Abstract
Currently, there is no effective antidote to prevent skin injuries by sulfur mustard (SM) and nitrogen mustard (NM), which are vesicating agents with potential relevance to chemical warfare, terrorist attacks, or industrial/laboratory accidents. Our earlier report has demonstrated the therapeutic efficacy of silibinin, a natural flavanone, in reversing monofunctional alkylating SM analog 2-chloroethyl ethyl sulfide-induced toxic effects in mouse skin. To translate this effect to a bifunctional alkylating vesicant, herein, efficacy studies were carried out with NM. Topical application of silibinin (1 or 2 mg) 30 min after NM exposure on the dorsal skin of male SKH-1 hairless mice significantly decreased NM-induced toxic lesions at 24, 72 or 120 h post-exposure. Specifically, silibinin treatment resulted in dose-dependent reduction of NM-induced increase in epidermal thickness, dead and denuded epidermis, parakeratosis and microvesication. Higher silibinin dose also caused a 79% and 51% reversal in NM-induced increases in myeloperoxidase activity and COX-2 levels, respectively. Furthermore, silibinin completely prevented NM-induced H2A.X phosphorylation, indicating reversal of DNA damage which could be an oxidative DNA damage as evidenced by high levels of 8-oxodG in NM-exposed mouse skin that was significantly reversed by silibinin. Together, these findings suggest that attenuation of NM-induced skin injury by silibinin is due to its effects on the pathways associated with DNA damage, inflammation, vesication and oxidative stress. In conclusion, results presented here support the optimization of silibinin as an effective treatment of skin injury by vesicants.
Keywords: Nitrogen mustard, skin, silibinin, SKH-1 hairless mice, microvesication, oxidative DNA damage
Introduction
Sulfur mustard (SM) is a primary vesicant that was first used as a chemical warfare agent in World War I, which remains a threat even today for both military conflicts and terrorist activities (Wattana and Bey, 2009). SM exposure causes severe injuries to skin, ocular and respiratory tissues, and this toxicity can be fatal (Graham et al., 2005; Kehe and Szinicz, 2005; Shakarjian et al., 2010). As a powerful vesicant, early toxic effects of SM include severe inflammation, apoptotic cell death, DNA damage and epidermal-dermal separation in the affected skin tissue (Shakarjian et al., 2010; Firooz et al., 2011). Histopathologically, severe toxic effects of both SM and nitrogen mustard (NM) on skin epidermis include parakeratosis, hyperkeratosis, hyperplasia, necrosis, acanthosis and multinucleated giant cells (Naraghi et al., 2005; Ghabili et al., 2010; Benson et al., 2011). Early studies with the SM analogs 2-chloroethyl ethyl sulfide (CEES) and NM from our laboratory and elsewhere have shown that pathways related to DNA damage, oxidative stress and inflammation are involved in their skin injury effects (Sabourin et al., 2002; Kehe et al., 2009; Pal et al., 2009; Tewari-Singh et al., 2010; Inturi et al., 2011; Jain et al., 2011a; Jain et al., 2011b). Accordingly, both antioxidants, such as AEOL10150, glutathione, sulforaphane, butylated hyroxyanisole, ebselen and others (Paromov et al., 2007; Laskin et al., 2010; Tewari-Singh et al., 2011; Tewari-Singh et al., 2014a) and anti-inflammatory drugs (Nyska et al., 2001; Dachir et al., 2004; Chang et al., 2014) have been evaluated to treat vesicant-induced skin injuries and have shown some promise. However, more efforts are needed to identify novel agents to rescue vesicants-induced skin injuries, specifically in the mass causality scenario.
Silibinin is a natural flavonoid derived from the seeds of milk thistle which is widely consumed as a dietary supplement in hepatic disorders (Ferenci et al., 1989; Pares et al., 1998). Extensive studies from our laboratory have established its anticancer efficacy against various types of epithelial cancer (Singh and Agarwal, 2005; Kavitha et al., 2014; Narayanapillai et al., 2014), and silibinin has also shown strong activity against ultraviolet B radiation-induced DNA damage and apoptotic cell death in both epithelial cell culture and SKH-1 hairless mouse skin (Dhanalakshmi et al., 2005; Narayanapillai et al., 2014). In addition, based on our finding that silibinin reverses SM analog CEES-induced skin toxicity (Tewari-Singh et al., 2012) and that it possesses strong antioxidant and anti-inflammatory activities whereby it targets multiple oxidative stress-induced signaling pathways involved in inflammation and DNA repair, here we assessed silibinin efficacy to attenuate NM-induced skin injuries employing recently established biomarkers for NM-induced skin injury in SKH-1 hairless mice (Tewari-Singh et al., 2012; Tewari-Singh et al., 2013; Jain et al., 2014a; Tewari-Singh et al., 2014b). Overall, our results show a strong effect of silibinin treatment in ameliorating NM-induced injuries including DNA damage, apoptotic cell death, microvesication, inflammation and histopathological damage.
Materials and Methods
Materials
Silibinin and NM (mechlorethamine hydrochloride) were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies for phospho H2A.X S139, 8-oxodG and cyclooxygenase-2 (COX-2) were from Cell Signaling (MA, USA), JalCA (Japan) and Cayman chemicals (Ann Arbor, USA), respectively. DAB detection system was from Vector Labs (CA, USA). Dead-end colorimetric TUNEL system was from Promega (Madison, WI). All other chemicals were from Sigma-Aldrich.
Animals and experiment design
Four to five weeks old male SKH-1 hairless mice were purchased from Charles River Laboratories (Wilmington, MA) and maintained according to the IACUC-approved animal protocol from the University of Colorado Denver. Animals were acclimatized for one week prior to NM exposure. All experiments were performed according to the approved animal protocol. The dorsal skin of male SKH-1 hairless mice (n=5 per group) was exposed to 3.2mg NM in 200µl acetone, and after 30 min, these mice were topically treated with 1 or 2mg of silibinin in 200µl acetone or only 200µl acetone. Control mice received only 200µl acetone or 2mg of silibinin topically in 200µl acetone. Mice were sacrificed at 24, 72 and 120h following these treatments, and dorsal skin tissue was collected and fixed in 10% formalin for histopathological and immunohistochemical analysis or snap frozen in liquid nitrogen for western blot analysis (Tewari-Singh et al., 2012; Jain et al., 2014a).
H&E staining and histopathological analysis
To prepare blocks for tissue sectioning, skin tissue was fixed in 10% formalin and processed through ascending concentrations of ethanol, cleared with xylene, and finally embedded in paraffin. The 5µm thick sections were cut using a Leica microtome, processed for H&E staining, and microscopically (Zeiss microscope, Germany) evaluated for histopathological features such as epidermal thickness, parakeratosis, epidermal denuding, epidermal death and microvesication, as described previously (Tewari-Singh et al., 2014b).
TUNEL staining
Apoptotic cell death in 5µm dorsal skin sections was detected employing TUNEL staining according to the manufacturer’ protocol as described earlier (Jain et al., 2014b). Positive apoptotic dead cells were visualized as brown nuclei after DAB (3,3′-diaminobenzidine) reaction, quantified in 10 randomly selected fields at 400× magnification, and the percent apoptotic cell death was calculated by number of apoptotic cells/total number of cells×100.
Western blot analysis
Tissue lysates were prepared by homogenizing 100mg of dorsal skin samples in tissue lysis buffer as published earlier (Jain et al., 2014b). After estimating the protein by Lowry’s method, 50µg of protein from each sample was run on SDS-PAGE and separated proteins were transferred onto nitrocellulose membrane by western blotting. Membranes were incubated with primary antibody for phospho-H2A.X Ser139 (1:500 dilution) or COX-2 (1:500 dilution) overnight at 4°C. After washing three times, membranes were incubated with appropriate secondary antibody for 1h, washed, and visualized by either enhanced chemiluminescence detection system (GE Healthcare Life Sciences, Pittsburgh, PA)/ or using Odyssey™ Infrared Imager (LI-COR Biosciences Lincoln, NE).. Equal protein loading was confirmed by stripping the membrane and reprobing for β-actin. Band density from each group was determined by densitometry analysis using image J software.
Myeloperoxidase (MPO) activity assay
A Fluoro MPO kit from Cell Technology (Mountain View, CA) was employed to detect MPO activity in the frozen skin tissue samples according to the recommended protocol as detailed earlier (Tewari-Singh et al., 2012a).
Immunohistochemistry (IHC)
Dorsal skin sections (5µm) were deparaffinized, rehydrated and incubated in sodium citrate buffer (pH 6) at sub-boiling temperature (85°C) for antigen retrieval. After blocking endogenous peroxidase activity and nonspecific binding with hydrogen peroxide and blocking solution respectively, sections were incubated overnight with rabbit polyclonal 8-oxodG antibody (1:2000 dilution) at 4°C in a humidified chamber. After washing, sections were incubated with biotin conjugated anti-rabbit antibody for 1h and then with streptavidin–HRP conjugates for another hour. Thereafter, DAB detection system was used according to the vendor’s protocol for immune-positivity reaction and color development, and slides were mounted for microscopic observation. Brown colored positive cells were counted in 15 randomly selected fields using 400× magnification and the percent of 8-oxodG positive cells were calculated using the formula as described above. All the H&E and IHC stained slides were analyzed and representative pictures were taken using a Zeiss Axioscope 2 microscope (Carl Zeiss, Inc., Germany).
Statistical analysis
Statistically significant differences between groups were determined by one-way ANOVA using SigmaStat 3.5 software (Jandel Scientific, San Rafael, CA) and the Tukey test for multiple comparisons, and a p-value of <0.05 was considered significant. Data are represented as mean±SEM from 4–5 replicate animals.
Results
Silibinin decreased NM-induced epidermal thickness, necrosis and apoptotic cell death
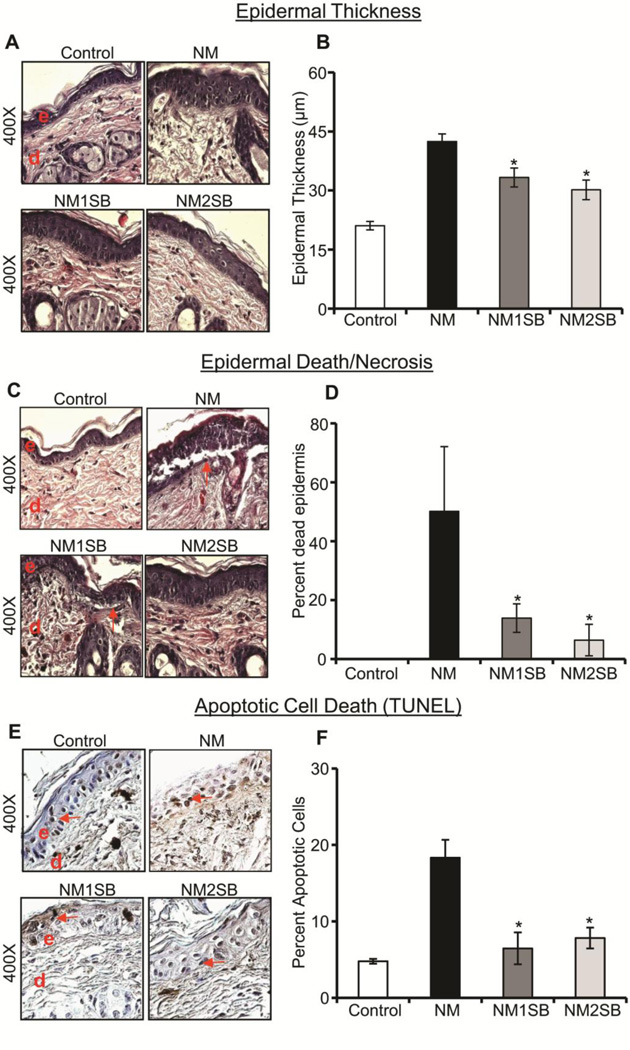
Epidermal thickness, death/necrosis and apoptotic cell death are primary injury end points that were established in our earlier studies; significantly increased epidermal thickness and apoptotic cell death were observed at an early time point (24h) and epidermal death/necrosis was observed at later time point (72h) after NM or CEES exposure of dorsal mouse skin (Jain et al., 2011b; Jain et al., 2014a). In silibinin efficacy study, its treatment at 1 or 2 mg dose per mouse at 30 min following NM exposure resulted in a strong decrease in NM-induced epidermal thickness (Fig. 1A). Quantitative analysis of H&E stained skin tissues sections showed that silibinin produced a significant dose-dependent reduction (42 and 57%, respectively) in NM-induced epidermal thickness (Fig. 1B). Similarly, silibinin also significantly reversed, in a dose-dependent manner, the NM-induced epidermal cell death in skin sections by 72% (1mg dose) and 87% (2mg dose) at 120h time point (Fig. 1C and D). Further analysis of apoptotic cell death in skin epidermis by the TUNEL staining method also showed a strong reduction in NM-induced apoptotic cell death by silibinin, as evidenced by a decrease in brown-stained nuclei (Fig. 1E). Overall, both silibinin doses significantly reversed the NM-induced apoptotic cell death by 88 and 78% (Fig. 1F). There was no significant effect on epidermal thickness, necrosis and apoptotic cell death in silibinin alone group (data not shown).
Figure 1. Effect of silibinin treatment on NM-induced epidermal thickness, death/necrosis and apoptotic cell death.
Following NM exposure and silibinin treatments, dorsal skin was used for both H&E staining and TUNEL analysis. (A) and (C) are representative pictures of epidermal thickness and epidermal death/necrosis from H&E stained skin sections, and (E) shows representative pictures from TUNEL staining at 24h. (B), (D) and (F) Depict quantitative data of epidermal thickness, epidermal death/necrosis and TUNEL positive cells, respectively. Data presented are the mean ± SEM from 4–5 animals. *, p<0.05 compared to NM exposed group. e, epidermis; d, dermis; red arrows, TUNEL positive cells.
Silibinin treatment decreased NM-induced epidermal denudation and parakeratosis
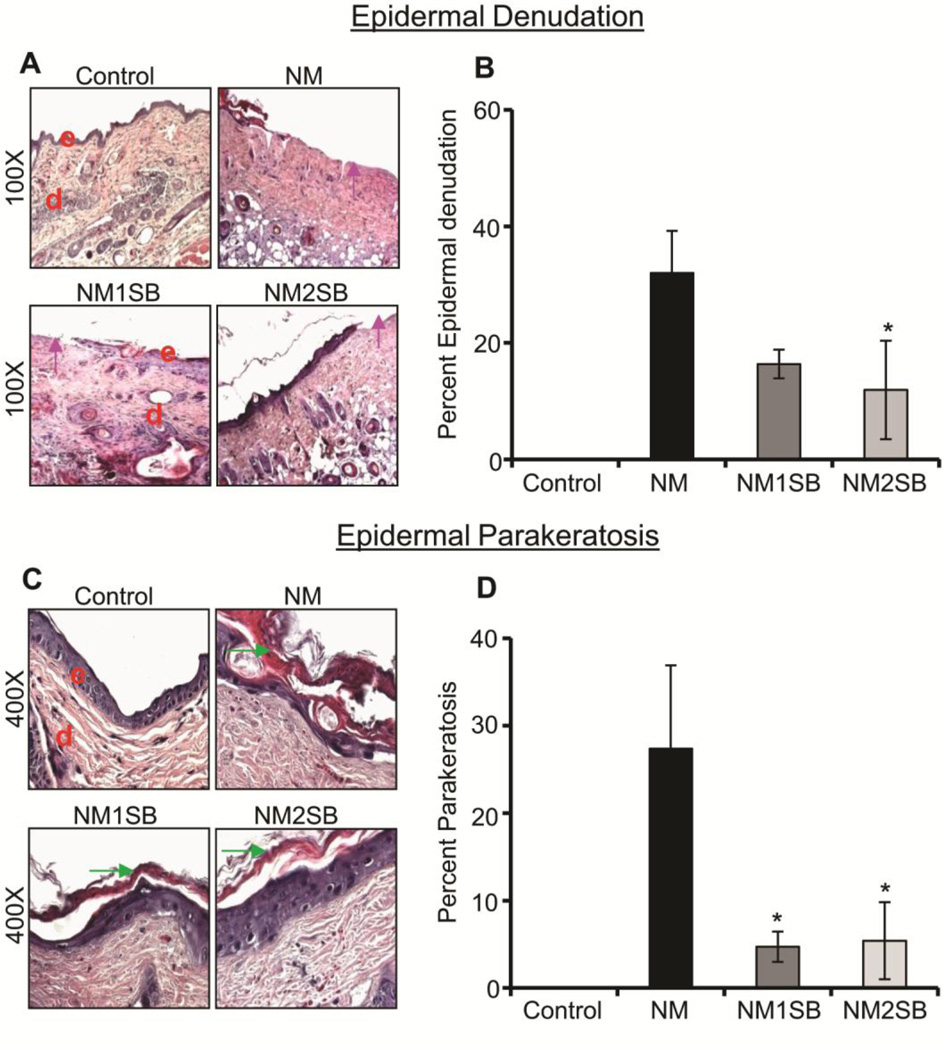
In our recently published study with NM, we have also established clinically relevant biomarkers such as epidermal denudation, epidermal parakeratosis, scab formation, hyperkeratosis and acanthosis that occur in a time-dependent manner in the SKH-1 hairless mouse model (Tewari-Singh et al., 2014b). Importantly, in the present study, silibinin treatment reversed some of these NM-induced clinically relevant biomarkers at different times after NM exposure (Fig. 2). Specifically, silibinin decreased epidermal denudation (loss of epidermis) at 72h time by 49 and 63% (Fig. 2A and B). Epidermal parakeratosis is clinically defined as abnormal retention of nucleated keratinocytes in the stratum corneum, and NM exposure induced parakeratosis in 27% of the length of the total skin section length at 72h, which was significantly reduced (80–83%) by the silibinin treatments at both doses (Fig. 2C and D). Epidermal denudation and parakeratosis effects were not observed in silibinin alone group (data not shown).
Figure 2. Effect of silibinin treatment on NM-induced epidermal denudation and parakeratosis.
H&E stained dorsal skin sections were analyzed for epidermal denudation and epidermal parakeratosis at 72h. (A) and (C) are representative pictures of epidermal denudation and epidermal parakeratosis, respectively. (B) and (D) Depict quantitative data for epidermal denudation and epidermal parakeratosis, respectively. Data presented are the mean ±SEM from 4–5 animals. *, p<0.05 compared to NM exposed group. e, epidermis; d, dermis; pink arrow, epidermal denuding; green arrow, epidermal parakeratosis.
Silibinin treatment decreased NM-induced microvesication
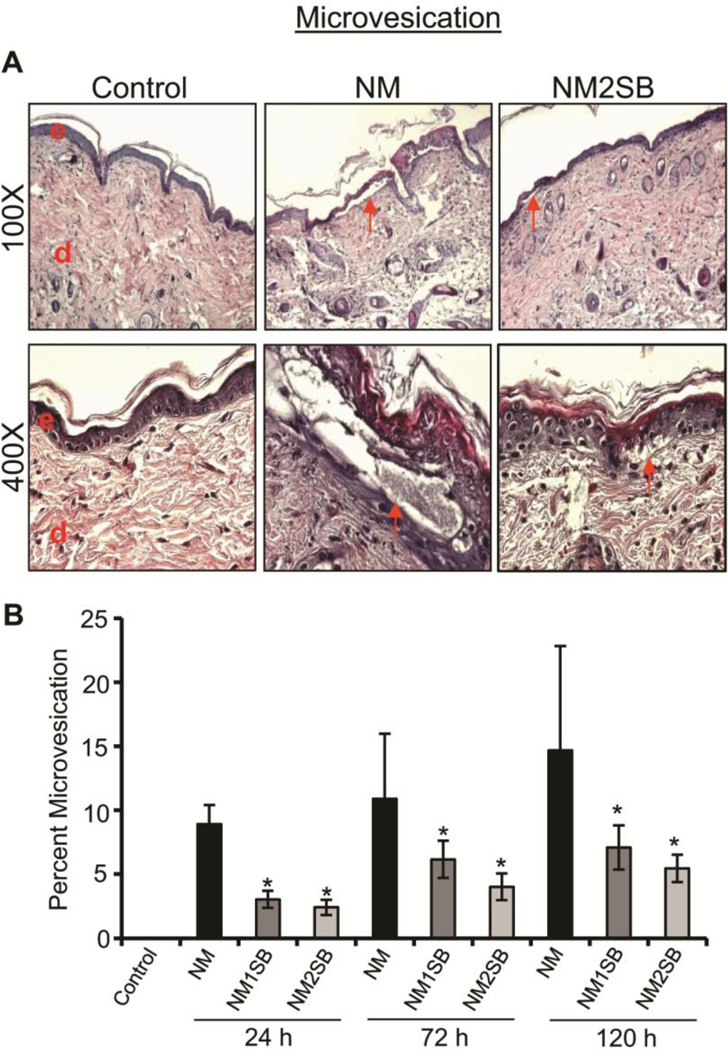
Previously studies have shown that SM causes blister formation in humans and micro-blister formation in mouse models (Shakarjian et al., 2010; Jain et al., 2011b); both are related to dermal-epidermal separation. Consistent with this, both NM and CEES exposures have been shown to result in high incidences of microvesication in SKH-1 hairless and C57BL/6 haired mice at 24, 72 and 120h after exposure (Jain et al., 2011b; Jain et al., 2014a). Accordingly, herein we quantified the extent of microvesication throughout the skin sections following NM exposure without or with silibinin treatments. As shown in Fig. 3A, NM exposure induced epidermal-dermal separation, this was inhibited by silibinin. Quantitatively, NM exposure of mouse skin resulted in 9–15% microvesication at 24–72h; however, treatments with both doses of silibinin significantly reversed the incidence of microvesication at all three time points (Fig. 3B). Microvesication was not induced in silibinin alone group (data not shown).
Figure 3. Effect of silibinin treatment on NM-induced microvesication.
H&E stained sections were analyzed for epidermal-dermal separation (microvesication). (A) Representative pictures of epidermal-dermal separation at 100X and 400X from control, NM exposed, and SB-treated groups. (B) Depicts the percent length of microvesication calculated as detailed in Materials and Methods. Data presented are the mean ± SEM from 4–5 animals. *, p<0.05 compared to NM exposed group. e, epidermis; d, dermis; red arrows, microvesication.
Silibinin treatment decreased NM-induced H2A.X phosphorylation, COX-2 level, MPO activity and oxidative DNA damage
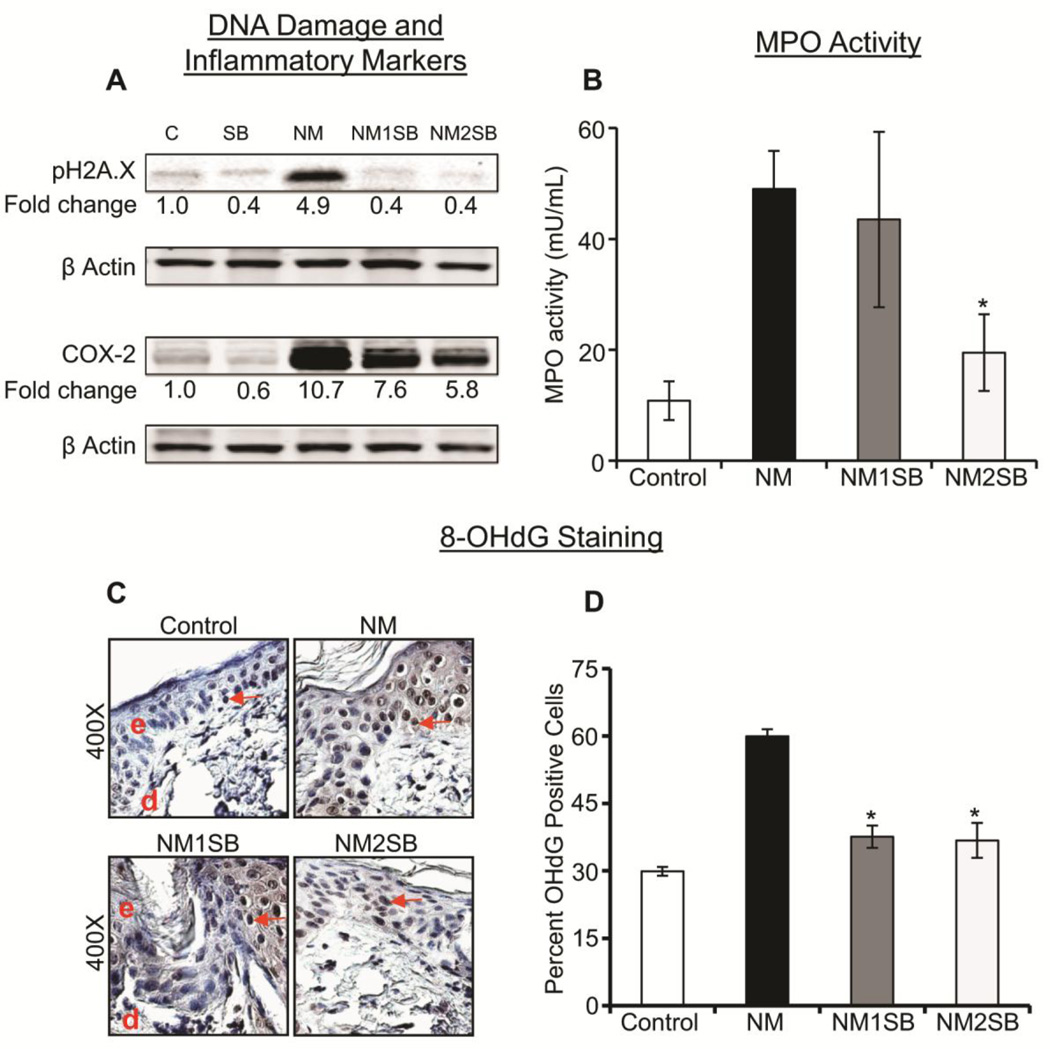
Recently, we have shown that the SM analog CEES and the primary vesicating agent NM exposures result in DNA damage as detected by an increased expression of Ser139 phospho-H2A.X as well as increased expression of inflammatory mediators such as COX-2. It was previously reported that silibinin decreases these events in CEES- and radiation-induced skin injuries (Singh and Agarwal, 2005; Jain et al., 2011a). Therefore, we also assessed the effect of silibinin on these two pathways, and found that both 1 and 2mg doses of silibinin strongly reversed NM-induced increases in the phosphorylation of pH2A.X at Ser139 at 72h after exposure (Fig. 4A). Similarly, silibinin treatments also caused 32 and 51% reversal in the NM-induced increase in COX-2 expression at 120 after exposure (Fig. 4A). MPO activity, an indicator of neutrophil infiltration and an important injury marker following vesicant exposure, as reported by us (Jain et al., 2014b; Jain et al., 2014a), was also assessed to relate inflammatory response of NM in mouse skin and silibinin effect on it. As shown in Fig. 4B, NM exposure of mouse skin resulted in a robust increase in MPO activity, which was reversed by ~50% at the higher dose (2mg) of silibinin. Silibinin alone group did not show a significant increase in MPO activity as compared to vehicle control group (data not shown). Recent studies by us and others have shown that CEES and SM exposures result in oxidative DNA damage (DNA oxidation) in epidermal cell lines and in mouse skin, and that silibinin was very effective in attenuating CEES and other agents caused oxidative stress and subsequent oxidative DNA damage (Tewari-Singh et al., 2012). Consistent with these findings, in the presents study, we found that NM exposure results in a strong oxidative DNA damage, analyzed by IHC for the expression of 8-oxodG (a marker for oxidative DNA damage), and that silibinin treatments at 1 and 2mg doses resulted in 74–77% reversal in NM-induced increases in DNA oxidation, as measured by detection of 8-oxodG positive cells (Fig. 4C and D).
Figure 4. Effect of silibinin treatment on NM-induced H2A.X phosphorylation, COX-2 level, MPO activity and oxidative DNA damage.
Dorsal skin samples were collected following various treatments, and employed for western blot and immunohistochemical analyses. (A) Tissue lysates were analyzed by western blotting to observe the expression of Ser139 phospho-H2A.X at 72h and COX-2 at 120h post NM exposure, as detailed in Materials and Methods. For loading control, membranes were stripped and reprobed for β-actin. The intensity of bands was analyzed by densitometric analysis using image J software. (B) The frozen tissue from 120h time point was subjected to MPO assay as detailed under materials and methods. Oxidative DNA damage was visualized at 24h; as 8-oxodG staining by IHC analysis as detailed in Materials and Methods. (C) Representative images of 8-oxodG IHC staining, along with (D) quantitative data for 8-oxodG positive cells. Data presented are the mean ± SEM from 4–5 animals. *, p<0.05 compared to NM exposed group. e, epidermis; d, dermis; red arrows, 8-oxodG positive cells.
Discussion
Cutaneous manifestations of vesicants exposure include edema, blister formation, ulceration, necrosis and desquamation (Momeni et al., 1992; Smith et al., 1998), and currently, decontamination is the best available intervention for skin injury (Casillas et al., 2000; Graham et al., 2009). Previous reports from our laboratory and other investigators have shown that SM, CEES or NM exposure causes skin injury by triggering complex signal transduction pathways related to DNA damage, cell death, oxidative stress, apoptosis and inflammation (Jowsey et al., 2009; Kehe et al., 2009; Pal et al., 2009; Tewari-Singh et al., 2010; Inturi et al., 2011; Jain et al., 2011a). These recent advances in our understanding of the various molecular mechanisms of vesicants-induced skin injuries suggest that, to develop effective antidotes, agents are needed that target multiple mechanisms and pathways. Consistent with this notion, the results of the present study clearly showed that through its pleiotropic mechanisms of action, silibinin strongly reverses the toxic effects of exposure to the primary vesicant NM. Notably, silibinin has already been shown efficacious in CEES-induced skin toxicity through targeting cellular signaling pathways including DNA damage, oxidative stress and inflammation (Tewari-Singh et al., 2012). Furthermore, silibinin has also shown its strong therapeutic effects against radiation-induced skin damages including skin cancer by targeting multiple signal transduction pathways (Dhanalakshmi et al., 2005; Narayanapillai et al., 2014). Corroborating these previous findings, our results herein show that silibinin inhibits NM-caused DNA damage and other skin toxicity manifestations including inflammation by targeting multiple pathways.
SM has been the most widely used chemical warfare agent, resulting in extensive casualties in both soldiers and civilians (Khateri et al., 2003; Kehe and Szinicz, 2005). It is a highly reactive compound, reacting with macromolecules of the cell to cause injury in multiple organs. Being a strong alkylating agent, SM causes DNA damage and double strand breaks, thereby causing cytotoxicity. Extensive DNA damage subsequently activates the DNA repair pathway, cell cycle arrest, and/or apoptosis and necrosis (Rosenthal et al., 1998). In the present study, we employed primary vesicating agent, NM, as a prototype of SM for laboratory studies. Our results show that, similar to SM, NM exposure of mouse skin induced H2A.X phosphorylation at ser139 suggesting cellular DNA damage, which is strongly reversed by silibinin treatments (Jain et al., 2011a). Previous studies have also suggested in addition to direct DNA damage, oxidative stress is involved in DNA damage resulting from CEES or NM exposure (Inturi et al., 2011; Jain et al., 2011a; Tewari-Singh et al., 2014a). Consistent with these results, we observed drastically increased levels of 8-oxodG in NM-exposed mouse skin samples; these increases were reversed by silibinin. Together, these results suggest that in addition to its direct DNA damaging effect, NM causes oxidative stress leading to oxidative DNA damage, and that both of these damages might be responsible of the observed H2A.X phosphorylation at ser139. Furthermore, these results suggest that the silibinin inhibition of NM-induced oxidative stress may be the cause for the observed reduction in H2A.X phosphorylation at ser139. Whether silibinin also attenuates the direct DNA-damaging effects of NM in mouse skin, remains to be studied in future studies.
In addition to its DNA damaging effects, we also observed that NM exposure causes various other injuries and pathological changes in the epidermis including epidermal thickness, epidermal-dermal separation, parakeratosis, denudation, necrosis and apoptosis, similar to those reported following SM exposure (Naraghi et al., 2005; Ghabili et al., 2010). Importantly, silibinin treatments strongly reversed all of these NM-induced skin pathologies. One possible explanation could be that following its skin exposure, NM-caused DNA damage results in skin cell death by various mechanisms and pathways leading to the observed pathological condition. On the other hand, silibinin, by inhibiting NM-caused DNA damage, prevents skin cell death and thus rescues skin from NM-induced toxic injuries. NM exposure is also known to activate inflammatory and vesicant mediators such as COX-2 and MMP-9 in skin, and these potentially play key roles in skin inflammation and blister formation. Consistent with these properties, silibinin reversed NM-induced inflammation by decreasing COX-2 expression, neutrophil infiltration, and MPO activity. In addition, silibinin treatment also decreased microvesication (epidermal-dermal separation), which is a prominent lesion after SM exposure (Tewari-Singh et al., 2012). In relation to its protective effect against this process, silibinin is also known to target vesicant-induced MMP-9 activation (Tewari-Singh et al., 2012). In conclusion, the results presented here demonstrate that silibinin is an effective treatment option for the rescue of vesicants-induced skin toxicity/injury by targeting signaling pathways related to DNA damage, apoptosis, inflammation and oxidative stress. Accordingly, in its further development, silibinin should be tested and optimized against SM-induced skin injury.
Highlights.
Silibinin treatment attenuated nitrogen mustard (NM)-induced skin injury.
Silibinin affects pathways associated with DNA damage, inflammation and vesication.
The efficacy of silibinin could also be associated with oxidative stress.
These results support testing and optimization of silibinin against SM-induced skin injury.
Acknowledgement
This work was supported by the Countermeasures Against Chemical Threats (CounterACT) Program, Office of the Director National Institutes of Health (OD) and the National Institute of Environmental Health Sciences (NIEHS), [Grant Number U54 ES015678]. The study sponsor (NIH) had no involvement in the study design; collection, analysis and interpretation of data; the writing of the manuscript; and the decision to submit the manuscript for publications.
Abbreviations
- 8-oxodG
8-oxo-7,8-dihydro-2ʹ-deoxyguanosine
- NM
nitrogen mustard
- IHC
immunohistochemistry
- MPO
myeloperoxidase
- SM
sulfur mustard
- COX-2
cyclooxygenase-2
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- CEES
2-chloroethylethyl sulfide
- AEOL 10150
Mn(III) tetrakis(N,Nʹ-diethylimidizolium-2-yl)porphyrin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benson JM, Seagrave J, Weber WM, Santistevan CD, Grotendorst GR, Schultz GS, March TH. Time course of lesion development in the hairless guinea-pig model of sulfur mustard-induced dermal injury. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2011;19:348–357. doi: 10.1111/j.1524-475X.2011.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casillas RP, Kiser RC, Truxall JA, Singer AW, Shumaker SM, Niemuth NA, Ricketts KM, Mitcheltree LW, Castrejon LR, Blank JA. Therapeutic approaches to dermatotoxicity by sulfur mustardIModulaton of sulfur mustard-induced cutaneous injury in the mouse ear vesicant model. J Appl Toxicol. 2000;20(Suppl 1):S145–S151. doi: 10.1002/1099-1263(200012)20:1+<::aid-jat665>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Chang YC, Wang JD, Hahn RA, Gordon MK, Joseph LB, Heck DE, Heindel ND, Young SC, Sinko PJ, Casillas RP, Laskin JD, Laskin DL, Gerecke DR. Therapeutic potential of a non-steroidal bifunctional anti-inflammatory and anti-cholinergic agent against skin injury induced by sulfur mustard. Toxicol Appl Pharmacol. 2014;280:236–244. doi: 10.1016/j.taap.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dachir S, Fishbeine E, Meshulam Y, Sahar R, Chapman S, Amir A, Kadar T. Amelioration of sulfur mustard skin injury following a topical treatment with a mixture of a steroid and a NSAID. J Appl Toxicol. 2004;24:107–113. doi: 10.1002/jat.955. [DOI] [PubMed] [Google Scholar]
- Dhanalakshmi S, Agarwal C, Singh RP, Agarwal R. Silibinin up-regulates DNA-protein kinase-dependent p53 activation to enhance UVB-induced apoptosis in mouse epithelial JB6 cells. J Biol Chem. 2005;280:20375–20383. doi: 10.1074/jbc.M414640200. [DOI] [PubMed] [Google Scholar]
- Ferenci P, Dragosics B, Dittrich H, Frank H, Benda L, Lochs H, Meryn S, Base W, Schneider B. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989;9:105–113. doi: 10.1016/0168-8278(89)90083-4. [DOI] [PubMed] [Google Scholar]
- Firooz A, Sadr B, Davoudi SM, Nassiri-Kashani M, Panahi Y, Dowlati Y. Long-term skin damage due to chemical weapon exposure. Cutaneous and ocular toxicology. 2011;30:64–68. doi: 10.3109/15569527.2010.529547. [DOI] [PubMed] [Google Scholar]
- Ghabili K, Agutter PS, Ghanei M, Ansarin K, Shoja MM. Mustard gas toxicity: the acute and chronic pathological effects. Journal of applied toxicology : JAT. 2010;30:627–643. doi: 10.1002/jat.1581. [DOI] [PubMed] [Google Scholar]
- Graham JS, Chilcott RP, Rice P, Milner SM, Hurst CG, Maliner BI. Wound healing of cutaneous sulfur mustard injuries: strategies for the development of improved therapies. J Burns Wounds. 2005;4:e1. [PMC free article] [PubMed] [Google Scholar]
- Graham JS, Stevenson RS, Mitcheltree LW, Hamilton TA, Deckert RR, Lee RB, Schiavetta AM. Medical management of cutaneous sulfur mustard injuries. Toxicology. 2009;263:47–58. doi: 10.1016/j.tox.2008.07.067. [DOI] [PubMed] [Google Scholar]
- Inturi S, Tewari-Singh N, Gu M, Shrotriya S, Gomez J, Agarwal C, White CW, Agarwal R. Mechanisms of sulfur mustard analog 2-chloroethyl ethyl sulfide-induced DNA damage in skin epidermal cells and fibroblasts. Free Radic Biol Med. 2011;51:2272–2280. doi: 10.1016/j.freeradbiomed.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Tewari-Singh N, Gu M, Inturi S, White CW, Agarwal R. Sulfur mustard analog, 2-chloroethyl ethyl sulfide-induced skin injury involves DNA damage and induction of inflammatory mediators, in part via oxidative stress, in SKH-1 hairless mouse skin. Toxicol Lett. 2011a;205:293–301. doi: 10.1016/j.toxlet.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Tewari-Singh N, Inturi S, Orlicky DJ, White CW, Agarwal R. Histopathological and immunohistochemical evaluation of nitrogen mustard-induced cutaneous effects in SKH-1 hairless and C57BL/6 mice. Exp Toxicol Pathol. 2014a;66:129–138. doi: 10.1016/j.etp.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Tewari-Singh N, Inturi S, Orlicky DJ, White CW, Agarwal R. Myeloperoxidase deficiency attenuates nitrogen mustard-induced skin injuries. Toxicology. 2014b;320:25–33. doi: 10.1016/j.tox.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Tewari-Singh N, Orlicky DJ, White CW, Agarwal R. 2-Chloroethyl ethyl sulfide causes microvesication and inflammation-related histopathological changes in male hairless mouse skin. Toxicology. 2011b;282:129–138. doi: 10.1016/j.tox.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jowsey PA, Williams FM, Blain PG. DNA damage, signalling and repair after exposure of cells to the sulphur mustard analogue 2-chloroethyl ethyl sulphide. Toxicology. 2009;257:105–112. doi: 10.1016/j.tox.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Kavitha CV, Deep G, Gangar SC, Jain AK, Agarwal C, Agarwal R. Silibinin inhibits prostate cancer cells- and RANKL-induced osteoclastogenesis by targeting NFATc1, NFkappaB, and AP-1 activation in RAW264.7 cells. Mol Carcinog. 2014;53:169–180. doi: 10.1002/mc.21959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehe K, Balszuweit F, Steinritz D, Thiermann H. Molecular toxicology of sulfur mustard-induced cutaneous inflammation and blistering. Toxicology. 2009;263:12–19. doi: 10.1016/j.tox.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Kehe K, Szinicz L. Medical aspects of sulphur mustard poisoning. Toxicology. 2005;214:198–209. doi: 10.1016/j.tox.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Khateri S, Ghanei M, Keshavarz S, Soroush M, Haines D. Incidence of lung, eye, and skin lesions as late complications in 34,000 Iranians with wartime exposure to mustard agent. J Occup Environ Med. 2003;45:1136–1143. doi: 10.1097/01.jom.0000094993.20914.d1. [DOI] [PubMed] [Google Scholar]
- Laskin JD, Black AT, Jan YH, Sinko PJ, Heindel ND, Sunil V, Heck DE, Laskin DL. Oxidants and antioxidants in sulfur mustard-induced injury. Annals of the New York Academy of Sciences. 2010;1203:92–100. doi: 10.1111/j.1749-6632.2010.05605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momeni AZ, Enshaeih S, Meghdadi M, Amindjavaheri M. Skin manifestations of mustard gas. A clinical study of 535 patients exposed to mustard gas. Arch Dermatol. 1992;128:775–780. [PubMed] [Google Scholar]
- Naraghi ZS, Mansouri P, Mortazavi M. A clinicopathological study on acute cutaneous lesions induced by sulfur mustard gas (yperite) European journal of dermatology : EJD. 2005;15:140–145. [PubMed] [Google Scholar]
- Narayanapillai S, Agarwal C, Deep G, Agarwal R. Silibinin inhibits ultraviolet B radiation-induced DNA-damage and apoptosis by enhancing interleukin-12 expression in JB6 cells and SKH-1 hairless mouse skin. Mol Carcinog. 2014;53:471–479. doi: 10.1002/mc.22000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyska A, Lomnitski L, Maronpot R, Moomaw C, Brodsky B, Sintov A, Wormser U. Effects of iodine on inducible nitric oxide synthase and cyclooxygenase-2 expression in sulfur mustard-induced skin. Archives of toxicology. 2001;74:768–774. doi: 10.1007/s002040000199. [DOI] [PubMed] [Google Scholar]
- Pal A, Tewari-Singh N, Gu M, Agarwal C, Huang J, Day BJ, White CW, Agarwal R. Sulfur mustard analog induces oxidative stress and activates signaling cascades in the skin of SKH-1 hairless mice. Free Radic Biol Med. 2009;47:1640–1651. doi: 10.1016/j.freeradbiomed.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pares A, Planas R, Torres M, Caballeria J, Viver JM, Acero D, Panes J, Rigau J, Santos J, Rodes J. Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double-blind, randomized and multicenter trial. J Hepatol. 1998;28:615–621. doi: 10.1016/s0168-8278(98)80285-7. [DOI] [PubMed] [Google Scholar]
- Paromov V, Suntres Z, Smith M, Stone WL. Sulfur mustard toxicity following dermal exposure: role of oxidative stress, and antioxidant therapy. J Burns Wounds. 2007;7:e7. [PMC free article] [PubMed] [Google Scholar]
- Rosenthal DS, Simbulan-Rosenthal CM, Iyer S, Spoonde A, Smith W, Ray R, Smulson ME. Sulfur mustard induces markers of terminal differentiation and apoptosis in keratinocytes via a Ca2+-calmodulin and caspase-dependent pathway. The Journal of investigative dermatology. 1998;111:64–71. doi: 10.1046/j.1523-1747.1998.00250.x. [DOI] [PubMed] [Google Scholar]
- Sabourin CL, Danne MM, Buxton KL, Casillas RP, Schlager JJ. Cytokine, chemokine, and matrix metalloproteinase response after sulfur mustard injury to weanling pig skin. J Biochem Mol Toxicol. 2002;16:263–272. doi: 10.1002/jbt.10050. [DOI] [PubMed] [Google Scholar]
- Shakarjian MP, Heck DE, Gray JP, Sinko PJ, Gordon MK, Casillas RP, Heindel ND, Gerecke DR, Laskin DL, Laskin JD. Mechanisms mediating the vesicant actions of sulfur mustard after cutaneous exposure. Toxicol Sci. 2010;114:5–19. doi: 10.1093/toxsci/kfp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RP, Agarwal R. Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur J Cancer. 2005;41:1969–1979. doi: 10.1016/j.ejca.2005.03.033. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Smith WJ, Hamilton T, Skelton HG, Graham JS, Okerberg C, Moeller R, Hackley BE., Jr Histopathologic and immunohistochemical features in human skin after exposure to nitrogen and sulfur mustard. The American Journal of dermatopathology. 1998;20:22–28. doi: 10.1097/00000372-199802000-00005. [DOI] [PubMed] [Google Scholar]
- Tewari-Singh N, Agarwal C, Huang J, Day BJ, White CW, Agarwal R. Efficacy of glutathione in ameliorating sulfur mustard analog-induced toxicity in cultured skin epidermal cells and in SKH-1 mouse skin in vivo. J Pharmacol Exp Ther. 2011;336:450–459. doi: 10.1124/jpet.110.173708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Gu M, Agarwal C, White CW, Agarwal R. Biological and molecular mechanisms of sulfur mustard analogue-induced toxicity in JB6 and HaCaT cells: possible role of ataxia telangiectasia-mutated/ataxia telangiectasia-Rad3-related cell cycle checkpoint pathway. Chem Res Toxicol. 2010;23:1034–1044. doi: 10.1021/tx100038b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Inturi S, Jain AK, Agarwal C, Orlicky DJ, White CW, Agarwal R, Day BJ. Catalytic antioxidant AEOL 10150 treatment ameliorates sulfur mustard analog 2-chloroethyl ethyl sulfide-associated cutaneous toxic effects. Free radical biology & medicine. 2014a;72:285–295. doi: 10.1016/j.freeradbiomed.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Jain AK, Inturi S, Agarwal C, White CW, Agarwal R. Silibinin attenuates sulfur mustard analog-induced skin injury by targeting multiple pathways connecting oxidative stress and inflammation. PLoS One. 2012;7:e46149. doi: 10.1371/journal.pone.0046149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Jain AK, Inturi S, White CW, Agarwal R. Clinically-relevant cutaneous lesions by nitrogen mustard: useful biomarkers of vesicants skin injury in SKH-1 hairless and C57BL/6 mice. PloS one. 2013;8:e67557. doi: 10.1371/journal.pone.0067557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Jain AK, Orlicky DJ, White CW, Agarwal R. Cutaneous injury-related structural changes and their progression following topical nitrogen mustard exposure in hairless and haired mice. PloS one. 2014b;9:e85402. doi: 10.1371/journal.pone.0085402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattana M, Bey T. Mustard gas or sulfur mustard: an old chemical agent as a new terrorist threat. Prehosp Disaster Med. 2009;24:19–29. doi: 10.1017/s1049023x0000649x. discussion 30-11. [DOI] [PubMed] [Google Scholar]