Abstract
Lower extremity peripheral artery disease (PAD) is frequently under-diagnosed, in part because of the wide variety of leg symptoms manifested by patients with PAD and in part because of the high prevalence of asymptomatic PAD. In primary care medical practices, 30% to 60% of PAD patients report no exertional leg symptoms and approximately 45–50% report exertional leg symptoms that are not consistent with classic intermittent claudication. The prevalence and extent of functional impairment and functional decline in PAD may also be underappreciated. Functional impairment and functional decline is common in PAD, even among those who are asymptomatic. Lower extremity ischemia is also associated with pathophysiologic changes in calf skeletal muscle including smaller calf muscle area, increased calf muscle fat content, impaired leg strength, and impaired metabolic function. People with severe PAD have poorer peroneal nerve conduction velocity compared to people with mild PAD or no PAD. The degree of ischemia-related pathophysiologic changes in lower extremity muscles and peripheral nerves of people with PAD are associated with the degree of functional impairment. New interventions are needed to improve functional performance and prevent mobility loss in the large number of PAD patients, including in those who are asymptomatic or who have exertional leg symptoms other than claudication.
Keywords: Peripheral artery disease, physical functioning, intermittent claudication, mobility
Lower extremity peripheral artery disease (PAD) affects 8 million men and women in the United States (U.S.) and more than 200 million men and women worldwide (1,2). Patients with PAD have a high prevalence of co-existing coronary artery and cerebrovascular atherosclerosis (1,3,4) and an increased risk of cardiovascular morbidity and mortality, compared to people without PAD (5,6). Risk factors for PAD include smoking, diabetes, hyperlipidemia, and hypertension (1,7).
PAD can be readily diagnosed in medical practice with the ankle brachial index (ABI), a ratio of Doppler recorded systolic pressures in the lower and upper extremities (8). People without PAD have ABI values ranging from 1.00 to 1.30 (8). An ABI < 0.90 is approximately 70% sensitive and 95% specific for PAD (8,9).
Patients with PAD experience calf muscle ischemia during walking activity, when metabolic demands exceed oxygen supply, and calf muscle reperfusion during rest, when blood supply increases sufficiently to meet calf muscle oxygen requirements. This phenomenon of ischemia-reperfusion generates reactive oxygen species, such as superoxide anion and hydrogen peroxide, that damage muscle fibers, impair mitochondrial function, and promote apoptosis (10–17). Thus, walking impairment in people with PAD is related to both reduced vascular perfusion, from atherosclerotic blockages in lower extremity arteries, and skeletal muscle damage, most likely from ischemia reperfusion injury to skeletal muscle.
Over a five year period, only one to two percent of people with PAD will develop critical limb ischemia or require lower extremity amputation (4). Yet, even among patients without critical limb ischemia, chronic leg ischemia in PAD is associated with pathophysiologic changes in the lower extremities, impaired quality of life, and mobility loss (18–23). Adverse lower extremity outcomes associated with PAD include ischemic leg pain during walking activity, reduced leg strength, impaired balance, slow walking speed, impaired calf skeletal muscle mitochondrial function, ischemic peripheral neuropathy, and functional decline and mobility loss (18–23). Awareness of these lower extremity consequences of PAD will help clinicians better recognize the manifestations of PAD and will help scientists better identify interventions to reverse ischemia-related functional decline and mobility loss in people with PAD. This review summarizes lower extremity manifestations of PAD in people without critical limb ischemia. The spectrum of leg symptoms, the prevalence and significance of asymptomatic lower extremity ischemia, the associations of ischemia with pathophysiologic changes in peripheral muscle and nerves, and the functional consequences of PAD are discussed.
The 2005 clinical practice guidelines for PAD recommend both treadmill exercise testing (Class I Recommendation, Level of Evidence-B) and six-minute walk testing (Class IIb Recommendation, Level of Evidence-B) to measure walking endurance in people with PAD (4). However, prospective natural history studies of lower extremity functioning in people with PAD have used serial six-minute walk testing to objectively document the changes in functional performance over time in people with PAD. Thus, available evidence about the natural history of functional decline in people with PAD is primarily from the six-minute walk test, rather than from treadmill testing.
Historical Perspective
The classic leg symptom of PAD, intermittent claudication, was originally described and characterized for the purposes of epidemiologic study by Dr. Geoffrey Rose, a London epidemiologist (24). The Rose Claudication Questionnaire was developed to facilitate the standardized measurement of the incidence, prevalence, and significance of claudication symptoms in epidemiologic population studies, and was based on observations of symptoms reported by patients with PAD identified in vascular surgery practices (24). Dr. Rose’s definition of intermittent claudication consisted of: Exertional calf pain that does not begin at rest, does not resolve during walking activity, and resolves within 10 minutes of rest (Table 1). The Rose Claudication Questionnaire was used to establish the incidence and consequences of intermittent claudication among more than 5,200 participants in the Framingham Study. The biennial incidence of intermittent claudication among participants in the Framingham Study was 7.1 per 1000 in men and 3.6 per 1000 in women (25). Kannel et al reported that men with intermittent claudication in the Framingham Study had an annual mortality rate of 39 per 1,000 vs. 10 per 1,000 among men without intermittent claudication (26). In the Framingham Study, women with intermittent claudication had a two-fold increased rate of mortality compared to women without intermittent claudication (26). Kannel et al concluded that because people with intermittent claudication could reduce their physical activity to avoid leg symptoms, the increased risk of mortality due to heart disease or stroke was a far greater concern for people with intermittent claudication (26). However, subsequent study has documented the adverse health consequences associated with reduced physical activity levels in people with PAD (27,28).
Table 1.
| Exertional Leg Pain that |
|---|
|
Criteria were defined based upon observations of patients diagnosed with PAD in a vascular surgery practice.
Most people with PAD do not have classic symptoms of intermittent claudication
Over the past 25–30 years it has been established that most people with PAD do not have the classical symptoms of intermittent claudication as originally defined by Dr. Rose (24,29–37). For example, in 1985, Criqui et al performed non-invasive vascular studies in 575 community dwelling men and women from Southern California who were participating in the Lipid Research Clinic study (29). Overall, 65 (11%) had lower extremity non-invasive studies that were consistent with PAD. Among the 65 participants with PAD, only six (9.2%) had classic symptoms of intermittent claudication (29). Thus, the sensitivity of the Rose claudication questionnaire for PAD in this population was 9.2%. Five of 510 participants without PAD also had symptoms of intermittent claudication, yielding a Rose claudication questionnaire specificity of 99%. Criqui et al also defined a leg symptom category, “possible claudication”. Participants with possible claudication had exertional calf pain that did not begin at rest but that otherwise did not meet criteria for intermittent claudication. Twenty percent of the 65 participants with PAD in the Lipid Research Clinical study had either classic Rose claudication symptoms or “possible claudication”. In contrast, just 4.1% of the 510 participants without PAD met criteria for classic Rose claudication symptoms or “possible claudication”. These findings were the first to demonstrate that a substantial proportion of individuals with PAD have exertional leg symptoms that are not consistent with classic intermittent claudication.
In 1991, Fowkes et al reported the prevalence of intermittent claudication in the Edinburgh Artery Study, in which 1,592 community dwelling men and women age 55–74 underwent an ABI and were administered the Rose Claudication Questionnaire (30). Of the 1,592 participants, nine percent had an ABI < 0.90, consistent with PAD. However, just 15% of those with PAD had classical symptoms of intermittent claudication and 35% of those with PAD reported no exertional leg symptoms, consistent with asymptomatic PAD. These results from the Edinburgh Artery Study were among the first to document the high prevalence of asymptomatic PAD. Fowkes et al concluded that the high prevalence of asymptomatic PAD is consistent with the high prevalence of asymptomatic atherosclerosis in other vascular beds, including asymptomatic coronary artery disease and asymptomatic cerebrovascular disease (30).
Since these original reports by Criqui et al and Fowkes et al, multiple studies have confirmed that most men and women with PAD do not have classic symptoms of intermittent claudication (18–20,31–37). In community dwelling populations and in primary care settings, approximately 10% of people with PAD have classic symptoms of intermittent claudication (Table 2). In comparison, in community dwelling populations, approximately 60% of people with PAD are asymptomatic and in primary care medical practices, 30% to 60% of PAD patients are asymptomatic (Table 2). The remainder has exertional leg symptoms that are not typical of intermittent claudication. In community dwelling settings, approximately 30% of people with PAD have exertional leg symptoms other than intermittent claudication and in primary care medical practices, approximately 50% of patients with PAD have exertional leg symptoms other than intermittent claudication.
Table 2.
Prevalence of Intermittent Claudication, Asymptomatic PAD, and Atypical Exertional Leg Symptoms in Defined Cohorts of People with Peripheral Artery Disease
| Study/year of publication | Characteristics of PAD Population | Total Sample Size/Prevalence of PAD | Mean Age in Years (standard deviation)* | Percent Female (%) | Prevalence of Claudication in PAD participants | Prevalence of Asymptomatic PAD | Prevalence of Atypical Exertional Leg Symptoms |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Community Dwelling Populations | |||||||
| Lipid Research Clinics (1985) (29) | Community dwelling men and women in Southern California. | N=575 total/N=65 (11%) with PAD | 66 Range 38–82 |
59% | N=6 (9.2%) | Not reported. | Not reported. |
| Cardiovascular Health Study (1993) (32) | Men and women age 65 and older from four United States Communities. | N=5,084 total/N=629 (12.4%) with ABI < 0.90 | 71.7 for those with ABI 0.90 to 1.50; 75.5 for those with ABI < 0.80. | :ABI < 0.80: 54.1% ABI 0.90 to 1.50: 60% |
2.0% overall | Not reported. | Not reported. |
| Women’s Health and Aging Study (2000) (34) | Community dwelling women age 65 and older with disability and without leg pain. | N=933 N=328 (35%) with ABI < 0.90 |
PAD: 77.4 (7.3); Non-PAD: 75.5 (6.9) | 100% | Not reported. | N=198 (60.3%) | Not reported. |
| Cardiovascular Health Study (2001) (33) | Men and women age 65 and older from four U.S. Communities. | N=5,888 overall N=723 with ABI < 0.90 (12%) |
Range from 72.7 (5.5) in people without leg pain to 74.9 (5.8) in those with intermittent claudication. | 54% | N= 62 (8.5%) | N=427 (59%) | N=234 (32%) |
|
| |||||||
|
Primary Care Medical Practices
| |||||||
| General Medicine Study (1999) (35) | Patients age 55 and older in a general medical practice with no prior history of PAD. | N=174 N=26 with PAD (14.9%) |
PAD-69 (6.5) Non-PAD-68 (7.2) |
PAD-31% Non-PAD-65%) |
N=1 (3.8%) | N=14 (43.8%) | N=2 (7.7%) |
| PARTNERS Study (2001) (36) | Primary care patients who were a) age 70 and older or b) age 50 to 69 with history of smoking or diabetes. | N=6,979 overall N=1,865 (27%) with ABI < 0.901 |
68.9 (10) among those without PAD. Mean age of PAD ranged from 70.8 (10.1) to 72.3 (9.1). |
52% overall | N=178 (9.5%) | N=572 (30.6%) | N=934 (50.1%) |
| Texas Primary Care Practices (37) | Patients age 50 and older in a Veterans Administration and two primary care practices in Houston. | N=403 total sample N=67 with PAD |
Non-PAD: 63.5 (7.3). PAD: 65.3 (7.0) |
Non-PAD: 53% PAD: 49% |
N=5 (8%) | N=31 (47%) | N=30 (45%) |
| Get ABI Study (2009) (31) | Patients age 65 and older in primary care medical practices in Germany. | N=6,880 total sample size N=1,429 (21%) with PAD |
Non-PAD: 72.2 + 5.1; PAD: 73.9 (5.6) | 58% | N=593 (41.5%) had any symptoms or had history of peripheral revascularization or amputation. | N=836 (58.5%) | Not reported. |
|
| |||||||
|
Non-invasive vascular laboratory
| |||||||
| WALCS Chicago cohort (2001) (18) | Consecutive patients with PAD in a non-invasive vascular laboratory. | 460 PAD participants | 71.9 (8.4) | 41% | N=150 (32%) | N=91 (20%) | N=219 (47.6%) |
Standard deviation provided when available.
Asymptomatic PAD and PAD with atypical symptoms may contribute to under-diagnosis of PAD
Asymptomatic leg ischemia and atypical leg symptoms may contribute to underdiagnoses of PAD. This phenomenon was illustrated in the PARTNERS study, in which 6,979 men and women in primary care practices across the United States were screened for PAD with the ABI. Participants in PARTNERS were age 70 and older or were age 50 to 69 with history of smoking or diabetes. Of the 6,979 patients screened with the ABI, 1,865 (29%) had an ABI < 0.90 consistent with PAD. Among those in the PARTNERS Study with PAD and no other clinically evident cardiovascular disease, 5.5% of those newly diagnosed with PAD had classical symptoms of intermittent claudication vs. 12.6% of those with previously diagnosed PAD (36). Overall, 48% of those for whom PAD was newly diagnosed in the PARTNERS study were asymptomatic vs. 25.8% of those with previously diagnosed PAD. Together, these data demonstrate that people with unrecognized PAD are more likely to be asymptomatic and less likely to have classic symptoms of intermittent claudication than people with previously established PAD.
Identifying PAD in people without classic intermittent claudication symptoms
PAD can be identified non-invasively with the ABI, a ratio of Doppler recorded systolic pressures in the lower and upper extremities (8). However, Medicare and most medical insurance companies do not reimburse for office-based ABI testing unless arterial waveforms are also measured and interpreted. Most medical practices do not routinely measure the ABI. The American Heart Association recommends ABI screening in patients with exertional leg symptoms, in patients with non-healing wounds, in all patients age 70 and older, and in patients age 50–69 with a history of diabetes or smoking (4). However, the U.S. Preventive Services Task Force recently concluded that there is insufficient evidence to recommend ABI screening in patients with PAD (38). Thus, identifying people with PAD who are asymptomatic or who have atypical leg symptoms remains challenging.
Types of Atypical Leg Symptoms Commonly Observed in PAD
Studies in defined clinical populations have characterized the types of leg symptoms other than intermittent claudication that are commonly observed in people with PAD (18,20,21,37,39,40). Two common types of atypical leg pain symptoms in PAD are leg pain on exertion and rest and leg pain/carry on. Leg pain on exertion and rest is exertional leg pain that sometimes begins at rest but is distinct from rest pain due to critical limb ischemia (18,20,21,37,39,40). Leg pain/carry on is exertional leg pain that does not cause the patient to stop walking (18,20,21,37,39,40). These leg symptom categories are important because they are associated with specific clinical characteristics and prognoses with regard to functional impairment and decline among people with PAD (Table 3). The Walking and Leg Circulation Study (WALCS) was a cohort of 460 prospectively followed men and women with PAD assembled from among consecutive patients diagnosed with PAD in three Chicago-area vascular laboratories. Of the 460 PAD participants in WALCS, 88 PAD participants (19%) reported exertional leg symptoms that sometimes began at rest (i.e. leg pain on exertion and rest) and 46 PAD participants (9%) reported exertional leg symptoms that did not stop them from walking (i.e. leg pain/carry on) (18). None of the PAD participants in WALCS had critical limb ischemia at study entry.
Table 3.
Atypical Leg Pain Categories Commonly Experienced in Patients with Peripheral Artery Disease
| Leg Pain/Carry On | Leg Pain on Exertion and Rest | |
|---|---|---|
| Leg Symptom Features | Exertional leg pain that does not stop the patient from continuing to walk. | Exertional leg pain that sometimes begins at rest among PAD patients without critical limb ischemia. |
| Prevalence among people with PAD | Approximately 10% | Approximately 20% |
| Clinical Characteristics as compared to people with intermittent claudication | -Fewer depressive symptoms -Higher ABI values. |
-Higher prevalence of diabetes -Higher prevalence of spinal stenosis -Reduced peripheral nerve sensation -Greater numbers of comorbidities. |
| Functional Impairment as compared to patients with classic intermittent claudication | -Data are mixed with some evidence for less functional impairment and some showing no difference compared to PAD patients with claudication. | -Greater functional impairment -Faster functional decline. |
Leg pain on exertion and rest
As compared to PAD patients with classical symptoms of intermittent claudication, PAD patients with leg pain on exertion and rest in the WALCS cohort had poorer lower extremity nerve sensation, a higher prevalence of diabetes, a higher prevalence of spinal stenosis, and a higher burden of comorbid disease (18). ABI values were higher among PAD participants with leg pain on exertion and rest, compared to those with intermittent claudication, suggesting less severe PAD among those with leg pain on exertion and rest. Despite this, PAD participants with leg pain on exertion and rest had poorer six-minute walk performance, poorer balance, and slower walking speed than PAD participants with intermittent claudication (18). In a separate study, Gardner et al, reported that PAD participants with leg pain on exertion and rest had poorer six-minute walk performance compared to PAD participants with intermittent claudication (40). Collins et al reported that PAD participants with leg pain on exertion and rest had poorer scores on the self-reported Walking Impairment Questionnaire domains of walking distance, walking speed, and stair climbing, compared to PAD participants with intermittent claudication (37). Comorbid diseases, particularly spinal stenosis and peripheral neuropathy, may contribute to the type of leg symptoms reported by people with PAD who have leg pain on exertion and rest. Jaffe et al reported that 75.7% of 107 patients found to have PAD in a non-invasive vascular laboratory also had lumbar degenerative disease on a magnetic resonance imaging or computed tomography test performed within six months of their lower extremity arterial testing (41). However, the extent to which comorbid disease causes atypical leg symptoms in patients with PAD has not been specifically delineated.
Leg pain/carry on
Compared to PAD participants with classical symptoms of intermittent claudication, PAD participants with leg pain/carry in the WALCS cohort had fewer depressive symptoms, a higher ABI, and a lower prevalence of prior lower extremity revascularization (18). PAD participants with leg pain/carry-on also had a better six-minute walk performance and were less likely to stop during the six-minute walk than those with intermittent claudication (18). However, Gardner et al reported no difference in treadmill walking or six-minute walk performance among PAD participants with leg pain/carry on, compared to PAD participants with intermittent claudication (40). Similarly, Collins et al reported no significant differences in the Walking Impairment Questionnaire scores in PAD participants with leg pain/carry on, compared to PAD participants with intermittent claudication, although the sample sizes in the study by Collins et al were very small (37).
Asymptomatic peripheral artery disease and functional impairment and decline
People with asymptomatic PAD have significantly greater functional impairment and faster functional decline than people without PAD (18–21,42). The Women’s Health and Aging Study (WHAS), a cohort of 933 community-dwelling women age 65 and older with disability, included 328 (35%) participants with an ABI < 0.90, consistent with PAD. Of WHAS participants with an ABI < 0.90, 63% reported no exertional leg pain and therefore were classified as asymptomatic (34). Among all of the WHAS participants who reported no exertional leg symptoms, women with ABI values < 0.90 performed more poorly on multiple subjective and objective measures of lower extremity functioning, than asymptomatic women with normal ABI values. Asymptomatic women with PAD had lower physical activity levels, were more likely to report difficulty walking up one flight of steps or walking ¼ mile, had slower walking velocity, were slower during a timed repeated stand from a seated position, and had poorer balance than asymptomatic women without PAD (34). In the WALCS cohort, asymptomatic PAD participants had an average annual decline in six-minute walk performance of −76.8 feet per year at two-year follow-up, compared to −8.7 feet per year among WALCS participants without PAD at baseline (20). Asymptomatic PAD participants in the WALCS cohort who did not develop leg symptoms during the six-minute walk test at baseline had an increased rate of mobility loss at five-year follow-up, compared to participants without PAD (hazard ratio = 2.94 (95% CI= 1.39–6.19)) (21).
Future study is needed to identify interventions that improve walking performance and prevent mobility loss in people with asymptomatic PAD. The two medications that are FDA approved for treating walking impairment in people with PAD, cilostazol and pentoxifylline, have been studied only in PAD patients with intermittent claudication symptoms. Only two randomized trials of PAD patients have included PAD patients who are asymptomatic (43,44). These two randomized trials suggest that exercise therapy is beneficial even within the subset of PAD patients who are asymptomatic. Because asymptomatic PAD patients do not have exertional leg pain, improvement in walking performance cannot be measured by patient report of improved leg symptoms. Objective outcomes such as a graded treadmill test, the six-minute walk test, or physical activity levels during daily life are helpful to objectively document improved walking performance in response to interventions.
Although the etiology of functional limitations in people with asymptomatic PAD has not been fully delineated, pathophysiologic skeletal muscle and peripheral nerve changes, including reduced calf muscle mass, increased calf muscle fat content, and reduced lower extremity peripheral nerve function, have been documented in PAD patients without exertional leg symptoms (18,42). For example, in the WALCS cohort, PAD participants who were asymptomatic had poorer lower extremity sensation, measured with a monofilament, than PAD participants with classic symptoms of intermittent claudication (18). In the WALCS II cohort, PAD participants who were asymptomatic and did not develop leg pain during the six-minute walk had significantly lower calf muscle area and increased calf muscle percent fat, compared to PAD participants with symptoms of intermittent claudication (42). Based on pathophysiologic abnormalities documented in calf skeletal muscle mitochondria of PAD patients with intermittent claudication (10–17), it is possible that impaired skeletal muscle mitochondrial function also contributes to functional impairment in people with asymptomatic PAD. However, mitochondrial function in calf skeletal muscle of PAD participants who are asymptomatic has not been studied.
Why do some PAD patients lack ischemic leg symptoms?
There are at least two potential explanations for absence of exertional leg symptoms in patients with PAD. First, some PAD patients limit their walking activity during daily life to avoid leg symptoms. This phenomenon is illustrated by people with PAD who report that they have no exertional leg symptoms but develop leg pain during a six-minute walk test (18). In the WALCS cohort, 42 of 91 PAD participants (46%) who reported absence of exertional leg symptoms developed leg symptoms during the six-minute walk test. In addition, asymptomatic PAD participants in the WALCS cohort were categorized according to their physical activity level. An asymptomatic/inactive group of PAD participants consisted of asymptomatic PAD participants who walked ≤ 6 blocks per week. An asymptomatic/active group of PAD participants consisted of asymptomatic people with PAD who walked more than six blocks per week (18). Thirty-three percent of PAD participants in the asymptomatic/active group developed leg pain during the six-minute walk test vs. 89% of PAD participants who were asymptomatic/inactive (18). In summary, asymptomatic PAD participants who were less physically active were more likely to develop leg symptoms during a six-minute walk test than asymptomatic PAD participants who were more physically active (18). A second potential explanation for asymptomatic PAD is that some PAD patients may slow their walking speed in order to avoid exertional leg symptoms (42).
Lower extremity functional impairment and decline in PAD
The natural history of lower extremity outcomes in people with PAD has been described as “benign” (45–47). This is in part because only a small proportion of patients with PAD develop gangrene, require amputation, or undergo surgical revascularization (45–47). Furthermore, in some studies of PAD patients identified from vascular surgery practices, patients with claudication report no change or even improvement in claudication symptoms over a five-year follow-up period (45–47). As described above, this phenomenon of stabilization or improvement in claudication symptoms is explained in part by reducing physical activity levels to avoid ischemic leg symptoms (18,34). Yet PAD patients with lower levels of physical activity have faster rates of functional decline and increased rates of mortality than those more physically active (27,28). For these reasons, improvement or absence of ischemic leg symptoms should not necessarily be equated with improvement in PAD.
The magnitude of functional impairment in people with PAD
In the WALCS cohort, the six-minute walk and other objective functional performance measures were assessed annually in 460 men and women with PAD and also in 240 without PAD, enabling objective assessment of functioning over time, independently of patient-reported leg symptoms. At baseline, patients with PAD achieved shorter distance in the six-minute walk test, had slower walking velocity, were more likely to stop to rest during the six-minute walk, had lower physical activity levels, and had poorer balance than people without PAD (18,19). People with more severe PAD, measured by lower ABI values, had even poorer functional performance than people with mild PAD (19). As compared to participants without PAD, those with an ABI < 0.50 walked 523 feet fewer and had slower four-meter walking velocity by 0.21 meters/second even after adjusting for age, sex, race, body mass index, smoking, and comorbidities (19). Fifty feet and 0.10 meters/second, respectively, represent clinically meaningful changes in six-minute walk and four-meter walking velocity (48). Therefore, these differences in walking endurance and walking speed between people with mild vs. severe PAD represent clinically important differences.
At two year follow-up in the WALCS cohort, participants with the most severe PAD (ABI < 0.50) had an average annual decline in six-minute walk of −73 feet/year, those with mild to moderate PAD (ABI 0.50 to 0.89) had an average annual decline in six-minute walk of −58.8 feet per year, and those without PAD (ABI 0.90–1.50) had an average annual decline in six-minute walk of −12.6 feet per year (20). Among participants who completed the six-minute walk without stopping at baseline, those with ABI < 0.50 at baseline were nearly 13-times more likely to become unable to walk for six-minutes without stopping at two-year follow-up (20). These associations were independent of age, sex, race, comorbidities, smoking history, and body mass index. In summary, PAD is associated with greater functional impairment than absence of PAD, and more severe PAD is associated with greater impairment and faster decline than less severe PAD.
Lower extremity ischemia and calf skeletal muscle
Although the pathophysiologic mechanisms causing functional impairment and functional decline in PAD are not fully delineated, available evidence suggests that both impaired vasculature and skeletal muscle pathophysiologic changes contribute to the lower extremity functional impairment and functional decline that is present in PAD. People with PAD have smaller calf muscle area and increased calf muscle percent fat, as measured by computed tomography (CT), than people without PAD (22). These ischemia-related adverse changes in calf skeletal muscle are independent of differences in physical activity level between people with mild vs. severe PAD. In 92 men and women with PAD without history of lower extremity revascularization whose left and right leg ABI values differed by > 0.20, consistent with clinically important differences in leg ischemia between the left and right legs, legs with more severe ischemia had a CT scan measured average calf muscle area of 5,283 mm2 and legs with highest ABI had a CT scan measured average calf muscle area of 5,511 mm2 (22). Similarly, average calf muscle percent fat was higher in the leg with more severe ischemia (11.4% vs. 9.5%). By comparing calf muscle characteristics between legs with higher vs. lower ABI, these analyses controlled fully for ischemia-associated differences in physical activity and demonstrate that greater ischemia is associated with smaller muscle area and higher calf muscle fat content (22).
Ischemia-related calf muscle pathophysiologic changes in people with PAD are likely to be in the biologic pathway of functional decline in people with PAD. In the WALCS II cohort, including 380 PAD participants who were followed prospectively after baseline measurement of calf muscle characteristics, PAD participants with greater CT-measured calf muscle percent fat and lower CT-measured calf muscle density at baseline each had an increased incidence of mobility loss at 2-year follow-up (49). PAD participants in the lowest tertile of calf muscle percent fat at baseline had a 0.18 hazard for developing mobility loss, compared to PAD participants in the highest tertile of calf muscle percent fat at baseline. PAD participants in the lowest tertile of calf muscle density had a 3.50-fold increased hazard of mobility loss at two-year follow-up, compared to PAD participants in the highest tertile of calf muscle density at baseline. These associations were independent of age, sex, race, comorbidities, smoking, BMI, and ABI (49). Together, these data demonstrate that ischemia related pathophysiologic changes in lower extremity calf skeletal muscle predict increased rates of mobility loss.
Mitochondrial impairment in lower extremity skeletal muscle of patients with PAD
PAD patients experience calf muscle ischemia during walking activity, when metabolic demands exceed oxygen supply. Patients with PAD experience calf muscle reperfusion during rest, when blood supply increases sufficiently to meet calf muscle oxygen requirements. This phenomenon of ischemia-reperfusion generates reactive oxygen species, such as superoxide anion and hydrogen peroxide, that can damage muscle fibers, impair mitochondrial function, and promote apoptosis (10–16,50,51). Electron microscopy of calf muscle from patients with PAD shows distorted mitochondria consistent with a severe qualitative dysfunction (52–55). Growing evidence also demonstrates a quantitative mitochondria impairment in calf muscle among individuals with PAD, resulting in reduced energy production (17,56,57).
In a study of 34 participants with PAD and 21 without PAD who underwent calf muscle biopsy, PAD participants had higher protein carbonyl content (695 grayscale units ± 1.32 vs. 486 grayscale units ± 135) and higher 4-hydroxy-2-nonenal (4-HNE) levels (436 grayscale units ± 119 vs. 261 grayscale units ± 101, P<.001) than those without PAD, indicating higher calf muscle levels of oxidative stress in the PAD participants (10). Higher quantities of oxidative stress were associated with fewer myofibers in participants with PAD (10). In two cross-sectional studies, poorer mitochondrial function, measured by respirometry from muscle biopsy specimens (58) or magnetic resonance spectroscopy (17), were associated with poorer treadmill walking in people with PAD.
Lower extremity nerve function in peripheral artery disease
Ischemia-related damage to lower extremity nerves may also cause functional impairment and functional decline in people with PAD. The inferior gluteal artery supplies the lumbosacral nerve plexus and the popliteal artery supplies the common peroneal nerve. Cross-sectional studies regarding PAD and lower extremity nerve function have reported conflicting results, with some studies demonstrating no difference in peroneal or femoral nerve function between people with vs. without PAD and another reporting poorer peroneal and tibial nerve function in people with PAD (59–61). However, these studies included small sample sizes. In a study of 25 patients with PAD and 37 age-matched controls without PAD, Weber et al identified an axonal lower extremity neuropathy affecting the sural, peroneal, and tibial nerves (61). However, this study included PAD participants with critical limb ischemia and the most severe findings were observed in PAD patients with critical limb ischemia-related rest pain (61).
The WALCS II cohort studied the association of PAD and lower extremity nerve function in 478 participants with PAD and 292 without PAD using lower extremity electrodiagnostic testing to measure peripheral nerve function (23). In participants without diabetes, severe PAD (ABI < 0.50) was associated with poorer peroneal nerve conduction velocity (NCV) compared to participants without PAD (ABI 0.90 to 1.50) and as compared to participants with mild PAD (ABI 0.70 to 0.90) or moderate PAD (ABI 0.50 to 0.70). In participants with diabetes, the presence of PAD was associated with poorer peroneal NCV and poorer sural nerve amplitude compared to absence of PAD, even after adjustment for known and potential confounders. The WALCS II cohort is the largest to evaluate the association of lower extremity ischemia with lower extremity peripheral nerve function. Results suggest that among people without critical limb ischemia, severe PAD (i.e. ABI < 0.50) is associated with impaired lower extremity peripheral nerve function (23). Additional findings from the WALCS II cohort demonstrated that greater impairment in lower extremity nerve function is associated with poorer six-minute walk performance and slower walking velocity among people with PAD (62). In summary, ischemia-related impairment in lower extremity nerve function may contribute to functional impairment and decline in people with PAD.
Improving functional impairment and preventing functional decline in people with PAD who did not have classical intermittent claudication symptoms
The development of therapeutic interventions to improve walking performance in PAD has focused on people with typical symptoms of intermittent claudication. There are no United States Food and Drug Administration (FDA) approved medications for treating walking impairment in PAD patients without intermittent claudication. However, two recent clinical trials of exercise included PAD patients without classical symptoms of intermittent claudication. First, the Study to Improve Leg Circulation (SILC) was specifically designed to determine whether supervised treadmill exercise and supervised resistance training, respectively, significantly improved six-minute walk performance as compared to a control group in PAD patients with and without classical symptoms of claudication (44). In the SILC trial, 156 patients with PAD were randomized to supervised treadmill exercise, supervised resistance training, or a control group, respectively. Of the 156 participants, 29 (19%) had classical symptoms of intermittent claudication and the remainder were asymptomatic or had exertional leg symptoms other than intermittent claudication. Supervised treadmill exercise significantly improved six-minute walk performance, treadmill walking performance, and quality of life in PAD participants with and without intermittent claudication. Supervised resistance training significantly improved treadmill walking performance and quality of life in PAD participants with and without intermittent claudication (44). Although the SILC trial did not have adequate statistical power to determine whether the exercise interventions were more effective in a single leg symptom group, compared to the others, the magnitude of improvement in study outcomes was similar between the PAD participants with and without intermittent claudication. The magnitude of improvement in response to study interventions was also similar between PAD participants who were asymptomatic and those with exertional leg symptoms. Second, the Group Oriented Arterial Leg Study (GOALS) randomized trial tested the ability of a home-based exercise intervention that employed a Group Mediated Cognitive Behavioral (GMCB) intervention to improve walking performance in people with PAD, including participants with and without symptoms of intermittent claudication (43). The GMCB home-based exercise intervention significantly improved walking endurance, measured by the six-minute walk and by treadmill walking performance. In subgroup analyses, the GMCB home-based walking exercise intervention improved six-minute walk performance in the subset of PAD participants who did not have classical symptoms of intermittent claudication. Together, these data demonstrate that walking exercise interventions benefit PAD patients even among PAD patients without classical symptoms of intermittent claudication (43,44). However, Medicare and most other medical insurance companies typically do not pay for supervised exercise programs in people with PAD.
Measuring change in walking performance and quality of life in people with PAD
Treadmill exercise testing is the most commonly used objective outcome measure in randomized trials studying interventions to improve walking performance in people with PAD (63). Treadmill testing is conducted in a tightly controlled setting and measures maximal walking performance. The six-minute walk test is an objective measure of walking endurance that simulates walking in the community because it is performed in a hall corridor. The six-minute walk test has been well validated in patients with PAD and improves in response to effective exercise interventions (43,44,64–66). The 2005 American Heart Association/American College of Cardiology clinical practice guidelines for PAD recommend both treadmill exercise testing (Class I recommendation, Level of Evidence-B) and six-minute walk testing (Class IIb recommendation, Level of Evidence-B) (4) for objective assessment of changes in walking performance in patients with PAD. Since 2005, new evidence has been accrued demonstrating the validity of the six-minute walk test as an objective measure of walking performance in PAD (43,44,48,64–68). Validated questionnaires for measuring change in patient-reported walking performance and/or quality of life include the Walking Impairment Questionnaire (69), the Short-Form-36 physical functioning score (70), the Peripheral Artery Questionnaire (71), and the PAD Quality of Life Questionnaire (72). These outcomes are not interchangeable. Each outcome measures a different aspect or dimension of walking performance or quality of life. PAD patients’ perceptions of walking performance do not fully correlate with objective measures of their walking performance. For example, in a randomized trial of cilostazol to improve walking performance in PAD patients with intermittent claudication, the correlation between improvement in treadmill walking performance and improvement in the patient-reported WIQ distance score was only 0.34 (73). In the Claudication: Exercise vs. Endovascular Revascularization (CLEVER) randomized trial in PAD patients with intermittent claudication, treadmill walking performance and patient-reported change in walking performance and quality of life were only modestly correlated among participants who received the exercise intervention (74). In summary, objective and subjective assessments of walking performance, quality of life, and physical activity used in randomized trials of therapeutic interventions measure distinct aspects of walking performance and functioning in people with PAD. Researchers and clinicians should be cognizant that these outcome measures are complementary and not inter-changeable.
In conclusion, most people with PAD do not have classical symptoms of intermittent claudication. Lower extremity pathophysiologic changes associated with PAD include reduced calf skeletal muscle mass, increased calf skeletal muscle fatty infiltration, reduced calf skeletal muscle density, impaired lower extremity peroneal nerve function, impaired calf muscle mitochondrial function, impaired functional performance, and increased rates of mobility loss and functional decline. Functional impairment and functional decline are observed even among PAD patients who are asymptomatic or who have atypical leg symptoms other than intermittent claudication. Future studies should identify therapeutic interventions to improve functional performance and prevent functional decline and mobility loss among people with PAD, including the large proportion without classical symptoms of intermittent claudication.
Supplementary Material
Figure 1.

Proposed biologic pathways for the association of lower extremity ischemia with mobility loss in peripheral artery disease. (Illustration Credit: Ben Smith).
Figure 2.
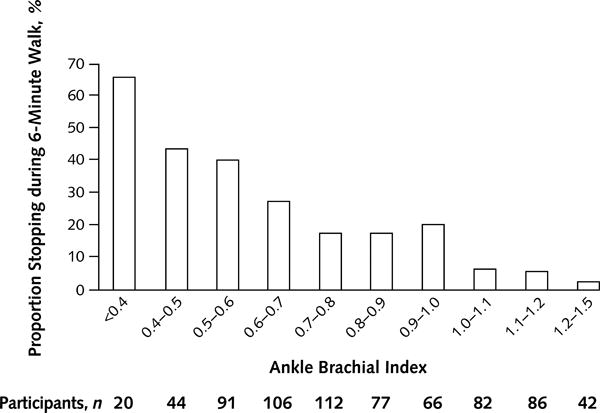
Association of the ankle brachial index with the six-minute walk in people with and without peripheral artery disease. (Re-published with permission from reference #19).
Table 4.
Pathophysiologic Findings in the Lower Extremities of PAD patients*
| Calf skeletal muscle |
|
| Peripheral nerve |
|
Results reported in Table 4 are limited to PAD patients without critical limb ischemia.
Acknowledgments
SOURCES OF FUNDING: Funded in part by the National Institute on Aging (R21AG047510) and by the National Heart Lung and Blood Institute (R01-HL107510).
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- CLEVER
Claudication: Exercise vs. Endovascular Revascularization
- GOALS
Group Oriented Arterial Leg Study
- GMCB
group mediated cognitive behavioral
- CT
computed tomography
- 4-HNE
4-hydroxy Nonenal
- FDA
Food and Drug Administration.
- SILC
Study to Improve Leg Circulation
- WHAS
Women’s Health and Aging Study
- WALCS
Walking and Leg Circulation Study
- PAD
peripheral artery disease
- ABI
ankle brachial index
- NCV
nerve conduction velocity
- ATP
adenosine triphosphate
- PARTNERS
PAD Awareness, Risk, and Treatment: New Resources for Survival
Footnotes
DISCLOSURES
None.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 3.McDermott MM, Liu K, Criqui MH, Ruth K, Goff D, Saad MF, Wu C, Homma S, Sharrett AR. Ankle-brachial index and subclinical cardiac and carotid disease: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 5.Ankle Brachial Index Collaboration. Fowkes FG, Murray GD, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heald CL, Fowkes FG, Murray GD, Price JF. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: Systematic review. Atherosclerosis. 2006;189:61–69. doi: 10.1016/j.atherosclerosis.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Joosten MM, Pai JK, Bertoia ML, Rimm EB, Spiegelman D, Mittleman MA, Mukamal KJ. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308:1660–1667. doi: 10.1001/jama.2012.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jönsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat-Jacobson D, American Heart Association Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 9.Lijmer JG, Hunink MG, Van Den Dungen JJ, Loonstra J, Smit AJ. ROC analyses of noninvasive tests for peripheral arterial disease. Ultrasound Med Biol. 1996;22:391–398. doi: 10.1016/0301-5629(96)00036-1. [DOI] [PubMed] [Google Scholar]
- 10.Weiss DJ, Casale GP, Koutakis P, Nella AA, Swanson SA, Zhu Z, Miserlis D, Johanning JM, Pipinos II. Oxidative damage and myofiber degeneration in the gastrocnemius of patients with peripheral arterial disease. J Transl Med. 2013;11:230. doi: 10.1186/1479-5876-11-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurke L, Marx A, Sutter PM, Stierli P, Harder F, Heberer M. Function of fast- and slow-twitch rat skeletal muscle following ischemia and reperfusion at different intramuscular temperatures. Eur Surg Res. 2000;32:135–141. doi: 10.1159/000008754. [DOI] [PubMed] [Google Scholar]
- 12.Gillani S, Cao J, Suzuki T, Hak DJ. The effect of ischemia reperfusion injury on skeletal muscle. Injury. 2012;43:670–675. doi: 10.1016/j.injury.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Pipinos II, Judge AR, Selsby JT, Zhu Z, Swanson SA, Nella AA, Dodd SL. The myopathy of peripheral arterial occlusive disease: Part 2. Oxidative stress, neuropathy, and shift in muscle fiber type. Vasc Endovascular Surg. 2008;42:101–112. doi: 10.1177/1538574408315995. [DOI] [PubMed] [Google Scholar]
- 14.Pipinos II, Swanson SA, Zhu Z, Nella AA, Weiss DJ, Gutti TL, McComb RD, Baxter BT, Lynch TG, Casale GP. Chronically ischemic mouse skeletal muscle exhibits myopathy in association with mitochondrial dysfunction and oxidative damage. Am J Physiol Regul Integr Comp Physiol. 2008;295:R290–R296. doi: 10.1152/ajpregu.90374.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pipinos II, Judge AR, Zhu Z, Selsby JT, Swanson SA, Johanning JM, Baxter BT, Lynch TG, Dodd SL. Mitochondrial defects and oxidative damage in patients with peripheral arterial disease. Free Radic Biol Med. 2006;41:262–269. doi: 10.1016/j.freeradbiomed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Pipinos II, Sharov VG, Shepard AD, Anagnostopoulos PV, Katsamouris A, Todor A, Filis KA, Sabbah HN. Abnormal mitochondrial respiration in skeletal muscle in patients with peripheral arterial disease. J Vasc Surg. 2003;38:827–832. doi: 10.1016/s0741-5214(03)00602-5. [DOI] [PubMed] [Google Scholar]
- 17.Anderson JD, Epstein FH, Meyer CH, Hagspiel KD, Wang H, Berr SS, Harthun NL, Weltman A, Dimaria JM, West AM, Kramer CM. Multifactorial determinants of functional capacity in peripheral arterial disease: uncoupling of calf muscle perfusion and metabolism. J Am Coll Cardiol. 2009;54:628–635. doi: 10.1016/j.jacc.2009.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR, Sharma L, Clark E, Gibson D, Martin GJ. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 19.McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, Chan C, Martin GJ, Schneider J, Pearce WH, Taylor LM, Clark E. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 20.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ, Clark E. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 21.McDermott MM, Ferrucci L, Liu K, Guralnik JM, Tian L, Liao Y, Criqui MH. Leg symptom categories and rates of mobility decline in peripheral arterial disease. J Am Geriatr Soc. 2010;58:1256–1262. doi: 10.1111/j.1532-5415.2010.02941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDermott MM, Hoff F, Ferrucci L, Pearce WH, Guralnik JM, Tian L, Liu K, Schneider JR, Sharma L, Tan J, Criqui MH. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2007;55:400–406. doi: 10.1111/j.1532-5415.2007.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDermott MM, Sufit R, Nishida T, Guralnik JM, Ferrucci L, Tian L, Liu K, Tan J, Pearce WH, Schneider JR, Sharma L, Criqui MH. Lower extremity nerve function in patients with lower extremity ischemia. Arch Intern Med. 2006;166:1986–1992. doi: 10.1001/archinte.166.18.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–648. [PMC free article] [PubMed] [Google Scholar]
- 25.Kannel WB. The demographics of claudication and the aging of the American population. Vasc Med. 1996;1:60–64. doi: 10.1177/1358863X9600100111. [DOI] [PubMed] [Google Scholar]
- 26.Kannel WB, Skinner JJ, Jr, Schwartz MJ, Shurtleff D. Intermittent claudication. Incidence in the Framingham Study. Circulation. 1970;41:875–883. doi: 10.1161/01.cir.41.5.875. [DOI] [PubMed] [Google Scholar]
- 27.Garg PK, Liu K, Tian L, Guralnik JM, Ferrucci L, Criqui MH, Tan J, McDermott MM. Physical activity during daily life and functional decline in peripheral arterial disease. Circulation. 2009;119:251–260. doi: 10.1161/CIRCULATIONAHA.108.791491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garg PK, Tian L, Criqui MH, Liu K, Ferrucci L, Guralnik JM, Tan J, McDermott MM. Physical activity during daily life and mortality in patients with peripheral arterial disease. Circulation. 2006;114:242–248. doi: 10.1161/CIRCULATIONAHA.105.605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Criqui MH, Fronek A, Klauber MR, Barrett-Connor E, Gabriel S. The sensitivity, specificity, and predictive value of traditional clinical evaluation of peripheral arterial disease: results from noninvasive testing in a defined population. Circulation. 1985;71:516–522. doi: 10.1161/01.cir.71.3.516. [DOI] [PubMed] [Google Scholar]
- 30.Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20:384–392. doi: 10.1093/ije/20.2.384. [DOI] [PubMed] [Google Scholar]
- 31.Diehm C, Allenberg JR, Pittrow D, Mahn M, Tepohl G, Haberl RL, Darius H, Burghaus I, Trampisch HJ, German Epidemiological Trial on Ankle Brachial Index Study Group Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. 2009;120:2053–2061. doi: 10.1161/CIRCULATIONAHA.109.865600. [DOI] [PubMed] [Google Scholar]
- 32.Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, Wofson SK. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 33.Newman AB, Naydeck BL, Sutton-Tyrrell K, Polak JF, Kuller LH, Cardiovascular Health Study Research Group The role of comorbidity in the assessment of intermittent claudication in older adults. J Clin Epidemiol. 2001;54:294–300. doi: 10.1016/s0895-4356(00)00308-5. [DOI] [PubMed] [Google Scholar]
- 34.McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: the women’s health and aging study. Circulation. 2000;101:1007–1012. doi: 10.1161/01.cir.101.9.1007. [DOI] [PubMed] [Google Scholar]
- 35.McDermott MM, Mehta S, Greenland P. Exertional leg symptoms other than intermittent claudication are common in peripheral arterial disease. Arch Intern Med. 1999;159:389–392. doi: 10.1001/archinte.159.4.387. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AH, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 37.Collins TC, Petersen NJ, Suarez-Almazor M. Peripheral arterial disease symptom subtype and walking impairment. Vasc Med. 2005;10:177–183. doi: 10.1191/1358863x05vm615oa. [DOI] [PubMed] [Google Scholar]
- 38.Lin JS, Olson CM, Johnson ES, Whitlock EP. The ankle-brachial index for peripheral artery disease screening and cardiovascular disease prediction among asymptomatic adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159:333–341. doi: 10.7326/0003-4819-159-5-201309030-00007. [DOI] [PubMed] [Google Scholar]
- 39.Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1:65–71. doi: 10.1177/1358863X9600100112. [DOI] [PubMed] [Google Scholar]
- 40.Gardner AW, Montgomery PS, Afaq A. Exercise performance in patients with peripheral arterial disease who have different types of exertional leg pain. J Vasc Surg. 2007;46:79–86. doi: 10.1016/j.jvs.2007.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ain DL, Slovut DP, Kamath R, Jaff MR. The association between peripheral artery and lumbar spine disease: a single-center study. Am J Med. 2012;125:411–415. doi: 10.1016/j.amjmed.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 42.McDermott MM, Guralnik JM, Ferrucci L, Tian L, Liu K, Liao Y, Green D, Sufit R, Hoff F, Nishida T, Sharma L, Pearce WH, Schneider JR, Criqui MH. Asymptomatic peripheral arterial disease is associated with more adverse lower extremity characteristics than intermittent claudication. Circulation. 2008;117:2484–2491. doi: 10.1161/CIRCULATIONAHA.107.736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDermott MM, Liu K, Guralnik JM, Criqui MH, Spring B, Tian L, Domanchuk K, Ferrucci L, Lloyd-Jones D, Kibbe M, Tao H, Zhao L, Liao Y, Rejeski WJ. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA. 2013;310:57–65. doi: 10.1001/jama.2013.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301:165–174. doi: 10.1001/jama.2008.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imparato AM, Kim GE, Davidson T, Crowley JG. Intermittent claudication: its natural course. Surgery. 1975;78:795–799. [PubMed] [Google Scholar]
- 46.McAllister FF. The fate of patients with intermittent claudication managed nonoperatively. Am J Surg. 1976;132:593–595. doi: 10.1016/0002-9610(76)90351-2. [DOI] [PubMed] [Google Scholar]
- 47.Boyd AM. The natural course of arteriosclerosis of the lower extremities. Proc R Soc Med. 1962;55:591–593. [PubMed] [Google Scholar]
- 48.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 49.McDermott MM, Ferrucci L, Guralnik J, Tian L, Liu K, Hoff F, Liao Y, Criqui MH. Pathophysiological changes in calf muscle predict mobility loss at 2-year follow-up in men and women with peripheral arterial disease. Circulation. 2009;120:1048–1055. doi: 10.1161/CIRCULATIONAHA.108.842328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jansson E, Johansson J, Sylvén C, Kaijser L. Calf muscle adaptation in intermittent claudication. Side-differences in muscle metabolic characteristics in patients with unilateral arterial disease. Clin Physiol. 1988;8:17–29. doi: 10.1111/j.1475-097x.1988.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 51.Clyne CA, Mears H, Weller RO, O’Donnell TF. Calf muscle adaptation to peripheral vascular disease. Cardiovasc Res. 1985;19:507–512. doi: 10.1093/cvr/19.8.507. [DOI] [PubMed] [Google Scholar]
- 52.Makris KI, Nella AA, Zhu Z, Swanson SA, Casale GP, Gutti TL, Judge AR, Pipinos II. Mitochondriopathy of peripheral arterial disease. Vascular. 2007;15:336–343. doi: 10.2310/6670.2007.00054. [DOI] [PubMed] [Google Scholar]
- 53.Marbini A, Gemignani F, Scoditti U, Rustichelli P, Bragaglia MM, Govoni E. Abnormal muscle mitochondria in ischemic claudication. Acta Neurol Belg. 1986;86:304–310. [PubMed] [Google Scholar]
- 54.Mäkitie J, Teräväinen H. Histochemical changes in striated muscle in patients with intermittent claudication. Arch Pathol Lab Med. 1977;101:658–663. [PubMed] [Google Scholar]
- 55.Pipinos II, Judge AR, Selsby JT, Zhu Z, Swanson SA, Nella AA, Dodd SL. The myopathy of peripheral arterial occlusive disease: part 1. Functional and histomorphological changes and evidence for mitochondrial dysfunction. Vasc Endovascular Surg. 2008;41:481–489. doi: 10.1177/1538574407311106. [DOI] [PubMed] [Google Scholar]
- 56.Pipinos II, Shepard AD, Anagnostopoulos PV, Katsamouris A, Boska MD. Phosphorus 31 nuclear magnetic resonance spectroscopy suggests a mitochondrial defect in claudicating skeletal muscle. J Vasc Surg. 2000;31:944–952. doi: 10.1067/mva.2000.106421. [DOI] [PubMed] [Google Scholar]
- 57.Kemp GJ, Roberts N, Bimson WE, Bakran A, Harris PL, Gilling-Smith GL, Brennan J, Rankin A, Frostick SP. Mitochondrial function and oxygen supply in normal and in chronically ischemic muscle: a combined 31P magnetic resonance spectroscopy and near infrared spectroscopy study in vivo. J Vasc Surg. 2001;34:1103–1110. doi: 10.1067/mva.2001.117152. [DOI] [PubMed] [Google Scholar]
- 58.Hou XY, Green S, Askew CD, Barker G, Green A, Walker PJ. Skeletal muscle mitochondrial ATP production rate and walking performance in peripheral arterial disease. Clin Physiol Funct Imaging. 2002;22:226–232. doi: 10.1046/j.1475-097x.2002.00423.x. [DOI] [PubMed] [Google Scholar]
- 59.Chopra JS, Hurwitz LJ. Femoral nerve conduction in diabetes and chronic occlusive vascular disease. J Neurol Neurosurg Psychiatry. 1968;31:28–33. doi: 10.1136/jnnp.31.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chopra JS, Hurwitz LJ. A comparative study of peripheral nerve conduction in diabetes and non-diabetic chronic occlusive peripheral vascular disease. Brain. 1969;92:83–96. doi: 10.1093/brain/92.1.83. [DOI] [PubMed] [Google Scholar]
- 61.Weber F, Ziegler A. Axonal neuropathy in chronic peripheral arterial occlusive disease. Muscle Nerve. 2002;26:471–476. doi: 10.1002/mus.10235. [DOI] [PubMed] [Google Scholar]
- 62.Garg PK, Liu K, Ferrucci L, Guralnik JM, Criqui MH, Tian L, Sufit R, Nishida T, Tao H, Liao Y, McDermott MM. Lower extremity nerve function, calf skeletal muscle characteristics, and functional performance in peripheral arterial disease. J Am Geriatr Soc. 2011;59:1855–1863. doi: 10.1111/j.1532-5415.2011.03600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hiatt WR, Rogers RK, Brass EP. The treadmill is a better functional test than the 6-minute walk test in therapeutic trials of patients with peripheral artery disease. Circulation. 2014;130:69–78. doi: 10.1161/CIRCULATIONAHA.113.007003. [DOI] [PubMed] [Google Scholar]
- 64.McDermott MM, Guralnik JM, Criqui MH, Liu K, Kibbe MR, Ferrucci L. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation. 2014;130:61–68. doi: 10.1161/CIRCULATIONAHA.114.007002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McDermott MM, Guralnik JM, Tian L, Ferrucci L, Liu K, Liao Y, Criqui MH. Baseline functional performance predicts the rate of mobility loss in persons with peripheral arterial disease. J Am Coll Cardiol. 2007;50:974–982. doi: 10.1016/j.jacc.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McDermott MM, Tian L, Liu K, Guralnik JM, Ferrucci L, Tan J, Pearce WH, Schneider JR, Criqui MH. Prognostic value of functional performance for mortality in patients with peripheral artery disease. J Am Coll Cardiol. 2008;51:1482–1489. doi: 10.1016/j.jacc.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McDermott MM, Ferrucci L, Liu K, Guralnik JM, Tian L, Kibbe M, Liao Y, Tao H, Criqui MH. Women with peripheral arterial disease experience faster functional decline than men with peripheral arterial disease. J Am Coll Cardiol. 2011;57:707–714. doi: 10.1016/j.jacc.2010.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gardner AW, Parker DE, Montgomery PS, Blevins SM. Step-monitored home exercise improves ambulation, vascular function, and inflammation in symptomatic patients with peripheral artery disease: a randomized controlled trial. J Am Heart Assoc. 2014;3:e001107. doi: 10.1161/JAHA.114.001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Regensteiner JG, Steiner JF, Panzer RJ. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol. 1990;2:142–152. [Google Scholar]
- 70.Ware J, Jr, Kosinski M, Keller SD. A 12-item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 71.Spertus J, Jones P, Poler S, Rocha-singh K. The peripheral artery questionnaire: a new disease-specific health status measure for patients with peripheral arterial disease. Am Heart J. 2004;147:301–308. doi: 10.1016/j.ahj.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 72.Treat-Jacobson D, Lindquist RA, Witt DR, Kirk LN, Schorr EN, Bronas UG, Davey CS, Regensteiner JG. The PADQOL: development and validation of a PAD-specific quality of life questionnaire. Vasc Med. 2012;17:405–415. doi: 10.1177/1358863X12466708. [DOI] [PubMed] [Google Scholar]
- 73.Regensteiner JG, Ware JE, McCarthy WJ, Zhang P, Forbes WP, Heckman J, Hiatt WR. Effect of cilostazol on treadmill walking, community-based walking ability, and health-related quality of life in patients with intermittent claudication due to peripheral arterial disease: meta-analysis of six randomized controlled trials. J Am Geriatr Soc. 2002;50:1939–1946. doi: 10.1046/j.1532-5415.2002.50604.x. [DOI] [PubMed] [Google Scholar]
- 74.Murphy TP, Reynolds MR, Cohen D, Regensteiner J, Massaro J, Cutlip D, Mohler E, Cerezo JV, Oldenburg N, Thum C, Goldberg S, Hirsch A. Correlation of patient-reported symptom outcomes and treadmill test outcomes after treatment for aortoiliac claudication. J Vasc Interv Radiol. 2013;24:1427–1435. doi: 10.1016/j.jvir.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


