Abstract
Inflammation is an integral component of autoimmune arthritis. The balance of pathogenic T helper 17 (Th17) and protective T regulatory (Treg) cells can influence disease severity, and its resetting offers an attractive approach to control autoimmunity. We determined the frequency of Th17 and Treg in the joints of rats with adjuvant arthritis (AA), a model of rheumatoid arthritis (RA). We also investigated the impact of Celastrol, a bioactive compound from the traditional Chinese medicine Celastrus that can suppress AA, on Th17/Treg balance in the joints. Celastrol treatment reduced Th17 cells but increased Treg in the joints, and it inhibited Th17 differentiation but promoted Treg differentiation in vitro by blocking the activation of pSTAT3. Furthermore, Celastrol limited the production of Th17-differentiating cytokines and chemokines (CCL3, CCL5). Thus, Celastrol suppressed arthritis in part by altering Th17/Treg ratio in inflamed joints, and it should be tested as a potential adjunct/alternative for RA therapy.
1. INTRODUCTION
Chronic inflammation is a hallmark of autoimmune diseases such as rheumatoid arthritis (RA), which is characterized by inflammatory cell infiltration into the synovium, synovial hyperplasia, angiogenesis, and cartilage and bone damage [1; 2; 3]. A variety of anti-inflammatory and disease-modifying anti-rheumatic drugs are available for the treatment of RA, but their prolonged use is frequently associated with severe adverse reactions. The new category of drugs, the biologics, such as antibodies and/or decoy receptors aimed at neutralizing the pro-inflammatory cytokines such as TNF-α and IL-6 have made a major impact on the management of RA [4; 5; 6]. However, about 30–40% of patients either fail to respond or become unresponsive over time to these newer medications, and there is increased risk of infections in patients treated with biologics. In addition, biologics are expensive. Thus, newer anti-inflammatory and antiarthritic therapeutic products are being sought. Natural products belonging to the traditional systems of medicine represent a promising resource in this regard [7]. However, for acceptance into the mainstream therapy, it is imperative that the mechanisms of action of herbal products for treatment of autoimmune diseases are better defined in context of the contemporary immune parameters.
The T cells play an important role in the disease process in autoimmunity: the T helper 17 cells (Th17) drives pathogenic inflammation [8; 9], whereas the T regulatory cells (Treg) have been shown to protect against autoimmune diseases [10; 11]. Two major challenges remain to be further addressed in autoimmunity: first, defining the dynamics of the cellular immune responses in the target organ, particularly the relative frequency of Th17 and Treg and the resulting Th17/Treg balance; and second, identifying novel therapeutic agents that can revert an imbalance between Th17 and Treg in the target organ.
In this study, we have examined the above-stated issues using Celastrol, a bioactive component of the traditional Chinese medicine Celastrus aculeatus Merr [12], in the rat adjuvant-induced arthritis (AA) model of human RA [13]. IL-17 plays a vital role in the pathogenesis of AA [13]. However, little is known about the relative frequency of Th17 and Treg in arthritic joints in rats with AA and the influence of anti-arthritic agents on these cellular parameters. We have previously shown that Celastrol possesses anti-arthritic activity as tested in the rat AA model [14]. Furthermore, it can inhibit IL-6 production and pSTAT3 activation implying that it might influence Th17 differentiation [14]. Accordingly, we hypothesized that Celastrol limits the progression of arthritis in part by altering the Th17/Treg balance in the target organ to facilitate immune regulation. In addition, Celastrol may influence T cell activation and cellular migration into the joints. Our results support these propositions.
2. MATERIALS AND METHODS
2.1 Induction and evaluation of adjuvant arthritis (AA)
Five week old inbred Lewis (RT.1l) rats (Harlan Laboratories, Inc.) were immunized subcutaneously (s.c.) at the base of the tail with 1 mg/rat heat-killed Mycobacterium tuberculosis H37Ra (Mtb) (Difco) in oil. The severity of arthritis was graded on the basis of erythema and swelling of the paws as described previously [13; 14].
2.2 Treatment of arthritic rats with Celastrol
Lyophilized Celastrol (EMD Millipore) was dissolved in dimethylsulfoxide (DMSO), diluted in PBS (6 µl of stock in 500 µl of PBS), and injected into arthritic rats (1 mg/kg/d) intraperitoneally (i.p.) from the onset of AA (about d 10) to d 18 as described in our previous study [14]. The corresponding control group received the vehicle, DMSO (1.2%) in PBS. (For simplicity, this vehicle is referred to as PBS.) All rats were evaluated regularly for the severity of arthritis.
2.3 Flow cytometric analysis of the target organ-infiltrating cells in rats with AA
The synovium-infiltrating cells (SIC) from arthritic Lewis rats treated with the vehicle (control) or Celastrol (test) were cultured in RPMI 1640 (Quality Biologics) supplemented with 10% fetal bovine serum (FBS), 1% L-glutamine, 1% penicillin/ streptomycin (all from Invitrogen), and 0.1% β-mercaptoethanol (Sigma). For cytokine measurements, cells were stimulated for 5 h with 50 ng/mL PMA and 2 µM ionomycin in the presence of Brefeldin A (BD GolgiPlug). Cells were then treated with 10% normal rat serum to block non-specific binding sites. Thereafter, the T cells were surface-stained with anti-CD3 APC, anti-CD4 PerCP eFlour 710, anti-CD8β FITC, and/or anti-CD25 PE (all from eBioscience). Antigen-presenting cells (APCs) and neutrophils were surface-stained with anti-CD45 APC, anti-CD11b/c PerCP eFlour 710, anti-rat granulocyte marker FITC, anti-MHCII FITC, anti-CD80 PE (all from eBioscience), and/or anti-CD86 PE (BD). For intracellular staining, cells were fixed and permeabilized using the BD Fixation/Permeabilization Kit (BD Bioscience) and stained using anti-rat/mouse IL-17A eFlour 450 (eBioscience) and anti-IFN-γ PE (Biolegend). Foxp3 was stained using anti-mouse/rat Foxp3 eFlour 450 (eBioscience) in Foxp3 buffer (eBioscience). Flow cytometric data was acquired on LSRII (BD Bioscience) with gating set on isotype controls, and analysis was performed with FlowJo software (Tree Star). Cell numbers were calculated from frequencies using live cell counts by Trypan blue dye and a hemocytometer.
2.4 CD4+ T cell differentiation assay
Naïve CD4+ T cells were isolated by disrupting spleens of 6- to 12-wk-old C57BL/6 mice. Cells were cultured in RPMI supplemented with 10% fetal calf serum, 2 mM glutamine, 100 iu/mL penicillin, 0.1 mg/mL streptomycin, and 2 µM β-mercaptoethanol. The T cells were purified with a Mouse CD4+ T Cell Isolation Kit with an AutoMacs (Miltenyi Biotec). Naive T cells were obtained by surface staining with anti-CD4, anti-CD62L, anti-CD44, and anti-CD25 (BD Bioscience). The CD4+CD62L+CD44−CD25− population was isolated by flow cytometric cell sorting with a Mo-Flo cell sorter (Dako). Cells were activated by plate-bound anti-CD3 (5 µg/mL) and soluble anti-CD28 (5 µg/mL) (BioXCell) for 3 d and cultured either under neutral conditions with no exogenous cytokines and anti-cytokine antibodies (Th0 conditions) or in the presence of polarizing cytokines. For Th17-generating conditions, cell cultures were supplemented with IL-6 (20 ng/ml), TGF-β-1 (2.5 ng/ml), anti-IFN-γ (10 µg/ml), and anti-IL-4 (10 µg/ml). For Treg-generating conditions, cultures were supplemented with TGF-β-1 (2.5 ng/ml) and IL-2 (10 ng/mL) with/ without anti-IL-6 (10 µg/ml). Celastrol was added to cells at the indicated concentrations. All cytokines were obtained from R&D Systems and all anti-cytokine antibodies were obtained from BioXCell. In brief, cells were stimulated for 2 h with PMA (50ng/mL) and ionomycin (500ng/mL, Sigma Aldrich) with the addition of brefeldin A (GolgiPlug; BD).
2.5 Quantitative real-time PCR for cytokines
Total RNA was prepared cells using TRIzol reagent following the method described earlier [14]. Then cDNA was prepared from RNA and amplified in an ABI PRISM 7900HT cycler using SYBR Green PCR Master Mix and appropriate primers. The mRNA levels were normalized to those of the hypoxanthine-guanine phosphoribosyltransferase (HPRT) gene, and the relative gene expression levels were determined.
2.6 Western blot analysis for cytokines and cytokine signaling
For detection of IL-1β, spleen adherent cells (SAC) from arthritic rats treated with Celastrol (test) or vehicle (control) were stimulated ex vivo with or without Mtb sonicate (10 µg/ml) for 6 h in a 6-well plate [14]. For in vitro treatment, SAC were stimulated in the presence or absence of Celastrol. The cell lysate was subjected to electrophoretic resolution and then transferred onto a PVDF membrane [14]. After blocking, the membrane was probed sequentially with rabbit anti-rat IL-1β and HRP-conjugated secondary anti-rabbit antibody. GAPDH was used as a protein- loading control. Protein bands were visualized with a chemiluminescent substrate and subjected to densitometry.
To determine IL-6-induced signaling via the JAK/STAT pathway, CD4+ T cells were isolated from inbred C57BL/6 mouse spleens using the EasySep CD4+ T cell isolation kit (Stemcell Technologies). Cells were cultured (2 × 106/mL) in the presence or absence of 300 nM Celastrol at 37°C for 8 h. Recombinant IL-6 (2 ng/mL) was added to the culture and incubated at 37°C in a water bath for 0, 15, 30, 60, or 90 min. Cell lysates were collected and intracellular levels of phosphorylated STAT proteins were identified by blotting with antibodies against pSTAT3, STAT3, pSTAT5, STAT5 (Cell Signaling Technologies).
2.7 Multiplex analysis for chemokines and cytokines
The preparation of synovium-infiltrating cells (SIC) from Celastrol-treated and vehicle-treated rats and their cultivation is described elsewhere [14]. The culture supernate was analyzed for cytokines (IL-1β and IL-6) and chemokines (CCL3 (MIP-1α) and CCL5 (RANTES)) using a Multiplex assay (Cytokine Core Facility, University of Maryland School of Medicine). The assays were read using the Luminex 100 system following the manufacturer’s instructions.
2.8 In vitro cell migration assay
Primary lymphocytes derived from the draining lymph nodes were suspended in serum-free RPMI medium (without any supplements) and then placed (2 × 106 cells/mL) in the top well of a Transwell chamber (6-well with an 8.0-µm pore size, Corning). RPMI containing 50 ng/ml chemokine (C-C motif) ligand 3 (CCL3; MIP-1α) or 5 (CCL5; RANTES) (Gemini Bio-Products) in the presence or absence of Celastrol (100, 200 and 300nM) was placed in the lower compartment. Cells were allowed to migrate through the membrane for 24 h at 37° C in an atmosphere of 5% CO2 and 95% air. Thereafter, the top chambers were discarded, and the migrated cells in the bottom chamber were quantified using a 1/10 dilution with Trypan Blue and a hemocytometer. The migration of cells in response to CCL3/ CCL5 in the presence of Celastrol was compared with that with the vehicle (control).
2.9 Lymph node cell (LNC) proliferation assay
A single-cell suspension of the draining LNCs of Celastrol- and vehicle-treated arthritic rats was prepared as described elsewhere [14]. These cells were cultured in serum-free HL-1 followed by pulsing with 1 μCi of [3H]-thymidine. The results were expressed as counts per minute (cpm).
2.10 NF-kB luciferase assay
NF-kB-induced gene induction was analyzed by performing a luciferase reporter assay in HEK293T cells. An expression construct containing Firefly luciferase and a promoter containing three copies of the NF-kB binding site (NF-kB luciferase) was used. Transfections of reporter plasmids into HEK293T cells were performed with the lipofectamine LTX (Invitrogen) transfection reagent. For all transfections, 200 ng of luciferase plasmid was used in each well of a 48-well plate with 1 µl of Lipofectamine LTX (Invitrogen). At 18 h post-transfection, cells were cultured with indicated concentrations of Celastrol or vehicle for 2 h prior to stimulation with 10 ng TNF-α (Sigma). After 6 h, cells were lysed and assayed for luciferase expression using Dual luciferase reporter system per manufacturer’s instructions (Promega). The percent reduction over the vehicle control was plotted in each figure. All transfections were performed in triplicate.
2.11 Statistical analysis
Results are expressed as mean ± SEM as calculated by GraphPad Prism 6 (GraphPad Software). Differences in the mean values were analyzed using a Student’s t test or one-way analysis of variance (ANOVA) for multiple comparisons. Significance was set as P<0.05.
3. RESULTS
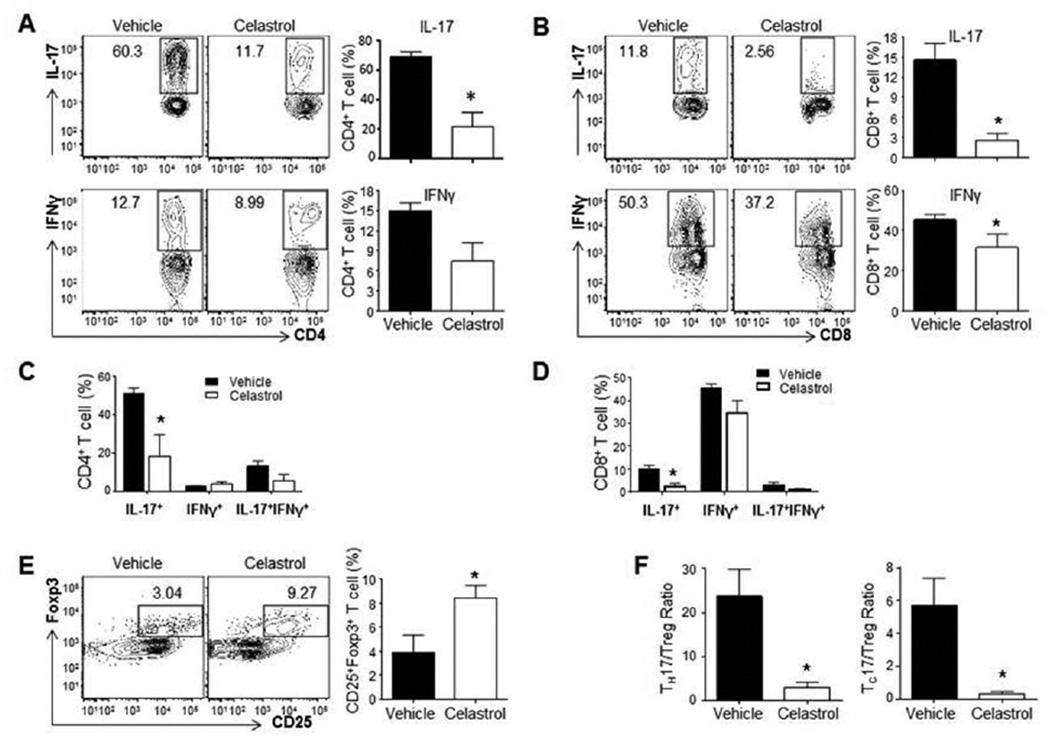
We determined the phenotype of defined subsets of T cells in the target organ (the inflamed joints) of Celastrol-treated versus vehicle-treated (control) arthritic rats. The dose of Celastrol used in these experiments is effective in modulating AA as reported in our previous study [14]. The number of CD4+ T cells producing IL-17 was significantly reduced from an average of 68.8% in vehicle-treated rats to 21.8% in Celastrol-treated animals (n=8, p<0.05) (Figure 1A, top right). This reduction was also seen in CD8+ T cells producing IL-17; an average reduction from 15.5% to 2.8% (p<0.05) for vehicle- to Celastrol-treated rats, respectively (Figure 1B, top right). We also observed a reduction in IFN-γ-producing CD4+ T cells in the synovial tissue in Celastrol-treated rats compared with control rats, but this difference was not significant (Figure 1A, bottom right). A reduction in IFN-γ+ CD8+ T cells was also seen in SIC of vehicle-treated over Celastrol-treated rats, 45.4% and 31.5%, respectively (p<0.05) (Figure 1B, bottom right). In the case of both CD4 and CD8 T cells, Celastrol-induced reduction in IL-17 production was primarily owing to a decrease in the frequency of Th17 instead of that of IL-17+, IFN-γ+ double producers (Figure 1C, D).
Figure 1. Celastrol treatment downregulates the frequency of IL-17-producing T cells, but upregulates Treg in the SIC of arthritic joints.
On d 18 after Mtb immunization of rats, SIC were collected from the joints of arthritic rats treated with Celastrol or vehicle (n= 8 each), and the cells analyzed by flow cytometry for IL-17-/IFN-γ -expressing CD4+ (A) and CD8+ (B) T cells. In each section (A/B), the left panel shows a representative profile, whereas the right panel shows the average (mean ± SEM) values of the group. Also depicted is the relative proportion of IL-17-producing only, IFN-γ-producing only, and IL-17-IFN-γ double producing cells among CD4 (C) and CD8 (D) T cells from SIC. Sections ‘C’ and ‘D’ correspond to sections ‘A’ and ‘B’, respectively. The frequency of Treg cells in the SIC (n= 8) was also determined (E). The Th17/Treg ratio (n = 8) (left panel) and Tc17/Treg ratio (n = 8) (right panel) are also shown (F). (Where indicated, the error bars represent SEM.)
Conversely, the Treg phenotype was significantly increased in the synovial tissue from Celastrol-treated over vehicle-treated rats, 8.4% over 3.9%, respectively (Figure 1E, right). The average ratio of IL-17-producing CD4+ T cells to Treg (Th17/Treg) in the synovium showed a significant reduction from 23.7 (vehicle) to 2.9 (Celastrol) (Figure 1F, left). A similar trend was observed for the ratio of IL-17-producing CD8+ T cells and Treg, with a reduction from 5.3 to 0.3 (P<0.05) (Figure 1F, right). Taken together, these results suggest that Celastrol attenuates autoimmune arthritis in part by reducing the ratio of Th17 to Treg in the cellular infiltrate of arthritic joints. Contrary to SIC, the draining lymph node cells (LNC) showed a marked reduction in Th17 cells but not Th1 and Treg in Celastrol-treated versus vehicle-treated rats (Figure 2). Thus, Celastrol-induced reduction in Th17 coupled with augmentation of Treg frequency is a unique feature of the target organ (SIC) affected in autoimmune arthritis.
Figure 2. Celastrol treatment downregulates the frequency of Th17, but has little effect on Th1 and Treg cells in the draining lymph nodes.
The draining lymph node cells were harvested on d 18 after disease induction from rats treated with Celastrol or vehicle (n = 3 rats per group). These cells were analyzed by flow cytometry for the frequency of CD4+ T cells expressing IL-17 (A), IFN-γ (B), or Foxp3 (C). In each row, the left panel shows a representative profile, whereas the right panel shows the average (mean ± SEM) values of the group.
We then tested the effects of Celastrol on changes intrinsic to the T cell differentiation process using mouse splenocytes. The cytokine cocktails for the differentiation of mouse and human naïve T cells are well-defined. In mice, the synergistic action of IL-6 and TGF-β in the presence of anti-IL-2 facilitates the differentiation of T cells into Th17 cells, driving RORγt expression and IL-17 production. In contrast, TGF-β and IL-2 drive the expression of Foxp3 and differentiation of Treg cells [15]. To ascertain if Celastrol had any direct inhibitory effect on Th17 differentiation, we cultured naïve CD4+ mouse T cells for 72 h with and without Celastrol under defined cytokine conditions. Treatment with Celastrol markedly decreased Th17 differentiation in a dose-dependent manner (Figure 3A, bottom panel). Moreover, RORγt, the transcription factor that regulates Th17 cell differentiation, was also reduced (Figure 3B). The dose-dependent inhibition of Th17 activation and differentiation by Celastrol was also seen in naïve human CD4+ T cells differentiated with IL-1β, IL-6, IL-23, and TGF-β (data not shown).
Figure 3. Celastrol inhibits the in vitro differentiation of Th17, but augments that of Treg.
(A) Naive CD4+ T cells were isolated from mouse splenocytes that had been polyclonally stimulated for 3 d with plate-bound anti-CD3 and soluble anti-CD28 in the presence or absence of different cytokines and varying concentrations of Celastrol as follows: TGF-β and IL-6 and anti-IL-2 antibody (bottom panel); IL-6 and TGFβ (middle panel); or IL-2 and TGFβ (top panel), as described in detail under Materials and Methods. In each case, flow cytometric analysis of intracellular staining of IL-17 and Foxp3 was performed. A representative profile for each experiment is shown in each section. (B) Intracellular staining for RORγt was performed in cells shown in the bottom panel of section ‘A’. A representative profile (left panel) and average (mean + SEM, n= 3) values of the group (right panel) are shown.
In contrast to its inhibitory effect on Th17, Celastrol augmented Treg differentiation from naïve T cells in the presence of IL-2 and TGF-β (Figure 3A, top panel). As Th17 and Treg differentiation is primarily separated by exogenous IL-6 and IL-2, we differentiated naïve mouse T cells with IL-6 and TGF-β but in the absence of anti-IL-2 to allow IL-2 produced by the activated T cells to influence the differentiation process (Figure 3A, middle panel).
The T cell differentiation assays (Figure 3) revealed that Celastrol can inhibit Th17 differentiation and promote Treg differentiation in the same culture. We hypothesized that this was in part due to inhibition of the IL-6 signaling pathway, which resulted in exogenous TGF-β and endogenous IL-2 mimicking the Treg differentiation environment. To test this, we incubated mouse CD4+ T cells with rIL-6 with and without Celastrol. The IL-6 receptor (IL-6R) consists of the IL-6Rα and gp130, and signals through a pSTAT3 homodimer with some ancillary pSTAT5. Our results show that Celastrol treatment resulted in a decrease in STAT3 and STAT5 phosphorylation (Figure 4A), as well as STAT3-induced expression of suppressor of cytokine signaling 3 (SOCS3) (Figure 4B). SOCS3 is a STAT3-induced gene that acts as a phosphatase and dephosphorylates STAT3 in a negative feedback-loop. The gene expression of SOCS3 was used to determine if the reduction in pSTAT3 caused by Celastrol treatment was due to a block in STAT3 signaling or an increase in SOCS3 expression. The decrease in SOCS3 expression and pSTAT3 suggests that Celastrol can directly inhibit Th17 differentiation and promote Treg differentiation in part by blocking IL-6R signaling pathway.
Figure 4. Celastrol inhibits IL-6 receptor signaling via reducing STAT3 signaling.
Mouse CD4+ T cells were treated with Celastrol (300 nM) for 8 h and then activated with IL-6 (2 ng/mL) for the indicated duration (minutes). (A) Cell lysates were collected and pSTAT3 and pSTAT5 were tested using a western blot assay. Total STAT3 and total STAT5 were used as protein-loading controls. The gel was then subjected to densitometry and the values were normalized to appropriate controls. (B) Total RNA isolated from cells treated as described above was tested for STAT3-induced gene SOCS3 by qRT-PCR (mean ± SEM, n = 3).
We next examined whether Celastrol had any influence on Th17-Treg differentiation through T cell-extrinsic mechanisms, specifically its effect on the pro-inflammatory cytokines that are relevant for both Th17 differentiation and autoimmune disease progression. Celastrol treatment of arthritic rats led to decreased production of IL-1β and IL-6 by both SAC and SIC compared to vehicle treatment, when tested ex vivo (Figure 5A). Similarly, SAC obtained from an untreated arthritic rat and then restimulated with Mtb sonicate in the presence of Celastrol in vitro showed reduction in both the gene expression (which cannot distinguish between pro and mature forms of IL-1β) (Figure 5B, top) and the level of protein corresponding to pro-IL-1β and IL-1β by western blot analysis (Figure 5B, bottom). However, SAC obtained from Celastrol-treated rats and restimulated with Mtb in vitro without subsequent exposure to Celastrol in vitro showed marked reduction in the expression of the active (mature) form of IL-1β, but relatively much less reduction in pro-IL-1β (Figure 5C, bottom), but without much effect on gene expression for IL-1β (Figure 5C, top). The above-mentioned reduced production of IL-1β and IL-6 following Celastrol treatment correlates with inhibition of NF-kB activity by Celastrol, as tested using an NF-kB reporter assay (Figure 5D). Furthermore, a reduction in the release of mature IL-1β following in vivo Celastrol treatment suggests that Celastrol might inhibit IL-1β maturation steps.
Figure 5. Celastrol reduces the production of IL-6 and IL-1β by SAC and SIC.
SAC and SIC from Mtb-immunized arthritic rats treated with Celastrol or the vehicle were harvested on day 18 of AA. (A) Cells were restimulated in vitro for 24 h with or without Mtb sonicate (10 µg/ml). Thereafter, cell-free culture supernatants were collected, and the levels of IL-1β and IL-6 were measured by a Multiplex assay. (B–C) SAC from untreated arthritic rats were restimulated in vitro for 6 h with Mtb sonicate in the presence or absence of Celastrol (100 or 300 nM) (n = 3 each) (B). Similarly, SAC from arthritic rats treated with Celastrol or the vehicle were restimulated in vitro for 6 h with or without Mtb sonicate (n = 3) (C). In each case (B/C), the total RNA prepared from cells was processed by quantitative RT-PCR to measure IL-1β and IL-6 mRNA expression (top panel), and values were normalized to the respective hypoxanthine-guanine phosphoribosyltransferase mRNA levels and expressed as relative message. In parallel (bottom panel), cell lysates were prepared from SAC and the levels of pro-IL-1β (p31) and IL-1β (p17) were analyzed by western blot. (D) NF-kB inhibition. HEK 293T cells were transfected with an expression construct containing Firefly luciferase and a promoter containing three copies of the NF-kB binding site (NF-kB luciferase). Cells were pretreated with indicated concentrations of Celastrol for 2 h followed by stimulation with TNF-α for 6 h. Luciferase activity was read and the results presented as percent inhibition over the medium control (mean ± SEM, n = 3). (E) SAC from an untreated arthritic rat were treated with Mtb sonicate as in ‘C’ but with or without an inflammasome inhibitor (Glyben, also known as Glibenclamide), a protease inhibitors (yVAD: caspase-1 inhibitor; MMP-9 inhibitor; AEBSF: serine protease inhibitor), or Celastrol for 24 h. The culture supernatants were analyzed for pro-IL-1β and mature IL-1β by western blot.
The release of mature IL-1β takes place via either an inflammasome-dependent pathway (e.g., through caspase-1) or an inflammasome-independent pathway (e.g., through serine proteases or MMP9). SAC obtained from an arthritic rat were restimulated for 24 h with Mtb sonicate in the presence or absence of an inflammasome inhibitor, a caspase-1 inhibitor, an MMP-9 inhibitor, or a serine protease inhibitor, separately. There was a marked reduction in the release of mature IL-1β in response to Mtb stimulation in cells treated with each of these inhibitors except MMP-9 inhibitor (Figure 5E). These findings suggest that IL-1β maturation in SAC following Mtb stimulation involves both inflammasome-dependent as well as inflammasome-independent mechanisms. Importantly, all these enzymes (caspase-1, serine protease, and MMP9) have been reported to be inhibited by Celastrol in in vitro systems by other investigators [16; 17] and us [14]. Taken together, our results show that Celastrol can inhibit the in vivo generation of cytokines (T cell-extrinsic effects) that are critical for Th17 differentiation and progression of autoimmune arthritis.
Two important aspects of T cell attributes in relation to autoimmune pathogenesis are cellular migration from the peripheral lymphoid tissues into the target organ and the antigen-induced T cell response in the lymphoid tissue. Therefore, we determined whether Celastrol might influence cellular migration and antigen-induced T cell response. Examination of the SIC showed a decrease in the frequency and the number of neutrophils and CD4+ T cells in the joints of Celastrol-treated rats compared to vehicle-treated controls (Figure 6A). This corresponded with a reduction in the production of IL-17 production by Th17 cells and pro-inflammatory chemokines CCL3 and CCL5 by the SIC in Celastrol-treated group compared to the control group (Figure 6B). Further, Celastrol also inhibited the chemotactic migration of lymph node cells (LNC) in vitro (Figure 6C). In addition to reducing the cellular migration of LNC, Celastrol markedly reduced the T cell proliferative response to arthritis-related antigens (e.g., Mtb, mycobacterial hsp65 (Bhsp65), and rat hsp65 (Rhsp65)), but the response to an irrelevant control antigen was unaffected (Figure 6D). Taken together, these results suggest that Celastrol has the potential to inhibit the expansion of antigen-reactive T cells and the migration of inflammatory cells into an arthritic joint.
Figure 6. Celastrol limits the inflammatory cell infiltration into the joints and the chemotactic migration of cells in vitro, and inhibits antigen-induced T cell proliferation.
(A) Neutrophils (CD45+ CD11b/c+ Rat Granulocyte Marker+) and T cells (CD3+) were isolated from SIC from arthritic joints and enumerated by flow cytometry. The Th1, Th17, and Treg cells were detected by intracellular staining for IFN-γ, IL-17, and Foxp3, respectively. Data from 4–5 independent experiments (n = 3 rats per group) are presented. (B) Total SIC from arthritic rats treated with Celastrol or vehicle were stimulated ex vivo with Mtb sonicate. Chemokines in cell supernatants were measured by Multiplex assay. (C) LNC derived from untreated arthritic rats were allowed to migrate using a Transwell membrane in the presence of CCL3 or CCL5 (50 ng/mL) and Celastrol at the indicated concentrations. Cells treated with vehicle served as controls for Celastrol-treated cells (n = 3). (Where indicated, the error bars represent SEM.). (D) Celastrol limits T cell proliferative responses to arthritis-related antigens in lymph nodes. Ex vivo T cell recall responses to arthritis-related antigens were measured using LNC of Celastrol-/vehicle-treated rats. The draining LNC from these rats were harvested 18 d after Mtb-immunization and tested in a proliferation assay using the indicated recall antigens (mean ± SEM, n = 3). (Keyhole limpet hemocyanin, KLH; Mycobacterium tuberculosis H37Ra, Mtb; Mycobacterial heat shock protein 65, Bhsp65; and Rat heat shock protein 65, Rhsp65.).
4. DISCUSSION
The results of our study highlighted the influence of a traditional Chinese medicinal product, Celastrol, in reducing Th17 and increasing Treg frequency in the inflamed target organ, the joints, in a model of experimental arthritis. Given the functional attributes of Th17 and Treg, this change in their balance favors an anti-inflammatory/ immunomodulatory local environment in the inflamed tissue. Therefore, we suggest that a reduction in Th17/Treg ratio by Celastrol in part contributes to the anti-arthritic activity of this herbal product. The resetting of Th17/Treg balance by Celastrol is a novel finding in regard to the mechanism of action of this natural product.
Our findings in the AA model are supported by those in RA patients and other experimental models of this disease. For example, the number of IL-17 producing T cells are increased in the synovial fluid of RA patients and have an inverse relationship with that of Treg cells [18]. The reversal of the Th17/Treg imbalance in the peripheral blood mononuclear cells has been observed in clinical trials using conventional single therapy tocilizumab (human anti-IL-6) [19]. Similarly, vasoactive intestinal peptide and grape seed proanthocyanidin extract have been shown to alter the Th17/Treg ratio in splenocytes in mice with collagen-induced arthritis (CIA) [20; 21]. In another study on AA but in Wistar rats, Celastrol was found to be more effective than Digoxin in suppressing arthritis [16]. Further, Digoxin, a known inhibitor of RORγt, was found to reduce the frequency of IL-17-producing cells in the spleen of treated rats. The above studies highlight the utility of natural products for the treatment of experimental arthritis. Earlier, an extract of Tripterygium wilfordii and its bioactive component triptolide were shown to be effective in the treatment of RA [22], and snake venom has been shown to suppress adjuvant arthritis [23], further reinforcing the potential of the vast resource of natural products for the treatment of RA.
Using a T cell differentiation assay, we showed that Celastrol can directly reduce the frequency of IL-17-producing and RORγt-expressing T cells but augment the frequency of Foxp3-expressing T cells. The cytokine cocktail required for rat Th17/Treg differentiation has not been reported in the literature. Accordingly, we tested Celastrol on mouse CD4+ T cells. Celastrol was able to reduce the frequency of IL-17-producing mouse CD4+ T cells. We also showed one potential mechanism of Celastrol, namely reduction in pSTAT3 (T cell-intrinsic effect), which plays an important role in modulating Th17/Treg balance [24]. Many Th17-specific inhibitors have been developed, but have not been tested on Treg [25]. In contrast, a molecule like Celastrol can reduce Th17, while enhancing Treg differentiation, potentially controlling arthritis via two synergistic mechanisms. Our findings on this reciprocal effect of Celastrol on Th17 and Treg are supported by similar results using STA-21, a STAT3 specific inhibitor, in the CIA model of RA [26].
The primary difference in the differentiation of Th17 and Treg is the presence or absence, respectively of the pro-inflammatory cytokine IL-6 and/or IL-1β [27; 28]. Therefore, these two cytokines, which are produced by myeloid cells (macrophage and dendritic cells) during inflammatory conditions, are valid targets for influencing T cell subset differentiation. In this regard, our results showed that Celastrol treatment can significantly reduce the levels of IL-6 and IL-1β produced by myeloid cells (e.g., spleen adherent cells), offering a T cell-extrinsic mechanism of Celastrol-induced inhibition of Th17 differentiation under inflammatory conditions. Additional support is derived from our results along with those of others [29] showing that Celastrol has an inhibitory effect on NF-kB, the master transcription factor that regulates the production of IL-6 and IL-1β. In the case of IL-1β, pro-IL-1β production is regulated by NF-κB, whereas production of mature IL-1β from pro-IL-1β involves the action of enzymes such as caspase-1, caspase-11, or others [30]. The reduction in pro-IL-1β by Celastrol can be explained by the inhibition of NF-kB [29], whereas the decrease in mature IL-1β points to a likely additional direct effect of Celastrol on the conversion of pro-IL-1β into mature IL-1β. We are currently examining the effect of Celastrol on enzymes involved in the generation of mature IL-1β.
The seeding of cells into the synovium of the joints results from T cell activation in the draining lymph nodes and subsequent migration into the joints. Our results suggest that Celastrol modulates both these processes. We observed reduced T cell proliferation in response to arthritis-related antigens, heat-shock protein 65. Furthermore, the influence of Celastrol on cellular migration is in part owing to reduction in the production of CCL3 (MIP-1α) and CCL5 (RANTES) by synovial-infiltrating cells as well as by a direct effect on the migration of T cells in response to the same chemokines provided exogenously. These results are supported by other studies showing that the blockade or inhibition of chemokines [31; 32; 33] and synovium-associated adhesion molecules [3] can suppress arthritis.
In summary, Celastrol can directly limit Th17 differentiation, but enhance Treg generation by preventing STAT3 activation. In addition, it can limit the production of IL-6 and IL-1β, thereby indirectly reducing Th17 differentiation. The reciprocal effect of Celastrol on Th17 and Treg is an advantage over compounds/drugs that inhibit Th17 only. Furthermore, Celastrol also targets cellular migration into the synovial tissue. We propose that Celastrol be further investigated for its efficacy against RA and its eventual use as an adjunct/ alternative to conventionally used anti-arthritic drugs.
HIGHLIGHTS.
-
*
T cell-based mechanism of anti-arthritic activity of Celastrol are not fully defined.
-
*
Celastrol treatment reduced Th17 but increased Treg frequency in arthritic joints
-
*
Celastrol influences T cell differentiation in vitro in support of in vivo findings
-
*
Celastrol also inhibits chemotactic migration of T cells
-
*
Celastrol should be tested as an adjunct to available drugs for arthritis therapy
ACKNOWLEDGEMENT
This work was supported by NIH grants R01 AT004321 and F31 AT007278. We thank Krystal Matthews for her help with the NF-kB reporter assay; Joao Pedra for advice in the testing of mature IL-1β; Qing Chen for help with testing of the composition of synovial-infiltrating cells; and Stefanie Vogel for providing the qRT-PCR facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors confirm that there are no conflicts of interest.
REFERENCES
- 1.Harris ED., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990;322:1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- 2.Scott DL, Smith C, Kingsley G. Joint damage and disability in rheumatoid arthritis: an updated systematic review. Clin Exp Rheumatol. 2003;21:S20–S27. [PubMed] [Google Scholar]
- 3.Lee DM, Kiener HP, Agarwal SK, Noss EH, Watts GF, Chisaka O, Takeichi M, Brenner MB. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315:1006–1010. doi: 10.1126/science.1137306. [DOI] [PubMed] [Google Scholar]
- 4.Burmester GR, Feist E, Dorner T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10:77–88. doi: 10.1038/nrrheum.2013.168. [DOI] [PubMed] [Google Scholar]
- 5.Sfikakis PP, Tsokos GC. Towards the next generation of anti-TNF drugs. Clin Immunol. 2011;141:231–235. doi: 10.1016/j.clim.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Choi SI, Brahn E. Rheumatoid arthritis therapy: advances from bench to bedside. Autoimmunity. 2010;43:478–492. doi: 10.3109/08916931003674717. [DOI] [PubMed] [Google Scholar]
- 7.Moudgil KD, Berman BM. Traditional Chinese medicine: potential for clinical treatment of rheumatoid arthritis. Expert Rev Clin Immunol. 2014;10:819–822. doi: 10.1586/1744666X.2014.917963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Berg WB, McInnes IB. Th17 cells and IL-17 a--focus on immunopathogenesis and immunotherapeutics. Semin Arthritis Rheum. 2013;43:158–170. doi: 10.1016/j.semarthrit.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Moudgil KD, Choubey D. Cytokines in autoimmunity: role in induction, regulation, and treatment. J Interferon Cytokine Res. 2011;31:695–703. doi: 10.1089/jir.2011.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shevach EM. Biological functions of regulatory T cells. Adv Immunol. 2011;112:137–176. doi: 10.1016/B978-0-12-387827-4.00004-8. [DOI] [PubMed] [Google Scholar]
- 11.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong L, Moudgil KD. Celastrus aculeatus Merr. suppresses the induction and progression of autoimmune arthritis by modulating immune response to heat-shock protein 65. Arthritis Res Ther. 2007;9:R70. doi: 10.1186/ar2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajaiah R, Puttabyatappa M, Polumuri SK, Moudgil KD. Interleukin-27 and interferon-gamma are involved in regulation of autoimmune arthritis. J Biol Chem. 2011;286:2817–2825. doi: 10.1074/jbc.M110.187013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkatesha SH, Yu H, Rajaiah R, Tong L, Moudgil KD. Celastrus-derived Celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses. J Biol Chem. 2011;286:15138–15146. doi: 10.1074/jbc.M111.226365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014 Jan 11; doi: 10.1016/j.autrev.2013.12.004. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Cascao R, Vidal B, Raquel H, Neves-Costa A, Figueiredo N, Gupta V, Fonseca JE, Moita LF. Effective treatment of rat adjuvant-induced arthritis by celastrol. Autoimmun Rev. 2012;11:856–862. doi: 10.1016/j.autrev.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang H, Chen D, Cui QC, Yuan X, Dou QP. Celastrol, a triterpene extracted from the Chinese "Thunder of God Vine, " is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006;66:4758–4765. doi: 10.1158/0008-5472.CAN-05-4529. [DOI] [PubMed] [Google Scholar]
- 18.Nistala K, Moncrieffe H, Newton KR, Varsani H, Hunter P, Wedderburn LR. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum. 2008;58:875–887. doi: 10.1002/art.23291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samson M, Audia S, Janikashvili N, Ciudad M, Trad M, Fraszczak J, Ornetti P, Maillefert JF, Miossec P, Bonnotte B. Brief report: inhibition of interleukin-6 function corrects Th17/Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64:2499–2503. doi: 10.1002/art.34477. [DOI] [PubMed] [Google Scholar]
- 20.Deng S, Xi Y, Wang H, Hao J, Niu X, Li W, Tao Y, Chen G. Regulatory effect of vasoactive intestinal peptide on the balance of Treg and Th17 in collagen-induced arthritis. Cell Immunol. 2010;265:105–110. doi: 10.1016/j.cellimm.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Park MK, Park JS, Cho ML, Oh HJ, Heo YJ, Woo YJ, Heo YM, Park MJ, Park HS, Park SH, Kim HY, Min JK. Grape seed proanthocyanidin extract (GSPE) differentially regulates Foxp3(+) regulatory and IL-17(+) pathogenic T cell in autoimmune arthritis. Immunol Lett. 2011;135:50–58. doi: 10.1016/j.imlet.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Goldbach-Mansky R, Wilson M, Fleischmann R, Olsen N, Silverfield J, Kempf P, Kivitz A, Sherrer Y, Pucino F, Csako G, Costello R, Pham TH, Snyder C, van der Heijde D, Tao X, Wesley R, Lipsky PE. Comparison of Tripterygium wilfordii Hook F versus sulfasalazine in the treatment of rheumatoid arthritis: a randomized trial. Ann Intern Med. 2009;151:229–240. W49–W51. doi: 10.7326/0003-4819-151-4-200908180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kou JQ, Han R, Xu YL, Ding XL, Wang SZ, Chen CX, Ji HZ, Ding ZH, Qin ZH. Differential Effects of Naja naja atra Venom on Immune Activity. Evid Based Complement Alternat Med. 2014;2014:287631. doi: 10.1155/2014/287631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durant L, Watford WT, Ramos HL, Laurence A, Vahedi G, Wei L, Takahashi H, Sun HW, Kanno Y, Powrie F, O'Shea JJ. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huh JR, Littman DR. Small molecule inhibitors of RORgammat: targeting Th17 cells and other applications. Eur J Immunol. 2012;42:2232–2237. doi: 10.1002/eji.201242740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JS, Kwok SK, Lim MA, Kim EK, Ryu JG, Kim SM, Oh HJ, Ju JH, Park SH, Kim HY, Cho ML. STA-21, a promising STAT3 inhibitor that reciprocally regulates Th17 and Treg, inhibits osteoclastogenesis and alleviates autoimmune inflammation. Arthritis Rheum. 2013 Dec 10; doi: 10.1002/art.38305. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda S, Saijo S, Murayama MA, Shimizu K, Akitsu A, Iwakura Y. Excess IL-1 Signaling Enhances the Development of Th17 Cells by Downregulating TGF-beta-Induced Foxp3 Expression. J Immunol. 2014;192:1449–1458. doi: 10.4049/jimmunol.1300387. [DOI] [PubMed] [Google Scholar]
- 29.Jin HZ, Hwang BY, Kim HS, Lee JH, Kim YH, Lee JJ. Antiinflammatory constituents of Celastrus orbiculatus inhibit the NF-kappaB activation and NO production. J Nat Prod. 2002;65:89–91. doi: 10.1021/np010428r. [DOI] [PubMed] [Google Scholar]
- 30.Dinarello CA. A clinical perspective of IL-1beta as the gatekeeper of inflammation. Eur J Immunol. 2010;41:1203–1217. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- 31.Szekanecz Z, Vegvari A, Szabo Z, Koch AE. Chemokines and chemokine receptors in arthritis. Front Biosci (Schol Ed) 2010;2:153–167. doi: 10.2741/s53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahrara S, Proudfoot AE, Park CC, Volin MV, Haines GK, Woods JM, Aikens CH, Handel TM, Pope RM. Inhibition of monocyte chemoattractant protein-1 ameliorates rat adjuvant-induced arthritis. J Immunol. 2008;180:3447–3456. doi: 10.4049/jimmunol.180.5.3447. [DOI] [PubMed] [Google Scholar]
- 33.Marotte H, Ruth JH, Campbell PL, Koch AE, Ahmed S. Green tea extract inhibits chemokine production, but up-regulates chemokine receptor expression, in rheumatoid arthritis synovial fibroblasts and rat adjuvant-induced arthritis. Rheumatology (Oxford) 2010;49:467–479. doi: 10.1093/rheumatology/kep397. [DOI] [PMC free article] [PubMed] [Google Scholar]