Abstract
In newborn mammals, most of the germ cell population rests in a pool of quiescent small follicles in the ovaries. Regularly throughout adulthood, a small percentage of these oocytes and follicles grows to a certain stage of development and then either degenerates or matures and ovulates. This entire process is under both exogenous and endogenous control. Recent work, including my laboratory’s, has clarified that cytokines and glycosaminoglycans are involved as exogenous and endogenous factors in ovarian follicular development, atresia, and maturation in mammals. The present article describes our contribution regarding the cytokines and ovarian glycosaminoglycans that act as intraovarian regulators of follicular development and oogenesis, including oocyte maturation, in mammals.
Keywords: follicular development, intraovarian regulators, oocyte maturation, oogenesis, ovarian glycosaminoglycans
Introduction
In the ovary, oocytes become surrounded by somatic cells to form the complex known as the follicle-enclosed oocyte. Subsequently, growth and differentiation are induced within the follicle. Follicular development is a continuous, selective process in mammals by which a species-specific number of oocytes from the pool of quiescent follicles progress to mature and ovulate. The meiotic division of oocytes begins prenatally in the follicles and stops at the diplotene stage of prophase I just before or immediately after birth. Oocytes remain under meiotic arrest until concentrations of the ovulatory gonadotropins (follicle-stimulating hormone [FSH] and luteinizing hormone [LH]) peak rapidly, thereby stimulating the resumption of meiosis in preovulatory follicles. After the FSH–LH surge, the oocyte resumes meiosis, proceeds through meiosis I, enters the second cycle of meiotic division, and arrests at metaphase II. This is the process called oocyte maturation. Persistent questions in mammals are how a small number of follicle-enclosed oocytes are recruited to survive during the process of follicular development (follicular selection), how meiosis is arrested at the diplotene stage, and how the resumption of oocyte meiosis (oocyte maturation) is triggered. I and my colleagues have identified the substances involved in follicular selection and in the arrest and resumption of oocyte meiosis. This article describes these achievements and proposed hypotheses regarding the roles of these factors in these processes.
1. The mechanism controlling selective follicular development in mammals
A small percentage of the ovarian follicles in fetuses and newborns are in the process of development.1) Although the number of oocytes drops steadily after birth, hundreds of thousands of oocytes are present at puberty, and only a species-specific proportion of these (less than 1%) will reach full maturation in adult mammals. The remaining follicles degenerate through a process termed “atresia”.2) In this manner, follicular development is a selective process through which a few oocytes are recruited to ovulate. An FSH inhibitor, the ovarian glycosaminoglycans (oGAGs), and angiogenic factors involved in follicular selection in mammals have been identified as described below.
1). An inhibitor of FSH suppresses follicular development.
All ovarian follicles are not equally responsive to the same cyclic condition, and local inductive factors for follicular selection are surmised to reside in individual follicles.1) These morphologic observations led to our identification of an FSH inhibitor in bovine and porcine follicular fluid.3–5) To this end, bovine follicular fluid was precipitated by using ammonium sulfate at 14.5% to 18.5%; the resulting fraction was separated into two peaks during Sephadex G-200 column chromatography. The second peak, detectable as a single band by polyacrylamide gel disc electrophoresis, contained all of the inhibitory activity necessary to suppress compensatory ovavian hypertrophy in mice.4,5) Serum FSH levels of unilaterally ovariectomized mice were lower in those given injections of the inhibitor compared with saline. In addition, the inhibitor suppressed FSH binding to granulosa cells in vitro.6)
These results suggested that this inhibitor has two modes of action: suppression of FSH levels in serum and suppression of FSH binding to granulosa cells. Although purification of the inhibitor from bovine follicular fluid was more difficult than that of the porcine inhibitor, their patterns of elution by column chromatography and disc electrophoresis, and their modes of action, are very similar, indicating their similar molecular weights and physiologic properties. However, the porcine inhibitor was almost twice as effective as the bovine form in suppressing compensatory ovarian hypertrophy in mice. In addition, immunohistochemistry revealed that the porcine protein was present at the granulosa cells in 62% of antral follicles but was absent from all other follicles, including those lacking an antrum.7) Our results suggest that the follicles containing this inhibitor in their follicular fluid are prevented from progressing developmentally and that this protein may be a local regulator of selective follicular growth. Consistent with our findings, inhibin was subsequently identified as a prominent peptide in the control of FSH secretion in the pituitary gland.8)
2). Capillary networks are associated with follicular development and atresia.
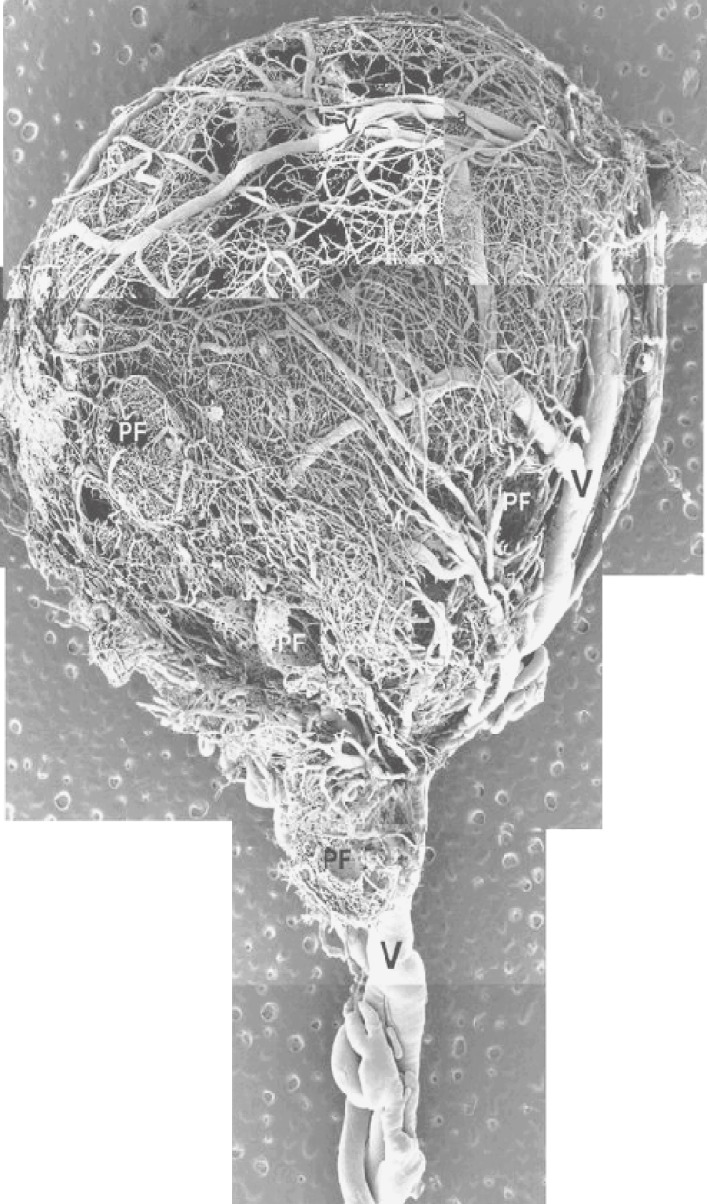
The angiogenesis of ovarian follicles plays an important role in folliculogenesis. A recent study using corrosion casts for scanning electron microscopy has revealed the microvascular structure of the ovary.9,10) For example, porcine ovaries have coiled arteries in the hilus and spiraling branches in the cortex. In addition, small arterioles originating from the cortical coiled arteries straighten before entering the vascular complexes of the follicles (Fig. 1).
Figure 1.
Vascular architecture of large (9.9 mm) porcine follicles. Several vascular plexuses of pre-antral follicles (PF) around that of large follicles were observed. Arterial (a) and venous (v, V) vessels are indicated.
Follicles 150 to 300 µm in diameter are surrounded by a polygonal meshwork comprising several large capillary meshes, but no basket-like structure is visible. In comparison, follicles 500 to 700 µm in diameter have a spherical meshwork comprising just a few capillaries, and the capillary network is arranged as a thin single layer of capillaries. The microvascular architecture of follicles of 1000 to 2000 µm in diameter contains three layers of vascular plexuses. The inner plexus consists of a small spherical basket-like capillary network that is not well developed. The middle layer consists of small arterioles and venules, and the outer layer is a coarse capillary plexus.9,10)
The recruitment, selection, and dominance of follicles occur at regular intervals, and only the follicle that is dominant during the follicular phase ovulates.11,12) The dominance of follicles is influenced by vascularization, and increased vascularity has been proposed to be the primary determinant of follicular dominance. Our studies have demonstrated that dominant follicles are more vascular than are non-dominant follicles.13–16) Specifically, dominant follicles have a more vascular theca than do other antral follicles; consequently uptake of serum gonadotropins is increased in dominant follicles.
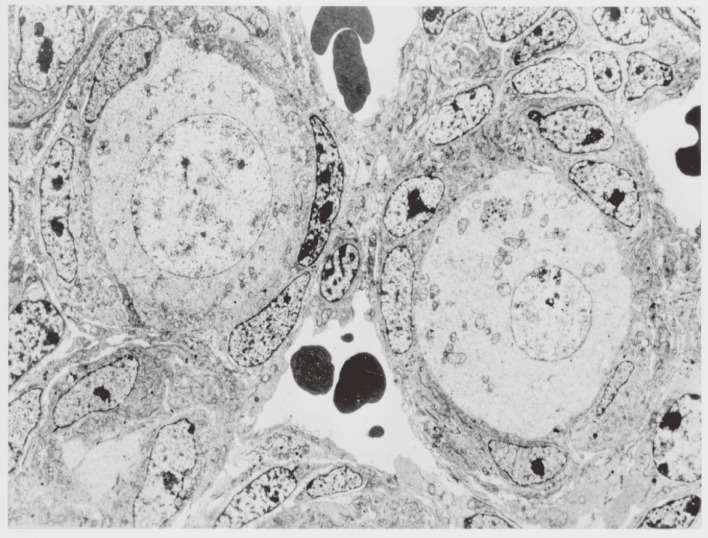
In addition, the follicular capillary network influences follicular development. In mice, oocytes grow from 10 to 50 µm in diameter during days 1 through 5 after birth. According to our observations, follicles containing oocytes 20 to 30 µm in diameter become surrounded by capillary networks (Fig. 2), suggesting that the association of follicles with capillaries promotes oocyte growth.17)
Figure 2.
Electron micrograph of oocytes and granulosa cells in follicle in the ovary of a 1-day-old newborn mouse. The follicles, which include oocytes larger than 20 µm in diameter, are surrounded by capillary networks (×1,000).
Follicles containing oocytes with germinal vesicles are surrounded by a few layers of basket-like capillary wreaths that lie adjacent to the follicular basement membrane. Just before ovulation in Graafian follicles, some thecal cells differentiate into hypertrophic cells and the follicular basement membrane fragments. Then, the capillaries within the theca interna dilate, become hyperpermeable, and appear to be injured. The capillary wreath extends into the follicle via the hypertrophied theca interna. After ovulation, the follicular wall becomes markedly edematous. Capillary branches invade the granulosa cell layer of the ruptured follicle from the region of extravasation to form intricate capillary networks.17)
The capillary network also influences follicular atresia. Decreased proliferation of thecal capillary endothelial cells leads to reduced thecal vasculature, which is one of the earliest events during follicular atresia. The inhibition of blood supply to ovarian follicles may trigger their atresia.13–15)
3). Ovaries contain glycosaminoglycans (GAGs) with angiogenic activity.
Given that gonadotropins induce rapid, striking follicular and vascular changes in the ovary and because the increase in vascularization may be dependent on the secretion of angiogenic factor(s), we injected mice with gonadotropins and examined their ovarian extracts for angiogenic activity.18,19) Specifically, we injected immature female mice with pregnant mare serum gonadotropin (PMSG) or human chorionic gonadotropin (hCG). The ovaries were excised 48 h after injection (that is, before ovulation) and homogenized in 0.5 M ammonium carbonate for 10 min. After freeze-thawing, the homogenates were centrifuged at 54,000 × g for 1 h, and the supernatant was lyophilized. The ovarian extracts were packed in polymer “sandwiches”.18) The thin, transparent film of Elvax 40 containing lyophilized ovarian extracts was cut into squares by using a razor blade. Each square of this slow-releasing polymer sandwich contained 2 or 6 mg of extract. To determine tissue response to the film, the films were implanted in the lateral wall of the sheath of the rectus abdominis muscle. On the 20th day after implantation, the abdominal wall was examined by means of stereomicroscopy for vascularization. Tissue samples, including the films, were excised, fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
The control (untreated) extract elicited neovascularizing reaction in 24% to 29% of mice, whereas those from hCG- and PMSG-treated mice induced neovascularization in 32% to 60% and 77% to 85%. At equivalent dose, the PMSG-treated extract had a greater angiogenic activity than did that from hCG-treated mice. Because neovascularization may occur in association with inflammation, the tissue samples were examined for signs of inflammatory reactions, and some specimens did contain inflammatory cells. When the number of macrophages and other inflammatory cells around the film exceeded that obtained with plain Elvax film (control), the response was considered as inflammation and not scored as neovascularization. Inflammatory reactions were present in 5% to 10% of the mice implanted with films containing ovarian extract.
Our finding that ovarian extract from untreated mice showed angiogenic activity (24% to 29%) suggests that the factor is present in the immature ovary. The greater potency of ovarian extracts prepared from PMSG- and hCG-treated mice indicates that hormonal administration enhances the angiogenic activity. However, note that this phenomenon is not a consequence of an inflammatory reaction. The PMSG used in the cited study18) can induce follicular growth because 5 to 10 IU of this hormone promotes the development of antral follicles in mice. Hence, the follicle-stimulating and angiogenic activities may be related. Ovarian extract prepared from hCG-treated mice induced neovascularization, although a higher dose was necessary. It is well known that hCG induces luteinization of follicular cells and carries intrinsic FSH activity. Taken together, these findings suggest that gonadotropins induce the production of an angiogenic factor that stimulates the proliferation of capillaries from the vascular wreath present in the theca layer and promotes follicular development. Our 1982 report describing the angiogenic activity in mouse ovaries stimulated later studies on ovarian angiogenesis. This angiogenic factor has been partially purified and identified to contain glycosaminoglycan-like substances,20) which have since been given the collective name “ovarian glycosaminoglycans” (oGAGs).
4). oGAGs potentiate the angiogenic activity of epidermal growth factor.
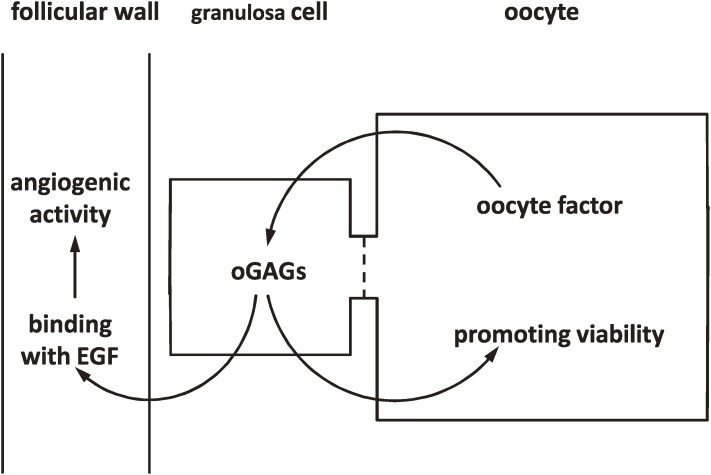
Epidermal growth factor (EGF) is produced by thecal and interstitial cells in the ovary21) and was shown in the 1980s to enhance the proliferation of vascular endothelial cells in vitro.22) In vivo, EGF also promotes neovascularization. For example, when EGF in a slow-release polymer composed of Elvax 40 was implanted in the rabbit cornea, capillary proliferation was induced.23) EGF therefore is considered to be a key angiogenic factor in the ovary. Furthermore, EGF is soluble in tissue fluid, can be translocated in tissues, and induces endothelial cells to proliferate and form capillaries. In the ovary, however, rapid and profound angiogenesis is restricted to a specific region (thecal layer).13) We have demonstrated that oGAGs from ovarian extracts potentiate the angiogenic activity of EGF in mice, suggesting that oGAGs potentiate endogenous EGF in inducing angiogenesis,24) so that the tissue specificity inducing angiogenesis may be due to the localization of ovarian components, including oGAGs and EGF, to a specific region (thecal layer) in ovaries allowing angiogenesis to occur.1,24) In the ovaries of 3-day-old newborn mice, oGAGs accumulate within follicles and the outer layer of growing follicles containing oocytes larger than 30 µm in diameter.25,26) It may be possible that oGAGs interact with EGF to induce vascularization, which accelerates the growth of the oocytes within the follicles. Indeed, histologic analysis has revealed well-developed blood vessels around follicles containing oocytes larger than 30 µm in diameter26) (Fig. 3).
Figure 3.
Schematic representation of the action of ovarian glycosaminoglycans (oGAGs) in ovarian follicles. Oocytes secrete an oocyte factor that stimulates the production and secretion of oGAGs in the granulosa cells. oGAGs accumulate in the intercellular space of granulosa cells and follicular wall. oGAGs in the follicular wall associate with epidermal growth factor (EGF) to induce focal accumulation of EGF and subsequently stimulate rapid and profound proliferation of follicular capillaries. oGAGs accumulated inside the follicles act directly on oocytes and promote viability. Follicles in which oGAGs function as depicted here survive and are recruited for further development.
The observation that granulosa cell proliferation is associated with improved rates of oocyte growth in vitro suggests that oocyte growth and follicular development are interrelated.27,28) Blood circulation perhaps initially affects granulosa cell proliferation and then affects oocyte growth. These lines of evidence appear to indicate that blood vessels influence oocyte and follicular growth directly and accelerate the selective growth of oocytes larger than 30 µm in diameter via granulosa cell proliferation in mice.
5). oGAGs are involved in oocyte survival and macrophage activation in apoptotic follicles.
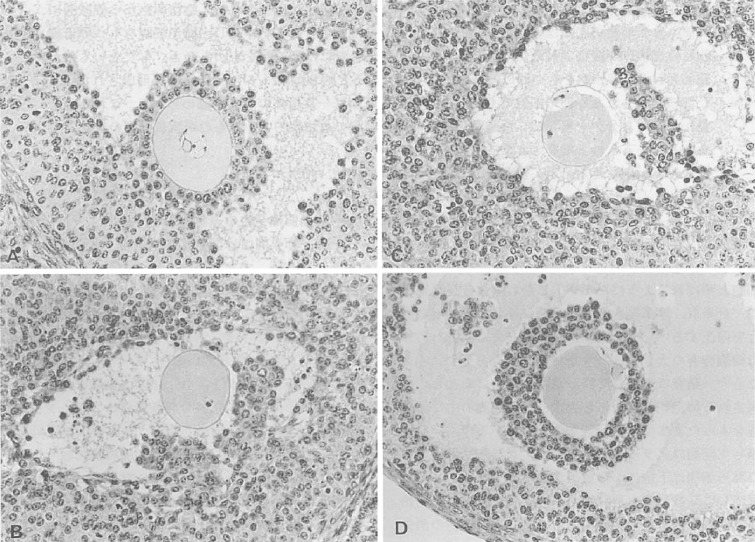
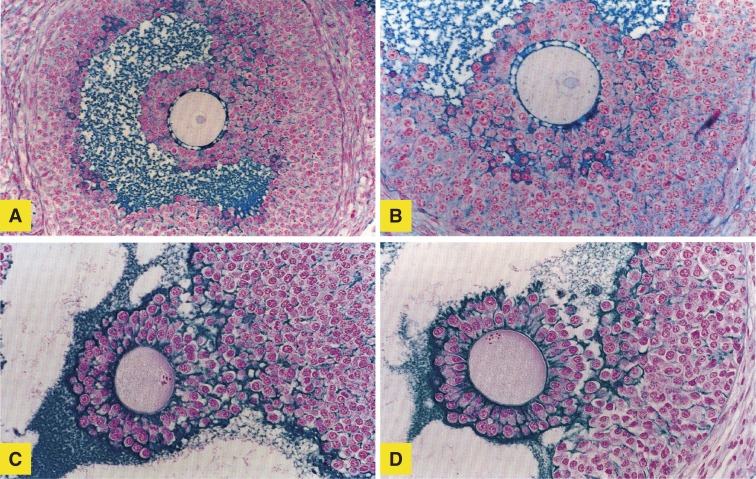
Studies of the effect of FSH on nuclear degeneration of oocytes revealed that the pattern of nuclear degeneration was strongly correlated with the developmental stage of the follicle or oocyte.29) Nuclear degeneration of oocytes was observed in 52.2% of preantral (types 4 through 5) follicles and in 39.2% of antral (types 6 through 7) follicles. Whereas degenerative changes, such as pyknosis and the disappearance of chromosomes, predominated in preantral follicles, about 80% of oocytes in antral follicles showed signs of induction of pseudo-maturation division (Fig. 4).
Figure 4.
Light micrographs of oocytes in vesicular follicles of 32- to 34-day-old mouse ovaries (original magnification, 80×). (A) The cumulus–oocyte complex with germinal vesicle attached to the granulosa cell layer; (B, C, D) Oocytes showing resumption of meiosis. Chromosomes at the (B) first and (C) second metaphase are visible at the surface of oocytes. The first polar body is seen in panels C and D. (D) The cumulus is intact, but the cumulus–oocyte complex has detached from the granulosa cell layer.
The administration of FSH significantly increased the numbers of antral follicles but not of preantral follicles and decreased the numbers of both preantral and antral follicles with degenerating oocytes. Numerous macrophages were identified immunohistochemically in the interstitial tissue around the follicles. Macrophages cluster around atretic follicles and are involved in their clean up of the atretic follicles.
CD44 functions as a sinaling receptor in a variety of cell types including macrophages.30) Then we next evaluated levels of CD44 mRNA, protein, and antigen glycosylation in macrophages during follicular atresia in pigs. Levels of CD44 protein in the follicle were increased, as was posttranslational polylactosamine modification of CD44 on macrophages at atretic follicles; these changes may be involved in the removal of apoptotic granulosa cells.31) Another series of our results32) suggested that the hyaluronan (HA) concentration in follicular fluids increases during atresia. In addition, hyaluronan synthase 1 may be the dominant enzyme in thecal cells that produces HA in pig ovaries and stimulates the removal of degenerating follicles and oocytes. FSH likely stimulates the clearance of atretic follicles via production of HA, which induces macrophage activity.32)
In addition, we noted that a significant number of oocytes underwent segmentation when cultured for 3 or more days in vitro. The observation that segmentation occurred more often with cumulus-free oocytes suggests that cumulus cells produce a component(s) that delays the degenerative process. In this regard, oGAGs isolated from porcine and bovine follicular fluid prevented segmentation in isolated mouse, porcine, and bovine oocytes cultured in vitro.33–36) The oocytes underwent segmentation, forming “blastomeres” or “cellular segments” containing none, a few, or several chromosomal fragments or clumps of chromatin material, according to staining with Hoechst dye.
Cumulus cells synthesize GAGs and proteoglycans to organize the cumulus matrices during cumulus expansion, and glucosamine is used to produce their components, especially HA and chondroitin sulfate. To determine whether changes in cumulus expansion are reflected in GAG synthesis, we measured [3H]-glucosamine incorporation into intact and oocytectomized cumulus–oocyte complexes (COCs) and demonstrated that 1) oocytectomy decreased cumulus expansion of porcine COCs, 2) GAG synthesis had a time-dependent profile with a dramatic rise after 16 h, 3) total GAG synthesis in intact COCs was reflective of HA rather than chondroitin sulfate, and 4) oocytectomy reduced this HA synthesis. These results suggest that an oocyte-dependent mechanism regulates HA synthesis by porcine COCs.37)
These findings imply that FSH-induced production of HA promotes oocyte survival and stimulates macrophages to degrade and remove atretic follicles. These data support the involvement of HA in both follicular development and atresia. Moreover, as mentioned earlier, GAGs (including HA) participate in the neovascularization of a specific region (theca layer) of growing follicles. Therefore, we propose that an oocyte-associated factor stimulates the production of oGAGs in the granulosa cells, which then accumulate in the theca layer and bind to EGF, subsequently increasing the concentration of EGF in the theca layer for rapid and profound neovascularization. In addition, granulosa-produced oGAGs feed back to the oocyte to support its survival and growth (Fig. 3).
6). Fragments of the vascular endothelial growth factor (VEGF) gene enhance thecal angiogenesis associated with follicular development.
During the 2000s, we continued our efforts to clarify the role of angiogenic factors in follicular development. During the 1990s, VEGF was identified as a potent angiogenic factor. We then showed that the local administration of VEGF during follicular development increased the number of oocytes ovulated in rats.38) These findings raised the possibility that the enhancement of circular concentrations of VEGF around the follicles could stimulate follicular development process.
To test this hypothesis, we performed in vivo injection of VEGF gene fragments into pig ovaries in an attempt to over-produce VEGF and enhance the thecal angiogenesis associated with follicular development.39–42) Histologic examination revealed that the vascular density in the theca interna of follicles in VEGF-treated ovaries increased two-fold compared with that in untreated ovaries. The pattern of perifollicular angiogenesis in the VEGF-treated group was essentially the same as that during follicular development after treatment with equine chorionic gonadotropin (eCG). In addition, whereas large follicles (diameter, >5 mm) protruded on the ovarian surface of eCG-treated gilts both with and without injection of VEGF gene fragments, the mean number of large follicles was significantly increased in the VEGF-treated ovaries. Furthermore, the large follicles on the surface of VEGF-treated ovaries were particularly rich in visible blood vessels containing erythrocytes. VEGF-treated ovaries were significantly heavier than were those from pigs treated with eCG only or left untreated. These changes clearly indicate the stimulation of thecal angiogenesis is involved in the follicular development. Because perifollicular vessels supply gonadotropins and many growth factors to the granulosa cells of follicles, increases in perifollicular angiogenesis are associated with the promotion of follicular development.
In addition to the stimulation of follicular development, the increase in vascular density around the antral follicles contributes to the inhibition of atresia. When placed in culture oocyte in early atretic follicles can regenerate, suggesting that the follicle remains in the atretic state due to a decrease in vascularity that limits access to nutrients, substrates, and trophic hormones.43) We found that VEGF-induced promotion of thecal vascularization contributed to the decrease in atretic follicles.44) In pig ovaries, as in those of other species, apoptotic cell death induces follicular atresia. Therefore, VEGF may suppress granulosa cell apoptosis and inhibit follicular atresia.
2. The intraovarian mechanism controlling the induction of oocyte maturation in mammals
In antral follicles, cumulus cells surround the oocyte; together, they form the COC. Cells in the innermost layer of the cumulus, the corona radiate, extend cytoplasmic processes through the intervening zona pellucida to the oolemma. Gap junctions occur where the processes of cumulus cells contact the oolemma. Shortly before ovulation, the physical integrity of the COC is disrupted. The cytoplasmic processes joining the cumulus cells to the oocyte retract, and the ionic coupling and metabolic cooperation between the cumulus cells and oocyte decrease greatly.44–46) Using dye coupling, we correlated the physical integrity between cumulus cells and an oocyte with the maturation stage of that oocyte.47) At the time of cumulus expansion, the oocytes in preovulatory follicles resume meiosis I after their activation by the ovulation-induced release of gonadotropins.48)
These intercellular communications are vital—first to keep the oocyte arrested at prophase I of meiosis and later to signal the oocyte to resume meiosis at the time of ovulation. This phenomenon, known as “cumulus expansion”, facilitates the detachment of the COC from the follicular wall, the extrusion of the COC during ovulation,49) and its capture by the oviductal fimbria;50) promotes the acrosomal reaction in spermatozoa;51) and transports the ovulated COC in the oviduct.52) Furthermore, we have clarified that granulosa cells express an inhibitor of the induction of meiotic resumption and that cumulus expansion interferes with this inhibitor to induce the resumption of meiosis in oocytes in mammals.53–56)
1). Two types of inhibitor for the resumption of meiosis in oocytes.
Isolated follicle-enclosed oocytes remain arrested in the dictyate stage. When released from the follicles, the oocytes undergo spontaneous maturation, indicating that an inhibitor of meiotic resumption within the follicles maintains the oocytes in the dictyate stage.53) Several maturation-preventing factors have been identified in follicular fluid and granulosa cells.54–59) The resumption of meiosis in cumulus-enclosed oocytes can be prevented by being in contact with the granulosa cell layer,54,55) and the meiotic-arresting activity of granulosa cells occurs only when they adhere to an oocyte surrounded by cumulus cells.55) A substance with maturation-preventing activity (designated as meiosis-arresting factor, MAF) has been isolated from the intercellular matrix and external surfaces of granulosa cells.55,56)
Other maturation-preventing factors in follicular fluid are oocyte maturation inhibitor57) and hypoxanthine,58) which acts only in combination with cAMP.60) Follicular fluid may contain another kind of nucleotide that has maturation-preventing activity when in combination with cAMP.59,60) Among these arresting factors, MAF is the most potent agent. Nonetheless, at least two types of factors—one peptide, the other non-peptide—appear to be necessary to sustain meiotic arrest. The small molecular size of the non-peptide factor enables it to translocate between cells through gap junctions.
Related to MAF, we have clarified that GAGs accumulated between the COC and granulosa cells prevent the action of MAF on COC.54) During cumulus expansion, cells in the periphery of the cumulus separate and begin to disperse as the oocyte undergoes maturation.61) The resumption of meiosis in oocytes surrounded by dispersed cumulus is not inhibited even when COCs are in contact with a layer of adherent granulosa cells. Therefore, the dispersion of the cumulus cells, due to the accumulation of GAGs in the intercellular matrix,62) promotes maturation. Gonadotropins (FSH and LH) stimulate the preovulatory follicle to produce GAGs, which accumulate on the surface of and between cumulus cells.62)
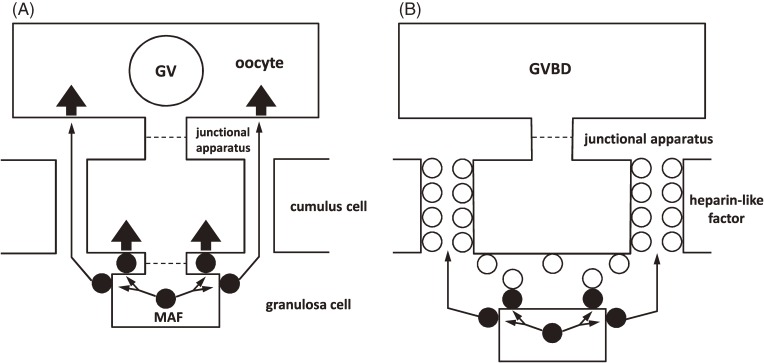
After cumulus expansion, the oocytes undergo maturation. Among the various GAGs, heparin and heparan sulfate interact with MAF and nullify its maturation-inhibiting activity.63) These combined data prompt the following hypothesis to explain the mechanism of meiotic arrest and the resumption of meiosis in mammalian oocytes (Fig. 5).
Figure 5.
Schematic representation of arrest and resumption of first meiosis in mammalian oocytes. (A) Meiosis arresting factor (MAF) (●), located on the surface of and between granulosa cells, initiates meiotic arrest by acting directly on the cumulus–oocyte complex. (B) Gonadotropins stimulates the production and accumulation of ovarian glycosaminoglycans (oGAGs) that surround the cumulus–oocyte complex. oGAGs (○) in intercellular matrices prevent the action of MAF on the oocyte and interferes with the flow of MAF, promoting the resumption of meiosis.
According to this hypothesis, MAF is located on the external surface of and in the intercellular spaces between the granulosa cells. Through direct contact between the granulosa cells and the COC, MAF sustains the oocyte in meiotic arrest. Gonadotropins stimulate the production and accumulation of GAGs in the intercellular space of the cumulus cells. The net result is a disruption of the interaction between the cumulus cells and the oocyte. In addition, GAGs bind MAF to prevent its translocation and subsequent action on the oocyte. In this manner, the oocyte is released from the arresting influence of MAF and resumes meiosis.
Related to the function of MAF, we have identified that, in culture, adrenomedullin (ADM), a multifunctional hormone that regulates cell growth, potently suppresses germinal vesicle breakdown (GVBD), the initial morphologic change in the induction of oocytes.64) Mouse cumulus cells but not the associated oocytes express ADM receptor and proteins that modify the activity of this receptor. Although ADM alone is insufficient to inhibit GVBD, ADM in the presence of nitric oxide significantly inhibits GVBD, and Akt blockade abrogates the ADM-dependent inhibition of GVBD. In addition, Akt expression and phosphorylation was inhibited by ADM, suggesting that Akt signaling in cumulus cells is responsible for GVBD. Immunohistochemical analysis revealed that ADM is localized in the granulosa cells of developed follicles, implying that ADM physiologically affects oocyte maturation in vivo.
2). The accumulation of GAGs between COCs and the granulosa cell layer influences the induction of oocyte maturation.
In preovulatory follicles, we noted that the majority of the cumulus cells associated with oocytes were round in shape.48) The region of heterologous cell interaction appeared to be distributed randomly over the entire surface of the oocyte, and oocyte microvilli were displaced to accommodate this contact. After gonadotropin administration, the cumulus cells of COCs in which the oocytes underwent GVBD became dispersed due to the deposition of copious amounts of intercellular material. Most of the processes of the cumulus cells that extended through the zona pellucida to the oocyte became undulated and withdrew. The contact between cumulus cells themselves decreased but was not completely eliminated; junctional apparatuses were still frequent between these projections and the oolemma at the points of contact.
The accumulation of intercellular material seems to be more active in the peripheral region of the cumulus than in its inner region. Cumulus expansion initially is due to the accumulation of GAGs at the periphery of the cumulus cell mass and then gradually involves its central portion. In the final stage, the attachment of the cumulus to the oocyte is disrupted, and cell-to-cell communication between cumulus and granulosa cells may start to disintegrate at the beginning of cumulus dispersion.65) These morphologic observations support our hypothesis regarding the release of oocytes from the inhibitory action of granulosa cells in the ovary.
Other morphologic studies demonstrated the collection of colloidal iron (Fig. 6) between COCs and the granulosa cell layer of mouse ovaries.65)
Figure 6.
Distribution of colloidal iron-positive substance (blue) in the Graafian follicular tissue at (A) 0, (B) 1, and (C, D) 3 h after administration of human chorionic gonadotropin. Note the presence of enlarged cells where the cumulus mass and granulosa cell layers intertwine and (C, D) the extensive accumulation of colloidal iron-positive substances in the spaces between the enlarged cells. Magnification, 150× (A); 200× (B–D).
Materials deposited between the cumulus cells of Graafian follicles in gonadotropin-treated mouse ovaries were stained with colloidal iron. Specifically, just before the induction of GVBD, colloidal iron-positive material was clearly visible along the periphery of the cumulus cell mass. Histochemical analysis suggests that this colloidal iron-positive material is composed of GAGs. The cells at the connection site between the COC and granulosa cell layer then became enlarged, and colloidal iron-positive substances were present in the intercellular spaces between these enlarged cells, and in the inner part of the cumulus mass. This histologic evidence supports the hypothesis shown in Fig. 5.
3). Follicular fluid and the has2 gene control cumulus expansion.
As mentioned earlier, the attachment of the COC to the granulosa cell layer inhibits the resumption of meiosis in oocytes, and the accumulation of GAGs between the COC and granulosa cell layer prevents the action of MAF and induces the resumption of meiosis in the oocytes in preovulatory follicles. Our next step was to attempt to clarify the mechanism underlying the control of cumulus expansion.
To this end, we sought to purify from porcine follicular fluid the active substance(s) that promote cumulus expansion in pig oocytes matured in vitro.66–68) The follicular fluid yielded four fractions after prolonged ultracentrifugation. COCs were then cultured in each of the reconstituted fractions. After 24 h of culture, oocytes that matured in fraction 1 showed marked expansion of the surrounding cumulus, as did oocytes cultured in complete pig follicular fluid; COCs cultured in the remaining fractions exhibited very little or no expansion. The degree of cumulus expansion did not differ whether the follicular fluid was collected from small, medium, or large follicles. Additional analysis of fraction 1 by HPLC gave several subfractions, one of which induced significantly greater expansion of the cumulus than did the others. The active factor(s) responsible for cumulus expansion was heat stable at 100 ℃ for 15 min, resistant to freezing and thawing, and did not become completely inactive after proteinase K digestion. The estimated molecular mass of the active factor is 6.5 kDa.
We confirmed the expression of hyaluronan synthase (has) family genes and of the CD44 gene in porcine cumulus cells and oocytes.69) has2 is expressed in cumulus cells, whereas has3 mRNA is expressed only in oocytes; has1 mRNA was not detected in COCs. To investigate whether the two has genes are involved in cumulus expansion, we used RT-PCR to confirm the expression of has2 mRNA in COCs and oocytes.69) Both eCG and porcine follicular fluid induced the expression of has2 mRNA.
Given the presence of CD44 in and on cumulus cells, we speculated that CD44 is involved not only in the retention of HA in the extracellular matrix but also in cumulus cell–matrix interactions as a signaling receptor for HA in porcine COCs during oocyte maturation.
4). Phosphorylation of Cx43 induced by the signal of the HA–CD44 interaction shuts down cell-to-cell communication in COCs.
Elucidation of the role of the HA–CD44 interaction during the induction of meiotic resumption is important in understanding the induction of oocyte maturation. Meiotic resumption in mammalian oocytes is accompanied by a substantial increase in the activity of several kinases. The central component of this activity is maturation-promoting factor (MPF), which is a serine–threonine protein kinase composed of a regulatory subunit (cyclin B) and a catalytic subunit (p34cdc2).70) In all species studied so far, MPF activity appears shortly before GVBD, peaks in metaphase I oocytes, decreases dramatically during the transition from metaphase I to metaphase II, and regains its maximal level in metaphase II oocytes.71) To elucidate the role of the HA–CD44 interaction in oocyte maturation, we examined its effect on MPF activity and GVBD in porcine oocytes.72)
COCs were cultured for 24 h with 6-diazo-5-oxo-1-norleucine (to inhibit HA synthesis) or anti-CD44 antibody (to inhibit HA binding).73) Oocytes from COCs showed a low level of MPF activity immediately after their collection from follicles. After 24 h in culture, MPF activity and GVBD rates in oocytes cultured in drug-free medium significantly increased compared with those before culture. In contrast, the exposure of COCs to 6-diazo-5-oxo-1-norleucine or anti-CD44 antibody during 24 h of culture significantly suppressed MPF activity and GVBD rates compared with those of untreated COCs. Consequently, the HA–CD44 interaction is required for the resumption of meiosis in porcine oocytes. CD44 is expressed in cumulus cells but not in oocytes.72) Given these results, the HA–CD44 interaction may promote the resumption of meiosis in porcine oocytes via the cumulus cells.
The coordinated function of COCs is mediated by cell-to-cell communication that involves gap junctions.74) Early studies showed that oocyte growth and development are strictly dependent on the supply of nutrients from the follicle cells. Later studies demonstrated that the meiotic maturation of oocytes is subject to regulation by the somatic compartment of the ovarian follicle.75) This control is mediated by the transmission of cAMP from cumulus cells, which maintains the oocyte in meiotic arrest.75) Cyclic AMP is synthesized in cumulus cells and transported into oocytes via the numerous gap junctions in COCs. Interruption of the gap junctions in COCs, which occurs in response to the preovulatory surge of gonadotropins, leads to a drop in the intraoocyte cAMP concentration followed by the resumption of meiosis.76) These reports led to the conclusion that the disruption of gap junctions in COCs induces the resumption of meiosis in oocytes.
Gap junctions are specialized regions in closely apposed membranes of neighboring cells; these junctions allow cells to exchange small molecules, thus coordinating cellular activities. Each gap junction channel comprises two symmetrical hemispheres (termed connexons) derived from two neighboring cells. The connexon is a hexagonal arrangement of six subunits of a protein named connexin (Cx). Connexins are encoded by members of a multigene family, are defined by their molecular weight, and share high homology. To date, at least 15 connexin genes have been reported in mammals, and seven genes (Cx26, Cx30, Cx32, Cx37, Cx43, Cx45, and Cx60) are expressed in the ovary.77) We consequently examined the effect of the HA–CD44 interaction on the expression of Cx43, the most abundant Cx in ovarian follicles78) and COCs.73) Exposure of COCs in vitro to 6-diazo-5-oxo-1-norleucine or anti-CD44 antibody had no effect on the expression of total Cx43 but significantly inhibited its tyrosine phosphorylation. Several laboratories have shown that when a tyrosine kinase (such as pp60Src) is active, Cx43 is phosphorylated on tyrosine residues and intercellular junctional communication is inhibited.79) Therefore, the HA–CD44 interaction is required to disrupt Cx43 gap junctional communication in COCs. Expression of Cx43 is suggested to be restricted to cumulus and granulosa cells, whereas oocytes exclusively produce Cx37.77)
In pigs, we demonstrated that localization of Cx43 is restricted to cumulus cells, with no Cx43 detected in the oocytes,73) and we used cell fractionation analysis to examine the cellular distribution of CD44 in COCs.73) CD44 was detected exclusively in the membrane fraction; no corresponding band was detected in the nuclear, cytoplasmic, or insoluble fractions. Moreover, indirect immunofluorescence analysis of COCs confirmed the localization of CD44 on or in the membrane of cumulus cells in every tomogram and the three-dimensional reconstruction of the confocal sections.73) The result of the immunohistochemical analysis confirmed the biochemical evidence showing CD44 localization in cumulus cells.72)
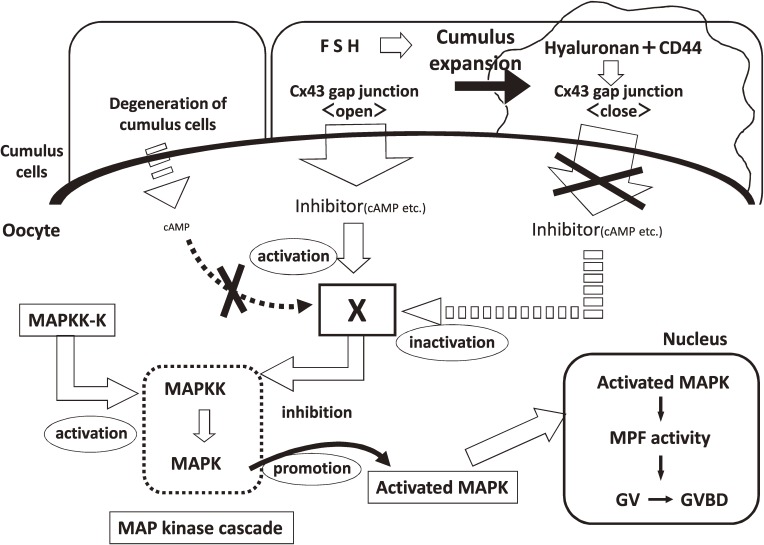
Collectively these findings, along with the abovementioned reports regarding cAMP and non-peptide inhibitors in oocytes, strongly suggest that the HA–CD44 interaction in cumulus cells induces disruption of the Cx43 gap junctions between cumulus cell and adjacent cumulus cell in COCs, inhibits the transport of cAMP and non-peptide inhibitors from cumulus cells into oocytes, and leads to the resumption of meiosis in oocytes (Fig. 7).
Figure 7.
Schematic representation of the mechanism involving non-peptide inhibitors that affect the arrest and resumption of oocyte meiosis in mammals. This mechanism is based on meiosis-arresting factor (Fig. 5). Degeneration of the cumulus in atretic follicles induces the activation of MAP kinase in the oocyte. Follicle-stimulating hormone induces cumulus expansion by stimulating hyaluronan synthesis. The hyaluronan–CD44 interaction in cumulus cells induces the disruption of the Cx43 gap junction in the cumulus–oocyte complex, thus inhibiting the transport of cAMP and non-peptide inhibitors from cumulus cells to the oocyte and leading to activation of the MAP kinase cascade in the oocyte. Inside the oocyte, activated MAPK translocates into the nucleus and induces germinal vesicle breakdown (GVBD). Shut down cell-to-cell communication in COCs is induced at the almost same time with the release of COCs from the influence of MAF of granulose cells.
As mentioned above, we have isolated MAF from the intercellular matrix and external surface of granulosa cells and proposed the hypothesis how MAF sustains the oocyte in meiotic arrest and how GAG releases the oocyte from arresting influence of MAF (Fig. 5). Our experiments shows that shut down cell-to-cell communication in COCs is induced at the almost same time with the release of COCs from the influence of MAF of granulosa cells.47,65)
5). Translocation of phosphorylated MAP kinase into GVs for the induction of meiotic resumption in mammalian oocytes.
During meiotic maturation, protein phosphorylation and dephosphorylation play key roles in a series of events including nuclear envelope breakdown, chromosome condensation, and cytoskeletal change.80) Mitogen-activated protein kinases/extracellular signal-regulated kinases (MAPKs/ERKs) are activated by phosphorylation on both tyrosine and serine–threonine residues by upstream kinases identified as a dual-specific MAPK kinase or MAPK/ERK kinase.81) The MAPK kinase cascade may play a critical role in the diverse intracellular signaling processes by which external signals trigger the G0/G1 transition of the cell cycle.82)
Cultured mouse oocytes resume their maturation within 1 h after culture. This rapid transition of the cell cycle could complicate the detection of changes in kinase activation. In contrast, meiosis in pig oocytes resumes after about 24 h of culture.83) This long duration of the GV stage facilitates analysis of the meiotic events occurring in the nuclei. We demonstrated that MAPK with molecular masses of 42 kDa and 44 kDa (ERK2 and ERK1, respectively) exist in porcine GV-stage oocytes and that their amounts do not change during oocyte maturation.83) MAPK activity was low in GV-stage oocytes, might require GVBD, remained high until metaphase II, and showed a transient slight decrease at the time of first polar body extrusion. These results indicate that the activation of MAPK is involved in the regulation of meiotic maturation in pig oocytes.
The cytoplasmic localization of MAPK kinase is determined by the nuclear export signal in its near-N-terminal region (residues 33 through 44), and inactive MAPK is localized to the cytoplasm through its specific association with MAPK kinase.84) The nuclear accumulation of MAPK is accompanied by dissociation of the complex between MAPK and MAPK kinase after activation of the MAPK pathway and is essential for the transduction of various extracellular signals to the nucleus.84) To investigate the possible function of MAPK as a transducer of the maturation-inducing signal in porcine immature oocytes, we clarified the subcellular localization of MAPK in porcine GV-stage oocytes by using immunofluorescence and immunoblotting.85,86) We showed that two MAPKs (ERK1 and ERK2) were inactive and entirely localized within the cytoplasm. However, phosphorylated MAPKs translocated into GVs just before GVBD. Furthermore, only the phosphorylated, active form of MAPK was detected in GVs, although both active and inactive ERK1/2 were present in the ooplasm after culture for 25 h (that is, just before GVBD). These results agree well with the localization mechanism mentioned earlier, and they strongly support the hypothesis that MAPK is involved in the transduction pathway of the maturation-inducing signal and that MAPK itself transmits these signals from the cytoplasm to GVs.
To test this hypothesis, immature GV-arrested oocytes were microinjected with active MAPK,85) but cytoplasmic injection of MAPK did not increase MAPK activity or accelerate GVBD. However, the MAPK we used was not specifically modified against dephosphorylation, so dephosphorylation may have prevented the injected active MAPK from increasing the total MAPK activity. Although cytoplasmic injection of MAPK did not induce an abrupt increase in MAPK activity and consequently prevent GVBD, a gradual and partial activation of MAPK occurred between 0 and 30 h of culture. This trend appears to support the proposal that the activation of MAPK occurs prior to and therefore does not require GVBD. However, it may be possible that GVBD is required for the full activation of MAPK: a positive feedback loop occurs between MAPK and maturation promoting factor,87,88) the activation of which is exactly analogous to the relation between MAPK and GVBD. It has been reported that the timing of MAPK activation varies in mammalian species, and that MAPK activation delayed compared with MPF activation in some culture conditions.89,90) However, our results show that, in porcine oocytes, inactive MAPK localizes in the cytosol of immature germinal-vesicle oocytes and that activated MAPK translocates into the GVs just before GVBD, indicating that MAPK mediates the maturation-inducing signal from the cytoplasm into the nucleus and induces meiotic resumption (Fig. 7). Our results also suggest a possible requirement for cytoplasmic maturation prior to MAPK activation. We consider that the putative MAPK-suppressing factor(s) shown in Fig. 6 would be disrupted or inhibited and the mechanism(s) for activating the MAPK cascade would be completed during cytoplasmic maturation.
Acknowledgements
I am deeply grateful for the continuous guidance and encouragement received from Prof. Emeritus Akira Iritani (Faculty of Agriculture at Kyoto University) and Dr. Samuel S. Koide (Center for Biomedical Research, The Population Council, The Rockefeller University) as well as for collaborations with colleagues and students at the Faculty of Agriculture, Kyoto University; The Rockefeller University; The Institute of Medical Science, The University of Tokyo; The Graduate School of Agricultural Science, Tohoku University; and the National Livestock Breeding Center of Japan. I also appreciate the financial support offered by the Ministry of Education, Culture, Sports, Science and Technology of Japan; The Rockefeller Foundation; The Japan Society for the Promotion of Science; and the Program for the Promotion of Basic and Applied research for Innovations in Bio-oriented Industry (Brain).
Abbreviations
- ADM
adrenomedullin
- COC
cumulus–oocyte complex
- Cx
connexin
- eCG
equine chorionic gonadotropin
- EGF
epidermal growth factor
- ERK
extracellular signal-regulated kinase
- FSH
follicle-stimulating hormone
- GAG
glycosaminoglycan
- GVBD
germinal vesicle breakdown
- HA
hyaluronan
- has
hyaluronan synthase
- hCG
human chorionic gonadotropin
- MAF
meiosis-arresting factor
- MAPK
mitogen-activated protein kinase
- MPF
maturation-promoting factor
- oGAG
ovarian glycosaminoglycan
- PMSG
pregnant mare serum gonadotropin
- VEGF
vascular endothelial growth factor
Profile
Eimei Sato was born in Hokkaido in 1948, graduated from Kyoto University in 1971, and started his research career in 1973 by studying mammalian oocyte maturation in culture at the Faculty of Agriculture (Kyoto University). He established initial in vitro culture system of domestic mammalian oocytes obtained at the slaugterhouse and subsequently expanded his research area to include intraovarian control mechanisms of ovarian follicular development and oogenesis. Sato obtained his doctoral degree from Kyoto University (1979) for identifying follicle-stimulating hormone (FSH) inhibitor and meiosis-arresting factor and demonstrating their roles as regulators of selective follicular growth and meiotic arrest in mammalian oocytes. He was appointed as Assistant Professor (1974) and Associate Professor (1988) of the Faculty of Agriculture (Kyoto University). During his stay in Kyoto, he completed a special postdoctoral Rockefeller Foundation fellowship and determined the angiogenic factor involved in mammalian follicular development. Subsequent appointments include Associate Professor at The Institute of Medical Science (The University of Tokyo, 1992) and Full Professor (1997) at the Graduate School of Agriculture Science (Tohoku University), and Distinguished Professor (2009) (Tohoku University), where he continued his research into the intraovarian control mechanisms of selective follicular development and meiotic arrest and the resumption of meiosis in oocytes. After retiring from Tohoku University in 2013, Sato became the President of National Livestock Breeding Center, Incorporated Administrative Agency, Japan. He served as president of the Japanese Society of Reproduction and Development (2003–2006), Japan Society of Fertilization and Implantation (2006–2010), and Japanese Society of Animal Science (2007–2009) and has been a member of the Science Council of Japan since 2011. In 2008, Sato was appointed as a foreign fellow of The Royal Swedish Academy of Agriculture and Forestry. Awards received include those from the Japanese Society of Animal Science (1991), Japan Society of Agricultural Science (2005), and the Japan Academy (2013) and the Medal with Purple Ribbon (2009).

References
- 1).Bjersing, L. (1982) Maturation, morphology, and endocrine function of the ovarian follicle. In Intraovarian Control Mechanisms (eds. Channing C.P. and Segal S.J.). Plenum Press, New York, pp. 1–14. [DOI] [PubMed] [Google Scholar]
- 2).Sato, E. and Miyoshi, K. (1998) Oogenesis in mammals: A 1997 perspective. In Reproductive Biology Update, Novel Tool for Assessment of Environmental Toxicity (eds. Manabe, N. et al.). Nakanishi Printings, Kyoto, pp. 93–103. [Google Scholar]
- 3).Sato E., Ishibashi T. (1977) Inhibition of compensatory ovarian hypertrophy in the mouse by the administration of the non-dialyzable fraction of bovine follicular fluid. Jpn. J. Zootech. Sci. 48, 782–783. [Google Scholar]
- 4).Sato E., Miyamolto H., Ishibashi T., Iritani A. (1978) Identification, purification and immunohistochemical detection of the inhibitor from porcine ovarian follicular fluid to compensatory ovarian hypertrophy in mice. J. Reprod. Fertil. 54, 263–267. [DOI] [PubMed] [Google Scholar]
- 5).Sato E., Ishibashi T., Iritani A. (1982) Purification and action sites of a follicle-stimulating hormone inhibitor from bovine follicular fluid. J. Anim. Sci. 55, 873–877. [DOI] [PubMed] [Google Scholar]
- 6).Sato E., Ishibashi T., Iritani A. (1980) Effect of inhibin-like substance isolated from porcine follicular fluid on the follicle-stimulating hormone (FSH) level in mouse serum and on FSH binding to porcine granulosa cells. Fertil. Steril. 34, 55–57. [DOI] [PubMed] [Google Scholar]
- 7).Sato E., Ishibashi T. (1982) Immunohistochemical localization of a follicle-stimulating hormone (FSH) inhibitor from bovine follicular fluid. Adv. Biosci. 34, 203–204. [Google Scholar]
- 8).Luisi S., Florio P., Reis F.M., Petraglia F. (2005) Inhibins in female and male reproductive physiology: role in gametogenesis, conception, implantation and early pregnancy. Hum. Reprod. Update 11, 123–135. [DOI] [PubMed] [Google Scholar]
- 9).Jiang J.Y., Macchiarelli G., Miyabayashi K., Sato E. (2002) Follicular microvasculature in the porcine ovary. Cell Tissue Res. 310, 93–101. [DOI] [PubMed] [Google Scholar]
- 10).Jiang J.Y., Shimizu T., Sasada H., Tsang B.K., Sato E. (2004) Increased ovarian follicular angiogenesis and dynamic changes of follicular vascular plexuses induced by equine chorionic gonadotropin in the gilt. Cell Tissue Res. 316, 349–357. [DOI] [PubMed] [Google Scholar]
- 11).Jablonka-Shariff A., Fricke P.M., Grazul-Bilska A.T., Reynoldes P.L., Redmer D.A. (1994) Size, number, cellular proliferation, and atresia of gonadotropin-induced follicles in ewes. Biol. Reprod. 51, 531–540. [DOI] [PubMed] [Google Scholar]
- 12).McNatty K.P., Gibb M., Dobson C., Thurley D.C. (1981) Evidence that changes in luteinizing hormone secretion regulate the growth of the preovulatory follicles in the ewe. J. Endocrinol. 90, 375–389. [DOI] [PubMed] [Google Scholar]
- 13).Jiang J.Y., Macchiarelli G., Tsang B.K., Sato E. (2003) Capillary angiogenesis and degeneration in bovine ovarian antral follicles. Reproduction 125, 211–223. [PubMed] [Google Scholar]
- 14).Jiang J.Y., Miyoshi K., Umezu M., Sato E. (1999) Superovulation of immature hypothyroid rdw rats by thyroxine therapy and the development of eggs after in vitro fertilization. J. Reprod. Fertil. 116, 19–24. [DOI] [PubMed] [Google Scholar]
- 15).Jiang J.Y., Umezu M., Sato E. (2000) Improvement of follicular development rather than gonadotrophin secretion by thyroxine treatment in infertile immature hypothyroid rdw rats. J. Reprod. Fertil. 119, 193–199. [DOI] [PubMed] [Google Scholar]
- 16).Jiang J.Y., Umezu M., Sato E. (2000) Characteristics of infertility and the improvement of fertility by thyroxine treatment in adult male hypothyroid rdw rats. Biol. Reprod. 63, 1637–1641. [DOI] [PubMed] [Google Scholar]
- 17).Sato E. (1995) Microvasculature in the mouse ovarian follicle demonstrated by a lectin angiography method. J. Anat. Embryol. 100 (Suppl. 1), 461–467. [PubMed] [Google Scholar]
- 18).Sato E., Ishibashi T., Koide S.S. (1982) Inducement of blood vessel formation by ovarian extracts from mice injected with gonadotropins. Experientia 38, 1248–1249. [DOI] [PubMed] [Google Scholar]
- 19).Sato E., Miyamoto H., Koide S.S. (1990) Hyaluronic acid-like substance from mouse ovaries with angiogenic activity. Z. Naturforsch. 45c, 873–880. [DOI] [PubMed] [Google Scholar]
- 20).Sato E. (1990) Mouse ovarian factors with angiogenic activity. Endocrinol. Japon. 37, 413–420. [DOI] [PubMed] [Google Scholar]
- 21).Berisha B., Schama D., Kosmann M., Amselgruber W., Einspanier R. (2000) Expression and localization of vascular endothelial growth factor during the final growth of bovine ovarian follicles. J. Endocrinol. 167, 371–382. [DOI] [PubMed] [Google Scholar]
- 22).Carpenter G., Cohen G. (1979) Epidermal growth factor. Annu. Rev. Biochem. 48, 193–216. [DOI] [PubMed] [Google Scholar]
- 23).Gospodarowicz D., Bialecki H., Thakral T.K. (1979) The angiogenic activity of the fibroblast and epidermal growth factor. Exp. Eye Res. 28, 501–514. [DOI] [PubMed] [Google Scholar]
- 24).Sato E., Tanaka T., Takeya T., Miyamoto H., Koide S.S. (1991) Ovarian glycosaminoglycans potentiate angiogenic activity of epidermal growth factor in mice. Endocrinology 128, 2402–2406. [DOI] [PubMed] [Google Scholar]
- 25).Shimoda K., Sato E., Tanaka T., Takeya T., Toyoda Y. (1993) Morphological differentiation of the microvasculature during follicular development, ovulation and luteinization of mouse ovaries. Dev. Growth Differ. 35, 431–437. [DOI] [PubMed] [Google Scholar]
- 26).Miyamoto Y., Nakayama T., Haraguchi S., Miyamoto H., Sato E. (1996) Morphological evaluation of microvascular networks and angiogenic factors in the selective growth of oocytes and follicles in the ovaries of mouse fetuses and newborns. Dev. Growth Differ. 38, 291–298. [DOI] [PubMed] [Google Scholar]
- 27).Carrol J., Whittingham D.G., Wood M.J. (1991) Effect of dibutyryl cyclic adenosine monophosphate on granulosa cell proliferation, oocyte growth and meiotic maturation in isolated mouse primary ovarian follicles cultured in collagen gels. J. Reprod. Fertil. 92, 197–207. [DOI] [PubMed] [Google Scholar]
- 28).Hirao Y., Kimura J., Miyano T., Kato S. (1993) Effect of PMSG on early oocyte growth and follicular development in newborn mouse ovaries cultured in vitro. J. Reprod. Dev. 39, 13–17. [Google Scholar]
- 29).Nakai T., Miyamoto Y., Nakayama T., Manabe N., Sato E. (1996) Histological profiles of nuclear degeneration of oocytes in the mouse ovaries and possible roles of follicle stimulating hormone for nuclear degeneration of oocytes in the atretic follicles. J. Mamm. Ova. Res. 13, 24–29. [Google Scholar]
- 30).Ilangumaran S., Borisch B., Hoessli D.C. (1999) Signal transduction via CD44: Role of plasma membrane microdomains. Leuk. Lymphoma 35, 455–469. [DOI] [PubMed] [Google Scholar]
- 31).Miyake Y., Matsumoto H., Yokoo M., Miyazawa K., Kimura N., Tunjung W.A.S., Shimizu T., Sasada H., Aso H., Yamaguchi T., Sato E. (2006) Expression and glycosylation with polylactosamine of CD44 antigen on macrophages during follicular atresia in pig. Biol. Reprod. 74, 501–510. [DOI] [PubMed] [Google Scholar]
- 32).Miyake Y., Sakurai M., Tanaka S., Tunjung W.A.S., Yokoo M., Matsumoto H., Aso H., Yamaguchi T., Sato E. (2009) Expression of hyaluronan synthase 1 and distribution of hyaluronan during follicular atresia in pig ovaries. Biol. Reprod. 80, 249–257. [DOI] [PubMed] [Google Scholar]
- 33).Sato E., Ishibashi T. (1988) Bovine ovarian glycosaminoglycans delaying the onset of spontaneous death of oocytes in culture. Jpn. J. Zootech. Sci. 59, 466–469. [Google Scholar]
- 34).Sato E., Ishibashi T., Koide S.S. (1987) Prevention of spontaneous degeneration of mouse oocytes in culture by ovarian glycosaminoglycans. Biol. Reprod. 37, 371–376. [DOI] [PubMed] [Google Scholar]
- 35).Sato E., Miyamoto H., Koide S.S. (1990) Glycosaminoglycans in porcine follicular fluid promoting viability of oocytes in culture. Mol. Reprod. Dev. 26, 391–397. [DOI] [PubMed] [Google Scholar]
- 36).Sato E., Inoue M., Takahashi Y., Toyoda Y. (1994) Glycosaminoglycans prevent induction of fragmentation of porcine oocytes stimulated by dibutyryl cyclic adenosine 3′, 5′-monophosphate in culture. 19, 29–36. [DOI] [PubMed] [Google Scholar]
- 37).Nakayama T., Inoue M., Sato E. (1996) Effect of oocytectomy on glycosaminoglycan composition during cumulus expansion of porcine cumulus-oocyte complexes cultured in vitro. Biol. Reprod. 55, 1299–1304. [DOI] [PubMed] [Google Scholar]
- 38).Iijima K., Jiang J.Y., Shimizu T., Sasada H., Sato E. (2005) Acceleration of follicular development by administration of vascular endothelial growth factor in cycling female rats. J. Reprod. Dev. 51, 161–168. [DOI] [PubMed] [Google Scholar]
- 39).Shimizu T., Iijima K., Ogawa Y., Miyazaki H., Sasada H., Sato E. (2008) Gene injections of vascular endothelial growth factor (VEGF) and growth differentiation factor-9 (GDF-9) stimulate ovarian follicular development in immature female rats. Fertil. Steril. 89 (Suppl. 1), 1563–1570. [DOI] [PubMed] [Google Scholar]
- 40).Shimizu T., Jiang J.Y., Iijima K., Miyabayashi K., Ogawa Y., Sasada H., Sato E. (2003) Induction of follicular development by direct single injection of vascular endothelial growth factor gene fragments into the ovary of miniature gilts. Biol. Reprod. 69, 1388–1393. [DOI] [PubMed] [Google Scholar]
- 41).Shimizu T., Jiang J.Y., Sasada H., Sato E. (2002) Change of messenger RNA expression of angiogenic factors and related receptors during follicular development in gilts. Biol. Reprod. 67, 1846–1852. [DOI] [PubMed] [Google Scholar]
- 42).Shimizu T., Yokoo M., Miyake Y., Sasada H., Sato E. (2004) Differential expression of bone morphogenetic protein 4–6 (BMP-4, -5, and -6) and growth differentiation factor-9 (GDF-9) during ovarian development in neonatal pigs. Domest. Anim. Endocrinol. 27, 397–405. [DOI] [PubMed] [Google Scholar]
- 43).Moor R.M., Speamark R.F. (1986) Cell signaling, permeability, and microvasculatory changes during antral follicle development in mammals. J. Dairy Sci. 69, 927–943. [DOI] [PubMed] [Google Scholar]
- 44).Guthrie H.D., Grimes R.W., Cooper B.S., Hammond J.M. (1995) Follicular atresia in pigs: Measurement and physiology. J. Anim. Sci. 73, 2834–2844. [DOI] [PubMed] [Google Scholar]
- 45).Eppig J.J. (1982) The relationship between cumulus cell-oocyte coupling, oocyte meiotic maturation, and cumulus expansion. Dev. Biol. 89, 268–272. [DOI] [PubMed] [Google Scholar]
- 46).Racowsky C., Satterlie R.A. (1985) Metabolic, fluorescent dye and electrical coupling between hamster oocytes and cumulus cells during meiotic maturation in vivo and in vitro. Dev. Biol. 108, 191–202. [DOI] [PubMed] [Google Scholar]
- 47).Kanayama I., Sato E., Nakayama T., Miyamoto H. (1995) Changes in dye coupling and physical integrity between oocyte and cumulus cells during oocyte maturation in mice. J. Mamm. Ova Res. 12, 101–106. [Google Scholar]
- 48).Kanayama I., Sato E., Shimoda K., Miyamoto H. (1990) Morphological differentiation of cumulus-oocyte complexes induced by the administration of gonadotropins in mice. Jpn. J. Vet. Sci. 52, 199–205. [DOI] [PubMed] [Google Scholar]
- 49).Tsafriri A. (1995) Ovulation as a tissue remodelling process. Adv. Exp. Med. Biol. 377, 121–140. [DOI] [PubMed] [Google Scholar]
- 50).Larsen W.J., Chen I., Powers R., Zhang H., Russell P.T., Chambers C., Hess K., Flick R. (1996) Cumulus expansion initiates physical and developmental autonomy of the oocytes. Zygote 4, 335–341. [DOI] [PubMed] [Google Scholar]
- 51).Mattioli M., Lueidi P., Barboni B. (1998) Expanded cumuli induce acrosome reaction in boar sperm. Mol. Reprod. Dev. 51, 445–453. [DOI] [PubMed] [Google Scholar]
- 52).Sato E., Ando N., Takahashi Y., Miyamoto H., Toyoda Y. (1995) Structural changes in the oviductal wall during the passage of unfertilized cumulus-oocyte complexes in mice. Anat. Rec. 241, 205–210. [DOI] [PubMed] [Google Scholar]
- 53).Sato E., Koide S.S. (1987) Biochemical transmitters regulating the arrest and resumption of meiosis in oocytes. Int. Rev. Cytol. 106, 1–33. [DOI] [PubMed] [Google Scholar]
- 54).Sato E., Ishibashi T. (1977) Meiotic arresting action of the substance obtained from cell surface of porcine ovarian granulosa cells. Jpn. J. Zootech. Sci. 48, 22–26. [Google Scholar]
- 55).Sato, E., Ishibashi, T. and Iritani, A. (1982) Meiotic arresting substance separated from porcine ovarian granulosa cells and hypothetical arresting mechanism of meiosis. In Intraovarian Control Mechanisms (eds. Channing, C.P. and Segal, S.J.). Plenum Press, New York, pp. 161–173. [DOI] [PubMed] [Google Scholar]
- 56).Sato E., Koide S.S. (1984) A factor from bovine granulosa cells preventing oocyte maturation. Differentiation 26, 59–62. [DOI] [PubMed] [Google Scholar]
- 57).Tsafriri, A. (1978) Oocyte maturation in mammals. In The Vertebrate Ovary (ed. Jones, R.E.). pp. 409–442. [Google Scholar]
- 58).Downs S.M., Coleman D.L., Ward-Bailey P.F., Eppig J.J. (1985) Hypoxanthine is the principal inhibitor of murine oocyte maturation in a low molecular weight fraction of porcine follicular fluid. Proc. Natl. Acad. Sci. U.S.A. 82, 454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Sato E., Wood H.N., Lynn D.G., Koide S.S. (1985) Modulation of oocyte maturation by cyclic adenosine 3,5-pyrophosphate. Cell Differ. 17, 169–174. [DOI] [PubMed] [Google Scholar]
- 60).Sato E., Koide S.S. (1984) Forskolin and mouse oocyte maturation in vitro. J. Exp. Zool. 230, 125–129. [DOI] [PubMed] [Google Scholar]
- 61).Dekel N., Lawrence T.S., Gilula N.B., Beers W.H. (1981) Modulation of cell-to-cell communication in the cumulus-oocyte complex and the regulation of oocyte maturation by LH. Dev. Biol. 80, 356–362. [DOI] [PubMed] [Google Scholar]
- 62).Nakayama T., Inoue M., Sato E. (1996) Effect of oocytectomy on glycosaminoglycan composition during cumulus expansion of porcine cumulus-oocyte complexes cultured in vitro. Biol. Reprod. 55, 1299–1304. [DOI] [PubMed] [Google Scholar]
- 63).Sato E., Ueno H., Koide S.S. (1986) Mouse oocyte maturation modulated by a granulosa cell factor and by heparin and heparan sulfate. Gamete Res. 13, 115–124. [Google Scholar]
- 64).Hiradate Y., Ohtake J., Hoshino Y., Tanemura K., Sato E. (2011) Adrenomedullin: A possible regulator of germinal vesicle breakdown. Biochem. Biophys. Res. Commun. 415, 691–695. [DOI] [PubMed] [Google Scholar]
- 65).Sato E., Inoue M., Toyoda Y. (1993) Morphological profiles of mouse ovarian follicles: Extensive accumulation of a strongly negative-charged substance at specific foci in follicular tissue during oocyte maturation. Arch. Histol. Cytol. 56, 293–302. [DOI] [PubMed] [Google Scholar]
- 66).Daen F.P., Sato E., Naito K., Toyoda Y. (1994) The effect of pig follicular fluid fractions on cumulus expansion and male pronucleus formation in porcine oocytes matured and fertilized in vitro. J. Reprod. Fertil. 101, 667–673. [DOI] [PubMed] [Google Scholar]
- 67).Daen F.P., Sato E., Nakayama T., Toyoda Y. (1995) Serum factor(s) stimulating cumulus expansion in porcine oocyte-cumulus complexes matured and fertilized in vitro. Cell Struct. Funct. 20, 223–231. [DOI] [PubMed] [Google Scholar]
- 68).Daen F.P., Miyoshi K., Sato E. (1997) Factor(s) in porcine follicular fluid arresting the induction of cumulus expansion of oocyte-cumulus complexes cultured in vitro. J. Reprod. Dev. 43, 165–170. [Google Scholar]
- 69).Kimura N., Konno Y., Miyoshi K., Matsumoto H., Sato E. (2002) Expression of hyaluronan synthases and CD44 messenger RNAs in porcine cumulus-oocyte complexes during in vitro maturation. Biol. Reprod. 66, 707–717. [DOI] [PubMed] [Google Scholar]
- 70).Labbe J.C., Picard A., Peaucellier G., Cavadore J.C., Nurse P., Doree M. (1989) Purification of MPF from starfish: Identification as the H1 histone kinase p34cdc2 and a possible mechanism for its periodic activation. Cell 57, 253–263. [DOI] [PubMed] [Google Scholar]
- 71).Naito K., Toyoda Y. (1991) Fluctuation of histone H1 kinase activity during meiotic maturation in porcine oocytes. J. Reprod. Fertil. 93, 467–473. [DOI] [PubMed] [Google Scholar]
- 72).Yokoo M., Miyahayashi Y., Naganuma T., Kimura N., Sasada H., Sato E. (2002) Identification of hyaluronic acid-binding protein and their expression in porcine cumulus-oocyte complexes during in vitro maturation. Biol. Reprod. 67, 1165–1171. [DOI] [PubMed] [Google Scholar]
- 73).Yokoo M., Sato E. (2004) Cumulus-oocyte complex interaction during oocyte maturation. Int. Rev. Cytol. 235, 251–291. [DOI] [PubMed] [Google Scholar]
- 74).Dekel, N. (1988) Spatial relationship of follicle cells in the control of the meiosis. In Meiotic Inhibition: Molecular Control of Meiosis (ed. Heseltine, I.). Alan R. Leiss, New York, pp. 87–101. [Google Scholar]
- 75).Brower P.T., Schultz R.M. (1982) Intercellular communication between granulosa cells and mouse oocytes: Existence and possible nutritional role during oocyte growth. Dev. Biol. 90, 144–153. [DOI] [PubMed] [Google Scholar]
- 76).Phillips D.M., Dekel N. (1991) Maturation of the rat cumulus-oocyte complex: Structure and function. Mol. Reprod. Dev. 28, 297–306. [DOI] [PubMed] [Google Scholar]
- 77).Simon A.M., Goodenough D.A., Li E., Paul D.L. (1997) Female infertility in mice lacking connexin 37. Nature 385, 525–529. [DOI] [PubMed] [Google Scholar]
- 78).Risek B., Guthrie S., Kumar N., Gilula N.B. (1990) Modulation of gap junction transcript and protein expression during pregnancy in the rat. J. Cell Biol. 110, 269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79).Lin R., Warn-Cramer B.J., Kurata W.E., Lau A.F. (2001) v-Src phospholylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. J. Cell Biol. 154, 815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80).Albertini D. (1992) Regulation of meiotic maturation in mammalian oocyte: interplay between exogenous cues and the microtubule cytoskeleton. Bioessay 14, 97–103. [DOI] [PubMed] [Google Scholar]
- 81).Matsuda S., Gotoh Y., Nishida E. (1993) Phosphorylation of Xenopus mitogen-activated protein (MAP) kinase kinase by MAP kinase kinase kinase and MAP kinase. J. Biol. Chem. 268, 3277–3281. [PubMed] [Google Scholar]
- 82).Nishida E., Gotoh Y. (1993) The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem. Sci. 4, 128–131. [DOI] [PubMed] [Google Scholar]
- 83).Inoue M., Naito K., Aoki F., Toyoda Y., Sato E. (1995) Activation of mitogen-activated protein kinase during meiotic maturation in porcine oocytes. Zygote 3, 265–271. [DOI] [PubMed] [Google Scholar]
- 84).Fukuda M., Gotoh Y., Nishida E. (1997) Interaction of MAP kinase with MAP kinase kinase: Its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO J. 16, 1901–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85).Inoue M., Naito K., Nakayama T., Sato E. (1998) Mitogen activated protein kinase translocates into the germinal vesicle and induces germinal vesicle breakdown in porcine oocytes. Biol. Reprod. 58, 130–136. [DOI] [PubMed] [Google Scholar]
- 86).Araki K., Naito K., Haraguchi S., Suzuki R., Yokoyama M., Inoue M., Aizawa S., Toyoda Y., Sato E. (1996) Meiotic abnormalities of c-mos knockout mouse oocytes: activation after first meiosis or enhance into third meiotic metaphase. Biol. Reprod. 55, 1315–1324. [DOI] [PubMed] [Google Scholar]
- 87).Gotoh Y., Nishida E. (1995) Activation mechanism and function of the MAP kinase cascade. Mol. Reprod. Dev. 42, 485–492. [DOI] [PubMed] [Google Scholar]
- 88).Hoshino Y., Sato E. (2008) Protein kinase B (PKB/Akt) is required for the completion of meiosis in mouse oocytes. Dev. Biol. 314, 215–223. [DOI] [PubMed] [Google Scholar]
- 89).Nebreda A.R., Ferby I. (2000) Regulation of the meiotic cell cycle in oocyte. Curr. Opin. Cell Biol. 12, 666–675. [DOI] [PubMed] [Google Scholar]
- 90).Chiri S., De Nadal C., Ciapa B. (1998) Evidence for MAP kinase activation during mitotic division. J. Cell Sci. 111, 2519–2527. [DOI] [PubMed] [Google Scholar]