPA treatment. Our data indicate that the mating index was similar across all treatment groups and generations, suggesting that in utero BPA exposure does not alter the mating behavior of the animals. In contrast, our data show that BPA (0.5 and 50 μg/kg/day) decreased the ability of mice to maintain pregnancy as they aged. Interestingly, the most profound decrease in gestational index was observed with the 0.5 μg/kg/day BPA treatment as females aged (both in the F1 and F2 generations at 9 months). It is possible that this dose of BPA is ovotoxic and that it may directly damage some of the germ cells during the exposure to BPA in utero that later result in unhealthy oocytes with epigenetic modifications that are gradually carried over subsequent generations. In support of this possibility are findings by Susiarjo et al. (2007) that showed that in utero BPA exposure interferes with early oocyte maturation and development of the F1 generation that results in an increased risk of producing aneuploid offspring. Because we did not collect tissues during pregnancy, we cannot determine what caused the pregnancy losses. Since most effects were observed in the F1 and F2 generations, we speculate that exposure to BPA caused abnormalities in ovary or uterus that later resulted in overall compromised reproductive capacity. Future studies should examine whether this is the case.
Interestingly, gestational indices in the F3 generation improved compared to the F1 and F2 generations. However, the fertility index in the 0.5 μg/kg/day BPA treatment group was severely reduced compared to all other treatment groups in the F3 generation at 9 months. These data suggest that fertility in the F3 generation is compromised at the level of becoming pregnant, but once the dam is pregnant, she is likely to maintain the pregnancy to term in the F3 generation.
Finally, our data show that BPA and DES treatment did not affect average litter size in those dams that were able to become pregnant and give birth. These findings are similar to a study by Tyl et al., who reported no significant differences in the number of pups born to BPA treated rats in any of the three generations (Tyl et al., 2002). Nevertheless, we did find some effects of BPA and DES on the percentage dead pups at PND 0. Using the F2 generation data, we could not perform statistical analysis due to a limited number of animals who gave birth at 9 months after in utero exposure to 0.5 μg/kg/day BPA (n = 1) and 20 μg/kg/day BPA (n = 2). However the percentage of dead pups was very high in 0.5 μg/kg/day BPA treatment group and relatively low in the 20 μg/kg/day BPA treatment group. Interestingly, in the F3 generation, the numbers of dead pups in the DES and BPA (20 and 50 μg/kg/day) treatment groups were significantly lower than the control group. We did not detect any histopathological or other particular problems in our colony that can explain these differences. These effects, at least in the F2 generation, are in contrast to the study by Tyl et al., who found no differences in pup survivability following BPA exposure (Tyl et al., 2002).
In conclusion, our data suggest that in utero BPA exposure may affect pubertal onset and reproductive capacity in the F1 generation of mice. Further, our data suggest that some of these effects may be transgenerational in nature. Specifically, in utero BPA exposure may cause transgenerational effects on the ability to become pregnant and to maintain pregnancy to term. Further studies should investigate the mechanism underlying the effects of BPA on female reproductive outcomes in the F1-F3 generations.
Highlights.
In utero BPA delayed vaginal opening in the F3 generation compared to control.
In utero BPA delayed estrus in the F3 generation compared to control.
In utero BPA reduced the ability of F1 and F2 female mice to maintain pregnancies.
In utero BPA compromised the ability of F3 female mice to become pregnant.
Some effects of in utero BPA may be transgenerational in nature.
Acknowledgements
This work was supported by: NIH PO1 ES022848 (JAF), EPA RD-83459301 (JAF), an Environmental Toxicology Fellowship (AZG), NIH T32 ES007326 (WW), and the Billie A. Field Fellowship in Reproductive Biology-UIUC (WW and AZG). The authors also wish to thank all the members of the Flaws’ lab for their assistance and constructive input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors have no conflicts of interest or any financial disclosures to declare.
References
- Anderson OS, Nahar MS, Faulk C, Jones TR, Liao C, Kannan K, Weinhouse C, Rozek LS, Dolinoy DC. Epigenetic responses following maternal dietary exposure to physiologically relevant levels of bisphenol A. Environmental and molecular mutagenesis. 2012;53:334–342. doi: 10.1002/em.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Science. Vol. 308. New York, N.Y.: 2005. Epigenetic transgenerational actions of endocrine disruptors and male fertility; pp. 1466–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, Leathers C, Skinner MK. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology. 2006;147:5515–5523. doi: 10.1210/en.2006-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudalia S, Berges R, Chabanet C, Folia M, Decocq L, Pasquis B, Abdennebi-Najar L, Canivenc-Lavier MC. A multi-generational study on low-dose BPA exposure in Wistar rats: effects on maternal behavior, flavor intake and development. Neurotoxicology and teratology. 2014;41:16–26. doi: 10.1016/j.ntt.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Cabaton NJ, Wadia PR, Rubin BS, Zalko D, Schaeberle CM, Askenase MH, Gadbois JL, Tharp AP, Whitt GS, Sonnenschein C, Soto AM. Perinatal exposure to environmentally relevant levels of bisphenol A decreases fertility and fecundity in CD-1 mice. Environmental health perspectives. 2011;119:547–552. doi: 10.1289/ehp.1002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environmental health perspectives. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M, Fujii S, Furukawa M, Kiguchi M, Ikka T, Harazono A. Rat two-generation reproductive toxicity study of bisphenol A. Reproductive toxicology (Elmsford, N.Y.) 2001;15:505–523. doi: 10.1016/s0890-6238(01)00160-5. [DOI] [PubMed] [Google Scholar]
- Fernandez M, Bianchi M, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a alters reproductive parameters and gonadotropin releasing hormone signaling in female rats. Environmental health perspectives. 2009;117:757–762. doi: 10.1289/ehp.0800267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama M, Choi EK, Wakitani S, Tachibana T, Khan H, Kusakabe KT, Kiso Y. Bisphenol-A (BPA) affects reproductive formation across generations in mice. The Journal of veterinary medical science / the Japanese Society of Veterinary Science. 2011;73:1211–1215. doi: 10.1292/jvms.11-0135. [DOI] [PubMed] [Google Scholar]
- Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reproductive toxicology (Elmsford, N.Y.) 2002;16:117–122. doi: 10.1016/s0890-6238(02)00006-0. [DOI] [PubMed] [Google Scholar]
- Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Human reproduction (Oxford, England) 2002;17:2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PloS one. 2013;8:e55387. doi: 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biology of reproduction. 2005;72:1344–1351. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- Michalowicz J. Bisphenol A--sources, toxicity and biotransformation. Environmental toxicology and pharmacology. 2014;37:738–758. doi: 10.1016/j.etap.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Miyawaki J, Sakayama K, Kato H, Yamamoto H, Masuno H. Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. Journal of atherosclerosis and thrombosis. 2007;14:245–252. doi: 10.5551/jat.e486. [DOI] [PubMed] [Google Scholar]
- Pepling ME. From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis (New York, N.Y. : 2000) 2006;44:622–632. doi: 10.1002/dvg.20258. [DOI] [PubMed] [Google Scholar]
- Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. The Journal of steroid biochemistry and molecular biology. 2011;127:27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Hotchkiss AK, Crofton KM, Gray LE., Jr. In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats. Toxicological sciences : an official journal of the Society of Toxicology. 2010;114:133–148. doi: 10.1093/toxsci/kfp266. [DOI] [PubMed] [Google Scholar]
- Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environmental health perspectives. 2002;110:A703–707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby MD. NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. Ntp cerhr mon. 2008;v:vii–ix. 1-64 passim. [PubMed] [Google Scholar]
- Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS genetics. 2007;3:e5. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyl RW, Myers CB, Marr MC, Sloan CS, Castillo NP, Veselica MM, Seely JC, Dimond SS, Van Miller JP, Shiotsuka RN, Beyer D, Hentges SG, Waechter JM., Jr. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicological sciences : an official journal of the Society of Toxicology. 2008;104:362–384. doi: 10.1093/toxsci/kfn084. [DOI] [PubMed] [Google Scholar]
- Tyl RW, Myers CB, Marr MC, Thomas BF, Keimowitz AR, Brine DR, Veselica MM, Fail PA, Chang TY, Seely JC, Joiner RL, Butala JH, Dimond SS, Cagen SZ, Shiotsuka RN, Stropp GD, Waechter JM. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicological sciences : an official journal of the Society of Toxicology. 2002;68:121–146. doi: 10.1093/toxsci/68.1.121. [DOI] [PubMed] [Google Scholar]
- Trasler JM. Gamete imprinting: setting epigenetic patterns for the next generation. Reproduction, Fertility and Development. 2005;18(2):63–69. doi: 10.1071/rd05118. [DOI] [PubMed] [Google Scholar]
- Wang W, Hafner KS, Flaws JA. In utero bisphenol A exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicology and applied pharmacology. 2014;276:157–164. doi: 10.1016/j.taap.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]