Abstract
Objective
Prevalence of hypertension was examined in a widely dispersed (45 110 km2) representative group of Ladakhi in Northern India. The influence of hypoxic environment of wide-ranged altitude (2600–4900 m) and lifestyle change on hypertension was studied.
Methods
2800 participants (age 20–94 years) were enrolled. Systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure of ≥90 mm Hg and/or taking current anti-hypertensive medicine was defined as hypertension. Height and weight for body mass index and SpO2 were examined. The rural population comprised six subdivisions with a distinct altitude, dietary and occupational pattern. Participants in the urban area of Leh consist of two groups, that is, migrants settled in Leh from the Changthang nomadic area, and dwellers born in Leh. The prevalence of hypertension in the two groups was compared with that in the farmers and nomads in rural areas. The effects of ageing, hypoxia, dwelling at high altitude, obesity, modernised occupation, dwelling in an urban area, and rural-to-urban migration to hypertension were analysed by multiple logistic regression.
Results
The prevalence of hypertension was 37.0% in all participants and highest in migrants settled in Leh (48.3%), followed by dwellers born in Leh town (41.1%) compared with those in rural areas (33.5). The prevalence of hypertension in nomads (all: 27.7%, Tibetan/Ladakhi: 19.7/31.9%)) living at higher altitude (4000–4900 m) was relatively low. The associated factors with hypertension were ageing, overweight, dwelling at higher altitude, engagement in modernised sedentary occupations, dwelling in urban areas, and rural-to-urban migration. The effects of lifestyle change and dwelling at high altitude were independently associated with hypertension by multivariate analysis adjusted with confounding factors.
Conclusions
Socioeconomic and cultural factors play a big role with the effect of high altitude itself on high prevalence of hypertension in highlanders in Ladakh.
Keywords: ALTITUDE MEDICINE, EPIDEMIOLOGY
Strengths and limitations of this study.
This study examined most of the socioeconomic environmental factors known to influence hypertension in a population of different distinct geographical subdivisions of a high-altitude region. Though we did not carry out a nutritional survey in all the participants, overweight was a decisive factor for hypertension according to lifestyle change.
This study showed the influence of ageing, overweight, modernised sedentary occupations, rural-to-urban migration and dwelling in urban areas to hypertension as well as the effect of altitude by multivariate analysis.
This study did not look into the genetic factors, as environmental and genetic factors may contribute to regional and racial variations of blood pressure and the prevalence of hypertension.
Introduction
Systemic arterial hypertension at high altitude has evoked great interest among high-altitude researchers as well as in sojourners and natives. There have been conflicting reports with investigators generally reporting a slight increase in the blood pressure level soon after arrival at high altitude1 2 and investigators reporting no such change3 4 or a decrease followed by an increase.5 6 There is no standard way of treating hypertension at high altitude for sojourners till now.7 8 Similar contradictory views also exist between the investigators of the two high-altitude continents regarding the blood pressure status of the high-altitude natives. Studies done in Spiti India (4000 m) show a lower prevalence of hypertension.9 Andean residents are reported to have low prevalence of hypertension1 10 11 while the prevalence of hypertension in Tibet Lhasa was found to be higher than that of Han migrants residing in Tibet.12 Recent reports showed that the prevalence of hypertension was higher in Tibetan highlanders13 14 than in Chinese lowlanders.15
The risk of developing hypertension may depend on socioeconomic factors, as well as geographic and racial differences. It is in the backdrop of this difference in opinion that we planned this study in Ladakh, one of the highest inhabited regions in the northernmost part of India. The population of the two districts of Ladakh (Leh and Kargil) was about 270 000 (Leh: 130 000, Kargil: 140 000) in 2011 by Census.16 77% of the population in Leh are Buddhists and 80% of the Kargil population are Muslims. Spread over 45 110 km2, sandwiched between Karakoram in the north and Trans-Himalaya in the south and 80% comprising of a rural population with many villages high up in the mountains remaining inaccessible during winter, logistics for conducting a comprehensive epidemiological study representative of the whole population is formidable. The purpose of the study is twofold: first, to determine the prevalence of hypertension in different geographical subdivisions of this widely dispersed high-altitude district (from a median high 2500∼ to very high ∼4500 m), and second, which factors among the altitude, occupation, socioeconomic and lifestyle play a predominant role in association with hypertension.
Methods
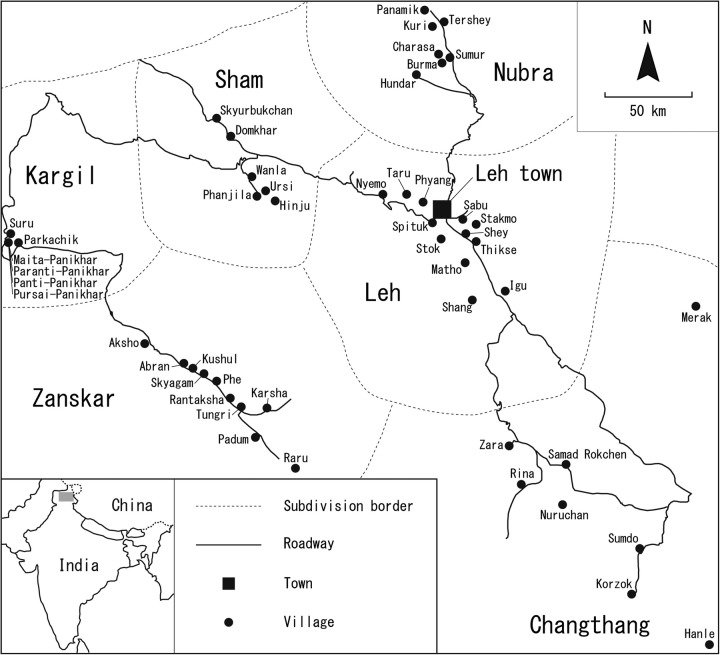
This cross-sectional epidemiological study was carried out from 2007 to 2011. A total of 2800 participants aged between 20 and 94 years were examined. Figure 1 shows the map of Ladakh region showing all the villages in the subdivisions where the study was conducted. A two-stage stratified sampling method was used to select a representative sample of the adult population over 20 years of age. The population was first stratified as urban versus rural and then in the rural sector into six geographical areas (subdivisions). Each geographical subdivision has different characteristics in altitude, occupation, dietary habits and socioeconomic conditions as well as separate administrative blocks (table 1). Migrants from the rural population now settled in the Leh town subdivision since the 1970s were included in the urban population as they have adopted a lifestyle similar to the city dwellers. The Tata Institute of Social Sciences Mumbai (TISS) and Ladakh Autonomous hill development council (LAHDC) conducted a house-to-house survey of the total population of Leh (urban population) in 2007 for developing Ladakhi villages in the region.17 Since the data from this census were the latest, we corrected and used this population survey list (age group 20–90 years) to draw our sample of urban population for the study. The list of 2000 eligible participants was representative of the age and gender structure of a Ladakhi family and they were invited as the volunteer participants to the research centre in Leh town. In the rural villages, all men and women aged 20 years or more were announced in the collaboration of health staff and village leaders. We carried out health checks on the volunteer participants in health centres or community halls in the rural villages.
Figure 1.
Map of Ladakh Region showing all the field sites. The map of Ladakh region showing all the villages in the subdivisions where the study was conducted.
Table 1.
Characteristics of the subdivisions
| Urban/rural | Subdivision | Altitude (metres above MSL) | Livelihood |
|---|---|---|---|
| Urban | Leh town (including colonies of migrants) | 3300–3600 | Urban lifestyle |
| Rural | Leh block villages | 3000–3700 | Farmer |
| Nubra | 2600–3000 | Farmer | |
| Kargil (Panikhar and Parkachik) | 2600–3100 | Farmer | |
| Sham | 2700–3900 | Farmer | |
| Zanskar | 3500–3900 | Farmer and cattle rearing | |
| Changthang | 4000–4900 | Livestock rearing nomads |
MSL, mean sea level.
There were no criteria for exclusion except absentees and critical and terminal illness patients who cannot report to the study centre to complete the study. Participants in Leh town subdivision were classified into two groups, that is, migrants settled in Leh town from Changthang area, and dwellers in Leh town. The former consisted of Tibetan and Ladakhi nomads. The latter consisted of Tibetans born in Leh, and other Ladakhi people, almost of whom were born in Leh, including some migrants from rural areas (non-Changthang).
The rural population was subdivided into six subdivisions as each subdivision had distinct characteristics which could influence the outcome.
Leh block subdivision comprises nearly 12 villages within 40 km of Leh town at an altitude varying between 3000 and 3700 m. The occupations of those living here are a mix of farming and modernised sedentary work. Nubra subdivision is in the north of Ladakh about 120 km from Leh after crossing Khardong Pass (5400 m), one of the highest motorable roads in the world. We studied the population of seven villages here. The subdivision is located on the banks of Shyok and Nubra rivers between the Karokoram and Ladakh ranges of mountains. People are predominantly farmers and the altitude of the valley generally is around 2600–3000 m. Kargil subdivision (Panikhar and Parkachik) is a green belt in Kargil district and is a fertile farming area on the Suru river. However, fruit trees are not cultivated here. The population is mainly Muslim and the altitude is 2600–3100 m. We studied the population of six villages representative of this subdivision. Sham (Khalse) subdivision is wide-ranged in altitude (2700–3900 m), generally more fertile and many of the villages have fruit trees like apricot, apple and almond. We studied six representative villages in this subdivision. Zanskar subdivision is a remote region on the trans Himalayan range of mountain which remains closed from the rest of the world for 6 months in a year due to heavy snowfall. Though people do farming, yet the harsh weather is not conducive for productive farming. Fresh fruit and vegetables are very meagre here. People rear cattle, which forms their secondary source of income by selling dairy products. The altitude of the subdivision is 3500–3900 m. We studied 10 villages representative of this subdivision. Changthang subdivision is the biggest and highest plateau (Altitude 4000–4900 m). The population is generally nomadic, moving from pasture to pasture every 3 months along with their cattle and livestock and living in Yak wool woven tents. Life is very hard for them because of the high altitude and severe cold. Farming is not possible, and fresh fruit and vegetables fruits are not available to them throughout the year. Meat, barley flour and local tea are their staple diet. We studied six villages representative of the subdivision.
The occupation was interviewed from all the participants and classified into four groups: farmer, nomad, sedentary worker and others (housewife, manual labourer, monk, retired sedentary worker and no job). A full-time housewife was regarded as a housewife. A housewife who also worked as a nomad or farmer was classified as a nomad or farmer. People engaged in work closely associated with an urban lifestyle are classified into sedentary workers consisting of office worker, business person, shopkeeper, taxi driver, government officer, travel agent, teacher and so on.
The procedure for obtaining informed consent was approved by the Institutional review board of the Ladakh institute of prevention and the District ethical committee, Leh, Ladakh and Research Institute for Humanity and Nature, Kyoto, Japan. The participants attended the village medical aid centre or the village community centre. Anthropometric measurements including weight and height were obtained using standard techniques. The body mass index (BMI) was calculated using the formula, weight(kg)/(height(m))2. Blood pressure was measured in an arm using an automatic device (HEM 7000; OMRON Life Science Co. Ltd, Kyoto, Japan) based on the cuff oscillometric principle, and its accuracy has been validated in previous studies.18–20 Oxyhaemoglobin saturation (SpO2) was measured by a pulse oximeter (PULSOX-300; KONICA MINOLTA Co. Ltd, Tokyo, Japan). Blood pressure and SpO2 were measured twice after taking at least a 5 min rest in a sitting position and the mean of systolic blood pressure (SBP), diastolic blood pressure (DBP) and SpO2 was calculated. SBP ≥140 mm Hg and/or DBP of ≥90 mm Hg and/or taking current anti-hypertensive medicine was defined as hypertension.21 The mean rate of current antihypertensive medication was 2.1%.
The age of the participants was confirmed with reference to a carefully prepared cross tabulation correlating their date of birth with the animal year, which the rural population always remembered, and to historical sentinel events in case of elderly participants.
Statistical analysis
χ2 Test, Student's t-test and one-way analysis of variance were conducted for the analysis of the prevalence rate of hypertension or overweight (BMI ≥25), mean SBP, DBP, BMI and SpO2. The associations of hypertension with the above confounding factors including altitude, ageing, sex, obesity, occupation and dwelling area were analysed by multiple logistic regression. Hypertension as the dependent variable was defined as SBP ≥140 mm Hg and/or DBP of ≥90 mm Hg and/or taking current antihypertensive medicine.21 SPSS V.17.0 (SPSS Inc., Chicago, Illinois, USA) was used for the analysis. A statistically significant level was p<0.05.
Results
A total of 2800 participants aged between 20 and 94 years were examined between 2007 and 2011.
Table 2 shows the characteristics of all variables and those associated with hypertension were overviewed. We found a 37.0% crude prevalence rate in the total Ladakhi population of both men and women. Male and older people, as well as those with overweight, had more prevalence of hypertension, but SpO2 was not associated with hypertension. Dwelling at an altitude of 3000–3999 m had more prevalence of hypertension compared with altitude below 3000 or above 4000 m. People dwelling in urban areas had more prevalence of hypertension compared with those in rural areas. Nomads had lower prevalence of hypertension compared with farmers or sedentary workers.
Table 2.
Characteristics of all variables and those associated with hypertension in Ladakh region
| All | Hypertension (+) | Hypertension (−) | p Value | |
|---|---|---|---|---|
| n | 2800 | 1037 | 1763 | |
| Per cent | 37.0 (35.2 to 38.8) | 63.0 (61.2 to 64.8) | ||
| Male (%) | 44.3 (41.8 to 46.8) | 46.9 (43.9 to 49.9) | 42.8 (40.5 to 45.1) | 0.03 |
| age (years) | 53.8±15.0 | 60.1±13.8 | 50.1±14.4 | <0.0001 |
| weight (kg) | 55.3±11.1 | 57.4±12.2 | 54.1±10.2 | <0.0001 |
| BMI | 22.6±3.6 | 23.6±3.9 | 22.0±3.3 | <0.0001 |
| Overweight (BMI ≥25) (%) | 24.4 (22.8 to 26.0) | 34.9 (32.0 to 37.8) | 18.2 (16.4 to 20.0) | <0.0001 |
| SpO2 (%) | 89.7±5.2 | 89.5±5.4 | 89.8±5.2 | ns |
| SpO2 <89 (%) | 32.5 (30.8 to 34.2) | 32.1 (29.3 to 34.9) | 32.7 (30.5 to 34.9) | ns |
| SBP (mm Hg) | 130.9±23.2 | 153.8±19.9 | 117.5±11.7 | <0.0001 |
| DBP (mm Hg) | 82.5±13.4 | 94.6±11.2 | 75.4±8.5 | <0.0001 |
| Altitude (m) | 3514.4±432.2 | 3524.6±388.6 | 3508.3±455.9 | ns |
| n | ||||
| Altitude (n=2800), m | Per cent | Per cent | <0.0001 | |
| 2500–2999 | 417 | 27.1 (22.8 to 31.4) | 72.9 (68.6 to 77.2) | |
| 3000–3499 | 428 | 37.4 (32.8 to 42.0) | 62.6 (58.0 to 67.2) | |
| 3500–3999 | 1604 | 40.8 (38.4 to 43.2) | 59.2 (56.8 to 61.6) | |
| 4000–4499 | 174 | 30.5 (23.7 to 37.3) | 69.5 (62.7 to 76.3) | |
| 4500–4999 | 177 | 32.2 (25.3 to 39.1) | 67.8 (60.9 to 74.7) | |
| Dwelling area (n=2800) | Per cent | Per cent | <0.0001 | |
| Rural areas | 1798 | 33.5 (31.3 to 35.7) | 66.5 (64.3 to 68.7) | |
| Leh block (3000–3700 m) | 349 | 33.0 (28.1 to 37.9) | 67.0 (62.1 to 71.9) | |
| Nubra (2600–3000 m) | 248 | 27.8 (22.2 to 33.4) | 72.2 (66.6 to 77.8) | |
| Kargil (2600–3100 m) | 115 | 24.3 (16.5 to 32.1) | 75.7 (67.9 to 83.5) | |
| Sham (2700–3900 m) | 451 | 39.2 (34.7 to 43.7) | 60.8 (56.3 to 65.3) | |
| Zanskar (3500–3900 m) | 284 | 36.3 (30.7 to 41.9) | 63.7 (58.1 to 69.3) | |
| Changthang (4000–4900 m) | 351 | 31.3 (26.4 to 36.2) | 68.7 (63.8 to 73.6) | |
| Urban area: Leh town (3300–3600 m) | 1002 | 43.4 (40.3 to 46.5) | 56.6 (53.5 to 59.7) | |
| Dwellers in Leh town* | 683 | 41.1 (37.4 to 44.8) | 58.9 (55.2 to 62.6) | |
| Migrants from Changthang | 319 | 48.3 (42.8 to 53.8) | 51.7 (46.2 to 57.2) | |
| Occupation (n=2800) | Per cent | Per cent | <0.0001 | |
| Farmer | 1247 | 36.6 (33.9 to 39.3) | 63.4 (60.7 to 66.1) | |
| Nomad | 220 | 27.7(21.8 to 33.6) | 72.3 (66.4 to 78.2) | |
| Sedentary worker | 549 | 37.3 (33.3 to 41.3) | 62.7 (58.7 to 66.7) | |
| Others | 784 | 40.2 (36.8 to 43.6) | 59.8 (56.4 to 63.2) | |
| Housewife | 325 | 42.5 (37.1 to 47.9) | 57.5 (52.1 to 62.9) | |
| Manual labourer | 63 | 14.3 (5.7 to 22.9) | 85.7 (77.1 to 94.3) | |
| Monk | 157 | 36.9 (29.4 to 44.4) | 63.1 (55.6 to 70.6) | |
| No job | 138 | 44.2 (35.9 to 52.5) | 55.8 (47.5 to 64.1) | |
| Retired sedentary | 101 | 48.5 (38.8 to 58.2) | 51.5 (41.8 to 61.2) | |
Mean±SD, % (95% CI).
p; χ2 Test for the comparison of the rate of variables, and Student's t test for the comparison of the mean of variables between hypertension and non-hypertension.
*Almost born in Leh with some migrants from no-Changthang areas.
BMI, body mass index; DBP, diastolic blood pressure; ns, not significant; SBP, systolic blood pressure; SpO2, oxyhaemoglobin saturation measured by a pulse oximeter.
Table 3 shows the participants surveyed and the prevalence rates of hypertension, mean SBP, DBP, BMI, rate of overweight (BMI ≥25) and mean SpO2 according to sex and age groups in Ladakh region. Prevalence rates of hypertension, mean SBP and DBP increased significantly with ageing in men and women. Up to the age of 60 years, men tend to have higher blood pressure than women; however, there were no significant differences between men and women aged 60 years or above. The prevalence of overweight was highest (28.5%) in the 40–59 age group and men had a higher prevalence rate of overweight than women up to 75 years. Mean SpO2 decreased significantly with ageing in both men and women.
Table 3.
Prevalence of hypertension and related variables according to sex and age groups in Ladakh region
| Age group (years) |
||||||
|---|---|---|---|---|---|---|
| 20–39 | 40–59 | 60–74 | 75– | p† | All | |
| Male (n) | 217 | 489 | 396 | 138 | 1240 | |
| Female (n) | 288 | 709 | 448 | 115 | 1560 | |
| All (n) | 505 | 1198 | 844 | 253 | 2800 | |
| Hypertension (%) | ||||||
| Male | 18.4 (13.2–23.6) | 34.2 (30.0–38.4) | 48.2 (43.3–53.1) | 63.8 (55.8–71.8) | <0.0001 | 39.2 (36.5–41.9)* |
| Female | 12.5 (8.7–16.3) | 29.9 (26.5–33.3) | 50.4 (45.8–55.0) | 67.0 (58.4–75.6) | <0.0001 | 35.3 (32.9–37.7) |
| All | 15.1 (12.0–18.2) | 31.6 (29.0–34.2) | 49.4 (46.0–52.8) | 65.2 (59.3–71.1) | <0.0001 | 37.0 (35.2–38.8) |
| SBP (mm Hg) | ||||||
| Male | 122.2±14.3**** | 127.7±18.0* | 138.9±22.6 | 149.0±26.1 | <0.0001 | 132.7±21.7*** |
| Female | 116.0±14.2 | 125.3±19.5 | 138.8±25.5 | 153.7±32.8 | <0.0001 | 129.5±24.2 |
| All | 118.7±14.5 | 126.3±18.9 | 138.8±24.2 | 151.1±29.4 | <0.0001 | 130.9±23.2 |
| DBP (mm Hg) | ||||||
| Male | 78.5±11.4 | 83.4±12.5*** | 85.2±12.8 | 87.5±14.2 | <0.0001 | 83.6±12.9*** |
| Female | 76.4±11.9 | 80.9±12.0 | 84.5±14.9 | 88.4±17.3 | <0.0001 | 81.7±13.7 |
| All | 77.3±11.7 | 81.9±12.3 | 84.9±14.0 | 87.9±15.7 | <0.0001 | 82.5±13.4 |
| BMI | ||||||
| Male | 22.4±3.2*** | 23.3±3.6* | 23.2±3.4**** | 22.4±3.4 | 0.0017 | 23.0±3.5**** |
| Female | 21.4±3.3 | 22.8±3.7 | 22.1±3.7 | 22.1±3.7 | <0.0001 | 22.3±3.7 |
| All | 21.8±3.3 | 23.0±3.7 | 22.6±3.6 | 22.3±3.5 | <0.0001 | 22.6±3.6 |
| BMI ≥25 (%) | ||||||
| Male | 22.6 (17.0–28.2)* | 31.7 (27.6–35.8)* | 28.4 (24.0–32.8)** | 19.6 (13.0–26.2) | 0.0098 | 27.7 (25.2–30.2)*** |
| Female | 14.6 (10.5–18.7) | 26.2 (23.0–29.4) | 19.5 (15.8–23.2) | 20.9 (13.5–28.3) | 0.0003 | 21.8 (19.8–23.8) |
| All | 18.0 (14.6–21.4) | 28.5 (25.9–31.1) | 23.6 (20.7–26.5) | 20.2 (15.3–25.1) | <0.0001 | 24.4 (22.8–26.0) |
| SpO2 (%) | ||||||
| Male | 90.8±4.4* | 90.4±4.6 | 89.1±5.3** | 89.0±5.4** | <0.0001 | 89.9±5.0 |
| Female | 91.6±3.6 | 90.3±4.8 | 87.7±6.4 | 86.6±6.5 | <0.0001 | 89.5±5.0 |
| All | 91.2±4.0 | 90.4±4.7 | 88.3±5.9 | 87.9±6.0 | <0.0001 | 89.7±5.2 |
| SpO2 <89 (%) | ||||||
| Male | 25.7 (19.9–31.5)* | 29.0 (25.0–33.0) | 37.1 (32.3–41.9)** | 39.4 (31.2–47.6)* | 0.0029 | 32.2 (29.6–34.8) |
| Female | 17.7 (13.3–22.1) | 27.2 (23.9–30.5) | 46.3 (41.7–50.9) | 52.2 (43.1–61.3) | <0.0001 | 32.8 (30.5–35.1) |
| All | 21.1 (17.5–24.7) | 27.9 (25.4–30.4) | 42.0 (38.7–45.3) | 45.2 (36.1–54.3) | <0.0001 | 32.5 (30.8–34.2) |
p†; χ2 Test for the comparison of the prevalence of hypertension and BMI ≥25 (%) among the 4 age groups, and ANOVA for the comparison of mean of SBP, DBP, BMI and SpO2 among the 4 age groups in the whole population (n=2800).
*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001: χ2 test for the comparison of the prevalence of hypertension and BMI ≥25 (%) and SpO2 <89 (%) between men and women, and Student's t test for the comparison of mean of SBP, DBP, BMI and SpO2 between men and women in each age group.
ANOVA, analysis of variance;BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; SpO2, oxyhaemoglobin saturation measured by a pulse oximeter.
Table 4 shows the crude and age-standardised prevalence rates of hypertension, and overweight (%) in seven subdivisions in Ladakh region in each age group. As the mean age was different among the participants of the seven subdivisions (ANOVA, analysis of variance; p<0.0001), age-standardised prevalence rates were calculated.
Table 4.
Prevalence of hypertension and related variables in different age groups in each subdivision in Ladakh region
| Age group (years) |
All | |||||
|---|---|---|---|---|---|---|
| 20–39 | 40–59 | 60–74 | 75– | p Value | ||
| Leh (n=1002) (mean 51.9±15.5 years) | n=223 | n=447 | n=245 | n=87 | ||
| Hypertension, % (Age-standardised prevalence rate, %) |
19.7 (14.5–24.9) | 41.6 (37.0–46.2) | 58.8 (52.6–65.0) | 70.1 (60.5–79.7) | <0.0001 | 43.4 (40.3–46.5) 45.5 (42.4–48.6) |
| BMI ≥25 (%) | 20.6 (15.3–25.9) | 40.7 (36.1–45.3) | 34.8 (28.8–40.8) | 26.4 (17.1–35.7) | <0.0001 | 33.6 (30.7–36.5) |
| Leh block (n=349) (mean 55.6±16.1 years) | n=60 | n=127 | n=123 | n=39 | ||
| Hypertension,% (Age-standardised prevalence rate,%) |
6.7 (0.4–13.0) | 22.0 (14.8–29.2) | 48.0 (39.2–56.8) | 61.5 (46.2–76.8) | <0.0001 | 33.0 (28.1–37.9) 30.7 (25.9–35.5) |
| BMI ≥25 (%) | 30.0 (18.4–41.6) | 34.6 (26.3–42.9) | 35.0 (26.6–43.4) | 23.1 (9.9–36.3) | ns | 32.7 (27.8–37.6) |
| Nubra (n=248) (mean 50.5±15.5 years) | n=78 | n=88 | n=64 | n=18 | ||
| Hypertension, % (Age-standardised prevalence rate,%) |
11.5 (4.4–18.6) | 29.5 (20.0–39.0) | 37.5 (25.6–49.4) | 55.6 (32.6–78.6) | 0.0001 | 27.8 (22.2–33.4) 31.0 (25.2–36.8) |
| BMI ≥25 (%) | 9.0 (2.6–15.4) | 17.0 (9.2–24.8) | 14.1 (5.6–22.6) | 16.7 (0–33.9) | ns | 13.4 (9.2–17.6) |
| Kargil (n=115) (mean 51.9±13.5 years) | n=25 | n=46 | n=42 | n=2 | ||
| Hypertension, % (Age-standardised prevalence rate, %) |
16.0 (1.6–30.4) | 19.6 (8.1–31.1) | 33.3 (19.0–47.6) | 50.0 (0–100) | ns | 24.3 (16.5–32.1) 24.6 (16.7–32.5) |
| BMI ≥25 (%) | 16.0 (1.6–30.4) | 2.2 (0–6.4) | 9.5 (0.6–18.4) | 0 | ns | 7.8 (2.9–12.7) |
| Sham (n=451) (mean 56.2±13.8 years) | n=62 | n=189 | n=150 | n=50 | ||
| Hypertension, % (Age-standardised prevalence rate, %) |
9.7 (2.3–17.1) | 31.2 (24.6–37.8) | 50.7 (42.7–58.7) | 72.0 (59.6–84.4) | <0.0001 | 39.2 (34.7–43.7) 36.9 (32.4–41.4) |
| BMI ≥25 (%) | 11.3 (3.4–19.2) | 19.6 (13.9–25.3) | 17.5 (11.4–23.6) | 14.0 (4.4–23.6) | ns | 17.1 (13.6–20.6) |
| Zanskar (n=284) (mean 59.5±12.8 years) | n=10 | n=115 | n=127 | n=32 | ||
| Hypertension, % (Age-standardised prevalence rate, %) |
20.0 (0–44.8) | 25.2 (17.3–33.1) | 42.5 (33.9–51.1) | 56.3 (39.1–73.5) | <0.01 | 36.3 (30.7–41.9) 32.1 (26.7–37.5) |
| BMI ≥25 (%) | 30.0 (1.6–58.4) | 13.9 (7.6–20.2) | 13.4 (7.5–19.3) | 15.6 (3.0–28.2) | ns | 14.4 (10.3–18.5) |
| Changthang (n=351) (mean 52.9±13.6 years) | n=47 | n=186 | n=93 | n=25 | ||
| Hypertension,% (Age-standardised prevalence rate, %) |
14.9 (4.7–25.1) | 22.6 (16.6–28.6) | 49.5 (39.3–59.72) | 60.0 (40.8–79.2) | <0.0001 | 31.3 (26.4–36.2) 32.6 (27.7–37.5) |
| BMI ≥25 (%) | 12.8 (3.2–22.4) | 24.7 (18.5–30.9) | 16.1 (8.6–23.6) | 16.0 (1.6–30.4) | ns | 20.2 (16.0–24.4) |
p; χ2 Test for the comparison of the prevalence of hypertension and BMI >25 (%) among the four age groups in each subdivision.
BMI, body mass index; ns, not significant.
Leh town subdivision, which is inhabited by an urban population, had a higher crude prevalence rate of hypertension (43.4%) with age-standardised prevalence rate (45.5%) than any other subdivisions comprising a rural population (crude; 24.3–39.1, age-standardised; 24.6–36.8) (ANOVA, p<0.0001). The prevalence of hypertension, especially in the younger age group of 40–59 years, was extremely high in Leh town (41.6%) compared with other rural subdivisions (19.6–30.7%) (ANOVA, p<0.0001). Also in the old population above 60 years, the prevalence of hypertension was highest in Leh town (61.7%) compared with other rural subdivisions (34.1–56.0%) (ANOVA, p=0.0001). There was no significant difference in prevalence of hypertension in the young age group of 20–39 among the seven subdivisions (ANOVA; ns, not significant). Prevalence rates of hypertension increased significantly with ageing in all subdivisions (ANOVA, p<0.01∼ p<0.0001) except Kargil. Prevalence of overweight (BMI≥25) was highest in the middle age group of 40–59 in Leh town subdivision.
Table 5 shows the prevalence rate of hypertension at different altitude levels according to age and occupation group. Up to the altitude of 4000 m, the prevalence of hypertension rose with altitude and the participants surveyed at altitude ranging from 3500 to 3999 m had a higher prevalence rate of hypertension (40.8%) than the other altitude ranges in all participants (ANOVA, p<0.0001). In the age group of 20–59 years, people at altitude ranging from 3000 to 3499 m had a higher prevalence rate of hypertension than others, while in the age group of 60–74 years, up to the altitude of 4499 m, the prevalence rate of hypertension rose with altitude, and people at altitude ranging from 4000 to 4499 m had the highest prevalence rate of hypertension (55.8%) (ANOVA, p<0.05). In the age group of 75 years and more, the prevalence of hypertension was highest and there was no difference among altitude levels.
Table 5.
Prevalence of hypertension and related variables according to altitude, age and occupation in Ladakh region
| Altitude (metres above MSL) |
p Value | |||||
|---|---|---|---|---|---|---|
| 2500–2999 m | 3000–3499 m | 3500–3999 m | 4000–4499 m | 4500–4999 m | ||
| All | n=417 | n=428 | n=1604 | n=174 | n=177 | |
| Hypertension (%) | 27.1 (22.8–31.4) | 37.4 (32.8–42.0) | 40.8 (38.4–43.2) | 30.5 (23.7–37.3) | 32.2 (25.3–39.1) | <0.0001 |
| SBP | 126.3±21.6 | 128.9±19.9 | 132.8±24.0 | 129.7±23.9 | 130.9±23.8 | <0.0001 |
| DBP | 80.8±12.2 | 83.5±13.1 | 83.3±13.7 | 80.0±13.7 | 79.2±12.8 | <0.0001 |
| BMI | 21.8±3.1 | 22.7±3.7 | 22.8±3.6 | 22.4±3.6 | 22.6±3.7 | <0.0001 |
| BMI ≥25 (%) | 16.6 (13.0–20.2) | 25.0 | 27.1 | 20.1 | 20.3 | <0.0001 |
| SpO2 | 90.2±4.7 | 90.6±4.2 | 90.0±5.2 | 86.1±5.8 | 86.7±5.7 | <0.0001 |
| SpO2 <89 (%) | 26.2 (22.0–30.4) | 23.6 (19.6–27.6) | 28.8 (26.6–31.0) | 67.1 (60.1–74.1) | 68.2 (61.3–75.1) | <0.0001 |
| 20–39 years | n=119 | n=128 | n=211 | n=30 | n=17 | |
| Hypertension (%) | 10.1 (4.7–15.5) | 22.7 (15.4–30.0) | 13.3 (8.7–17.9) | 10.0 (0–20.7) | 23.5 (3.3–43.7) | <0.05 |
| BMI ≥25 (%) | 11.8 (6.0–17.6) | 17.2 (10.7–23.7) | 23.2 (17.5–28.9) | 6.7 (0–15.6) | 23.5 (3.3–43.7) | <0.05 |
| SpO2 <89 (%) | 15.4 (8.9–21.9) | 16.4 (10.0–22.8) | 14.5 (9.7–19.3) | 69.0 (52.4–85.6) | 100.0 | <0.0001 |
| 40–59 years | n=155 | n=197 | n=660 | n=77 | n=109 | |
| Hypertension (%) | 27.1 (20.1–34.1) | 41.1 (34.2–48.0) | 32.4 (28.8–36.0) | 15.6 (7.5–23.7) | 27.5 (19.1–35.9) | <0.001 |
| BMI ≥25 (%) | 20.6 (14.2–27.0) | 32.5 (26.0–39.0) | 30.2 (26.7–33.7) | 28.6 (18.5–38.7) | 22.0 (14.2–29.8) | ns |
| SpO2 <89 (%) | 22.6 (16.0–29.2) | 24.1 (18.1–30.1) | 20.5 (17.4–23.6) | 55.8 (44.7–66.9) | 67.9 (59.1–76.7) | <0.0001 |
| 60–74 years | n=114 | n=81 | n=556 | n=52 | n=41 | |
| Hypertension (%) | 38.6 (29.7–47.5) | 44.4 (33.6–55.2) | 52.3 (48.1–56.5) | 55.8 (42.3–69.3) | 41.5 (26.4–56.6) | <0.05 |
| BMI ≥25 (%) | 15.9 (9.2–22.6) | 23.5 (14.3–32.7) | 26.5 (22.8–30.2) | 15.4 (5.6–25.2) | 17.1 (5.6–28.6) | ns |
| SpO2 <89 (%) | 36.6 (27.8–45.4) | 30.4 (20.4–40.4) | 40.2 (36.1–44.3) | 78.8 (67.7–89.9) | 56.1 (40.9–71.3) | <0.0001 |
| 75 years | n=29 | n=22 | n=177 | n=15 | n=10 | |
| Hypertension (%) | 51.7 (33.5–69.9) | 63.6 (43.5–83.7) | 68.4 (61.6–75.2) | 60.0 (35.2–84.8) | 60.0 (29.6–90.4) | ns |
| BMI ≥25 (%) | 17.2 (3.5–30.9) | 9.1 (0–21.1) | 22.6 (16.4–28.8) | 20.0 (0–40.2) | 10.0 (0–28.6) | ns |
| SpO2 <89 (%) | 48.3 (30.1–66.5) | 36.4 (16.3–56.5) | 41.5 (34.2–48.8) | 80.0 (59.8–100) | 70.0 (41.6–98.4) | <0.05 |
| Farmer | n=348 | n=178 | n=620 | n=81 | n=20 | |
| Hypertension (%) | 27.6 (22.9–32.3) | 33.7 (26.8–40.6) | 41.3 (37.4–45.2) | 40.7 (30.0–51.4) | 55.0 (33.2–76.8) | <0.001 |
| BMI ≥25 (%) | 14.7 (11.0–18.4) | 12.4 (7.6–17.2) | 15.6 (12.7–18.5) | 19.8 (11.1–28.5) | 20.0 (2.5–37.5) | ns |
| SpO2 <89 (%) | 23.8 (19.3–28.3) | 24.4 (18.1–30.7) | 41.5 (37.6–45.4) | 85.0 (77.2–92.8) | 94.7 (84.9–100) | <0.0001 |
| Nomad | n=67 | n=145 | ||||
| Hypertension (%) | 22.4 (12.4–32.4) | 29.0 (21.6–36.4) | ns | |||
| BMI ≥25 (%) | 25.4 (15.0–35.8) | 17.9 (11.7–24.1) | ns | |||
| SpO2 <89 (%) | 46.3 (34.4–58.2) | 63.9 (56.1–71.7) | <0.05 | |||
| Sedentary worker | n=33 | n=176 | n=340 | |||
| Hypertension (%) | 21.2 (7.3–5.1) | 40.9 (33.6–48.2) | 38.8 (33.6–44.0) | ns (0.09) | ||
| BMI ≥25 (%) | 21.2 (7.3–5.1) | 35.2 (28.1–42.3) | 36.6 (31.5–41.7) | |||
| SpO2 <89 (%) | 42.4 (25.5–59.3) | 20.2 (14.3–26.1) | 15.7 (11.8–19.6) | <0.001 | ||
p; χ2 Test for the comparison of the prevalence of hypertension, BMI ≥25 (%) and SpO2 <89 (%) among the five altitude groups.
BMI, body mass index; DBP, diastolic blood pressure; MSL, mean sea level; SBP, systolic blood pressure; SpO2, oxyhaemoglobin saturation measured by a pulse oximeter.
According to occupation group, the prevalence of hypertension rose closely with altitude remarkably in agriculture (p<0.001), mildly in sedentary workers (p=0.09) and insignificantly in nomads.
Table 6 shows the prevalence rate of hypertension at different altitude levels in each subdivision. In the only Sham subdivision, where altitude ranging is as wide as 2700–3900 m, the prevalence rate of hypertension increased (29.1, 36.2, 46.4%, p=0.0067) in accord with the elevation of altitude (2500–2999, 3000–3499, 3500–3999 m) in spite of the decrease in overweight (23.3, 18.9, 12.6%, p=0.040) with the altitude. In the other subdivisions, there was no difference in the prevalence rate of hypertension among different altitudes.
Table 6.
Prevalence of hypertension and overweight in different altitude levels in each subdivision in Ladakh region
| Altitude (metres above MSL) |
p Value | |||||
|---|---|---|---|---|---|---|
| 2500–2999 m | 3000–3499 m | 3500–3999 m | 4000–4499 m | 4500–4999 m | ||
| Leh (3300–3600 m) | ||||||
| n | 189 | 813 | ||||
| Hypertension (%) | 42.9 (35.8–50.0) | 43.5 (40.1–46.9) | ns | |||
| BMI ≥25 (%) | 32.8 (26.1–39.5) | 33.7 (30.5–36.9) | ns | |||
| Leh block (3000–3700 m) | ||||||
| n | 49 | 300 | ||||
| Hypertension (%) | 28.6 (15.9–41.3) | 33.7 (28.4–39.0) | ns | |||
| BMI ≥25 (%) | 40.8 (35.8–63.8) | 31.3 (26.1–36.5) | ns | |||
| Nubra (2600–3000 m) | ||||||
| n | 248 | |||||
| Hypertension (%) | 27.8 (22.2–33.4) | |||||
| BMI ≥25 (%) | 13.7 (9.4–18.0) | |||||
| Kargil (2600–3100 m) | ||||||
| n | 52 | 63 | ||||
| Hypertension (%) | 19.2 (8.5–29.9) | 28.6 (17.4–39.8) | ns | |||
| BMI ≥25 (%) | 15.4 (5.6–25.2) | 1.6 (0–4.7) | <0.01 | |||
| Sham (2700–3900 m) | ||||||
| n | 117 | 127 | 207 | |||
| Hypertension (%) | 29.1 (20.9–37.3) | 37.0 (28.6–45.4) | 46.4 (39.6–53.2) | <0.01 | ||
| BMI ≥25 (%) | 23.3 (15.6–31.0) | 18.9 (12.1–25.7) | 12.6 (8.1–17.1) | <0.05 | ||
| Zanskar (3500–3900 m) | ||||||
| n | 284 | |||||
| Hypertension (%) | 36.3 (30.7–41.9) | |||||
| BMI ≥25 (%) | 14.4 (10.3–18.5) | |||||
| Changthang (4000–4900 m) | ||||||
| n | 174 | 177 | ||||
| Hypertension (%) | 30.5 (23.7–37.3) | 32.2 (25.3–39.1) | ns | |||
| BMI ≥25 (%) | 20.1 (14.1–26.1) | 20.3 (14.4–26.2) | ns | |||
p; χ2 Test for the comparison of the prevalence of Hypertension and BMI ≥25 (%) among the altitude groups.
BMI, body mass index; MSL, mean sea level; ns, not significant.
Table 7 shows the prevalence rate of hypertension and overweight in people with different occupations. In the age group of 40–59 years, sedentary workers had the highest prevalence of hypertension (48.3%) and obesity (43.9%), while nomads (hypertension/obesity; 19.6%/22.5%) and manual labourers (11.3%/20.8%) had a lower prevalence of hypertension compared with other workers (27.3–36.1%/20.1–61.1%) (ANOVA, p<0.0001). In the other age groups, there was no or little significant difference in the prevalence of hypertension among different occupations.
Table 7.
Prevalence of hypertension and overweight in people with different occupations in each age group in Ladakh region
| 20–39 years |
40–59 years |
60–74 years |
75 years |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Hypertension | BMI ≥25 | n | Hypertension | BMI ≥25 | n | Hypertension | BMI ≥25 | n | Hypertension | BMI ≥25 | |
| Per cent | Per cent | Per cent | Per cent | Per cent | Per cent | Per cent | Per cent | |||||
| Farmer | 171 | 12.9 (7.9–17.9) | 12.3 (7.4–17.2) | 476 | 26.3 (22.3–30.3) | 16.0 (12.7–19.3) | 465 | 47.3 (42.8–51.8) | 15.9 (12.6–19.2) | 135 | 65.9 (57.9–73.9) | 14.1 (8.2–20.0) |
| Nomad | 4 | 0 | 25.0 (0–67.4) | 146 | 19.9 (13.4–26.4) | 23.3 (16.4–30.2) | 54 | 40.7 (27.6–53.8) | 11.1 (2.7–19.5) | 16 | 62.5 (38.8–86.2) | 25.0 (3.8–46.2) |
| Sedentary worker | 204 | 19.5 (14.1–24.9) | 19.1 (13.7–24.5) | 277 | 48.0 (42.1–53.9) | 44.0 (38.2–49.8) | 61 | 45.9 (33.4–58.4) | 39.3 (27.0–51.6) | 7 | 42.9 (6.2–79.6) | 42.9 (6.2–79.6) |
| Others | 126 | 10.3 (5.0–15.6) | 23.8 (16.4–31.2) | 299 | 30.8 (25.6–32.1) | 36.5 (23.2–36.0) | 264 | 55.7 (49.7–61.7) | 36.4 (30.6–42.2) | 95 | 66.3 (56.8–75.8) | 26.3 (17.4–35.2) |
| Housewife | 44 | 13.6 (3.5–23.7) | 25.0 (12.2–37.8) | 157 | 29.0 (21.9–36.1) | 36.3 (28.8–43.8) | 98 | 57.1 (47.3–66.9) | 25.5 (16.9–34.1) | 26 | 84.6 (70.7–98.5) | 23.1 (12.1–31.7) |
| Manual labourer | 1 | 0 | 0 | 53 | 11.3 (2.8–19.8) | 20.8 (9.9–31.7) | 9 | 33.3 (2.5–64.1) | 11.1 (0–31.6) | 0 | ||
| Monk | 43 | 4.7 (0–11.0) | 30.2 (16.5–43.9) | 36 | 36.1 (20.4–51.8) | 61.1 (45.2–77.0) | 57 | 56.1 (43.2–69.0) | 52.6 (39.6–65.6) | 21 | 52.4 (31.0–73.8) | 38.1 (17.3–58.9) |
| No job | 37 | 13.5 (2.5–24.5) | 16.2 (4.3–28.1) | 25 | 36.0 (17.2–54.8) | 36.0 (17.2–54.8) | 44 | 61.4 (47.0–75.8) | 38.6 (24.2–53.0) | 32 | 62.5 (45.7–79.3) | 28.1 (12.5–43.7) |
| Retired sedentary | 1 | 0 | 0 | 28 | 35.7 (18.0–53.4) | 35.7 (18.0–53.4) | 56 | 51.8 (38.7–64.9) | 41.1 (28.2–54.0) | 16 | 62.5 (38.8–86.2) | 12.5 (0–28.7) |
| p Value | ns (0.05) | ns (0.07) | <0.0001 | <0.0001 | ns (0.07) | <0.0001 | ns | <0.05 | ||||
p; χ2 Test for the comparison of the prevalence of hypertension and BMI ≥25 (%) among the four occupation groups: farmer, nomad, sedentary worker and others.
BMI, body mass index.
Table 8 shows the prevalence of hypertension and overweight comparing among dwellers in rural areas and Leh town and rural-to-urban migrants. The prevalence of hypertension and overweight was highest in migrants settled in Leh (hypertension/overweight; 48.3%/40.9%) followed by dwellers in Leh town (41.1%/30.2%) compared with those in rural areas (33. 5%/15.3–19.3%). The percentage of engagement in occupations was shown in each participant group.
Table 8.
Prevalence of hypertension and related variables in different dwelling areas in Ladakh region
| n | Rural areas | Urban: Leh town |
p Value | |
|---|---|---|---|---|
| Dwellers in Leh town | Migrants from Changthang | |||
| 1798 | 683 | 319 | ||
| Age (years) | 54.9±14.6 | 49.0±15.9 | 58.2±12.3 | <0.0001 |
| Hypertension (%) | 33.5 (31.3–35.7) | 41.1 (37.4–44.8) | 48.3 (42.8–53.8) | <0.0001 |
| BMI ≥25 (%) | 19.3 (17.5–21.1) | 30.2 (26.8–33.6) | 40.9 (35.5–46.3) | <0.0001 |
| SpO2 | 88.8±5.6 | 90.7±4.2 | 92.3±3.2 | <0.0001 |
| SpO2 <89 (%) | 40.6 (38.3–42.9) | 21.7 (18.6–24.8) | 10.1 (6.8–13.4) | <0.0001 |
| Altitude (m) | 3543.2±534.1 | 3449.0±86.9 | 3491.9±39.6 | <0.0001 |
| Occupation (%) | ||||
| Farmer | 66.8 (64.6–69.0) | 6.3 (4.5–8.1) | 0.9 (0–1.9) | |
| Nomad | 12.0 (10.5–13.5) | 0 | 1.3 (0.1–2.5) | |
| Sedentary | 8.0 (6.7–9.3) | 47.0 (43.3–50.7) | 26.6 (21.8–31.4) | |
| Others | 13.2 (11.6–14.8) | 46.7 (43.0–50.4) | 71.2 (66.2–76.2 | |
| Housewife | 2.6 (1.9–3.3) | 27.2 (23.9–30.5) | 28.8 (23.8–33.8) | |
| Manual labourer | 0 | 0.9 (0.2–1.6) | 17.9 (13.7–22.1) | |
| Monk | 6.8 (5.6–8.0) | 5.0 (3.4–6.6) | 0.3 (0–0.9) | |
| No job | 1.5 (0.9–2.1) | 5.9 (4.1–7.7) | 22.3 (17.7–26.9) | |
| Retired sedentary | 2.3 (1.6–3.0) | 7.8 (5.8–9.8) | 1.9 (0.4–3.4) | |
p; χ2 Test for the comparison of the prevalence of hypertension and BMI≥25 (%) among the three groups, and ANOVA for the comparison of mean age among the three groups.
ANOVA, analysis of variance; BMI, body mass index; SpO2, oxyhaemoglobin saturation measured by a pulse oximeter.
There was a difference in the prevalence of hypertension between Tibetan and Ladakhi nomads. The lowest prevalence of hypertension in spite of a higher prevalence of overweight was shown in Tibetan nomads (n=76) (hypertension/overweight; 19.7%/39.5%) compared with Ladakhi nomads (n=144) (31.9%/10.4%) living at higher altitude (4000–4900 m).
The effects of altitude, occupation and dwelling area on hypertension were analysed in all the participants by multiple logistic regression adjusted with age, sex and overweight in models 1–3 (table 9). In model 1, the altitude ranges of 3000–3499 (OR 1.78) and 3500–3999 (OR 1.42) were significantly associated with high prevalence of hypertension compared with 2500–2999 (m) adjusted with age, sex and obesity. However, the higher range of 4000–4499 or 4500– was not associated with hypertension. In model 2 with further adjustment by occupation, the altitude ranges of 3000–3499 (OR 1.62) and 3500–3999 (OR 1.34) and the highest range of 4500– (OR 2.57) became significantly associated with hypertension. Sedentary workers had a higher association (OR 1.56) compared with farmers, while nomads had a lower association (OR 0.42). In model 3, with further adjustment by dwelling area, the altitude range of 3000–3499 (OR 1.44) and the highest altitude range of 4500– (OR 2.69) kept significant association with hypertension independent of occupation and dwelling area. People dwelling in Leh town (OR 1.92) and migrants from Changthang (OR 1.70) were significantly associated with a high prevalence of hypertension compared with those dwelling in rural areas.
Table 9.
The effect of altitude, occupation and dwelling area on hypertension adjusted with age, sex and overweight by multiple logistic regression analysis
| n | Model-1 |
Model-2 |
Model-3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | CI | p Value | OR | CI | p Value | OR | CI | p Value | ||
| Age (year) | ||||||||||
| 20–39 | 505 | 1.00 | 1.00 | 1.00 | ||||||
| 40–59 | 1198 | 2.43 | 1.84 to 3.22 | <0.0001 | 2.78 | 2.08 to 3.71 | <0.0001 | 2.85 | 2.12 to 3.83 | <0.0001 |
| 60–74 | 844 | 5.66 | 4.24 to 7.55 | <0.0001 | 6.93 | 5.09 to 9.43 | <0.0001 | 7.20 | 5.26 to 9.86 | <0.0001 |
| 75– | 253 | 11.40 | 7.89 to 16.46 | <0.0001 | 14.45 | 9.82 to 21.26 | <0.0001 | 14.71 | 9.93 to 21.79 | <0.0001 |
| Male (vs female) | 1240 (1560) | 1.02 | 0.86 to 1.21 | ns | 0.95 | 0.80 to 1.13 | ns | 1.00 | 0.84 to 1.20 | ns |
| BMI ≥25 (vs BMI <25) | 683 (2117) | 2.60 | 2.14 to 3.13 | <0.0001 | 2.51 | 2.07 to 3.05 | <0.0001 | 2.52 | 2.08 to 3.06 | <0.0001 |
| Altitude (m) | ||||||||||
| 2500–2999 | 417 | 1.00 | 1.00 | 1.00 | ||||||
| 3000–3499 | 428 | 1.78 | 1.30 to 2.44 | <0.001 | 1.62 | 1.17 to 2.23 | <0.01 | 1.44 | 1.04 to 2.01 | <0.05 |
| 3500–3999 | 1604 | 1.42 | 1.10 to 1.83 | <0.01 | 1.34 | 1.02 to 1.75 | <0.05 | 1.16 | 0.88 to 1.54 | ns |
| 4000–4499 | 174 | 1.01 | 0.67 to 1.53 | ns | 1.37 | 0.87 to 2.15 | ns | 1.40 | 0.88 to 2.20 | ns |
| 4500– | 177 | 1.19 | 0.79 to 1.79 | ns | 2.57 | 1.41 to 4.68 | <0.01 | 2.69 | 1.48 to 4.90 | <0.01 |
| Occupation | ||||||||||
| Farmer | 1247 | 1.00 | 1.00 | |||||||
| Nomad | 220 | 0.42 | 0.24 to 0.72 | <0.01 | 0.37 | 0.22 to 0.64 | <0.001 | |||
| Sedentary worker | 549 | 1.56 | 1.20 to 2.02 | <0.001 | 1.02 | 0.74 to 1.40 | ns | |||
| Others | 784 | 0.99 | 0.80 to 1.23 | ns | 0.68 | 0.52 to 0.90 | <0.01 | |||
| Dwelling area | ||||||||||
| Rural areas | 1798 | 1.00 | ||||||||
| Dwellers in Leh town | 683 | 1.92 | 1.45 to 2.55 | <0.0001 | ||||||
| Migrants from Changthang | 319 | 1.70 | 1.21 to 2.38 | <0.01 | ||||||
Model-1: The effect of altitude on hypertension adjusted with age, sex and overweight.
Model-2: The effect of altitude and occupation on hypertension adjusted with age, sex and overweight.
Model-3: The effect of altitude, occupation and dwelling area on hypertension adjusted with age, sex and overweight.
BMI, body mass index; ns, not significant.
Discussion
In the current study, we found that one-third of the population is at a higher risk of hypertension. As table 3 shows, the prevalence of hypertension tends to increase with age in both genders. Average SBP and DBP in men less than 60 years of age was found to be higher than in age-matched women. This is consistent with the prevalence of adult hypertension in a US population,22 in a south Indian Chennai urban population study23 and in rural and urban communities of Rajasthan.24 The cause of lower blood pressure in women below 60 years may be due to hormonal effects in women during this age, that is, premenopausal women having a lower arterial blood pressure than age-matched men.25 This may also be due to the effect of obesity, as the prevalence of overweight in men was higher in people under 75 years compared with women. The epidemiology of hypertension on the Tibetan plateau carried out by Sun and shinfu,12 however, reports a higher prevalence of hypertension in women in all age groups. This difference in results might be influenced by there being more females in their cohort, as well as possible differences in obesity that are not shown in their report.
Though age-standardised prevalence of hypertension in Leh block (30.7%) was not high compared with other rural areas (24.6–36.8%), higher prevalence of hypertension in Leh town (45.5%) and higher prevalence of overweight in Leh block (32.7%) and Leh town (33.6%) were found compared with other rural areas (overweight; 7.8–20.2%). The high prevalence of overweight may be brought about because Leh block is somehow a more developed subdivision than the others in this study. Urbanisation can change the lifestyle of the people and their diet habits, which may result in obesity and high prevalence of hypertension. Dietary quantity intake as assessed by our nutritionist (Y.K) by a 24 h recall method showed that energy intake was higher in Leh town (2305 kcal in men and 1933 kcal in women) as compared to higher altitude at Changthang (2029 kcal in men and 1802 kcal in women). Variety of food intake as assessed by 11-item Food Diversity Score Kyoto (FDSK-11) was higher in Leh (6.7±1.8) as compared to higher altitude Changthang (6.1±1.5).26–28 Economic conditions, traditional food culture and a harsh environment with limitation of resources affect energy intake and food diversity. In urban Leh and Leh block, the economic condition of the population is better. Bread, mutton, rice, pulses, vegetables, thukpa and eggs are the main dietary foods, with snacks of sweet tea, biscuits, and fast food. Such a diet increases their calories, resulting in high BMI, and increases their salt intake, contributing to the higher prevalence of hypertension. One of the villages in Leh block, Stok, was a study centre in the Indian component of the Intersalt study,29 an international study to determine the relationship of blood pressure with dietary ingredients, particularly sodium and potassium. Urinary sodium (means (and SD) calculated for men aged 20–39, men aged 40–59, women aged 20–39, and women aged 40–59 and then averaged over age and sex groups) was 203.7 mmol/24 h (75.0) and urinary potassium was 47.0 (19.2) mmol/24 h with a poor potassium sodium ratio. Although the data pertain to the year 1988, there is every reason to surmise that the situation which persists as a condition of socioeconomic improvement without parallel improvement in health awareness prevails even today. There is a recent report on the effect of using a low-sodium, high-potassium salt substitute for Tibetan highlanders with hypertension.30
Domkhar valley in Sham subdivision situated along the Domkhar stream is about 25 km long and divided into three hamlets of different altitudinal contour and diversified environment. Paba, rice, bread, thukpa, sku, kholak and the local beverage chang are the main diets. Meat is rarely available. Fresh fruit is available in plenty in lower Domkhar and at some places in middle Domkhar, but none in upper Domkhar due to its high-altitude location (Altitude 3800 m). Prevalence of hypertension is very high here (39.1%) among the rural subdivisions. The prevalence of hypertension, especially in Sham subdivision, was as high as that in Leh town in the old age group above 60 years (Sham: 56.0% vs Leh town: 61.7%) and in the higher altitude level of 3500–3999 (Sham: 46.4%, Leh town: 43.5%) in spite of a much lower rate of overweight in Sham (17.1%) compared with Leh town (33.6%). Different from people in the Leh block subdivision, people in Sham had much poorer availability of foods for a long time until recently and they may have vulnerability to the recent quick change of dietary habits, especially in older people and those dwelling in remote areas at higher altitude. We showed the high prevalence of impaired glucose tolerance (35%) in old people in Domkhar compared with Tibetan people in Qinghai, China in the previous report. We also suggested that there may be a vulnerability to glucose intolerance brought on by recent changes in lifestyle in people with long-term backgrounds of economically traditional lifestyles with limited food resources.31
Mutton, rice, momo (mutton), thukpa (comprising of Atta, vegetable mostly dry and dry cheese), kholak (Barley flour with local tea) and paba (a mix of barley flour, wheat flour and grounded pea cooked in plain water with salt added to taste) are the main diets of the Changthang population in both Ladakhis and Tibetans. Taking snacks is not in their food culture, and nor are modern snack items available at that remote high-altitude region. A relatively lower prevalence of hypertension was observed in Changthang Tibetan natives (19.7%) and Changthang Ladakhis (31.9%) living at higher altitude (4000–4900 m).
Zanskar subdivision, located at an intermediate high altitude (3500–3900 m), has a population mainly concerned with farming and cattle rearing. Butter tea, local beverage chang, thukpa, barley flour kholak, rice and pulses are the main dietary foods; meat is rarely eaten. Fresh fruit and vegetables are usually not available in Zanskar and Changthang. The crude prevalence of hypertension in Zanskar appears to be high (36.3%) but age-standardised prevalence (32.1%) was the same as in other rural areas, as the mean age was highest in Zanskar (table 4).
Modernised sedentary workers, rural-to-urban migrants, and dwelling in urban area population (Altitude 3300–3600 m) had a higher prevalence of hypertension and increased BMI as compared to the rural population. Previous reports support our hypothesis of highlanders’ vulnerability to hypertension by socioeconomic globalisation.12–14 31–34 A higher prevalence of hypertension was reported in Tibetans compared with immigrant Hans in the Tibetan plateau, with the prevalence being greater in the urban population around Lhasa than in the rural population.12 In another report, a longitudinal survey was carried out in the prevalence of hypertension in people over 15 years in different ethnic groups in China in 1991 and 2002. The prevalence of hypertension in Tibetan people increased from 17.8% (in 1991) to 24.7% (in 2002), which was the highest compared with the other seven ethnic groups including Han (from 11.3% to 16.2%).32 A recent report showed that the prevalence of hypertension (SBP≥140 or DBP ≥90 or treatment) in 1289 Tibetan highlanders (Lhasa and suburbs; 3700–4200 m) aged 18 and more was 39%.13 Another report showed that the prevalence of hypertension in 692 Tibetan highlanders (rural area of Lhasa; 3700 m) aged 30–80 years was 37% (SBP≥130 or DBP ≥85 or treatment).14 The prevalence of hypertension was close to our result of 37% and higher than that of Chinese lowlanders aged 20 years and more (27% in 2007–2008).15 Blood pressure in 332 highlanders in Leh (13–81 years old, mean 50 years) was compared with those in U town, Hokkaido, Japan (24–79 years, mean 56.8) in 2004. Higher DBP and a larger increase in blood pressure with age were observed in people living at a high altitude, as compared with Japanese living at a low altitude.35 Younger people, but not adults and elderly people, among Tibetan immigrants from Leh to the lowlands in India were reported to have higher blood pressure compared with those living in the highlands. Measurements of adiposity had a significant effect on BP.33 The prevalence of hypertension was higher (72.7%) in Tibetan highlanders in Shangrila (Altitude: 3300 m) compared with lowlanders in Jing Hong (57.0%) and Tosa (59.9%). There was a significant association between living in an urban area with a higher prevalence of hypertension and obesity in younger people under 60 years compared with those living in a rural area.34 Younger people may be more vulnerable to hypertension by a quick modernised lifestyle change. Also in our report, a higher prevalence of hypertension in Leh town (44.7%) was observed, especially in the middle-aged group of 40–59 years, compared with other areas (19.6–30.7%).
A higher OR of altitudes from 3000 to 3999 m compared with an altitude below 3000 m was observed after adjustment with age, sex and overweight. One reason may be socioeconomic factor, as this altitude level was compatible with that of the urban area of Leh town and urban dwellers had a higher rate of hypertension and obesity by lifestyle change compared with rural dwellers. Another reason may be the effect of high altitude itself, as the dwellers in Sham subdivision at the altitude of 3000–3999 m had a higher prevalence of hypertension in spite of a lower prevalence of overweight compared with those dwelling below 3000 m. The highest prevalence in older people was shown at a higher altitude over 4000 m. Moreover, the prevalence of hypertension rose closely with altitude remarkably in farmers (p<0.001), mildly in sedentary workers (p=0.09) and insignificantly in nomads (table 5). That is the reason why the higher altitude range of 3000–3999 and 4500– (OR 2.18) kept significant association with hypertension after adjustment with age, occupation or dwelling area by the multivariate analysis, which also supports the effect of high altitude itself to hypertension.
The limitation of this paper is that it did not look into the genetic factors, as environmental and genetic factors may contribute to regional and racial variations of blood pressure and prevalence of hypertension. Genetic evidence for high-altitude adaptation in Tibetan people was reported recently.36 37 A relatively lower prevalence of hypertension in spite of a higher one of overweight in Changthang Tibetan natives (hypertension/overweight; 19.7% vs 31.9%/39.5% vs 10.4%) compared with Changthang Ladakhi living at higher altitude (4000–4900 m) was observed in our report. The association between the hypoxic adaptation gene and hypertension should be studied further. The strength of this study is that it looked into most of the environmental factors known to influence hypertension in the population of different distinct geographical subdivisions of a high-altitude region. This study showed the influence of ageing, overweight, modernised sedentary occupations and rural-to-urban migration and dwelling in urban areas to hypertension as well as the effect of high altitude on hypertension by multivariate analysis.
The conclusion reached is that like everywhere else in the world, hypertension prevalence in a high-altitude population has multifactorial aetiology. Our study shows that age, gender, socioeconomic factors, culture, race and changing lifestyle play a big role with the effect of high altitude itself on the high prevalence of hypertension.
Acknowledgments
The authors thank all the participants for their consent and participation. The authors thank our LIP staff, Mr Tsering Motup, Mrs Rigzin Dolma, Miss Ishey Lhamo (Laboratory Technicians), Mrs Sherab Dolma, Miss Rinchen Dolma (Office secretaries) and Miss kunznag Dolma (ECG technician and instrument upkeep staff) for their quality work while travelling to all the difficult subdivisions of Ladakh. The authors thank Mr Chewang Motup and Mrs Yangdu Motup of Rimo Expedition and Mr None P Wangchuk of LIP for their invaluable help in logistics.
Footnotes
Contributors: TN, TS, KO were involved in the study concept and design. TN, NT, NA, PT, IA, TC, VKS, PR, SBS, YK, EF, KS, MI, RS, MN, TY, TT, KO, KO were involved in the field study and data acquisition. TS, VKS, TN, YK, KM, KO were involved in the analysis and interpretation of the data. TN was involved in the drafting of manuscript. KO, TS, VKS were involved in the critical revision of manuscript.
Funding: TN received research support from the Tata Institute of Social Sciences Mumbai India, Research Institute for Humanity and Nature, Kyoto, Japan, DIHAR (Defence Institute of High Altitude Research), and DIPAS (Defence Institute of Physiology & Allied Sciences). KO received research support from High Altitude Project in Research Institute for Humanity and Nature, Kyoto, Japan.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Institutional review board of Ladakh institute of prevention and the District ethical committee, Leh, Ladakh and Research Institute for Humanity and Nature, Kyoto, Japan.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Siques P, Brito J, Banegas JR et al. Blood pressure responses in young adults first exposed to high altitude for 12 months at 3550 m. High Alt Med Biol 2009;10:329–35. 10.1089/ham.2008.1103 [DOI] [PubMed] [Google Scholar]
- 2.Parati G, Bilo G, Faini A et al. Changes in 24 h ambulatory blood pressure and effects of angiotensin II receptor blockade during acute and prolonged high-altitude exposure: a randomized clinical trial. Eur Heart J 2014;35:3113–22. 10.1093/eurheartj/ehu275 [DOI] [PubMed] [Google Scholar]
- 3. Monge MC, Monge CC. High altitude diseases; mechanism and management. Springfield, IL: Charles C Thomas, 1966:62–70. [Google Scholar]
- 4.Somers VK, Mark AL, Abboud FM. Potentiation of sympathetic nerve responses to hypoxia in borderline hypertensive subjects. Hypertension 1988;11:608–12. 10.1161/01.HYP.11.6.608 [DOI] [PubMed] [Google Scholar]
- 5.Levine BD, Zuckerman JH, deFilippi CR. Effect of high-altitude exposure in the elderly: the Tenth Mountain Division study. Circulation 1997;96:1224–32. 10.1161/01.CIR.96.4.1224 [DOI] [PubMed] [Google Scholar]
- 6.Baggish AL, Wolfel EE, Levine BD. Cardiovascular system. In: Swenson ER, Bartsch P, eds. High altitude: human adaptation to hypoxia. New York: Springer, 2014:103–40. [Google Scholar]
- 7.Luks AM. Should travelers with hypertension adjust their medications when traveling to high altitude? High Alt Med Biol 2009;10:11–15. 10.1089/ham.2008.1076 [DOI] [PubMed] [Google Scholar]
- 8.Velasco A, Vongpatanasin W, Levine BD. Treating hypertension at high altitude: the quest for a magic bullet continues. Eur Heart J 2014;35:3083–4. 10.1093/eurheartj/ehu366 [DOI] [PubMed] [Google Scholar]
- 9.Dasgupta DJ. Study of blood pressure of a high altitude community at Spiti (4000m). Indian Heart J 1986;38:134–7. [PubMed] [Google Scholar]
- 10.Baker PT. The biology of high altitude peoples. Cambridge, New York: Cambridge University Press, 1978:335–6. [Google Scholar]
- 11.Toselli S, Tarazona-Santos E, Pettener D. Body size, composition, and blood pressure of high-altitude Quechua from the Peruvian Central Andes (Huancavelica, 3,680m). Am J Hum Biol 2001;13:539–47. 10.1002/ajhb.1086 [DOI] [PubMed] [Google Scholar]
- 12.Sun SF. Epidemiology of hypertension on the Tibetan Plateua . Hum Biol 1986;58:507–15. [PubMed] [Google Scholar]
- 13.Chen W, Liu Q, Wang H et al. Prevalence and risk factors of chronic kidney disease: a population study in the Tibetan population. Nephrol Dial Transplant 2011;26:1592–9. 10.1093/ndt/gfq608 [DOI] [PubMed] [Google Scholar]
- 14.Sherpa LY, Deji, Stigum H, Chongsuvivatwong V et al. Prevalence of metabolic syndrome and common metabolic components in high altitude farmers and herdsmen at 3700 m in Tibet. High Alt Med Biol 2013;14:37–44. 10.1089/ham.2012.1051 [DOI] [PubMed] [Google Scholar]
- 15.Gao Y, Chen G, Tian H et al. , China National Diabetes and Metabolic Disorders Study Group . Prevalence of hypertension in China: a cross-sectional study. PLoS ONE 2013;8:e65938 10.1371/journal.pone.0065938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Census Organization of India. The Indian Census 2011. http://www.census2011.co.in/census/district/621-leh.html, http://www.census2011.co.in/census/district/622-kargil.html
- 17. Tata institute of social sciences and Ladakh automonous hill development council. Micro level plans (MLP 2010)- Leh District, District report, p11–17. http://download.tiss.edu/fap/gyurja/gyurja_reports/mlp_combined_1.pdf.
- 18.Imai Y, Abe K, Sasaki S et al. Clinical evaluation of semiautomatic and automatic devices for home blood pressure measurement: comparison between cuff-oscillometric and microphone methods. J Hypertens 1989;7:983–90. 10.1097/00004872-198912000-00009 [DOI] [PubMed] [Google Scholar]
- 19.Imai Y, Satoh H, Nagai K et al. Characteristics of a community-based distribution of home blood pressure in Ohasama in northern Japan. J Hypertens 1993;11:1441–9. 10.1097/00004872-199312000-00017 [DOI] [PubMed] [Google Scholar]
- 20.Okumiya K, Matsubayashi K, Wada T et al. A U-shaped association between home systolic blood pressure and four-year mortality in community-dwelling older men. J Am Geriatr Soc 1999;47:1415–21. [DOI] [PubMed] [Google Scholar]
- 21.Chobanian AV, Bakris GL, Black HR et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–52. 10.1161/01.HYP.0000107251.49515.c2 [DOI] [PubMed] [Google Scholar]
- 22.Burt VI, Whelton P, Rocella EJ et al. Prevalence of hypertension in the US adult population: Results of the Third National Health and Nutrition examination survey, 1988–1991. Hypertension 1995;25:305–13. 10.1161/01.HYP.25.3.305 [DOI] [PubMed] [Google Scholar]
- 23.Shantirani CS, Pradeepa R, Deepa R et al. Prevalence and risk factors of hypertension in a selected south Indian population- The Chennai urban population study. J Assoc Physicians India 2003;51:20–7. [PubMed] [Google Scholar]
- 24.Gupta R, Sharma AK, kapoor A et al. Epidemiological studies and treatment of hypertension in rural and urban community of Rajasthan. JAPI 1997;45:863–4. [PubMed] [Google Scholar]
- 25.Dubey RK, Oparil S, Imthurn B et al. Sex hormones and hypertension. Review. Cardiovasc Res 2002;53:688–708. 10.1016/S0008-6363(01)00527-2 [DOI] [PubMed] [Google Scholar]
- 26.Kimura Y, Fukitomi E, Ishikawa M et al. Association between total energy intake and diabetes in Ladakh. Himalayan Study Monograph 2013;14:39–45. [Google Scholar]
- 27.Kimura Y, Matsubayashi K, Sakamoto R et al. Food diversity and its relation to health of highlanders–comparison of urban and rural settings in Qinghai and Ladakh. Himalayan Study Monograph 2012;13:86–93. [Google Scholar]
- 28.Kimura Y, Wada T, Ishine M et al. Food diversity is closely associated with activities of daily living, depression, and quality of life in community-dwelling elderly people. J Am Geriatr Soc 2009;57:922–4. 10.1111/j.1532-5415.2009.02235.x [DOI] [PubMed] [Google Scholar]
- 29.Intersalt cooperative research group. INTERSALT: an international study of electrolyte excretion and blood pressure. Result for 24 hour urinary sodium and potassium excretion. BMJ 1988;297:319–28. 10.1136/bmj.297.6644.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao X, Yin X, Li X et al. Using a low-sodium, high-potassium salt substitute to reduce blood pressure among Tibetans with high blood pressure: a patient-blinded randomized controlled trial. PLoS ONE 2014;9:e110131 10.1371/journal.pone.0110131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okumiya K, Sakamoto R, Kimura Y et al. Diabetes mellitus and hypertension in elderly highlanders in Asia. J Am Geriatr Soc 2010;58:1193–5. 10.1111/j.1532-5415.2010.02862.x [DOI] [PubMed] [Google Scholar]
- 32.Hu YS, Yao CH, Wang WZ et al. Survey on the prevalence of hypertension in different ethnic groups in China in 2002. Wei Sheng Yan Jiu 2006;35:573–5. [PubMed] [Google Scholar]
- 33.Vikal T, Ranjan G. Blood pressure variation among Tibetans at different altitudes. Ann Hum Biol 2007;34:470–83. 10.1080/03014460701412284 [DOI] [PubMed] [Google Scholar]
- 34.Okumiya K, Ishine M, Kasahara Y et al. The effects of socioeconomic globalization on health and aging in highlanders compared to lowlanders in Yunnan, China, and Kochi, Japan. Ecol Res 2011;26:1027–38. 10.1007/s11284-010-0784-1 [DOI] [Google Scholar]
- 35.Otsuka K, Norboo T, Otsuka Y et al. Effect of aging on blood pressure in Leh, Ladakh, a high-altitude (3524m) community, by comparison with a Japanese town. Biomed Pharmacother 2005;59(Suppl 1):S54–7. 10.1016/S0753-3322(05)80011-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beall CM, Cavalleri GL, Deng L. Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci USA 2010;107:11459–64. 10.1073/pnas.1002443107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonson TS, Yang Y, Huff CD. Genetic evidence for high-altitude adaptation in Tibet. Science 2010;329:72–5. 10.1126/science.1189406 [DOI] [PubMed] [Google Scholar]