Abstract
Platelet-derived growth factor receptor α (PDGFRα) is an isoform of the PDGFR family of tyrosine kinase receptors involved in cell proliferation, survival, differentiation, and growth. In this review, we highlight the role of PDGFRα and the current evidence of its expression and activities in liver development, regeneration, and pathology—including fibrosis, cirrhosis, and liver cancer. Studies elucidating PDGFRα signaling in processes ranging from profibrotic signaling, angiogenesis, and oxidative stress to epithelial-to-mesenchymal transition point toward PDGFRα as a potential therapeutic target in various hepatic pathologies, including hepatic fibrosis and liver cancer. Furthermore, PDGFRα localization and modulation during liver development and regeneration may lend insight into its potential roles in various pathologic states. We will also briefly discuss some of the current targeted treatments for PDGFRα, including multireceptor tyrosine kinase inhibitors and PDGFRα-specific inhibitors.
Key words: Platelet-derived growth factor (PDGF), Liver development, Liver regeneration, Hepatic fibrosis, Cirrhosis, Hepatocellular carcinoma, Cholangiocarcinoma, β-Catenin, NF-κB, Transforming growth factor-β (TGF-β), Oxidative stress, Sorafenib, IMC-3G3, APA5
INTRODUCTION
Chronic liver disease is a significant cause of morbidity worldwide. In the US alone, around 5.5 million Americans suffer from hepatic fibrosis and cirrhosis (1). Hepatic fibrosis, a manifestation of chronic liver disease, is a wound-healing response that results in excessive, dysregulated collagen deposition from activation of hepatic stellate cells (HSCs). This could be a result of inflammation and the release of numerous paracrine and autocrine growth factors and inflammatory chemokines from injured hepatocytes, resident macrophages, infiltrating inflammatory cells, and HSCs themselves. Hepatic fibrosis can result from a variety of injurious stimuli to the liver, including chronic hepatitis B virus or hepatitis C virus infection, chronic alcohol exposure, nonalcoholic steatohepatitis, primary biliary cirrhosis, or autoimmune hepatitis (2). The convergence of each of these injurious stimuli on a similar fibrotic injury response has made the identification of therapeutic targets to prevent or reverse fibrosis a priority. Importantly, early fibrosis is potentially reversible if hepatic injury can be curbed or repair enhanced (3–5). However, failure to curb hepatic injury in the setting of fibrosis may eventually lead to the development of cirrhosis, setting the stage for liver failure or (in a subset of patients) liver cancer.
Hepatocellular carcinoma (HCC) is the most common type of liver cancer (83% of all cases) and is the fifth most common neoplasm worldwide (6). It is a disease of grim prognosis with a 5-year survival rate of only 8.9% in the US (6). Currently nonpalliative treatments for cirrhosis and HCC are limited to radiofrequency ablation, transarterial chemoembolization, resection, and liver transplantation (7). The latter is the most effective but is associated with high morbidity, high cost, lifelong immunosuppressive therapy, and a shortage of donor organs. Thus, there is a strong need for the identification of new therapeutic targets associated with the pathogenesis of these conditions as well as effective methods of inhibition.
The development of tyrosine kinase inhibitors (TKIs) has been an important avenue for the research of new treatments for fibrosis and advanced liver disease. Many of the most successful inhibitors to date have cotargeted the components of the platelet-derived growth factor (PDGF) family of ligands and their receptors. While the β isoform of this receptor, platelet-derived growth factor receptor β (PDGFRβ), has been at the forefront of PDGF signaling in the liver due to its important roles in nonparenchymal cells (NPCs), especially myofibroblast activation during fibrosis (8,9), several new studies have shown an emerging role of platelet-derived growth factor receptor α (PDGFRα) in liver pathophysiology that may identify this receptor as an important therapeutic target.
In the current review, we highlight the role of PDGFRα and the current evidence of its expression and activities in various aspects of liver pathobiology, including liver development, regeneration, fibrosis, cirrhosis, and liver cancer. Finally, we briefly discuss some of the current targeted treatments for PDGFRα, including multireceptor TKIs and PDGFRα-specific inhibitors, which may eventually have translational applications in a number of liver diseases.
PDGF SIGNAL TRANSDUCTION
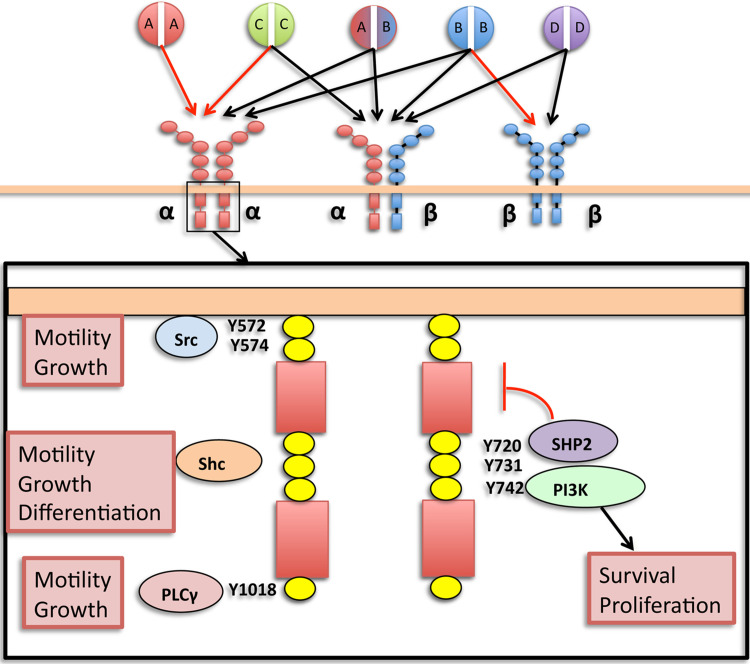
PDGFs are cysteine-knot-type growth factors that have been identified as four different disulfide-bonded polypeptide chains (A, B, C, D), which form five known dimer configurations: AA, AB, BB, CC, DD (see Fig. 1) (10–13). Each of these ligand dimers binds differentially to PDGFRs: type III receptor tyrosine kinases (RTKs) that possess five extracellular IgG domains and an intracellular kinase domain separated by a transmembrane helix (14). PDGFRs exist as α or β monomers in the plasma membrane, which are bound by dimeric PDGF ligands simultaneously to form αα, αβ, and ββ receptor dimers and, upon binding, trigger reciprocal tyrosine phosphorylation of specific residues of each receptor (15,16). Phosphorylation of tyrosine residues in the kinase domain increases catalytic efficiency and serves as binding sites for signaling molecules, including other kinases as well as nonenzymatic adaptor molecules.
Figure 1.
PDGFRα signaling. Differential binding of PDGF ligands to PDGFRs highlighting key tyrosine phosphorylation residues of the intracellular kinase domain of PDGFRα homodimer and their downstream effectors (boxed insert). Red arrows: known in vivo ligand binding. Black arrows: documented in vitro ligand binding only. Red bracket: autoinhibitory activity (SHP2). Not included are Tyr754 and Tyr849, which are also signaling tyrosine residues.
PDGFRα and PDGFRβ have distinct but overlapping sets of ligands and downstream effectors. While the differences between PDGFRα and PDGFRβ function in various cell types are likely primarily due to their spatiotemporal pattern of expression, there are some discrete differences between α and β forms as Crk binding is specific to PDGFRα, and PDGFRαβ heterodimers confer increased mitogenicity compared to α and β homodimers due to sustained activation of Ras and Erk2 (17). However, the physiologic roles of PDGFRαβ dimers are not yet clear. Downstream effectors of PDGFRα signaling include enzymes such as PI3K, MAPK, PLCγ, Src, and Shp-2, as well as nonenzymatic adaptor molecules such as Crk, Shc, and Grbs. These downstream mediators are important for a variety of cell processes including proliferation, cell survival, cell growth, and differentiation (see Fig. 1). Specific downstream mediators and tyrosine residue phosphorylation sites involved in PDGFR signaling have been previously reviewed (11,16).
PDGFRα IN LIVER PHYSIOLOGY
PDGFRα in Liver Development
Studies of both PDGFRα and PDGFRβ have demonstrated that they are essential in embryonic development. Mice lacking either PDGFRα or PDGFRβ are embryonic lethal (18,19), with PDGFRα homozygous null mutant embryos showing incomplete cephalic closure and apoptosis of migrating neural crest cells, as well as skeletal and vascular abnormalities. Within embryonic livers, PDGFRα is present in important mesenchymal and mesothelial subpopulations that modify the microenvironment to support developmental processes. For example, PDGFRα may mark an important population of mesenchymal progenitor cells that promote hepatoblast differentiation through direct contact and growth factor secretion. These cells, isolated by expression of Dlk-1 and PDGFRα from embryonic day 13.5 (E13.5) murine livers, show direct and indirect effects on hepatoblast maturation through direct contact and Transwell coculture experiments, respectively (20). This study is consistent with previous evidence of mesenchymal stem cell isolation using PDGFRα (21), as well as mesenchymal-supported hepatoblast maturation (22). Thus, PDGFRα may mark a small, but active, subpopulation of mesenchymal stem/progenitor cells that indirectly influence the development of hepatoblasts in fetal liver development.
Consistent with a supportive role of PDGFRα+ cells in hepatoblast development, PDGFRα+ stromal cells in murine fetal liver were also found to be necessary for erythropoiesis (23). In this study, it was shown that the PDGFRα+ fraction of murine fetal liver is necessary for the expansion of erythrocyte progenitor colonies in vitro, while maternal injection of anti-PDGFRα monoclonal antibody led to inhibition of erythropoiesis. In addition, exogenous PDGF-AA and PDGF-BB stimulated erythropoietin (EPO) production in fetal liver cells. These studies demonstrate an important role of PDGFRα signaling in EPO production and hematopoiesis in the liver, though a specific relationship between PDGFRα signaling and EPO production was not elucidated in this study.
While traditionally considered a receptor of mesenchymal cells, we observed both cytoplasmic and perinuclear expression of PDGFRα in a subset of epithelial cells during mouse embryonic liver development with peak expression from E10 to E12 (24,25). In contrast to the mesenchymal cell profiles from isolated PDGFRα+ cells reported by others (20), we show that a subset of HNF4α+ hepatoblasts from embryonic liver tissue expresses PDGFRα and that inhibition of PDGFRα signaling in embryonic liver cultures results in decreased survival and proliferation of these cells. This could be a cumulative effect of PDGFRα suppression in various aforementioned cell types. Following this midgestational period, PDGFRα expression dramatically decreases throughout murine fetal liver development and remains low in adult murine liver.
In combination with previous findings that PDGFRα marks a population expressing mesenchymal markers, the finding of PDGFRα in a subset of hepatoblasts brings to light the possibility that this receptor may be expressed in epithelia developing from a mesenchymal subpopulation—a process known as mesenchymal-to-epithelial transition. Such an occurrence has been previously reported in mouse hepatic stem cells in vivo, which coexpress markers of both epithelial (CK8/18) and mesenchymal (vimentin) markers at similar embryonic time points (26). In fact, the mesenchymal population characterized in this study was isolated based on intermediate expression of Dlk-1, a known marker of hepatoblasts and (at low expression) also a marker of mesothelial precursors (27). The contribution of mesenchyme to a subset of hepatoblasts and eventually to hepatocytes was also supported more recently by the fact that vascular endothelial growth factor receptor 2 (VEGFR2)—a known mesodermal marker—was also expressed in hepatic progenitors capable of contributing to a substantial portion of adult parenchyma shown by lineage-tracing studies (28).
PDGFRα expression was also identified in mesothelial and submesothelial cells of E12.5 murine livers, which are proposed to be precursors of HSCs (29). As with the abovementioned studies, PDGFRα was used primarily as an identifying marker, and a specific role of PDGFRα signaling was not elucidated. In the case of PDGFRα expression in mesothelial and submesothelial cells of the liver, it can be speculated that PDGFRα plays a proproliferative response, which may be important for expansion of this HSC precursor population during development.
The presence of PDGFRα in mesenchymal, mesothelial, and epithelial cells of the developing liver may provide insight on its importance in adult liver pathophysiology. For example, the expression of PDGFRα in mesothelial precursors of HSCs, including “submesothelial cells” and their transitional cell counterparts (29), as well as its potential expression in a subset of hepatoblasts, may signify that PDGFRα is serving as part of a modulatory proliferative transcription program, which is upregulated in liver development and pathology, while being suppressed in quiescent, nonproliferative states. Further investigation of the effects of PDGFRα inhibition in an in vivo or ex vivo developmental context will help to shed light on the function of this receptor in supporting hepatoblast maturation, erthyropoiesis, or mesothelial/submesothelial migration and HSC formation. Eventually, since tumorigenesis often represents reawakening of the developmental programs that may contextually encompass epithelial-to-mesenchymal transition (EMT), PDGFRα modulation may provide novel therapeutic opportunities in HCC.
PDGFRα in Liver Regeneration
Our lab has previously investigated the role of PDGFRα in liver regeneration using the well-known two thirds partial hepatectomy (PH) model in which two thirds of the liver mass is surgically removed and compensatory regeneration is subsequently studied at discrete, well-characterized time points (30). In control mice, PDGFRα activation was evident at 3 h, although its total levels were unequivocally elevated at 24 h. For further studies, we first generated mice lacking PDGFRα in hepatocytes (albumin-cre excision of floxed Pdgfra). These mice were indistinguishable from their littermates at baseline. When subjected to PH, PDGFRα knockout (KO) mice showed initial delay in Akt signaling by 3 h post-PH that was soon offset by upregulation of epidermal growth factor receptor (EGFR) and hepatocyte growth factor (HGF) receptor Met. Both EGFR and Met have been shown to be crucial mediators of normal liver regeneration (31). In combination with previous findings of Pdgfra and Pdgfa upregulation in rats during shRNA-mediated inhibition of EGFR following 24-h PH, our results suggest a potential reciprocal regulation between PDGFRα and EGFR (32). These studies exemplify the well-known phenomenon of growth factor signaling compensation in liver regeneration (30). Rather than diminish the importance of the PDGFRα signaling axis in hepatocyte regeneration in this model, these results attest to the signaling “flexibility” that is a well-recognized theme in PH. Similar to most growth factors in liver regeneration following PH, ligands of PDGFRα appear to play a significant, but replaceable, role.
PDGF ligands, including ligands for PDGFRα, are generally known for their mitogenic effects in mesenchymal-derived stromal cells of the liver. However, there is important evidence that hepatocytes themselves may respond to PDGFs. A recent study that examines the effect of growth factors on murine hepatocytes reveals a modest but significant and direct mitogenic effect of PDGF-AB on primary murine hepatocytes (33). The importance of this finding is underscored by the fact that prior to this study, only HGF and ligands of EGFR were identified as direct mitogens on primary hepatocytes in chemically defined medium (30). Evidence of PDGF-induced mitogenesis of hepatocytes in vitro or in vivo in the context of liver regeneration is sparse at this time. However, due to the increasing emergence of PDGFRα signaling as a therapeutic target in pathologic liver states (see below), the elucidation of regenerative hepatocyte PDGFRα signaling may be important to fully interpret the effects of therapeutic PDGFRα inhibition. Together, these studies suggest that PDGFRα signaling may occur in the hepatic parenchyma during liver regeneration—possibly contributing to mitogenesis. This is in contrast to models of chronic liver injury (discussed below) where PDGFRα seems to be located primarily in the NPCs.
PDGFRα IN LIVER PATHOLOGY
PDGFRα in Hepatic Fibrosis
Hepatic fibrosis is a complex process that involves many cell types within the liver (3). In many scenarios, it is initiated by apoptosis and necrosis of hepatocytes in the setting of chronic liver injury, which activates quiescent HSCs through the release of apoptotic bodies, reactive oxygen species (ROS), and the activation of Kupffer cells (34). The main mediators of fibrosis are activated myofibroblasts—the source of collagen and fibrous scar formation—arising from activated HSCs in the space of Disse (35). While myofibroblasts are the primary mediators of fibrosis (36), hepatocytes continue to play an important role through apoptosis, release of cytokines and growth factors to influence myofibroblast activation (37,38), and altered proliferation (39,40).
The role of PDGFRα signaling in the setting of fibrosis is still a matter of debate, as many studies present compelling data leading to differing conclusions on its contributions and relative importance compared to its related isoform PDGFRβ in HSC activation and proliferation. In the following sections, we discuss some of the evidence for the localization and function of PDGFRα in the fibrotic liver, highlighting conflicting results and interpretations in the literature.
Relative Contributions of PDGFRα Versus PDGFRβ in HSC Activation: Reconciling the Evidence
Though PDGFRβ has long been established as a functional marker of activated HSCs (9), PDGFRα has only recently emerged as a potential mediator of HSC activation in hepatic fibrosis. Early studies of PDGFR isoforms in HSC emphasized the importance of PDGFRβ due to the upregulation of this isoform at mRNA and protein level in contrast to the constant levels of PDGFRα observed following carbon tetrachloride (CCl4) or bile duct ligation (BDL)-mediated injury in rats (8). Over the next couple of decades, PDGFRα expression in HSCs of fibrotic livers became increasingly clear. PDGFRα mRNA is highly expressed in α-smooth muscle actin (α-SMA)-positive NPCs of cirrhotic human livers localized in the perisinusoidal region (41). This study also showed that PDGFRα is upregulated in stromal and sinusoidal cells in human livers during cirrhosis and reported a strong correlation between expression of PDGFRα and PDGFRβ in human livers to the histology activity index (Knodell’s score) and type III collagen deposition (41). These findings were subsequently affirmed when PDGFRα upregulation was also observed in whole cell lysates of rat livers treated with CCl4 (42) and has most recently been confirmed in the murine BDL (43) and CCl4 models (44). The exception of this trend is a study in BDL rats indicating a potential difference in PDGFRα signaling role in toxic and cholestatic fibrosis models (discussed further below) (45).
Findings from studies of PDGF signaling in isolated rat HSCs and culture-activated myofibroblasts indicate that the PDGFRαα homodimer is not likely to be the primary PDGFR isoform involved in HSC activation/proliferation as evident from studies showing that culture-activated HSCs showed selective proliferative response to PDGF-B and PDGF-D isoforms and lacked mitogenic response to PDGF-AA (specific for αα homodimer, see Fig. 1) (45). There is however some discrepancy between findings in this model system, as an earlier study showed a small, but significant (two- to threefold), proliferative effect of PDGF-AA on culture-activated HSCs (46). Of particular importance is a study that noted a comparable level of PDGF-AA-induced mitogenicity in HSC lines isolated from patients (47). This study also showed that PDGF-AA may help activated HSCs overcome proliferative inhibition from ECM molecules such as collagen I. It is worth mentioning that part of the discrepancy between the mitogenic responses of HSCs to PDGF-AA between studies may be related to the specific concentration of ligand used. The studies showing mitogenicity of PDGF-AA in rat- (46) and human-derived HSCs (47) both showed maximal proliferative stimulation of HSCs at 10 ng/ml PDGF-AA. In contrast, the study of rat culture-activated HSCs, which showed no effect of PDGF-AA only utilized a single and higher concentration (50 ng/ml).
Despite the relatively minor role of PDGFRα in proliferation of culture-activated HSCs, Hayes et al. recently showed that PDGFRα is upregulated in HSCs following CCl4-mediated fibrosis in mice and that activation of PDGFRα may contribute to hepatic fibrosis, since fibrosis was reduced following CCl4-mediated injury in mice heterozygous for PDGFRα (44). While previous studies have reported PDGFRα expression in HSC from animals (8,42) and patients (41), the study from Hayes et al. is the first report indicating that genetic reduction of PDGFRα signaling in vivo reduces hepatic fibrosis in chronic liver injury, thus paving the way forward for possible therapeutic inhibition.
What are possible explanations for the seeming discrepancies of the profibrotic contribution of PDGFRα signaling between culture-activated HSCs and murine PDGFRα heterozygotes? The answer is unclear at the moment but may involve one or more factors, including (i) an effect on PDGFRαβ heterodimer expression, (ii) a lesser role of PDGFRαα signaling in HSC activation/proliferation, and (iii) differences in receptor isoform signaling function. With regard to (i), PDGFRαβ heterodimer is not known to interact with PDGF-AA (Fig. 1) but still requires PDGFRα expression. If PDGFRαβ is playing an active role in HSC activation, PDGFRα might only contribute to HSC proliferation and myofibroblast activation through its ability to complex with the β receptor. This explanation is consistent with the findings of close PDGFRα and PDGFRβ colocalization in fibrotic livers (41,44), as well as the presence of PDGFRα phosphorylation in chronic liver injury [(43), unpublished observations].
While PDGFRαβ heterodimer function is a plausible explanation for these studies, PDGFRα is still likely to contribute to HSC activation through (ii) its homodimer form as PDGF-AA (a ligand specific to PDGFRαα homodimer) did show a significant, albeit lesser, effect on proliferation/intracellular calcium in culture-activated HSCs. Furthermore, transgenic mice overexpressing PDGF-A in hepatocytes spontaneously develop fibrosis (48). This study lends strong support to the notion that PDGF-AA/PDGFRαα signaling alone is at least sufficient to initiate hepatic fibrosis in mice—though whether hepatic fibrosis is propagated by active PDGFRαα signaling or is reliant on the subsequent autocrine or paracrine release of other factors (such as other PDGF isoforms) is not assessed in this study.
Finally, future investigations should consider that (iii) downstream signaling functions of PDGFRs in HSCs might be isoform specific. Studies of chemically defined mouse embryonic fibroblasts have shown that PDGFRαα, αβ, and ββ activate distinct downstream signaling pathways (49). Primary cell culture studies to determine differences of PDGFRα and PDGFRβ signaling in genetically PDGFR-defined HSCs or myofibroblasts may ultimately be necessary to fully understand the specific roles of PDGF/PDGFR in HSC activation, survival, or proliferation.
The development of several transgenic murine models overexpressing specific forms of PDGF in hepatocytes under albumin promoter-controlled cre recombinase support a potential role of PDGFRα in the development of fibrotic changes in the liver. In addition to transgenic overexpression of PDGF-A (described above), PDGF-B overexpression in hepatocytes leads to development of spontaneous fibrosis in mice (48,50). Similarly, the overexpression of PDGF-C, a known ligand of both PDGFRα and PDGFRβ in transgenic mice leads to the spontaneous development of liver fibrosis, steatosis, and HCC (51). Both PDGFRβ and PDGFRα were upregulated in whole liver lysates in this model. In addition, PDGF-C overexpression in hepatocytes causes expansion of NPC populations including sinusoidal endothelial cells and activated HSCs (52), supporting potential PDGFRα expression in both of these populations. It should be noted, however, that neutralization of PDGF-C in other murine strains by genetic knockout or neutralizing antiserum does not confer protection to BDL-induced liver injury (43). Data from this study indicate that PDGF-C may primarily mediate its fibrotic effects through PDGFRβ rather than PDGFRα, as PDGFRβ mRNA and total/phosphorylated protein level—not PDGFRα—is exclusively upregulated in response to PDGF-C neutralization. These authors confirm that this is not due to differential expression of other PDGF isoforms. Thus, at least in the context of murine experimental biliary fibrosis, it appears that PDGFRβ, not PDGFRα, is the primary activated receptor in response to PDGF-C in a pathophysiological (non-overexpressed) setting. Nevertheless, PDGFRα is still substantially upregulated and phosphorylated in these settings, indicating activation of this receptor in biliary fibrosis.
Evidence suggesting the presence of PDGFRα in HSCs and activated myofibroblasts sheds new light on much of the current literature regarding PDGFR signaling in HSCs and activated myofibroblasts in hepatic fibrosis/cirrhosis—the majority of which focus exclusively on assessment of PDGF-BB/PDGFRβ signaling. In light of the fact that PDGF-BB activates both PDGFRα and PDGFRβ, much of the data can be reinterpreted to consider a potential contribution of PDGFRα isoform.
TGF-β/PDGFRα Cross Talk in HSCs
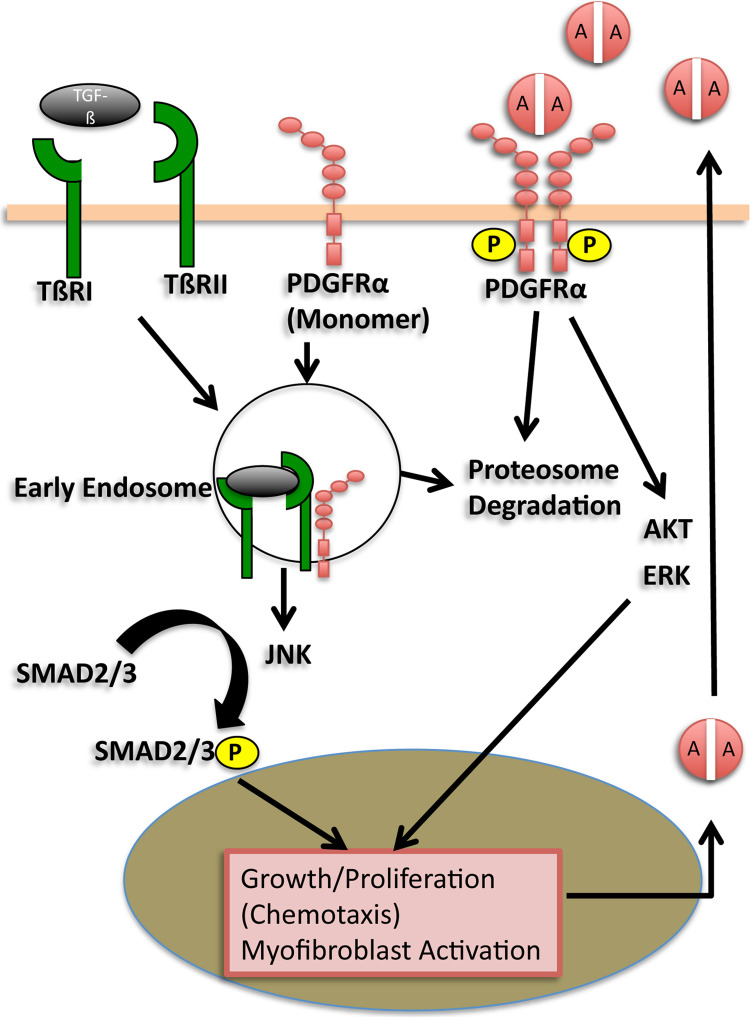
Thus far, we have primarily considered only ligand-dependent mechanisms of PDGFR activation in hepatic fibrosis. However, a recent study sheds new light on a potential ligand-independent role of PDGFRα in HSCs. Liu et al. show compelling evidence that PDGFRα appears to be necessary for SMAD2 signaling downstream of transforming growth factor-β (TGF-β) receptor in human HSCs in vitro (53). This was demonstrated through the shRNA knockdown of PDGFRα in human HSCs and HSC cell line LX-2, which led to a decreased RNA expression of TGF-β receptor I (TβRI) and SMAD2 phosphorylation activity of TGF-β receptor II (TβRII). SMAD-2 is a key mediator of fibrosis in myofibroblasts in the setting of acute and chronic liver injury (54), indicating a potential role of PDGFRα in this important arm of TGF-β signaling. This study brings to light a novel mechanism of indirect PDGFRα activation triggered by interaction of PDGFRα with TβRII (summarized in Fig. 2). The modulation of PDGFRα expression in response to TGF-β is consistent with previous findings in other fibroblast populations including scleroderma skin fibroblasts (55). Though PDGFRα activation in the absence of direct ligand binding has been previously reported (56), this is the first report indicating that PDGFRα is necessary for a major fibrotic signaling pathway in the liver. Combined with previous studies showing TGF-β-induced PDGFRα in a Ras-mutant murine hepatocyte model of EMT (see “PDGFRα in EMT” below), there may be a reciprocal regulation between TGF-β and PDGF signaling.
Figure 2.
TGF-βR/PDGFRα signaling cross talk in HSC. Ligand-dependent and -independent signaling pathways are shown. During ligand-independent signaling, PDGFRα is recruited to TβRI/TβRII complexes by TGF-β stimulation. Through interaction with TβRII, PDGFRα promotes internalization and trafficking of TGF-β receptors into the early endosomes, where phosphorylation of SMADs occurs and TGF-β signaling is activated. Knockdown of PDGFRα blocks endocytosis of TGF-β receptors, thereby inhibiting phosphorylation of SMADs. Activation of SMAD2/3 has been shown to lead to the upregulation of PDGF-A mRNA, which may indicate an autocrine mechanism of PDGFRα activation in HSCs. Abbreviations: TGF-β receptor II (TβRII). Adapted from Liu et al. (53).
Experimental RTK inhibitors often function by preventing the activating interaction of ligands and their receptors, either by binding ligands or receptors themselves to prevent phosphorylation. The findings by Liu et al. suggest that PDGFRα may function in chronic liver injury not only through RTK autophosphorylation following ligand binding but also through a ligand-independent mechanism involving monomeric PDGFRα. Further investigation of the extent of the latter form of PDGFRα signaling in vivo will be particularly relevant to predict the effectiveness of targeted PDGFRα inhibitors, which may only prevent ligand binding. Ligand-independent PDGFRα activation has been shown previously in the setting of proliferative vitreal retinopathy in which mitochondrial ROS triggers the activation of Src family kinases (SFKs) leading to phosphorylation of monomeric PDGFRα (57). In another example, the PDGFRα-specific inhibitor IMC-3G3 (discussed further below) failed to inhibit bone marrow-induced Akt activation in metastatic prostate cells in vitro and in vivo as a result of ligand-independent transactivation of PDGFRα (58,59). Despite these examples, exclusive monomeric activation of PDGFRα during liver injury is unlikely due to the overwhelming evidence that PDGF ligands play a central role in the initiation and progression of fibrosis (25,48,50,51,60). Thus, it is likely that ligand-independent PDGFRα activation through TGF-β signaling arm is only one mode of activation of downstream PDGFRα signaling.
While the full nature of PDGFRα signaling in this model is not elucidated in this study, previous studies suggesting PDGF-induced activation of SMADs may offer insight. Treatment of rat primary HSC in culture with TGF-β results in a selective increase of PDGF-A mRNA expression (61), which may implicate an autocrine activation of PDGFRα in HSC (see Fig. 2). It has previously been shown that cotreatment of cultured HSC with TGF-β and PDGF (unspecified isoform) leads to c-Jun N-terminal kinase (JNK)-mediated activation of SMAD2/3(62). In vitro, high TGF-β1 concentrations in a study of aortic smooth muscle cells and fibroblasts were shown to cause differential modulation of PDGF-AA (increased) and PDGFRα (decreased) (63) responsible for an inhibition of PDGF-AA-mediated growth. In light of the study by Liu et al., it is interesting to speculate that in addition to transcriptional regulation of PDGF-A and PDGFRα by TGF-β, posttranscriptional regulation may also be occurring via direct binding and internalization of PDGFRα by ligand-activated TβRs as in aortic smooth muscle cells and fibroblasts of the aforementioned study.
PDGFRα Inportal Myofibroblast Activation During Cholestatic Liver Injury
PDGFRα expression in cholestatic liver injury was initially reported in myofibroblasts isolated from mice subjected to BDL at various time points (8). However, in contrast to PDGFRβ, PDGFRα mRNA was not upregulated following BDL. Similarly, a more recent study of PDGFR and PDGF expression following BDL in rats shows that PDGFRα protein expression remains relatively unchanged, or only slightly elevated (45), in contrast to increased PDGFRβ expression.
Portal fibroblasts (PFs) are thought to play an important role in the initiation of fibrosis following cholestasis, particularly in early response to biliary injury (64). The question of whether PFs possess PDGFRs and are responsive to PDGF signaling is unclear at this time due to contradictory reports. PDGF-BB-mediated expansion of isolated peribiliary fibroblasts from rats that have undergone BDL express functional PDGFRβ that contributes to myofibroblastic differentiation as measured by α-SMA expression (65). In addition, peribiliary myofibroblast conversion as measured by α-SMA was reduced upon treatment with PDGFR inhibitor STI571 (Gleevec). On the other hand, primary rat PFs isolated by Wells et al. show no mitogenic activity in response to PDGF-BB stimulation in vitro (66). Li et al. demonstrate an interesting dichotomous effect of PDGF-BB on isolated rat PFs: exposure to PDGF-BB inhibited differentiation of PFs as measured by α-SMA but promoted proliferation (67) on collagen I-coated polyacrylamide gel supports. Finally, a study of murine BDL-derived activated PFs demonstrated that these fibroblasts were unresponsive to PDGF in contrast to HSCs (64).
Thus, the range of PDGFs and their receptors expressed in PFs during biliary fibrosis will require further investigation in order to elucidate the potential autocrine or paracrine mechanisms of PDGF signaling in this population. While PDGFRα and PDGFRβ have been previously reported in isolated HSCs during cholestatic liver injury (8), the absence or presence of PDGFRα in PFs prior to myofibroblastic changes remains unknown, as (to our knowledge) only PDGFRβ has been confirmed in isolated PFs (65). This will be an interesting question to address in future studies, since PFs actively contribute to the myofibroblast population in early cholestatic injury (64) and biliary fibrosis and can be attenuated by targeted inhibition of PDGF signaling (68).
PDGFRα Cellular Localization: Expression Patterns in Chronic Liver Injury
As an autocrine and paracrine signal receptor, insight on the actions of PDGFRα signaling may be elucidated by its cellular localization in normal and pathogenic liver states. Localization of PDGFRα is most clearly demonstrated in NPCs of the liver including HSC and EC. Early reports of PDGFRα localization in normal and cirrhotic human livers identify PDGFRα expression in stromal cells of portal tracts as well as some sinusoidal EC and EC of the centrilobular veins (8,41). Another group reported that mice with thioacetamide (TAA)-induced liver injury showed upregulated PDGFRα localizing in a sinusoidal pattern and in NPC (60). Consistent with a sinusoidal pattern of expression in cirrhosis, PDGFRα is overexpressed in EC of HCC associated with high metastatic potential (69) and increased recurrence in patients. This is in line with evidence that tumor fibroblasts may become resistant to anti-VEGF therapy through the expression of PDGF-C (70). While one recent study denied the expression of PDGFRα in EC during CCl4-mediated liver injury in mice (44), it should be noted that this conclusion was based on lack of colocalization with CD31 (PECAM), whose expression is low in liver sinusoidal EC (LSEC) following CCl4 treatment and thus may not be a sensitive marker in this model (71).
Currently, the cellular localization of PDGFRα in hepatocytes during chronic liver injury is unclear. In situ immunostaining of human normal and cirrhotic liver shows no PDGFRα expression in hepatocytes (41). A recent study also reported an absence of hepatocyte PDGFRα in a murine CCl4 model (44). In contrast, PDGFRα is reportedly upregulated in regenerating rat hepatocytes following CCl4-mediated fibrosis (42), and contrary to the reported findings of other labs, we have found a low level expression of PDGFRα in hepatocytes of human and murine liver (25). Further support for the presence of PDGFRα in hepatocytes stems from the finding that isolated murine hepatocytes proliferate in response to PDGF-AB in a chemically defined serum-free growth media, and PDGF-AA or PDGF-BB exposure increases bromodeoxyuridine staining in these hepatocyte cultures (33). In contrast, another group has reported that primary hepatocytes in culture do not respond to PDGF-CC (51). It should be noted that hepatocytes are heterogenous and different subpopulations (e.g., periportal vs. pericentral) may express different receptors due to their differing metabolic roles or depending on the zonality of liver injury. Therefore some subpopulations of hepatocytes may specifically upregulate PDGFRα/PDGF-A signaling over others, as was evidenced in rat livers subjected to CCl4 in which pericentral hepatocytes selectively expressed PDGF-A (42).
It remains undetermined whether a potential upregulation of PDGFRα in hepatocytes would be a reparative or pathologic response in chronic liver injury. Considering that hepatocyte survival and proliferation play crucial roles in liver regeneration and fibrosis, and detrimental roles in injury sequelae such as liver cancer, these findings warrant further investigation of PDGFRα signaling in hepatocytes. Elucidating the contribution of hepatocyte PDGFRα activation in disease pathogenesis, the signaling arms activated and their downstream cellular events will not only improve understanding of the pathobiology of this disease process but will also be relevant in validating PDGFRα as a therapeutic target. For these studies, hepatocyte-specific conditional knockouts of PDGFRα may lend themselves well (24).
PDGF Sources in Injured Liver
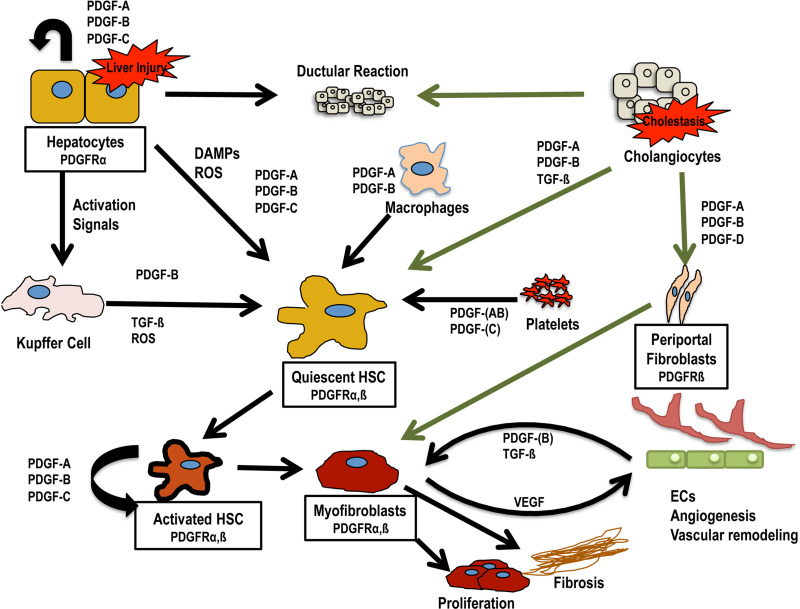
During liver injury, PDGFs are secreted by both resident and infiltrating cells of the liver including hepatocytes, Kupffer cells, cholangiocytes, infiltrating macrophages, and HSC themselves (summarized in Fig. 3, Table 1). PDGFs from all of these sources likely converge on HSCs to trigger their activation and myofibroblast conversion, as well as proliferation and migration. PDGFs are also likely to exert autocrine effects on cell populations that express PDGFRs in addition to PDGF ligands, such as hepatocytes and activated HSCs. Kupffer cells and infiltrating macrophages are considered one of the primary sources of PDGFs involved in activating HSC. PDGF-B is expressed by infiltrating macrophages and Kupffer cells in patients with chronic hepatitis/cirrhosis, the expression of which correlates with inflammation and severity of fibrosis (72). Hepatocytes influence HSC activation via activation of Kupffer cells as well as directly through the secretion of PDGFs and other signals during liver injury. Freshly isolated rat hepatocytes have been reported to express mRNA for PDGF-A and PDGF-C, while PDGF-B mRNA was present in low amounts, and PDGF-D mRNA was absent (61).
Figure 3.
PDGF sources and cell interactions in injured liver. Shown are known or predicted (in parentheses) sources of PDGF secretion during liver injury and potential interactions between resident and infiltrating liver cell populations during toxic or cholestatic liver injuries. Curved arrows represent potential autocrine stimulation. Green arrows represent events specific for cholestatic liver injury. Abbreviations: damage-associated molecular patterns (DAMPs), hepatic stellate cell (HSC), platelet-derived growth factor (PDGF), reactive oxygen species (ROS), transforming growth factor-β (TGF-β).
Table 1.
Expression Patterns of PDGFs/PDGFRs in Normal and Pathologic Liver States
| Cell Type | Normal Liver | Biliary Liver Injury | Toxic Liver Injury | HCC | CCA | Ref. |
|---|---|---|---|---|---|---|
| Stellate cells (myofibroblasts only) |
α, β A, B, D† |
α, β A, B, D |
α, β (D) |
(α, β) | NR | 8,42,44,45,53,60–62 |
| Portal fibroblasts (peribiliary myofibroblasts only) |
NR | β B, D |
(β) | NR | NR | 45,65,75 |
| Kupffer cells | NR | NR | B | NR | NR | 42,72,134 |
| Endothelial cells | α, β | α, β | α* | α, β | A | 8,42,45,116,135 |
| Hepatocytes | – A, B†, C |
NR | α*
A, B†, C |
α | α | 25,42,60,61 |
| Bile duct epithelia | – | A, B, D | D | – | A | 42,75,116,136 |
| Platelets | NR | NR | AB, C | NR | NR | 74 |
| Infiltrating macrophages | NR | NR | A,B | NR | NR | 72,134 |
Summary of PDGF and PDGFR isoform expression in select liver cells in normal and pathologic states. A, B, C, and D represent various PDGF ligands; α/β are PDGF receptors. Expression specific to activated forms (myofibroblasts) is shown in parentheses. NR, PDGF/PDGFR expression not reported in the evaluated literature. –: of PDGF/PDGFR expression specifically reported.
Discrepancy between studies.
Predicted expression only.
Perhaps one of the most important sources of PDGFs in fibrosis is from HSCs themselves. Freshly isolated rat HSCs express PDGF-A, B, and low levels of D, while transdifferentiated myofibroblasts (HSC plated for 8 days) also express PDGF-C (61). Platelets are also known to be important secretory sources of many molecules and growth factors involved in liver regeneration including PDGFs (73). Supporting this, freeze-dried platelets storing growth factors including PDGF are able to promote hepatocyte proliferation in mice (74).
The sources of PDGFs are most likely determined by the origin of the liver injury. In contrast to the lack of cholangiocyte PDGF positivity in tissue specimens from cirrhotic patients (41), cholangiocytes from patients with biliary atresia do demonstrate strong expression of PDGF-AA and PDGF-BB (75).
PDGFRα in HCC
During chronic liver injury, the persistence of hepatocyte cell death and fibrotic response can lead to cirrhosis characterized by the presence of regenerative nodules disrupting normal architecture, altered blood flow, and portal hypertension (76). In a subset of cirrhosis patients, hyperplastic nodules undergo increasing genomic stability as a result of unrelenting hepatocyte necrosis and proliferation, eventually forming dysplastic nodules and ultimately HCC (6).
Studies from our lab and others suggest a role of PDGFRα dysregulation in hepatocarcinogenesis [full review in (77)]. We have previously shown that the majority of human HCCs overexpress PDGFRα and that a subset of these tumors also upregulate PDGF-AA and PDGF-CC expression (25). This study also demonstrates that several human and rat cell lines of hepatoma and HCC also express increased levels and activation of PDGFRα, and in vitro inhibition of PDGFRα in these human cell lines using IMC-3G3 (Olaratumab, described below)—a monoclonal antibody inhibitor of human PDGFRα—led to significant decreases in DNA synthesis and cell survival. PDGFRα overexpression was also detected in 46/63 (73.0%) patients who did not undergo neoadjuvant chemo/radiotherapy (78). In this study, a significant clinical correlation was found between vascular invasion in resected HCCs that overexpress PDGFRα as well as those that overexpress PDGFRβ compared to those that did not. In addition, the coexpression of PDGFRα, PDGFRβ, and VEGF was identified by multivariate analysis to be an independent prognostic factor of disease-free and overall survival in this cohort. Furthermore PDGFRA is upregulated in K19-positive HCCs from patients, which are associated with increased tumor size, microvascular invasion, metastasis, and poor differentiation (79). Lastly, a study found high intratumoral expression of PDGFRα and PDGFRβ in a small subset of HCCs, which were independently associated with poor overall survival (80). The seeming discrepancy between the number of patients expressing “high” levels of PDGFRα in this study compared to other studies in which the majority of patients overexpress PDGFRα (25,78) may be explained by the categorization of patients into “high” or “low” expression groups, in which only tissues staining with the highest intensity on a five-tier scale were categorized as “high”—rather than direct comparison of PDGFRα upregulation compared to adjacent normal liver tissue.
Findings in patients are corroborated in preclinical animal models of HCC. Mice lacking the secreted proteoglycan decorin—a tumor suppressor inhibiting both EGFR and the hepatocyte growth factor receptor Met—have dysregulated PDGFRα signaling in TAA-induced hepatocarcinogenesis leading to more severe cirrhosis and HCC (60). This was possibly due to impaired sequestration of secreted PDGF ligands by decorin in the ECM and increased production of PDGF.
These findings suggest that unregulated PDGFRα signaling is pathogenic and may promote hepatocarcinogenesis. The number of studies suggesting a role of PDGFRα in promoting hepatocarcinogenesis has been a driving impetus for the further study of specific roles of PDGFRα in liver cancer. Some of the potential modes of action and regulation of PDGFRα in HCC are discussed in the following sections.
Potential Roles of PDGFRα in Tumor Biology: Modulation of Angiogenesis and Hypoxic Response in Chronic Liver Injury and Liver Cancer
HCC is a highly vascularized tumor for which PDGFRs represent potential alternative targets to supplement traditional VEGFR inhibitors. While PDGFRβ has been the most well-documented PDGF receptor for angiogenic effects including vessel stability (81) and maturation (82), there is evidence for a role of PDGFRα in angiogenesis as well.
Studies have shown that specific PDGFRα blockade results in the downregulation of angiogenic factors, which may be an important mode of growth inhibition in tumors (83). Furthermore PDGFRα is a cotarget of several antiangiogenic drugs (84), some with antifibrogenic effects (85). PDGFRα expression in endothelial cells (ECs) (86,87) and vascular smooth muscle cells (88), as well as liver sinusoidal ECs (8), has been reported. In addition, the presence of PDGFRα in liver ECs in liver cancer is strongly supported by findings in HCC (described below) indicating its upregulation in pathologic angiogenesis.
There is substantial evidence that PDGFRα signaling in HCC is associated with metastasis and tumor progression, mediated at least in part by pathologic angiogenesis. PDGFRα is overexpressed in ECs of HCC associated with high metastatic potential in a murine xenograft model and increased recurrence of HCC in patients (69). In fact, PDGFRα is one of the only known tumor EC markers in HCC whose expression level roughly correlates with metastasis. Higher tumor recurrence rate and lower survival in human HCCs expressing high PDGFRA was reaffirmed in a study by Zhu et al. (89). This study also employed a murine xenograft model using an HCC cell line and transfected human umbilical vein endothelial cells (HUVECs) to show evidence that tumor progression may be the result of dysregulation of PDGFRA by BRCA1, which is in turn regulated by microRNA 146a (MiR-146a). This study shows a potential regulatory mechanism of PDGFRα expression in ECs of HCC and introduces a new potential therapeutic target upstream of PDGFRα (MiR-146a).
While the precise role of PDGFRα/PDGF-A signaling in HCC progression is unknown, studies indicate that this signaling arm is likely to be an important escape pathway for pathologic angiogenesis in the setting of HCC. One murine HCC model showed increased PDGF-A expression in the liver following drug resistance development to IFN-α (90), an antiviral with known antiangiogenic effects (91). This is consistent with evidence that tumor fibroblasts may become resistant to anti-VEGF therapy through the expression of PDGF-C (70). Furthermore, it has been shown that VEGFA can activate PDGFRα and PDGFRβ, likely due to the close homology between PDGFR and VEGFR (92). Thus, PDGFRα signaling may be an important alternative therapeutic target in addition to VEGFRs and may explain why sorafenib (a multityrosine kinase receptor inhibitor targeting VEGFRs and PDGFRs) is currently the only clinically approved targeted therapy for HCC (93,94) (discussed further below).
PDGF signaling is also important for communication between HSCs and ECs of the liver during angiogenesis to coordinate the formation and stabilization of neovessels. Cotransplantation of Ras-transformed hepatocytes with myofibroblasts in a murine model of HCC enhances tumor growth in a PDGF-dependent manner (95). Studies in rats undergoing BDL demonstrate that PDGF-BB promotes HSC-driven vascular tube formation through ephrinB2 signaling (96). The authors in this study hypothesize that this phenomenon is responsible for a decrease in portal pressure in BDL rats following imatinib treatment. Given the known expression of PDGFRα on HSCs (41,44), and HCC-associated ECs (69), it is possible that PDGFRα activation by PDGF ligands may play an active role in these processes.
Hypoxia is a well-known driver of pathologic angiogenesis. Though the specific response of PDGFRα in hypoxic liver tissue has not been reported, potential activity of this receptor can be gleaned from hypoxia-induced modulation of PDGF ligands, especially PDGF-A. PDGF-A as well as PDGF-B are downregulated in HIF-1α-deficient mice, signifying a link between hypoxia and the release of these profibrogenic mediators (97). HIF-1α/β in hepatocytes in vitro do not appear to significantly affect the production of PDGF-A or PDGF-B mRNA but, rather, promote other angiogenic factors including VEGF. Combined with this group’s previous findings in HIF-1α-deficient mice, these data indicate that HIF-1 is regulating PDGF-A and -B expression in NPCs. HIF-2α is also shown to be a likely mediator of this effect (98). Investigation of PDGFR localization and activation in response to hypoxia will be an important complement to studies of HIF-induced ligand production in order to discern the precise effects of PDGFRs in hypoxic response and angiogenesis.
Oxidative Stress in HCC: Role of NF-κB/PDGFRα Signaling Axis in the Absence of β-Catenin
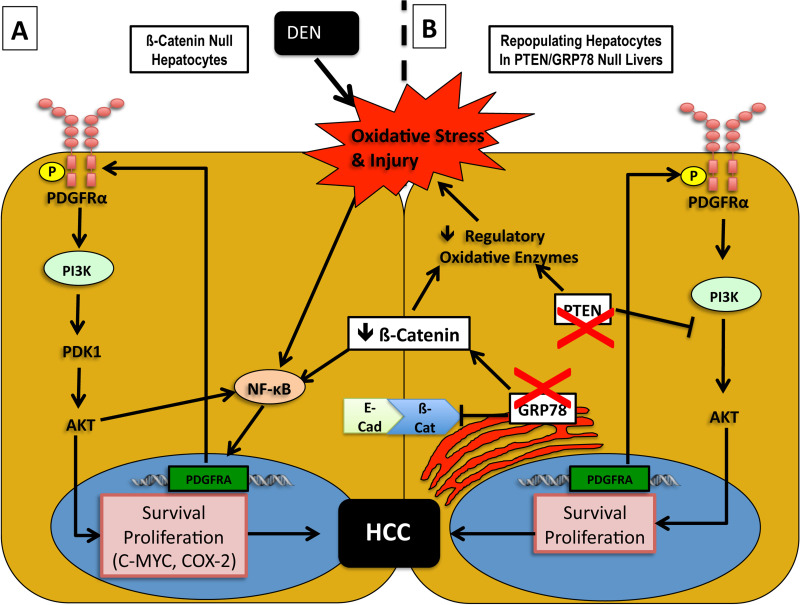
Chronic oxidative stress through mitochondrial dysfunction and failure to regulate reactive oxygen species (ROS) production has been shown to be an important modulator of liver injury and cause of DNA damage leading to HCC [for review, see (99)] and may be a stimulus for pathologic PDGFRα activation in HCC. This was demonstrated in our previous studies of N-nitrosodiethylamine (DEN)-induced hepatocarcinogenesis (100) as well as DEN treatment followed by continuous phenobarbitol treatment (DEN/PB) (101) in hepatic β-catenin knockout (KO) mice. Though β-catenin signaling is generally considered to be protumorigenic (102,103), β-catenin deficiency in hepatocytes in this model resulted in a paradoxical increase in susceptibility to DEN-induced tumor formation. Further analysis revealed increased PDGFRα and downstream phosphoinositide 3-kinase (PIK3CA)/Akt signaling responsible for compensatory hepatocyte growth and tumorigenesis. We showed that PDGFRα-mediated signaling was dependent on the induction of nuclear factor κ-light-chain enhancer of activated B cells (NF-κB). In another study, we identified the mechanism of increased NF-κB activity in β-catenin KO mice to be due to lack of inhibitory β-catenin–p65 complex in hepatocytes, which lowers the threshold of NF-κB activation to the observed oxidative stress due to DEN (104). We show that NF-κB upregulates PDGFRα in Hep3B hepatoma cells in vitro, contributing to cell proliferation. Based on this evidence, we hypothesize that NF-κB upregulation of PDGFRA serves as part of a prosurvival/proliferative response in hepatocytes in response to chronic high levels of oxidative stress (findings summarized in Fig. 4).
Figure 4.
Regulatory networks of β-catenin and PDGFRα modulation in malignant hepatocytes exposed to oxidative stress. (A) β-Catenin-deficient hepatocytes suffer from increased susceptibility to oxidative stress due to the inability to upregulate regulatory antioxidative enzymes. As a result, DEN induced genotoxic injury sustained by hepatic inflammation. The increased injury induces cytoprotective NF-κB signaling, which might also be complemented by the lack of β-catenin–p65 interactions that are known to sequester NF-κB to prevent its activation. This might, in turn, play a role in PDGFRα overexpression as PDGFRA promoter is known to contain NF-κB binding sequences. In the backdrop of injury, inflammation, and activation of NPCs, PDGF production induces activation of PDGFRα and downstream PIK3CA signaling, which leads to tumorigenesis through increased expression of targets such as c-Myc and COX-2. (B) Increased malignancy in hepatocytes due to downregulation of β-catenin and upregulation of PDGFRα was also observed in an albumin-cre-mediated PTEN/GRP78 double knockout mouse model. In this model, loss of β-catenin activity may be mediated by loss of GRP78, which increases free pools of cytoplasmic β-catenin by disrupting E-cadherin/β-catenin junction complexes at the cell membrane as well as sequestering APC (not shown), which normally forms part of the canonical inhibitory complex associated with β-catenin. PTEN loss in these hepatocytes also contributes to increased susceptibility to oxidative stress due to its normal role in maintaining regulatory antioxidative enzyme expression. Abbreviations: adenomatous polyposis coli (APC), cyclooxygenase 2 (COX2), diethylnitrosamine (DEN), glucose-regulated protein 78 (GRP78), phosphatase and tensin homolog (PTEN), phosphatidylinositol-4,5-bisphosphate 3 kinase (PIK3CA), nuclear factor κ-light-chain enhancer of activated B cells (NF-κB), nonparenchymal cells (NPCs).
In the latter study, we observed major PDGFRα and modest PDGFRβ upregulation in livers from β-catenin KO mice subjected to DEN/PB for 8 months, with increased production of PDGF-C and PDGFRα phosphorylation. In combination with in vitro data showing β-catenin knockdown with CTNNB1 siRNA is necessary for PDGF-CC-induced mitogenicity in Hep3B hepatoma cells, this study suggests an autocrine escape pathway via activation of PDGFRα. Also, since PDGFRα upregulation upon β-catenin suppression may be contributing to resistance to anti-β-catenin therapy, there may be a benefit to sequential inhibition of the two pathways.
Interestingly, other models of murine HCC have recapitulated a pattern of β-catenin downregulation and PDGFRα upregulation. Chen et al. show that Alb-cre-mediated knockout of phosphatase and tensin homolog (PTEN) and glucose-regulated protein 78 (GRP78) results in the development of both HCC and cholangiocarcinoma (CCA) associated with β-catenin downregulation and PDGFRα upregulation (105). This is most likely due to incomplete GRP78 knockout, providing repopulating liver progenitor cells and hepatocytes with a growth advantage. It is possible therefore that PDGFRα upregulation is occurring in the repopulating hepatocyte population under oxidative cell stress, similar to the findings in our previous study (100). A potential mechanism for the downregulation of β-catenin in tumor cells lacking GRP78 is the loss of a recently reported function of GRP78 in the disruption of E-cadherin/β-catenin junctional complexes at the cell membrane, as well as the sequestration of adenomatous polyposis coli (APC), which forms part of the cytoplasmic inhibitory complex of β-catenin (106) (see Fig. 4).
PDGFRα upregulation in response to chronic oxidative stress is consistent with effects seen in other tissues under pathologic conditions. For example, tissues from patients with idiopathic pulmonary fibrosis (IPF) show upregulation and colocalization of both PDGFRα and β with oxidized peroxiredoxin (Prx) II and markers of oxidative stress in parenchyma and hyperplastic epithelia (107). Upstream regulation of PDGFRα by NF-κB has also been reported in mouse interstitial lung fibroblasts (108) and rat lung myofibroblasts (109). Generation of ROS has also been shown to indirectly activate PDGFRα in proliferative vitreo retinopathy (PRV) through Src family kinase (SFK)-mediated phosphorylation of the intracellular region of the receptor followed by subsequent PI3K/Akt activation and downstream p53 suppression (110). Therefore, it is apparent that PDGFRα is commonly upregulated in response to oxidative stress and may serve as a compensatory survival response to the harsh oxidative microenvironment of HCCs.
PDGFRα in EMT
In the setting of chronic liver injury and cirrhosis in which HCC arises, hepatocytes begin to lose specific qualities of epithelial cells, including cellular junctions, apical/basal polarity, and the expression of differentiated hepatocyte markers, while acquiring migratory and invasive capabilities and reacquiring undifferentiated hepatocyte markers [reviewed in (111)]. This process, known as EMT, is known to occur in epithelial cells of other tumors as well. PDGFRα/PDGF-A signaling is strongly implicated in EMT during the pathogenesis of HCC in multiple models of neoplastic murine hepatocytes.
As mentioned above, β-catenin deficiency in albumin-expressing cells of the liver is associated with activation of PDGFRα signaling in DEN and DEN/PB models of HCC (100,101). Interestingly, β-catenin activation is implicated to be under reciprocal regulation of PDGF signaling due to studies in Ras-mutant murine models of hepatocyte neoplasia and EMT in which Ras- and TGF-β-induced PDGF signaling plays a role in the nuclear translocation of β-catenin (112). Inhibition of PDGFRα signaling through overexpression of dominant negative PDGFRA in these cells resulted in a reduction in cell motility in vitro and total tumor volume in vivo, indicating that PDGFRα may likely play a role in HCC hepatocyte proliferation, likely via β-catenin. This finding is consistent with the inhibitory activity of β-catenin from STI-571 (Gleevec), a multi-TKI with known inhibitory activity of PDGFRα and β, in several cancer cell lines (113). The relevance of this model was further supported by its use in a murine model of HCC in which it was shown that cotransplantation of Ras-transformed hepatocytes with myofibroblasts enhances tumor growth in a PDGF-dependent manner (95). Given the potential role of PDGFRα signaling in HCC-associated EMT, it is interesting to speculate that the poor prognosis in PDGFRα+ HCC is due to increased metastasis secondary to the activation of an EMT transcriptional program, pushing malignant hepatocytes to a poorly differentiated mesenchymal state.
Cholangiocarcinoma
Cholangiocarcinoma (CCA) is the second most common form of liver cancer (after HCC) and is a rapidly fatal malignant tumor of the biliary ducts (114). PDGF-A and PDGFRα are both upregulated in livers of human patients with CCA, and PDGF-A positivity showed prognostic significance due to decreased survival, increased staging, and increased metastasis (115). These patients also showed increased serum levels of PDGF-A. In addition, PDGFRα inhibition via sunitinib treatment in CCA cell lines in vitro showed reduced cell viability and migration as well as reduced phospho-PDGFRα and phospho-AKT.
The highest incidence of CCA is found in Northeast Thailand due to infection by liver fluke infection Opisthorchis viverrini. PDGF-A and PDGFRA mRNA and protein overexpression was observed in a hamster model of O. viverrini-associated CCA as well as in eight of 10 (PDGF-A overexpression) and four of 10 (PDGFRA overexpression) patients from Thailand with O. viverrini-associated CCA (116).
Therapeutic Inhibition of PDGF Receptors in Liver Disease
RTKs are critical pharmacologic targets. Evidence from the development of both small molecule TKIs as well as monoclonal antibody inhibitors support a role of PDGFRα and PDGFRβ in cancer and liver injury states, such as fibrosis and cirrhosis (94,117). PDGFRs are cotargeted by several small molecule pharmacologic agents, such as imatinib, sunitinib, and sorafenib, which are multi-TKIs, each targeting a discrete set of tyrosine kinases (118).
Many multi-TKIs that target PDGFRs appear to have activity against both α and β isoforms (69,119,120). As such, it is often difficult to delineate whether specific effects of these inhibitors stem from inhibition of PDGFRα, PDGFRβ, or both receptors. Nevertheless, preclinical and clinical studies of multi-TKIs provide important evidence that PDGFRα is a potential therapeutic target in cancer. Imatinib has shown activity in gastrointestinal stromal tumors (GISTs), which do not express mutations in c-KIT. This activity is likely due to demonstrated inhibition of PDGFRα, which is mutated in many GISTs with normal c-KIT (121) and shares an adjacent chromosomal location on human chromosome 4 as well as close amino acid homology with c-KIT. Sorafenib, a multikinase inhibitor targeting Raf, VEGFRs, and PDGFRα/β, is currently the only chemotherapeutic that has been shown to be effective in the treatment of HCC (93,94). Sorafenib has also been shown to have beneficial effects in animal models of hepatic fibrosis and portal hypertension. Partial portal vein ligation (PPVL) in rats, a model of portal hypertension, showed a decrease in portal pressure and splanchnic inflammation as well as a decrease in TGF-β, TGF-βR1, and TIMP2, potentially leading to reduced fibrogenesis (122). Sorafenib also reduced intrahepatic fibrosis, inflammation, and neovascularization in rats undergoing BDL.
A major impetus for the development of PDGFR inhibitors stems from their role in angiogenesis, as described in the preceding sections. Rats subjected to PPVL experienced decreased splanchnic neovascularization, pericyte coverage of new vessels, portal pressure, superior mesenteric artery blood flow, and resistance when treated with a combination of VEGFR inhibitor rapamycin and PDGFR inhibitor Gleevec compared to treatment with either agent alone (123). Beneficial effects of combined VEGF/PDGF signaling inhibition in portal hypertension are supported by subsequent studies showing improved hemodynamics in PPVL rats treated with Sorafenib (124).
Development of PDGFRα-Targeting Monoclonal Antibody Inhibitors: IMC-3G3
The development of specific inhibitors of PDGFRα has shown promising results in preclinical and clinical studies. IMC-3G3 (Olaratumab, ImClone Systems) is a humanized monoclonal antibody therapy that exclusively targets PDGFRα and is currently being tested in clinical trials (125). IMC-3G3 has been shown to have antitumor properties in several preclinical studies [full review available (125)], including lung cancer xenografts (126), glioblastoma and leiomyosarcoma xenografts (127), ovarian carcinoma (128), and uterine cancer (83).
The development of IMC-3G3 provides important proof-of-concept evidence of the potential effectiveness of specifically targeting PDGFRα signaling in chronic liver diseases, including cirrhosis and HCC. We have previously shown that IMC-3G3 decreases proliferation of various hepatoma and HCC cell lines (25). In addition, our previous studies in β-catenin KO mice suggest that PDGFRα may be an important cotherapeutic target in β-catenin inhibition of HCC (100). Based on the high frequency of PDGFRα overexpression (25,78) and the relative success of Sorafenib (a PDGFRα coinhibitor) in human HCCs, blockade of PDGFRα signaling may indeed have therapeutic value. In addition to IMC-3G3, other PDGFRα-specific inhibitors are available for use in preclinical models, such as the murine PDGFRα inhibitor APA5 (129–132). Combined with the potential for genetic modulation of PDGFRα demonstrated in recent studies (24,44), an abundance of potential preclinical models for PDGFRα modulation are available and await future investigation in liver regeneration, injury, and cancer.
CONCLUSION
In this review, we have aimed to evaluate the evidence of an active signaling role of PDGFRα in various aspects of liver physiology and pathology. While the specific contributions of PDGFRα continue to be investigated further, we conclude that PDGFRα inhibition may be a viable therapeutic strategy for specific hepatic pathologies, including hepatic fibrosis and HCC, which should be tested directly through the use of several clinical and experimental inhibitors. We feel that, currently, cell-specific studies of PDGFRα designed to assess the role of this receptor in individual cell populations in the liver warrant more in-depth investigations. Different resident cell populations may play potentially antagonistic biological roles such as pro- or antifibrotic roles in the setting of chronic liver injury (133). It is therefore conceivable that biologic endpoints of PDGFRα signaling (survival, proliferation, differentiation, motility) may contribute to injury progression in specific cells while ameliorating injury in others. In addition, our knowledge of the downstream targets of PDGFRα signaling especially in liver pathophysiology is rather limited and elucidation of these will be important to understand the specific role of PDGFRα. Studies of PDGFRα modulation in liver injury and liver cancer continue to uncover new signaling networks and shed light on regulatory networks and roles of PDGFRα signaling that will be important for the understanding of these conditions with eventual therapeutic implications.
ACKNOWLEDGMENTS
We would like to acknowledge the support of NIH grant R01DK095498 and Endowed Chair for Experimental Pathology to S.P.S.M. We would also like to acknowledge the support of the Angiogenesis Training Program (T32HL094295) to A.K.
REFERENCES
- 1. Action Plan for Liver Disease Research: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); 2004.
- 2. Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005; 115:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol 2011; 6:425–456. [DOI] [PubMed] [Google Scholar]
- 4. Ellis EL, Mann DA. Clinical evidence for the regression of liver fibrosis. J Hepatol 2012; 56:1171–1180. [DOI] [PubMed] [Google Scholar]
- 5. Friedman SL, Bansal MB. Reversal of hepatic fibrosis—Fact or fantasy? Hepatology 2006; 43:S82–88. [DOI] [PubMed] [Google Scholar]
- 6. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat Rev Cancer 2006; 6:674–687. [DOI] [PubMed] [Google Scholar]
- 7. Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut 2014; 63:844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong L, Yamasaki G, Johnson RJ, Friedman SL. Induction of beta-platelet-derived growth factor receptor in rat hepatic lipocytes during cellular activation in vivo and in culture. J Clin Invest 1994; 94:1563–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonner J. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 2004; 15:255–273. [DOI] [PubMed] [Google Scholar]
- 10. Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 2008; 22:1276–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heldin CH, Ostman A, Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta 1998; 1378:F79–113. [DOI] [PubMed] [Google Scholar]
- 12. Birge RB, Kalodimos C, Inagaki F, Tanaka S. Crk and CrkL adaptor proteins: Networks for physiological and pathological signaling. Cell Commun Signal 2009; 7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eriksson A, Nanberg E, Ronnstrand L, Engstrom U, Hellman U, Rupp E, et al. Demonstration of functionally different interactions between phospholipase C-gamma and the two types of platelet-derived growth factor receptors. J Biol Chem 1995; 270:7773–7781. [DOI] [PubMed] [Google Scholar]
- 14. Chen PH, Chen X, He X. Platelet-derived growth factors and their receptors: Structural and functional perspectives. Biochim Biophys Acta 2013; 1834:2176–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bishayee S, Majumdar S, Khire J, Das M. Ligand-induced dimerization of the platelet-derived growth factor receptor. Monomer-dimer interconversion occurs independent of receptor phosphorylation. J Biol Chem 1989; 264:11699–11705. [PubMed] [Google Scholar]
- 16. Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev 2004; 15:205–213. [DOI] [PubMed] [Google Scholar]
- 17. Ekman S, Thuresson ER, Heldin CH, Rönnstrand L. Increased mitogenicity of an alphabetaheterodimeric PDGF receptor complex correlates with lack of RasGAP binding. Oncogene 1999; 18:2481–2488. [DOI] [PubMed] [Google Scholar]
- 18. Soriano P. The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development 1997; 124:2691–2700. [DOI] [PubMed] [Google Scholar]
- 19. Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev 1994; 8:1888–1896. [DOI] [PubMed] [Google Scholar]
- 20. Ito K, Yanagida A, Okada K, Yamazaki Y, Nakauchi H, Kamiya A. Mesenchymal progenitor cells in mouse foetal liver regulate differentiation and proliferation of hepatoblasts. Liver Int 2013. [DOI] [PubMed] [Google Scholar]
- 21. Diarmaid DH, Yo M, Satoru M, Kunimichi N, Daisuke A, Sadafumi S, et al. Isolation of mouse mesenchymal stem cells on the basis of expression of Sca-1 and PDGFR-α. Nat Protoc 2012; 7:2103–2111. [DOI] [PubMed] [Google Scholar]
- 22. Toshitaka H, Hideaki F, Tetsuro H, Kentaro Y, Hisaya A, Shinji B, et al. Thy1-positive mesenchymal cells promote the maturation of CD49f-positive hepatic progenitor cells in the mouse fetal liver. Hepatology 2004; 39. [DOI] [PubMed] [Google Scholar]
- 23. Li WL, Yamada Y, Ueno M, Nishikawa S, Nishikawa S, Takakura N. Platelet derived growth factor receptor alpha is essential for establishing a microenvironment that supports definitive erythropoiesis. J Biochem 2006; 140:267–273. [DOI] [PubMed] [Google Scholar]
- 24. Awuah PK, Nejak-Bowen KN, Monga SP. Role and regulation of PDGFRalpha signaling in liver development and regeneration. Am J Pathol 2013; 182:1648–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stock P, Monga D, Tan X, Micsenyi A, Loizos N, Monga SP. Platelet-derived growth factor receptor-alpha: A novel therapeutic target in human hepatocellular cancer. Mol Cancer Ther 2007; 6:1932–1941. [DOI] [PubMed] [Google Scholar]
- 26. Li B, Zheng Y-W, Sano Y, Taniguchi H. Evidence for mesenchymal−epithelial transition associated with mouse hepatic stem cell differentiation. PLoS One 2011; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanaka M, Okabe M, Suzuki K, Kamiya Y, Tsukahara Y, Saito S, Miyajima A. Mouse hepatoblasts at distinct developmental stages are characterized by expression of EpCAM and DLK1: Drastic change of EpCAM expression during liver development. Mech Dev 2009; 126. [DOI] [PubMed] [Google Scholar]
- 28. Goldman O, Han S, Sourrisseau M, Dziedzic N, Hamou W, Corrneo B, et al. KDR identifies a conserved human and murine hepatic progenitor and instructs early liver development. Cell Stem Cell 2013; 12:748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Asahina K, Tsai SY, Li P, Ishii M, Maxson RE Jr., Sucov HM, Tsukamoto H. Mesenchymal origin of hepatic stellate cells, submesothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatology 2009; 49:998–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Michalopoulos GK. Liver regeneration. J Cell Physiol 2007; 213:286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kang L-II, Mars WM, Michalopoulos GK. Signals and cells involved in regulating liver regeneration. Cells 2011; 1:1261–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paranjpe S, Bowen WC, Tseng GC, Luo J-HH, Orr A, Michalopoulos GK. RNA interference against hepatic epidermal growth factor receptor has suppressive effects on liver regeneration in rats. Am J Pathol 2010; 176:2669–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bowen WC, Michalopoulos AW, Orr A, Ding MQ, Stolz DB, Michalopoulos GK. Development of a chemically defined medium and discovery of new mitogenic growth factors for mouse hepatocytes: Mitogenic effects of FGF1/2 and PDGF. PLoS One 2014; 9:e95487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prosser CC, Yen RD, Wu J. Molecular therapy for hepatic injury and fibrosis: Where are we? World J Gastroenterol 2006; 12:509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brenner DA. Molecular pathogenesis of liver fibrosis. Trans Am Clin Climatol Assoc 2009; 120:361–368. [PMC free article] [PubMed] [Google Scholar]
- 36. Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol 2010; 7:425–436. [DOI] [PubMed] [Google Scholar]
- 37. Constandinou C, Henderson N, Iredale JP. Modeling liver fibrosis in rodents. Methods Mol Med 2005; 117:237–250. [DOI] [PubMed] [Google Scholar]
- 38. Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008; 134:1655–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oe H, Kaido T, Furuyama H, Mori A, Imamura M. Simultaneous transfer of vascular endothelial growth factor and hepatocyte growth factor genes effectively promotes liver regeneration after hepatectomy in cirrhotic rats. Hepatogastroenterology 2004; 51:1641–1647. [PubMed] [Google Scholar]
- 40. Suarez-Cuenca JA, Chagoya de Sanchez V, Aranda-Fraustro A, Sanchez-Sevilla L, Martinez-Perez L, Hernandez-Munoz R. Partial hepatectomy-induced regeneration accelerates reversion of liver fibrosis involving participation of hepatic stellate cells. Exp Biol Med (Maywood) 2008; 233:827–839. [DOI] [PubMed] [Google Scholar]
- 41. Pinzani M, Milani S, Herbst H, DeFranco R, Grappone C, Gentilini A, et al. Expression of platelet-derived growth factor and its receptors in normal human liver and during active hepatic fibrogenesis. Am J Pathol 1996; 148:785–800. [PMC free article] [PubMed] [Google Scholar]
- 42. Borkham-Kamphorst E, Kovalenko E, van Roeyen CR, Gassler N, Bomble M, Ostendorf T, et al. Platelet-derived growth factor isoform expression in carbon tetrachloride-induced chronic liver injury. Lab Invest 2008; 88:1090–1100. [DOI] [PubMed] [Google Scholar]
- 43. Martin IV, Borkham-Kamphorst E, Zok S, van Roeyen CR, Eriksson U, Boor P, et al. Platelet-derived growth factor (PDGF)-C neutralization reveals differential roles of PDGF receptors in liver and kidney fibrosis. Am J Pathol 2013; 182:107–117. [DOI] [PubMed] [Google Scholar]
- 44. Hayes BJ, Riehle KJ, Shimizu-Albergine M, Bauer RL, Hudkins KL, Johansson F, et al. Activation of platelet-derived growth factor receptor alpha contributes to liver fibrosis. PLoS One 2014; 9:e92925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Borkham-Kamphorst E, Roeyen CRC, Ostendorf T, Floege J, Gressner AM, Weiskirchen R. Pro-fibrogenic potential of PDGF-D in liver fibrosis. J Hepatol 2007; 46:10641074. [DOI] [PubMed] [Google Scholar]
- 46. Pinzani M, Knauss TC, Pierce GF, Hsieh P, Kenney W, Dubyak GR, Abboud HE. Mitogenic signals for platelet-derived growth factor isoforms in liver fat-storing cells. Am J Physiol 1991; 260:91. [DOI] [PubMed] [Google Scholar]
- 47. Geremias AT, Carvalho MA, Borojevic R, Monteiro AN. TGF beta1 and PDGF AA override collagen type I inhibition of proliferation in human liver connective tissue cells. BMC Gastroenterol 2004; 4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thieringer F, Maass T, Czochra P, Klopcic B, Conrad I, Friebe D, et al. Spontaneous hepatic fibrosis in transgenic mice overexpressing PDGF-A. Gene 2008; 423:23–28. [DOI] [PubMed] [Google Scholar]
- 49. Wu E, Palmer N, Tian Z, Moseman AP, Galdzicki M, Wang X, et al. Comprehensive dissection of PDGF-PDGFR signaling pathways in PDGFR genetically defined cells. PLoS One 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Czochra P, Klopcic B, Meyer E, Herkel J, Garcia-Lazaro JF, Thieringer F, et al. Liver fibrosis induced by hepatic overexpression of PDGF-B in transgenic mice. J Hepatol 2006; 45:419–428. [DOI] [PubMed] [Google Scholar]
- 51. Campbell JS, Hughes SD, Gilbertson DG, Palmer TE, Holdren MS, Haran AC, et al. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc Natl Acad Sci USA 2005; 102:3389–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Campbell JS, Johnson MM, Bauer RL, Hudkins KL, Gilbertson DG, Riehle KJ, et al. Targeting stromal cells for the treatment of platelet-derived growth factor C-induced hepatocellular carcinogenesis. Differentiation 2007; 75:843–852. [DOI] [PubMed] [Google Scholar]
- 53. Liu C, Li J, Xiang X, Guo L, Tu K, Liu Q, et al. PDGF receptor-alpha promotes TGF-beta signaling in hepatic stellate cells via transcriptional and posttranscriptional regulation of TGF-beta receptors. Am J Physiol Gastrointest Liver Physiol 2014; 307:G749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Matsuzaki K. Smad phosphoisoform signals in acute and chronic liver injury: Similarities and differences between epithelial and mesenchymal cells. Cell Tissue Res 2012; 347:225–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yamakage A, Kikuchi K, Smith EA, LeRoy EC, Trojanowska M. Selective upregulation of platelet-derived growth factor alpha receptors by transforming growth factor beta in scleroderma fibroblasts. J Exp Med 1992; 175:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lei H, Velez G, Kazlauskas A. Pathological signaling via platelet-derived growth factor receptor {alpha} involves chronic activation of Akt and suppression of p53. Mol Cell Biol 2011; 31:1788–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lei H, Kazlauskas A. A reactive oxygen species-mediated, self-perpetuating loop persistently activates platelet-derived growth factor receptor alpha. Mol Cell Biol 2014; 34:110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dolloff NG, Russell MR, Loizos N, Fatatis A. Human bone marrow activates the Akt pathway in metastatic prostate cells through transactivation of the alpha-platelet-derived growth factor receptor. Cancer Res 2007; 67:555–562. [DOI] [PubMed] [Google Scholar]
- 59. Russell MR, Liu Q, Fatatis A. Targeting the {alpha} receptor for platelet-derived growth factor as a primary or combination therapy in a preclinical model of prostate cancer skeletal metastasis. Clin Cancer Res 2010; 16:5002–5010. [DOI] [PubMed] [Google Scholar]
- 60. Baghy K, Horvath Z, Regos E, Kiss K, Schaff Z, Iozzo RV, Kovalszky I. Decorin interferes with platelet-derived growth factor receptor signaling in experimental hepatocarcinogenesis. FEBS J 2013; 280:2150–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Breitkopf K, Roeyen C, Sawitza I, Wickert L, Floege J, Gressner AM. Expression patterns of PDGF-A, -B, -C and -D and the PDGF-receptors alpha and beta in activated rat hepatic stellate cells (HSC). Cytokine 2005; 31:349–357. [DOI] [PubMed] [Google Scholar]
- 62. Yoshida K, Matsuzaki K, Mori S, Tahashi Y, Yamagata H, Furukawa F, et al. Transforming growth factor-beta and platelet-derived growth factor signal via c-Jun N-terminal kinase-dependent Smad2/3 phosphorylation in rat hepatic stellate cells after acute liver injury. Am J Pathol 2005; 166:1029–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Battegay EJ, Raines EW, Seifert RA, Bowen-Pope DF, Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell 1990; 63:515–524. [DOI] [PubMed] [Google Scholar]
- 64. Keiko I, Chunyan J, Mingjun Z, Min C, Thomas Joseph M-M, Tae Jun P, et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci 2014; 111:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kinnman N, Francoz C, Barbu V, Wendum D, Rey C, Hultcrantz R, et al. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest 2003; 83:163–173. [DOI] [PubMed] [Google Scholar]
- 66. Wells RG, Kruglov E, Dranoff JA. Autocrine release of TGF-beta by portal fibroblasts regulates cell growth. FEBS Lett 2004; 559:107–110. [DOI] [PubMed] [Google Scholar]
- 67. Zhaodong L, Jonathan AD, Erick PC, Masayuki U, Jean S, Rebecca GW. Transforming growth factor-β and substrate stiffness regulate portal fibroblast activation in culture. Hepatology 2007; 46(4):1246–1256. [DOI] [PubMed] [Google Scholar]
- 68. Borkham-Kamphorst E, Herrmann J, Stoll D, Treptau J, Gressner AM, Weiskirchen R. Dominant-negative soluble PDGF-beta receptor inhibits hepatic stellate cell activation and attenuates liver fibrosis. Lab Invest 2004; 84:766–777. [DOI] [PubMed] [Google Scholar]
- 69. Zhang T, Sun HC, Xu Y, Zhang KZ, Wang L, Qin LX, et al. Overexpression of platelet-derived growth factor receptor alpha in endothelial cells of hepatocellular carcinoma associated with high metastatic potential. Clin Cancer Res 2005; 11:8557–8563. [DOI] [PubMed] [Google Scholar]
- 70. Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z, et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell 2009; 15:21–34. [DOI] [PubMed] [Google Scholar]
- 71. Neubauer K, Ritzel A, Saile B, Ramadori G. Decrease of platelet-endothelial cell adhesion molecule 1-gene-expression in inflammatory cells and in endothelial cells in the rat liver following CCl4-administration and in vitro after treatment with TNFα. Immunol Lett 2000; 74:153164. [DOI] [PubMed] [Google Scholar]
- 72. Ikura Y, Morimoto H, Ogami M, Jomura H, Ikeoka N, Sakurai M. Expression of platelet-derived growth factor and its receptor in livers of patients with chronic liver disease. J Gastroenterol 1997; 32:496501. [DOI] [PubMed] [Google Scholar]
- 73. Takeshi N, Soichiro M, Kiyoshi F, Nobuhiro O. Role of platelets in chronic liver disease and acute liver injury. Hepatol Res 2014; 44:165–172. [DOI] [PubMed] [Google Scholar]
- 74. Hoshi R, Murata S, Matsuo R, Myronovych A, Hashimoto I, Ikeda H, Ohkohchi N. Freeze-dried platelets promote hepatocyte proliferation in mice. Cryobiology 2007; 55:255–260. [DOI] [PubMed] [Google Scholar]
- 75. Faiz Kabir Uddin Ahmed A, Ohtani H, Nio M, Funaki N, Iwami D, Kumagai S, et al. In situ expression of fibrogenic growth factors and their receptors in biliary atresia: Comparison between early and late stages. J Pathol 2000; 192:73–80. [DOI] [PubMed] [Google Scholar]
- 76. Mormone E, George J, Nieto N. Molecular pathogenesis of hepatic fibrosis and current therapeutic approaches. Chem Biol Interact 2011; 193:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Oseini AM, Roberts LR. PDGFRalpha: A new therapeutic target in the treatment of hepatocellular carcinoma? Expert Opin Ther Targets 2009; 13:443–454. [DOI] [PubMed] [Google Scholar]
- 78. Chen L, Shi Y, Jiang CY, Wei LX, Lv YL, Wang YL, Dai GH. Coexpression of PDGFR-alpha, PDGFR-beta and VEGF as a prognostic factor in patients with hepatocellular carcinoma. Int J Biol Markers 2011; 26:108–116. [DOI] [PubMed] [Google Scholar]
- 79. Govaere O, Komuta M, Berkers J, Spee B, Janssen C, de Luca F, et al. Keratin 19: A key role player in the invasion of human hepatocellular carcinomas. Gut 2014; 63:674–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Patel SH, Kneuertz PJ, Delgado M, Kooby DA, Staley CA 3rd, El-Rayes BF, et al. Clinically relevant biomarkers to select patients for targeted inhibitor therapy after resection of hepatocellular carcinoma. Ann Surg Oncol 2011; 18:3384–3390. [DOI] [PubMed] [Google Scholar]
- 81. Zhang J, Cao R, Zhang Y, Jia T, Cao Y, Wahlberg E. Differential roles of PDGFR-alpha and PDGFR-beta in angiogenesis and vessel stability. FASEB J 2009; 23:153–163. [DOI] [PubMed] [Google Scholar]
- 82. Jain RK. Molecular regulation of vessel maturation. Nat Med 2003; 9:685–693. [DOI] [PubMed] [Google Scholar]
- 83. Roh JW, Huang J, Hu W, Yang X, Jennings NB, Sehgal V, et al. Biologic effects of platelet-derived growth factor receptor alpha blockade in uterine cancer. Clin Cancer Res 2014; 20:2740–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fernandez M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol 2009; 50:604–620. [DOI] [PubMed] [Google Scholar]
- 85. Tugues S, Fernandez-Varo G, Munoz-Luque J, Ros J, Arroyo V, Rodes J, et al. Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology 2007; 46:1919–1926. [DOI] [PubMed] [Google Scholar]
- 86. Cao R, Brakenhielm E, Li X, Pietras K, Widenfalk J, Ostman A, et al. Angiogenesis stimulated by PDGF-CC, a novel member in the PDGF family, involves activation of PDGFR-alphaalpha and -alphabeta receptors. FASEB J 2002; 16:1575–1583. [DOI] [PubMed] [Google Scholar]
- 87. Marx M, Perlmutter RA, Madri JA. Modulation of platelet-derived growth factor receptor expression in microvascular endothelial cells during in vitro angiogenesis. J Clin Invest 1994; 93:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kitami Y, Inui H, Uno S, Inagami T. Molecular structure and transcriptional regulation of the gene for the platelet-derived growth factor alpha receptor in cultured vascular smooth muscle cells. J Clin Invest 1995; 96:558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhu K, Pan Q, Zhang X, Kong L-QQ, Fan J, Dai Z, et al. MiR-146a enhances angiogenic activity of endothelial cells in hepatocellular carcinoma by promoting PDGFRA expression. Carcinogenesis 2013; 34:2071–2079. [DOI] [PubMed] [Google Scholar]
- 90. Zhang J-BB, Sun H-CC, Jia W-DD, Zhuang P-YY, Qian Y-BB, Zhu X-DD, et al. Up-regulation of platelet-derived growth factor-A is responsible for the failure of re-initiated interferon alpha treatment in hepatocellular carcinoma. BMC Cancer 2011; 12:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. von Marschall Z, Scholz A, Cramer T, Schäfer G, Schirner M, Oberg K, et al. Effects of interferon alpha on vascular endothelial growth factor gene transcription and tumor angiogenesis. J Natl Cancer Inst 2003; 95:437–448. [DOI] [PubMed] [Google Scholar]
- 92. Ball SG, Shuttleworth CA, Kielty CM. Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J Cell Biol 2007; 177:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378–390. [DOI] [PubMed] [Google Scholar]
- 94. Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 2013; 144:512–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zijl FV, Mair M, Csiszar A, Schneller D, Zulehner G, Huber H, et al. Hepatic tumor–stroma crosstalk guides epithelial to mesenchymal transition at the tumor edge. Oncogene 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Semela D, Das A, Langer D, Kang N, Leof E, Shah V. Platelet-derived growth factor signaling through ephrin-b2 regulates hepatic vascular structure and function. Gastroenterology 2008; 135:671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Moon J-OO, Welch TP, Gonzalez FJ, Copple BL. Reduced liver fibrosis in hypoxia-inducible factor-1alpha-deficient mice. Am J Physiol Gastrointest Liver Physiol 2009; 296:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Copple BL, Bustamante JJ, Welch TP, Kim ND, Moon J-OO. Hypoxia-inducible factor-dependent production of profibrotic mediators by hypoxic hepatocytes. Liver Int 2009; 29:1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Marra M, Sordelli IM, Lombardi A, Lamberti M, Tarantino L, Giudice A, et al. Molecular targets and oxidative stress biomarkers in hepatocellular carcinoma: An overview. J Transl Med 2010; 9:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhang X-F, Tan X, Zeng G, Misse A, Singh S, Kim Y, et al. Conditional β-catenin loss in mice promotes chemical hepatocarcinogenesis: Role of oxidative stress and platelet-derived growth factor receptor α/phosphoinositide 3-kinase signaling. Hepatology 2010; 52:954–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Awuah PK, Rhieu BH, Singh S, Misse A, Monga SP. β-Catenin loss in hepatocytes promotes hepatocellular cancer after diethylnitrosamine and phenobarbital administration to mice. PLoS One 2011; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA 1998; 95:8847–8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Colnot S, Decaens T, Niwa-Kawakita M, Godard C, Hamard G, Kahn A, et al. Liver-targeted disruption of Apc in mice activates beta-catenin signaling and leads to hepatocellular carcinomas. Proc Natl Acad Sci USA 2004; 101:17216–17221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nejak-Bowen K, Kikuchi A, Monga SP. Beta-catenin-NF-kappaB interactions in murine hepatocytes: A complex to die for. Hepatology 2013; 57:763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chen WT, Zhu G, Pfaffenbach K, Kanel G, Stiles B, Lee AS. GRP78 as a regulator of liver steatosis and cancer progression mediated by loss of the tumor suppressor PTEN. Oncogene 2014; 33:4997–5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Li Z, Wang Y, Wu H, Zhang L, Yang P, Li Z. GRP78 enhances the glutamine metabolism to support cell survival from glucose deficiency by modulating the β-catenin signaling. Oncotarget 2014; 5:5369–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Vuorinen K, Ohlmeier S, Leppäranta O, Salmenkivi K, Myllärniemi M, Kinnula VL. Peroxiredoxin II expression and its association with oxidative stress and cell proliferation in human idiopathic pulmonary fibrosis. J Histochem Cytochem 2008; 56:951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. McGowan SE, McCoy DM. Fibroblasts expressing PDGF-receptor-alpha diminish during alveolar septal thinning in mice. Pediatr Res 2011; 70:44–49. [DOI] [PubMed] [Google Scholar]
- 109. Lindroos PM, Rice AB, Wang YZ, Bonner JC. Role of nuclear factor-kappa B and mitogen-activated protein kinase signaling pathways in IL-1 beta-mediated induction of alpha-PDGF receptor expression in rat pulmonary myofibroblasts. J Immunol 1998; 161:3464–3468. [PubMed] [Google Scholar]
- 110. Moysidis SN, Thanos A, Vavvas DG. Mechanisms of inflammation in proliferative vitreoretinopathy: From bench to bedside. Mediators Inflamm 2011; 2012:815937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Franziska van Z, Gudrun Z, Michaela P, Doris S, Christoph K, Mara H, et al. Epithelial-mesenchymal transition in hepatocellular carcinoma. Future Oncol 2009; 5:1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Fischer ANM, Fuchs E, Mikula M, Huber H, Beug H, Mikulits W. PDGF essentially links TGF-β signaling to nuclear β-catenin accumulation in hepatocellular carcinoma progression. Oncogene 2006; 26:3395–3405. [DOI] [PubMed] [Google Scholar]
- 113. Zhou L, An N, Haydon RC, Zhou Q, Cheng H, Peng Y, et al. Tyrosine kinase inhibitor STI-571/Gleevec down-regulates the beta-catenin signaling activity. Cancer Lett 2003; 193:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: Systematic review and meta-analysis. JAMA Surg 2014. [DOI] [PubMed] [Google Scholar]
- 115. Boonjaraspinyo S, Boonmars T, Wu Z, Loilome W, Sithithaworn P, Nagano I, et al. Platelet-derived growth factor may be a potential diagnostic and prognostic marker for cholangiocarcinoma. Tumour Biol 2012; 33:1785–1802. [DOI] [PubMed] [Google Scholar]
- 116. Boonjaraspinyo S, Wu Z, Boonmars T, Kaewkes S, Loilome W, Sithithaworn P, et al. Overexpression of PDGFA and its receptor during carcinogenesis of Opisthorchis viverrini-associated cholangiocarcinoma. Parasitol Int 2012; 61:145–150. [DOI] [PubMed] [Google Scholar]
- 117. Thabut D, Routray C, Lomberk G, Shergill U, Glaser K, Huebert R, et al. Complementary vascular and matrix regulatory pathways underlie the beneficial mechanism of action of sorafenib in liver fibrosis. Hepatology 2011; 54:573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lorusso PM, Eder JP. Therapeutic potential of novel selective-spectrum kinase inhibitors in oncology. Expert Opin Investig Drugs 2008; 17:1013–1028. [DOI] [PubMed] [Google Scholar]
- 119. Roskoski R. Sunitinib: A VEGF and PDGF receptor protein kinase and angiogenesis inhibitor. Biochem Biophys Res Commun 2007; 356:323328. [DOI] [PubMed] [Google Scholar]
- 120. Abraham J, Chua YX, Glover JM, Tyner JW, Loriaux MM, Kilcoyne A, et al. An adaptive Src-PDGFRA-Raf axis in rhabdomyosarcoma. Biochem Biophys Res Commun 2012; 426:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y, Kitamura Y. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology 2003; 125:660–667. [DOI] [PubMed] [Google Scholar]
- 122. Rosmorduc O, Housset C. Hypoxia: A link between fibrogenesis, angiogenesis, and carcinogenesis in liver disease. Semin Liver Dis 2010; 30:258–270. [DOI] [PubMed] [Google Scholar]
- 123. Fernandez M, Mejias M, Garcia-Pras E, Mendez R, Garcia-Pagan JC, Bosch J. Reversal of portal hypertension and hyperdynamic splanchnic circulation by combined vascular endothelial growth factor and platelet-derived growth factor blockade in rats. Hepatology 2007; 46:1208–1217. [DOI] [PubMed] [Google Scholar]
- 124. Reiberger T, Angermayr B, Schwabl P, Rohr-Udilova N, Mitterhauser M, Gangl A, Peck-Radosavljevic M. Sorafenib attenuates the portal hypertensive syndrome in partial portal vein ligated rats. J Hepatol 2009; 51:865–873. [DOI] [PubMed] [Google Scholar]
- 125. Shah GD, Loizos N, Youssoufian H, Schwartz JD, Rowinsky EK. Rationale for the development of IMC-3G3, a fully human immunoglobulin G subclass 1 monoclonal antibody targeting the platelet-derived growth factor receptor alpha. Cancer 2010; 116:1018–1026. [DOI] [PubMed] [Google Scholar]
- 126. Gerber DE, Gupta P, Dellinger MT, Toombs JE, Peyton M, Duignan I, et al. Stromal platelet-derived growth factor receptor alpha (PDGFRalpha) provides a therapeutic target independent of tumor cell PDGFRalpha expression in lung cancer xenografts. Mol Cancer Ther 2012; 11:2473–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Loizos N, Xu Y, Huber J, Liu M, Lu D, Finnerty B, et al. Targeting the platelet-derived growth factor receptor alpha with a neutralizing human monoclonal antibody inhibits the growth of tumor xenografts: Implications as a potential therapeutic target. Mol Cancer Ther 2005; 4:369–379. [DOI] [PubMed] [Google Scholar]
- 128. Matsuo K, Nishimura M, Komurov K, Shahzad MM, Ali-Fehmi R, Roh JW, et al. Platelet-derived growth factor receptor alpha (PDGFRalpha) targeting and relevant biomarkers in ovarian carcinoma. Gynecol Oncol 2014; 132:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Takakura N, Yoshida H, Kunisada T, Nishikawa S, Nishikawa S-I. Involvement of platelet-derived growth factor receptor-alpha in hair canal formation. J Invest Dermatol 1996; 107:770–777. [DOI] [PubMed] [Google Scholar]
- 130. Zymek P, Bujak M, Chatila K, Cieslak A, Thakker G, Entman ML, Frangogiannis NG. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J Am Coll Cardiol 2006; 48:23152323. [DOI] [PubMed] [Google Scholar]
- 131. Fruttiger M, Calver AR, Krüger WH, Mudhar HS, Michalovich D, Takakura N, et al. PDGF mediates a neuron-astrocyte interaction in the developing retina. Neuron 1996; 17:1117–1131. [DOI] [PubMed] [Google Scholar]
- 132. Liao C-H, Akazawa H, Tamagawa M, Ito K, Yasuda N, Kudo Y, et al. Cardiac mast cells cause atrial fibrillation through PDGF-A-mediated fibrosis in pressure-overloaded mouse hearts. J Clin Invest 2010; 120:242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Canbay A, Friedman S, Gores GJ. Apoptosis: The nexus of liver injury and fibrosis. Hepatology 2004; 39:273–278. [DOI] [PubMed] [Google Scholar]
- 134. Preisser L, Miot C, Le Guillou-Guillemette H, Beaumont E, Foucher ED, Garo E, et al. IL-34 and M-CSF are overexpressed in HCV fibrosis and induce pro-fibrotic macrophages which promote collagen synthesis by hepatic stellate cells. Hepatology 2014. [DOI] [PubMed] [Google Scholar]
- 135. Ross MA, Sander CM, Kleeb TB, Watkins SC, Stolz DB. Spatiotemporal expression of angiogenesis growth factor receptors during the revascularization of regenerating rat liver. Hepatology 2001; 34:1135–1148. [DOI] [PubMed] [Google Scholar]
- 136. Grappone C, Pinzani M, Parola M, Pellegrini G, Caligiuri A, DeFranco R, et al. Expression of platelet-derived growth factor in newly formed cholangiocytes during experimental biliary fibrosis in rats. J Hepatol 1999; 31:100–109. [DOI] [PubMed] [Google Scholar]