Abstract
Immunological memory is a hallmark of the adaptive immune system. However, the ability to “remember” and respond more robustly against a second encounter with the same pathogen has been described in organisms lacking T and B cells. Recently, natural killer (NK) cells have been shown to mediate antigen-specific recall responses in several different model systems. Although NK cells do not rearrange the genes encoding their activating receptors, NK cells experience a selective education process during development, undergo a clonal-like expansion during virus infection, generate long-lived progeny (i.e. memory cells), and mediate more efficacious secondary responses against previously encountered pathogen – all characteristics previously ascribed only to T and B cells in mammals. This review describes past findings leading up to these new discoveries, summarizes the evidence for and characteristics of NK cell memory, and discusses the attempts and future challenges to identify these long-lived memory NK cell populations in humans.
Introduction
The innate and adaptive immune systems have traditionally been segregated into well-defined compartments. Innate immunity features short-lived cells that respond rapidly and non-specifically against pathogen exposure. Re-encounter with the same pathogen is thought to result in a qualitatively and quantitatively identical response as the first encounter. In contrast, adaptive immunity consists of T and B cells, which respond more slowly, but with high specificity due to somatically rearranged genes that generate an infinitely diverse set of antigen receptors. The clonal expansion of a pool of antigen-specific effector T and B cells initiated by pathogen exposure results in a population of long-lived “memory” cells that are able to respond quicker and more robustly during subsequent encounters with the same pathogen. The lifespan of many innate immune cells are thought to be on the order of hours or days - relatively short compared to T and B cells, which persist for months to years - making immune memory unimportant, or unnecessary, for short-lived cells comprising the innate immune system such as granulocytes and dendritic cells. Although frequently discussed in isolation, these two sides of the immune system rarely act in isolation and the interplay between cells of the innate and adaptive immune arms contributes to the most productive overall responses against pathogen invasion.
Evidence for innate immune memory
Although immunological memory has been one of the classical hallmarks that distinguishes adaptive immune T and B cells from all other cells of the hematopoietic lineage, evidence has been found in invertebrates (such as crustaceans, flies, beetles, and mosquitoes) and more primitive species (such as tunicates, sea urchins, and sea sponges) that immune “memory” exists independently of lymphocytes possessing rearranged antigen receptors (1–3). Exposure of the copepod Macrocyclops albidus (a minute crustacean) to its natural pathogen, the parasitic tapeworm Schistocephalus solidus, results in resistance to challenge with an antigenically similar tapeworm (4), providing evidence for the early existence of innate immune memory. Drosophila melanogaster injected with a sublethal dose of Streptococcus pneumoniae or Beauveria bassiana (a natural fruitfly pathogen) also demonstrated resistance against subsequent bacterial challenge compared to flies that were unprimed with bacteria (5). The underlying mechanisms for the observed protection following priming required activation of the Toll pathway in phagocytes, which was suggested to mediate the secondary responses and resistance. Specific priming of resistance against its natural bacterial pathogen Bacillus thuringiensis and the common bacterium Escherichia coli was observed in the red flour beetle, Tribolium castaneum (6). Demonstrating specificity in this innate immune response, beetles previously primed with heat-killed bacteria were more likely to survive a subsequent exposure to the same bacteria that was used in priming, rather than exposure to a heterologous pathogen. Similarly, Anopheles gambiae mosquitoes (which are the major vector for malaria spread in Africa) pre-exposed to Plasmodium falciparum demonstrate enhanced immunity upon parasite re-infection (7). The protective effect was attributed to circulating granulocytes, whose numbers are rapidly increased upon primary infection. Together, these reports suggest that innate immune cells in many simpler organisms (which lack adaptive immune cells) can be primed by previous infections, and mount stronger secondary responses upon homologous pathogen challenge.
Evidence for NK cell memory
Natural killer (NK) cells have long been categorized as a component of innate immunity. However, several lines of developmental, phenotypic, and functional evidence suggest that NK cells are closely related to T and B cells mediating adaptive immunity (8–10), with the exception of recombination-activating gene (RAG)-mediated rearrangement of antigen receptor genes. First, similar to the generation of T and B lymphocytes, NK cells are derived from the common lymphoid progenitor (11). Second, like T and B cells, NK cells require cytokines of the IL-2 receptor common-gamma chain family, particularly IL-15, for their development, homeostasis, and survival (12). Third, like thymocytes and pre/pro-B cells, immature NK cells undergo an “education” process whereby only appropriately selected cells (i.e. tolerant to self) are able to be functional effectors in the periphery (13–17). Fourth, activated NK cells express many of the same surface receptors (CD25, CD43, CD44, CD69, CD122, Ly6C, CD62L, and KLRG1) as activated T cells (18, 19). Fifth, NK cells are functionally similar to T cells in their ability to produce IFN-gamma and TNF-alpha following activating receptor-mediated or cytokine-induced stimulation (18, 19). Sixth, NK cells are functionally related to CTL through their shared ability to mediate cytotoxicity via perforin and granzymes (18, 19). With the exception of antigen receptors generated from somatically rearranged genes, the evidence above suggests that NK cells are evolutionarily similar to T and B cells of adaptive immunity. In fact, immature T cells that lack one specific transcription factor (Bcl11b) have been shown to de-differentiate into NK-like precursor cells (20–22). Taken together, these observations suggest that NK cells may be developmentally and functionally more closely related to adaptive immune lymphocytes than innate immune cells. In line with their lymphocytic nature, recent studies have further demonstrated that NK cells can undergo a clonal-like expansion following virus infection in both humans and mice, and that previously primed NK cells can mediate secondary memory responses.
Early studies suggested the possibility that NK cell memory could exist. In a model of F1 hybrid resistance (where the parental bone marrow graft is rejected by F1 recipient mice), B10 x B10.D2 mice “primed” with parental B10 bone marrow cells rejected a second B10 graft more efficiently (23). When mice were primed with parental B10.D2 or a third party allogeneic bone marrow prior to the parental B10 graft, the rapid rejection was abrogated. At the time, NK cells had not yet been identified as the cells mediating hybrid resistance; however, a retrospective interpretation of these early experiments suggests relevance for NK cell memory in graft rejection. Recent studies have indicated that NK cell priming can also occur non-specifically during inflammation induced by TLR triggering on DCs or exposure to cytokines (24, 25). Thus, although NK cells were initially described by their ability to kill certain tumor cells without previous sensitization (26–30), NK cells become even more potent effectors when stimulated. A study using the MHC class I-deficient RMA-S tumor model showed that NK cells primed by tumors that lacked MHC class I were also more activated and mediated greater effector responses (31). A significant increase in IFN-γ secretion and cytotoxic activity was measured in the activated NK cells only following inoculation with RMA-S tumor cells and not with RMA tumor cells expressing MHC class I, suggesting that in addition to inflammatory signals, NK-sensitive tumor cell targets can prime resting NK cells for greater effector potential, similar to T cell priming.
A more recent study demonstrated that NK cells mediate contact hypersensitivity responses to chemical haptens in RAG-deficient mice (32). In mice that lack T and B cells, contact hypersensitivity responses to 2,4-dinitrofluorobenzene and oxazolone persisted for greater than 4 weeks, and the responses were only elicited by the hapten to which mice were originally exposed and not by a different hapten. In the control mice lacking T, B, and NK cells due to a genetic deficiency in the RAG genes and the IL-2 receptor common gamma gene, no contact hypersensitivity was measured in previously sensitized mice. Interestingly, the only population of hapten-specific memory NK cells that could transfer sensitivity resided in the liver. Further studies are required to determine why only liver NK cells mediated the secondary response and the nature of the receptor-hapten interaction driving the responses. Following this report, our group used the well-characterized NK cell response to mouse cytomegalovirus (MCMV) to determine whether immune memory could exist in virus-specific NK cells (33). NK cells in C57BL/6 mice bearing the Ly49H receptor have been shown to specifically recognize MCMV-infected cells expressing the viral glycoprotein m157 and undergo a “clonal-like” expansion (34–36). This proliferation was antigen-specific because infection of mice with a mutant MCMV lacking m157 could not drive Ly49H+ NK cell expansion (33). Using an adoptive transfer system, small numbers of Ly49H+ NK cells were observed to proliferate 100–1000 fold in lymphoid and non-lymphoid tissues in the recipient following MCMV infection, resulting in a long-lived pool of memory NK cells (Fig. 1A). This self-renewing population of NK cells was able to undergo secondary and even tertiary expansion following several rounds of adoptive transfer and virus infection (37). The memory NK cells recovered from previously infected mice several months later exhibited more robust effector functions ex vivo and were far more effective at protection against viral challenge compared to an equal number of resting NK cells from naïve mice, demonstrating a qualitatively different secondary response in NK cells that had previously encountered viral antigen (33). More recently, another report showed that memory-like NK cells could be induced in vitro by exposure to inflammatory cytokines such as IL-12 and IL-18 (38). Following adoptive transfer into recipient mice, these cytokine-activated NK cells were found to respond more robustly several weeks later (measured by IFN-γ production following activating receptor triggering) compared to resting NK cells. Altogether, these studies demonstrate that NK cells can persist for far longer than previously estimated (39–41), and have the potential to contribute alongside memory T and B cell responses during subsequent encounters with the same pathogen.
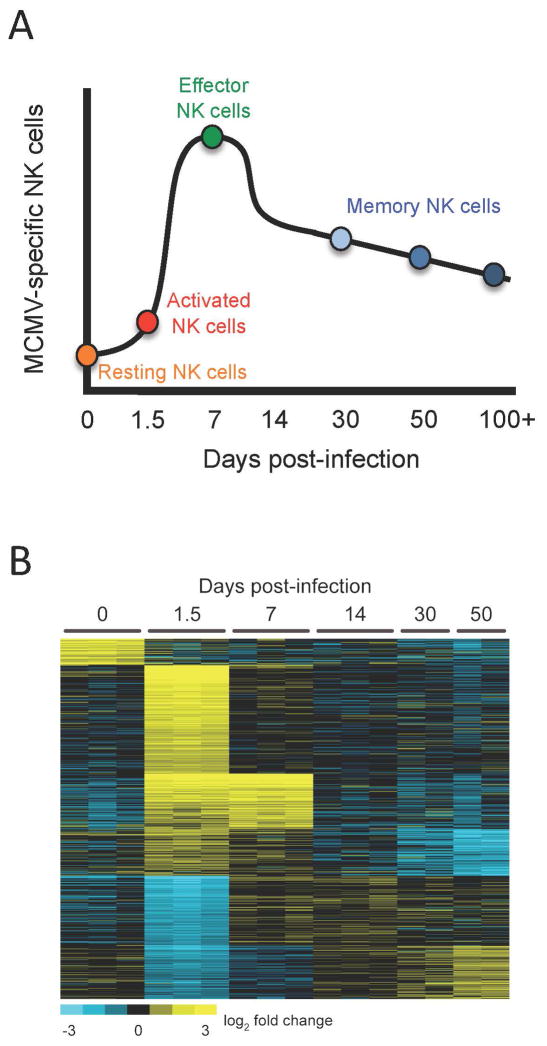
FIGURE 1.
(A) The virus-specific NK cell response to MCMV infection. During MCMV infection, resting Ly49H+ NK cells become activated and undergo an expansion phase resulting in the generation of more effector cells. The expansion phase is followed by the contraction of effectors resulting in long-lived memory NK cells months after initial infection. (B) The gene array profile of different stages of the Ly49H+ NK cell response to MCMV infection. Congenic Ly49H+ NK cells were adoptively transferred prior to MCMV infection, as previously described (33). The transcriptional signature of naïve (day 0), activated (day 1.5), effector (day 7), contracting (day 14), and memory (day 30 and 50) NK cells is unique at each time point following MCMV infection. Ly49H+ NK cells from 3 separate mice at each time point were individually sorted on a FACSAria for RNA isolation (except for day 30 and day 50 time points done in duplicate). Samples were hybridized on the MEEBO microarray platform against reference mouse RNA, as previously described (88). All microarray data are available through the Gene Expression Omnibus under accession number GSE25672.
Characteristics of NK cell memory
How do memory NK cells differ qualitatively from resting NK cells? We know that CD8+ T cells undergo a programmed differentiation during virus infection that results in dramatic changes in their gene expression profile at each stage (42). Similarly, we have recently used gene array technology to profile transcription in Ly49H+ NK cells at different stages following MCMV infection: resting, acute activation, expansion, contraction, and memory maintenance phases (Fig. 1B). Although much work remains to determine the relative contributions of specific genes during each particular phase of the NK cell response, the global signature confirms that the mRNA transcriptional profile of resting NK cells is different from that of memory NK cells (Fig. 1B). Furthermore, the profiles of resting and memory NK cells differ greatly from both early activated and clonally expanded Ly49H+ NK cells, demonstrating that NK cells at each phase of the response to MCMV exhibit a gene profile that is unique and stage-specific (Fig. 1B). The gene array “roadmaps” that immunologists studying memory T cell have generated (42–44), along with the identification of specific transcription factors for T cell differentiation, homeostasis, and survival (45), are useful tools that can guide the search for the factors that govern differentiation of NK cells following activation.
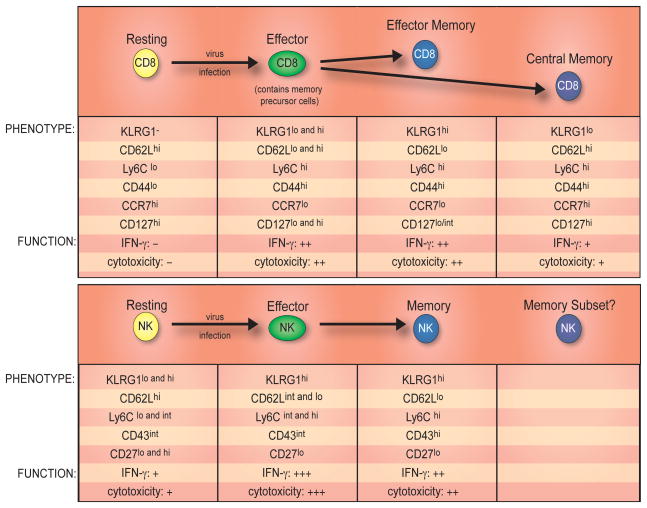
T cell studies have shown that during effector-to-memory cell differentiation different subsets of memory cells are generated, characterized by their anatomical location and expression of cytokine and homing receptors (46–48) (Fig. 2). Can the central memory versus effector memory T cell paradigm be found in memory NK cells as well? Memory NK cells generated during virus infection were all found to highly express KLRG1 (33), an inhibitory receptor recognizing e-cadherins, also predominantly expressed on effector memory CD8+ T cells (Fig. 2). Perhaps memory NK cells resemble this more terminally differentiated subset of memory T cells (Fig. 2). Unlike the overall maintenance of memory CD8+ T cell numbers, which “plateau” following contraction (49), the memory NK cell population contracts similarly to memory CD4+ T cell responses during LCMV infection (50). Although memory NK cells have been shown to self-renew (measured by BrdU uptake over the course of several days) (37), absolute numbers steadily decline and in the absence of secondary infection, memory NK cells in mice are difficult to detect 6 months after MCMV infection; however, this represents a relative time span equivalent to decades in humans (J.C.S. and L.L.L., unpublished observations). Whether the overall long-lived NK cell population experiences preferential survival of the progeny of a subset of memory cells remains to be addressed.
FIGURE 2.
A comparison of CD8+ T cell and NK cell differentiation and memory generation following viral infection. Phenotypic and functional descriptions of resting, effector, and memory T and NK cells are shown. (lo, low; int, intermediate; hi, high)
Like memory T cells, memory NK cells have been shown to both self-renew and mount multiple rounds of expansion and contraction in numbers (37). Future studies will determine whether differences exist in the maintenance of memory NK cells compared to T cells, and which cytokine signals (e.g. IL-7 and IL-15) and stromal cell interactions play an important role in the survival of long-lived NK cell populations. Many other outstanding tasks remain, the foremost of which is to identify a reliable and stable marker (or set of markers) to define memory NK cells, as has been identified for memory T cells (Fig. 2). This will allow direct analysis of long-lived NK cells within the endogenous NK cell population and circumvent the need for adoptive transfer to track antigen-experienced NK cells. Furthermore, we have observed that memory NK cells reside in both lymphoid and non-lymphoid tissue, but determining which soluble factors (cytokines and chemokines) modulate organ- and tissue-specific distribution and phenotype of memory NK cells is warranted. The contribution of stromal elements and other leukocytes (such as dendritic cells and CD4+ T helper cells) towards the generation of memory NK cells requires further elucidation. Lastly, the transcriptional control of NK cell differentiation and memory generation has not been well characterized, in contrast to the case with T cell differentiation. As more pathogen ligands recognized by NK cell receptors are characterized, additional infectious disease models will allow further analysis of the broader principles of NK cell activation, expansion, and memory.
Do memory NK cells exist in humans?
In humans, NK cells also play a crucial role in the response against CMV. In an early study, a patient selectively lacking NK cells, but having normal B and T cells, was found to suffer a life-threatening illness after infection with human CMV (HCMV) (51). Other reports of specific NK cell deficiencies (or NK cell functional deficiencies) in humans (52, 53) similarly describe overwhelming fatal infections during childhood or adolescence due to HCMV and other herpesviruses such as VZV and EBV, demonstrating the importance of NK cells in the immune response against certain viral infections. Functional Ly49 genes do not exist in humans, and thus far there is no evidence that members of the killer-cell immunoglobulin-like receptor (KIR) family, which encode human NK cell receptors analogous to activating and inhibitory Ly49 receptors in mice, can directly recognize HCMV-infected cells. Moreover, CMV is exquisitely species-specific; each CMV has co-evolved and adapted within its own specific mammalian host. The CMV genes that are involved in immune evasion mechanisms, such as genes responsible for downregulation of MHC class I and NKG2D ligands, and surface expression of decoy MHC ligands for NK inhibitory receptors have evolved independently in mouse and human CMV and are tailored to counter the host response (54). Currently, there is no known direct counterpart of the Ly49H-MCMV m157 interaction described between a human NK cell receptor and a HCMV protein, although evidence suggests the possibility of a viral ligand for CD94-NKG2C. Healthy blood donors that have previously been exposed to HCMV have an increased proportion of NK cells bearing the lectin-like heterodimeric receptor CD94-NKG2C compared to HCMV-seronegative donors (55). HCMV has also been reported to induce the expansion of CD94-NKG2C+ NK cells in healthy adults and children, as well as in HIV-infected and leukemia patients (56–59). Moreover, HCMV shapes the NK cell repertoire long after acute infection, as the percentage of CD94-NKG2C+ NK cells remains elevated even after therapeutic intervention and in asymptomatic HCMV+ donors that likely contracted the virus during childhood (55, 58). The expansion of CD94-NKG2C+ NK cells was observed in vitro when human NK cells were co-cultured with HCMV-infected fibroblasts, and was abrogated with a blocking CD94-specific mAb, supporting the involvement of a specific receptor-viral ligand interaction (60). Strikingly, a recent report showed a prolific expansion of CD94-NKG2C+ NK cells (greater than 80% of all NK cells) in an immunodeficient infant in which the NK cell response was carefully monitored during an acute infection with HCMV (61), comparable to the 100-fold expansion observed for mouse Ly49H+ NK cells during MCMV infection. Taken together, the evidence suggests that CD94-NKG2C receptor on human NK cells might represent the functional counterpart of the MCMV-specific Ly49H receptor in mice. This potential antigen-specific recognition might represent an ideal system in which to initiate the search for human memory NK cells.
Similar to Ly49H, the activating CD94-NKG2C receptor complex associates with the adapter protein DAP12 (62, 63). Both CD94-NKG2C and the highly related inhibitory CD94-NKG2A receptor complex recognize HLA-E as their ligand (64, 65). However, the nature of the HCMV-induced ligand that drives CD94-NKG2C+ NK cell expansion remains elusive. Is HLA-E presenting a processed viral peptide recognized by CD94-NKG2C, or does HCMV encode a CD94-NKG2C ligand expressed on the cell surface? Alternatively, could HCMV be inducing a host protein that is subsequently being recognized by CD94-NKG2C? Recently, a report hinted that CD94-NKG2C, but not CD94-NKG2A, binds weakly to the HCMV UL18 glycoprotein (65). Our group has also previously shown that expression of UL18 resulted in increased killing of HCMV-infected cells by human NK cell clones, but this appeared to not involve CD94 (66). Further investigation is warranted to conclusively determine whether a CD94-NKG2C - UL18 interaction is mediating expansion of this NK cell subset during HCMV infection.
The traditional cell surface phenotype defining human NK cells is absence of CD3 and expression of CD56, the 140-kDa isoform of neural cell adhesion molecule (NCAM) (67, 68). Two NK cell subsets have been characterized according to the cell surface density of CD56 and expression of CD16 (low-affinity Fcγ receptor IIIA, FcγRIIIa), with CD56dimCD16bright cells comprising ~90% and CD56brightCD16neg/dim cells constituting ~10% of NK cells in the blood (69). Human NK cells, however, are a heterogeneous population with respect to the expression of KIR, NKG2A, and natural cytotoxic receptors (NCR). Recently, our group and others have examined expression of CD57, a carbohydrate antigen that is expressed on subsets of human NK cells and T cells (70). CD57 is expressed only on a minor fraction of NK cells in fetal tissues or cord blood (which represent the most naïve NK cells in humans), and the percentage of NK cells and T cells expressing CD57 increases with age (71–73). A recent study showed that CD57 is a marker linked to cellular maturity of CD8+ T cells and NK cells, and that it correlates with high cytolytic potential (74). CD56dimCD57− NK cells can become CD56dimCD57+ after stimulation in vitro, during the reconstitution of the immune system in a humanized mouse model and in patients undergoing hematopoietic stem cell transplantation (75, 76). These studies suggest that as mature NK cells differentiate from CD56bright to CD56dim they lose expression of NKG2A, NCRs, CD27, and CD62L, while acquiring CD16, LIR-1 (also named CD85J and LILRB1), Siglec-9, and KIRs, and the final stage of activation involves the acquisition of CD57 (75–77).
Is the CD57 activation marker on human NK cells the equivalent of KLRG1 on the mouse NK cells that have become activated and differentiated into effectors? Because CD8+ T cells expressing CD57 were reported to possess shorter telomeres than CD57− cells and express an effector/memory phenotype (78, 79), it is possible that NK cells expressing CD57 might also represent NK cells that have previously been driven into clonal expansion by encounters with pathogens. The frequency of NK cells expressing CD57 varies in different adult blood donors. Moreover, the frequency of NK cells expressing CD57 within a given NK cell subset in an individual is not uniform; the percentages of CD57+ and CD57− NK cells within the KIR2D, KIR3D, and NKG2A NK cell subsets in a single individual vary considerably, suggesting that NK cells within these subsets exist at different stages of activation or differentiation, likely as a consequence of different exposure to environmental pathogens (75, 76). Further studies are necessary to determine whether the CD94-NKG2C+ NK cells that specifically expand during HCMV infection will upregulate CD57 expression, and whether CD57 will serve as a marker for NK cells that can form a long-lived memory cell population that respond against subsequent HCMV exposure.
As mentioned previously, memory NK cells in mice generated following MCMV infection produce higher amounts of cytokines and degranulate more robustly compared with resting NK cells (33). Surprisingly, the CD57+ NK cell population produced less IFNγ than CD57− NK cells in response to activation by cytokines (i.e. IL-12 and IL18), but had higher amounts of granzymes (75). Although they degranulated similarly in response to stimulation via the majority of activating receptors, CD57+ NK cells responded better to stimulation through CD16 compared to CD57− cells (75). It remains to be determined how CD57+ NK cells bearing a virus-specific receptor such as CD94-NKG2C will behave in vivo during a secondary infection or HCMV reactivation. Thus, although CD57 is a good candidate as a marker of highly mature NK cells that may have been driven to expand in response to pathogens, further studies are necessary to determine if CD57+ NK cells represent a long-lived memory cell population in humans.
Challenges and barriers
The study of human NK cells poses many challenges and barriers, not least of which are the logistical difficulties. First, trafficking of NK cells may contribute to their maturation, function, and longevity, but there is a dearth of information about human NK cells residing in different organs or at the site of infection as most human studies use NK cells from the peripheral blood. Second, there is high variability in the expression of NK cell receptors between individuals, in addition to the extensive polymorphism in the KIRs and their HLA ligands (80–82), thus making the number of donors or patients analyzed in any study crucial. Third, most of the functional data is obtained from ex vivo experiments or with cytokine-activated NK cells, which may alter surface receptor expression and cell function. Finally, assessing NK cell numbers and function in response to infection with pathogens is complicated by the fact that in most cases the dose, route, and time of pathogen exposure are unknown. In addition, treatment of infections with antibiotics and antiviral agents may adversely influence immune responses and NK cell responsiveness.
To demonstrate that a specific human NK cell population responds to a pathogen and that antigen-specific NK cells persist after infection, longitudinal studies are required. For HCMV, like most herpes viruses, the majority of the infections are asymptomatic and occur during childhood or adolescence, therefore determining when infection was initiated is difficult. HCMV persists in approximately 60% of the human population and once infected, the virus is never eliminated, but must constantly be restrained by the immune system. Although HCMV causes only subclinical disease in healthy humans, it can be life-threatening in newborns and immunocompromised or immunosuppressed individuals (83–85). In solid-organ transplant patients, where immunosuppressive drugs are used to prevent graft rejection, more than half suffer from clinical manifestations of CMV infection if they are not treated prophylactically with antiviral drugs (83). Therefore, given that many (~30% for the bone marrow transplant patients) of these people will demonstrate re-activation of HCMV, transplantation patients could pose an interesting cohort for longitudinal study of NK cells in response to HCMV infection in vivo. Even so, caveats due to immunosuppressive therapeutics administered to the transplant patients and/or the immature differentiation state of the NK cells in the bone marrow transplant patients will likely influence the NK cell response to reactivation or infection with HCMV, potentially confounding interpretation of such studies. Moreover, these patients are susceptible to opportunistic infections in addition to HCMV that may influence the NK cell response.
Conclusions
The study of NK cell memory in mice and humans is just beginning. The identification of these long-lived NK cells in mice opens up the possibility that similar populations exist in humans. As specific NK cell responses against many viruses (including herpesviruses, poxviruses, HIV, and influenza) have been described and more NK cell receptor-viral ligand interactions are being elucidated, NK cell subsets can be considered in the design of adoptive immunotherapy regimens against acute viral infection. Given the current interest in developing strategies to apply NK cells as therapeutic agents against a broad range of malignancies (86, 87), and approaches to augment NK cell function during chronic viral infections (such as HIV-1 and hepatitis C virus), NK cells have the potential to be exploited as another branch of immunity that can confer long-term protection through vaccination.
Acknowledgments
The authors thank Carrie Sun for generating the figures. We thank Sue Kaech and members of the Sun and Lanier labs for helpful discussions and review of this manuscript.
J.C.S. and S.L.V. are supported by the Cancer Research Institute and NIH grant AI085034 (J.C.S.). The Howard Hughes Medical Institute supports C.C.K. and J.L.D. L.L.L. is an American Cancer Society Professor and is supported by National Institutes of Health grants AI068129, CA095137, and AI066897.
Abbreviations used in this paper
- MCMV
mouse cytomegalovirus
- HCMV
human cytomegalovirus
- LCMV
lymphocytic choriomeningitis virus
- VZV
varicella zoster virus
- EBV
Epstein Barr virus
- HIV
human immunodeficiency virus
- KIR
killer-cell immunoglobulin-like receptor
- NCR
natural cytotoxicity receptor
- MHC
major histocompatibility complex
- HLA
human leukocyte antigen
References
- 1.Kurtz J. Specific memory within innate immune systems. Trends Immunol. 2005;26:186–192. doi: 10.1016/j.it.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Little TJ, Kraaijeveld AR. Ecological and evolutionary implications of immunological priming in invertebrates. Trends Ecol Evol. 2004;19:58–60. doi: 10.1016/j.tree.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Schmid-Hempel P. Natural insect host-parasite systems show immune priming and specificity: puzzles to be solved. Bioessays. 2005;27:1026–1034. doi: 10.1002/bies.20282. [DOI] [PubMed] [Google Scholar]
- 4.Kurtz J, Franz K. Innate defence: evidence for memory in invertebrate immunity. Nature. 2003;425:37–38. doi: 10.1038/425037a. [DOI] [PubMed] [Google Scholar]
- 5.Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 2007;3:e26. doi: 10.1371/journal.ppat.0030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth O, Sadd BM, Schmid-Hempel P, Kurtz J. Strain-specific priming of resistance in the red flour beetle, Tribolium castaneum. Proc Biol Sci. 2009;276:145–151. doi: 10.1098/rspb.2008.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigues J, Brayner FA, Alves LC, Dixit R, Barillas-Mury C. Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science. 2010;329:1353–1355. doi: 10.1126/science.1190689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 9.Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol. 2004;5:996–1002. doi: 10.1038/ni1114. [DOI] [PubMed] [Google Scholar]
- 10.Sun JC, Lanier LL. Natural killer cells remember: an evolutionary bridge between innate and adaptive immunity? Eur J Immunol. 2009;39:2059–2064. doi: 10.1002/eji.200939435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 12.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 13.Sun JC, Lanier LL. Tolerance of NK cells encountering their viral ligand during development. J Exp Med. 2008;205:1819–1828. doi: 10.1084/jem.20072448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tripathy SK, Keyel PA, Yang L, Pingel JT, Cheng TP, Schneeberger A, Yokoyama WM. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J Exp Med. 2008;205:1829–1841. doi: 10.1084/jem.20072446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson S, Johansson M, Rosmaraki E, Vahlne G, Mehr R, Salmon-Divon M, Lemonnier F, Karre K, Hoglund P. Natural killer cell education in mice with single or multiple major histocompatibility complex class I molecules. J Exp Med. 2005;201:1145–1155. doi: 10.1084/jem.20050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 20.Li P, Burke S, Wang J, Chen X, Ortiz M, Lee SC, Lu D, Campos L, Goulding D, Ng BL, Dougan G, Huntly B, Gottgens B, Jenkins NA, Copeland NG, Colucci F, Liu P. Reprogramming of T cells to natural killer-like cells upon Bcl11b deletion. Science. 2010;329:85–89. doi: 10.1126/science.1188063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Leid M, Rothenberg EV. An early T cell lineage commitment checkpoint dependent on the transcription factor Bcl11b. Science. 2010;329:89–93. doi: 10.1126/science.1188989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikawa T, Hirose S, Masuda K, Kakugawa K, Satoh R, Shibano-Satoh A, Kominami R, Katsura Y, Kawamoto H. An essential developmental checkpoint for production of the T cell lineage. Science. 2010;329:93–96. doi: 10.1126/science.1188995. [DOI] [PubMed] [Google Scholar]
- 23.Cudkowicz G, Stimpfling JH. Induction of Immunity and of Unresponsiveness to Parental Marrow Grafts in Adult F-1 Hybrid Mice. Nature. 1964;204:450–453. doi: 10.1038/204450a0. [DOI] [PubMed] [Google Scholar]
- 24.Gidlund M, Orn A, Wigzell H, Senik A, Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978;273:759–761. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- 25.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberg AH, Hudson L, Shen L, Roitt IM. Antibody-dependent cell-mediated cytotoxicity due to a “null” lymphoid cell. Nat New Biol. 1973;242:111–113. doi: 10.1038/newbio242111a0. [DOI] [PubMed] [Google Scholar]
- 27.Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975;16:230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- 28.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 29.Sendo F, Aoki T, Boyse EA, Buafo CK. Natural occurrence of lymphocytes showing cytotoxic activity to BALB/c radiation-induced leukemia RL male 1 cells. J Natl Cancer Inst. 1975;55:603–609. doi: 10.1093/jnci/55.3.603. [DOI] [PubMed] [Google Scholar]
- 30.Zarling JM, Nowinski RC, Bach FH. Lysis of leukemia cells by spleen cells of normal mice. Proc Natl Acad Sci U S A. 1975;72:2780–2784. doi: 10.1073/pnas.72.7.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glas R, Franksson L, Une C, Eloranta ML, Ohlen C, Orn A, Karre K. Recruitment and activation of natural killer (NK) cells in vivo determined by the target cell phenotype. An adaptive component of NK cell-mediated responses. J Exp Med. 2000;191:129–138. doi: 10.1084/jem.191.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 33.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 35.Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nat Immunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 36.Smith HR, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, Scalzo AA, Fremont DH, Yokoyama WM. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci U S A. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun JC, Beilke JN, Lanier LL. Immune memory redefined: characterizing the longevity of natural killer cells. Immunol Rev. 2010;236:83–94. doi: 10.1111/j.1600-065X.2010.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jamieson AM, Isnard P, Dorfman JR, Coles MC, Raulet DH. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J Immunol. 2004;172:864–870. doi: 10.4049/jimmunol.172.2.864. [DOI] [PubMed] [Google Scholar]
- 40.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med. 2003;197:967–976. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koka R, Burkett PR, Chien M, Chai S, Chan F, Lodolce JP, Boone DL, Ma A. Interleukin (IL)-15R[alpha]-deficient natural killer cells survive in normal but not IL-15R[alpha]-deficient mice. J Exp Med. 2003;197:977–984. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 43.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Rutishauser RL, Kaech SM. Generating diversity: transcriptional regulation of effector and memory CD8 T-cell differentiation. Immunol Rev. 2010;235:219–233. doi: 10.1111/j.0105-2896.2010.00901.x. [DOI] [PubMed] [Google Scholar]
- 46.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 47.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 48.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 49.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 50.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 51.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 52.Etzioni A, Eidenschenk C, Katz R, Beck R, Casanova JL, Pollack S. Fatal varicella associated with selective natural killer cell deficiency. J Pediatr. 2005;146:423–425. doi: 10.1016/j.jpeds.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 53.Orange JS. Human natural killer cell deficiencies. Curr Opin Allergy Clin Immunol. 2006;6:399–409. doi: 10.1097/ACI.0b013e3280106b65. [DOI] [PubMed] [Google Scholar]
- 54.Sun JC, Lanier LL. The Natural Selection of Herpesviruses and Virus-specific NK Receptors. Viruses. 2009;1:362–382. doi: 10.3390/v1030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guma M, Angulo A, Vilches C, Gomez-Lozano N, Malats N, Lopez-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 56.Guma M, Cabrera C, Erkizia I, Bofill M, Clotet B, Ruiz L, Lopez-Botet M. Human cytomegalovirus infection is associated with increased proportions of NK cells that express the CD94/NKG2C receptor in aviremic HIV-1-positive patients. J Infect Dis. 2006;194:38–41. doi: 10.1086/504719. [DOI] [PubMed] [Google Scholar]
- 57.Mela CM, Goodier MR. The contribution of cytomegalovirus to changes in NK cell receptor expression in HIV-1-infected individuals. J Infect Dis. 2007;195:158–159. doi: 10.1086/509811. author reply 159–160. [DOI] [PubMed] [Google Scholar]
- 58.Petersen L, Roug AS, Skovbo A, Thysen AH, Eskelund CW, Hokland ME. The CD94/NKG2C-expressing NK cell subset is augmented in chronic lymphocytic leukemia patients with positive human cytomegalovirus serostatus. Viral Immunol. 2009;22:333–337. doi: 10.1089/vim.2009.0032. [DOI] [PubMed] [Google Scholar]
- 59.Monsivais-Urenda A, Noyola-Cherpitel D, Hernandez-Salinas A, Garcia-Sepulveda C, Romo N, Baranda L, Lopez-Botet M, Gonzalez-Amaro R. Influence of human cytomegalovirus infection on the NK cell receptor repertoire in children. Eur J Immunol. 2010;40:1418–1427. doi: 10.1002/eji.200939898. [DOI] [PubMed] [Google Scholar]
- 60.Guma M, Budt M, Saez A, Brckalo T, Hengel H, Angulo A, Lopez-Botet M. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107:3624–3631. doi: 10.1182/blood-2005-09-3682. [DOI] [PubMed] [Google Scholar]
- 61.Kuijpers TW, Baars PA, Dantin C, van den Burg M, van Lier RA, Roosnek E. Human NK cells can control CMV infection in the absence of T cells. Blood. 2008;112:914–915. doi: 10.1182/blood-2008-05-157354. [DOI] [PubMed] [Google Scholar]
- 62.Smith KM, Wu J, Bakker AB, Phillips JH, Lanier LL. Ly-49D and Ly-49H associate with mouse DAP12 and form activating receptors. J Immunol. 1998;161:7–10. [PubMed] [Google Scholar]
- 63.Lanier LL, Corliss B, Wu J, Phillips JH. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity. 1998;8:693–701. doi: 10.1016/s1074-7613(00)80574-9. [DOI] [PubMed] [Google Scholar]
- 64.Braud VM, Allan DS, O’Callaghan CA, Soderstrom K, D’Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI, Phillips JH, Lanier LL, McMichael AJ. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 65.Kaiser BK, Pizarro JC, Kerns J, Strong RK. Structural basis for NKG2A/CD94 recognition of HLA-E. Proc Natl Acad Sci U S A. 2008;105:6696–6701. doi: 10.1073/pnas.0802736105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leong CC, Chapman TL, Bjorkman PJ, Formankova D, Mocarski ES, Phillips JH, Lanier LL. Modulation of natural killer cell cytotoxicity in human cytomegalovirus infection: the role of endogenous class I major histocompatibility complex and a viral class I homolog. J Exp Med. 1998;187:1681–1687. doi: 10.1084/jem.187.10.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lanier LL, Testi R, Bindl J, Phillips JH. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J Exp Med. 1989;169:2233–2238. doi: 10.1084/jem.169.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caligiuri MA, Murray C, Levine H, Longtine JA, Ritz J. Clonal evidence for the induction of NKH1 on activated human thymocytes. Functional changes associated with antigen expression. Eur J Immunol. 1989;19:1735–1739. doi: 10.1002/eji.1830190931. [DOI] [PubMed] [Google Scholar]
- 69.Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986;136:4480–4486. [PubMed] [Google Scholar]
- 70.Lanier LL, Le AM, Phillips JH, Warner NL, Babcock GF. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983;131:1789–1796. [PubMed] [Google Scholar]
- 71.Abo T, Miller CA, Balch CM. Characterization of human granular lymphocyte subpopulations expressing HNK-1 (Leu-7) and Leu-11 antigens in the blood and lymphoid tissues from fetuses, neonates and adults. Eur J Immunol. 1984;14:616–623. doi: 10.1002/eji.1830140707. [DOI] [PubMed] [Google Scholar]
- 72.Tilden AB, Grossi CE, Itoh K, Cloud GA, Dougherty PA, Balch CM. Subpopulation analysis of human granular lymphocytes: associations with age, gender and cytotoxic activity. Nat Immun Cell Growth Regul. 1986;5:90–99. [PubMed] [Google Scholar]
- 73.Merino J, Martinez-Gonzalez MA, Rubio M, Inoges S, Sanchez-Ibarrola A, Subira ML. Progressive decrease of CD8high+ CD28+ CD57− cells with ageing. Clin Exp Immunol. 1998;112:48–51. doi: 10.1046/j.1365-2249.1998.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chattopadhyay PK, Betts MR, Price DA, Gostick E, Horton H, Roederer M, De Rosa SC. The cytolytic enzymes granyzme A, granzyme B, and perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J Leukoc Biol. 2009;85:88–97. doi: 10.1189/jlb.0208107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF, Lanier LL. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK cell subset. Blood. 2010 doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bjorkstrom NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, Bjorklund AT, Flodstrom-Tullberg M, Michaelsson J, Rottenberg ME, Guzman CA, Ljunggren HG, Malmberg KJ. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK cell differentiation uncoupled from NK cell education. Blood. 2010 doi: 10.1182/blood-2010-04-281675. [DOI] [PubMed] [Google Scholar]
- 77.Beziat V, Descours B, Parizot C, Debre P, Vieillard V. NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Le Priol Y, Puthier D, Lecureuil C, Combadiere C, Debre P, Nguyen C, Combadiere B. High cytotoxic and specific migratory potencies of senescent CD8+ CD57+ cells in HIV-infected and uninfected individuals. J Immunol. 2006;177:5145–5154. doi: 10.4049/jimmunol.177.8.5145. [DOI] [PubMed] [Google Scholar]
- 79.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28−CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J Immunol. 1996;156:3587–3590. [PubMed] [Google Scholar]
- 80.Middleton D, Gonzelez F. The extensive polymorphism of KIR genes. Immunology. 2010;129:8–19. doi: 10.1111/j.1365-2567.2009.03208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McQueen KL, Parham P. Variable receptors controlling activation and inhibition of NK cells. Curr Opin Immunol. 2002;14:615–621. doi: 10.1016/s0952-7915(02)00380-1. [DOI] [PubMed] [Google Scholar]
- 82.Bashirova AA, Martin MP, McVicar DW, Carrington M. The killer immunoglobulin-like receptor gene cluster: tuning the genome for defense. Annu Rev Genomics Hum Genet. 2006;7:277–300. doi: 10.1146/annurev.genom.7.080505.115726. [DOI] [PubMed] [Google Scholar]
- 83.Fisher RA. Cytomegalovirus infection and disease in the new era of immunosuppression following solid organ transplantation. Transpl Infect Dis. 2009;11:195–202. doi: 10.1111/j.1399-3062.2009.00372.x. [DOI] [PubMed] [Google Scholar]
- 84.Reeves M, Sinclair J. Aspects of human cytomegalovirus latency and reactivation. Curr Top Microbiol Immunol. 2008;325:297–313. doi: 10.1007/978-3-540-77349-8_17. [DOI] [PubMed] [Google Scholar]
- 85.Britt W. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr Top Microbiol Immunol. 2008;325:417–470. doi: 10.1007/978-3-540-77349-8_23. [DOI] [PubMed] [Google Scholar]
- 86.Moretta A, Locatelli F, Moretta L. Human NK cells: from HLA class I-specific killer Ig-like receptors to the therapy of acute leukemias. Immunol Rev. 2008;224:58–69. doi: 10.1111/j.1600-065X.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 87.Vitale M, Della Chiesa M, Carlomagno S, Romagnani C, Thiel A, Moretta L, Moretta A. The small subset of CD56brightCD16− natural killer cells is selectively responsible for both cell proliferation and interferon-gamma production upon interaction with dendritic cells. Eur J Immunol. 2004;34:1715–1722. doi: 10.1002/eji.200425100. [DOI] [PubMed] [Google Scholar]
- 88.Kim CC, Parikh S, Sun JC, Myrick A, Lanier LL, Rosenthal PJ, DeRisi JL. Experimental malaria infection triggers early expansion of natural killer cells. Infect Immun. 2008;76:5873–5882. doi: 10.1128/IAI.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]