Abstract
Aim
To investigate whether ATP-sensitive potassium (KATP) channels modulate the tocolytic effect of β2-AR agonists (ritodrine and salmeterol) in early-pregnant (day 6) and late-pregnant (day 22) rat uterus in vitro, in order to examine the relation between the KATP channel sulphonylurea-binding regulatory subunit (SUR) expression and pharmacological reactivity of β2-AR agonists.
Methods
The tocolytic effects of ritodrine and salmeterol (10-10-10-5 M) on spontaneous rhythmic contractions were investigated cumulatively, alone, or in the presence of the KATP channel blocker glibenclamide (10-6 M) and the KATP channel opener pinacidil (10-9-10-7 M) after 5-min preincubation.
Results
β2-AR agonist induced myometrial relaxation was inhibited by glibenclamide and enhanced by pinacidil on day 6, when SUR1 expression levels were high. Neither glibenclamide nor pinacidil mediated tocolytic effect was measured on day 22.
Conclusion
Low expression of the KATP channels at the end of gestation may facilitate enhanced excitability and contractility in the rat myometrium. The combination of a betamimetic and a KATP channel opener will therefore not be of therapeutic relevance in the treatment of preterm delivery.
A number of agents have tocolytic effect, including β2-adrenergic receptor (β2-AR) agonists, magnesium sulfate, prostaglandin synthesis inhibitors, Ca2+-channel blockers, nitrogen monoxide donors, and oxytocin receptor antagonists (1). β2-AR agonists (such as salmeterol, terbutaline, fenoterol, hexoprenaline, and ritodrine) delay preterm labor for at least 48 hours, which is why they are the drugs of choice in the treatment of preterm labor (2). Of all tocolytics in use, β-mimetics have the most undesirable side-effect profile. The most serious reported side-effects associated with the administration of β2-AR agonists are pulmonary edema, hypotension, and tachycardia (3,4). Promising new therapeutic approach for the treatment of preterm delivery is the combination β2-AR agonists and 17alpha-hydroxyprogesterone (5) or Ca2+-channel blocker nifedipine (6).
Adenosine triphosphate (ATP)-sensitive potassium channels (KATP channels) are involved in β-AR agonists-induced smooth muscle relaxation in pulmonary vasorelaxation in the rat (7), vasodilatation in the rat diaphragmatic microcirculation (8), vasorelaxation in the rat mesenteric artery (9), detrusor muscle relaxation in the rat (10), and myometrial relaxation in non-pregnant buffaloes (11). KATP channels are formed by a combination of two types of subunits, the pore-forming inwardly rectifying subunit (Kir6x) and the sulphonylurea-binding regulatory subunit (SUR) (12). We earlier reported (13) that SUR subunits, SUR1 and SUR2, are both expressed in the rat uterus during gestation. SUR1 expression was elevated in the early pregnancy (day 6) and then dramatically decreased from day 8 to term, while the level of SUR2 subunit remained unchanged.
The aim of the present study was to investigate the role of the KATP channel in β2-AR agonist-induced myometrial relaxation. We studied the tocolytic effects of β2-AR agonists (salmeterol, ritodrine) in the presence of glibenclamide (KATP channel blocker) and pinacidil (KATP channel opener) in early pregnant (day 6) and late pregnant (day 22) rats in vitro, in order to clarify the relation between SUR1 expression and pharmacological reactivity of β2-AR agonists.
Materials and methods
Mating of the animals
The study was approved by the Hungarian Ethics Committee for Animal Research (registration number: IV/01758-2/2008) and the animals were treated in accordance with the European Communities Council Directives (86/609/ECC) and the Hungarian Act for the Protection of Animals in Research (XXVIII.tv.32.§). Sprague-Dawley rats (Charles-River Laboratories, Budapest, Hungary) were kept at 22 ± 3°C with relative humidity of 30%-70% and 12/12 h light/dark cycle. They were fed a standard rodent pellet diet (Charles-River Laboratories) with tap water available ad libitum.
Mature female (180-200 g) and male (240-260 g) rats were mated in a special mating cage with a metal door separating the rats of different sex, which is open when the rats are allowed to mate. Four to five hours after the potential mating, vaginal smears were taken from the female rats. The female rats in which sperm cells were microscopically detected (magnification of 1200 times) and those in whom smears could not have been taken because of a vaginal sperm plug were regarded as first-day pregnant animals (13).
Uterus preparation
The rats were euthanized by CO2 inhalation and the uteri were removed on the 6th and 22nd day of pregnancy. Muscle rings 5 mm long were sliced from the uterine horns and mounted vertically in an organ bath containing 10 mL of de Jongh solution (composition: 137 mM NaCl, 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 12 mM NaHCO3, 4 mM NaH2PO4, 6 mM glucose, pH = 7.4). The organ bath was maintained at 37°C, and carbogen (95% O2 + 5% CO2) was bubbled through it. After mounting, the rings were equilibrated for about 1 h before the experiments, with a solution change every 15 min. The initial tension of the preparation was set to about 1.25 g, which was relaxed to about 0.5 g at the end of equilibration. The tension of the myometrial rings was measured with a gauge transducer (SG-02; Experimetria Ltd, Budapest, Hungary) and recorded with a SPEL Advanced ISOSYS Data Acquisition System (Experimetria Ltd) (13).
In vitro studies
The tissue samples were incubated for 5 min and the tocolytic effect of β2-AR agonists ritodrine and salmeterol (10−10-10−5 M) on spontaneous rhythmic contractions was investigated cumulatively, alone, or in the presence of KATP channel blocker glibenclamide (10−6 M) or KATP channel opener pinacidil (10−9-10−7 M). Following the addition of each dose of β2-AR agonist, the changes were recorded for 300 s. Concentration-response curves were fitted and areas under curves (AUCs) were determined. Statistical analysis was carried out with the Prism 5.0 (Graphpad Software Inc., San Diego, CA, USA). From the AUC values, maximum inhibitory effects (Emax) of β2-AR agonists on a given day of pregnancy were calculated and the concentrations eliciting 50% of the maximum inhibition of uterine contraction (EC50) were calculated. Data were analyzed with the ANOVA Neuman-Keuls test. The alpha value was 0.05. The variances were constant and the distribution was normal.
Results
Both glibenclamide and pinacidil influenced the effect of ritodrine and salmeterol
Glibenclamide blocked the tocolytic effects of β2-AR agonists; the dose-response curves shifted to the right, and the EC50 values of β2-AR agonists significantly increased. Pinacidil enhanced the tocolytic effects of β2-AR agonists; the dose-response curves shifted to the left and the EC50 values of β2-AR agonists significantly decreased (Figure 1 and 2).
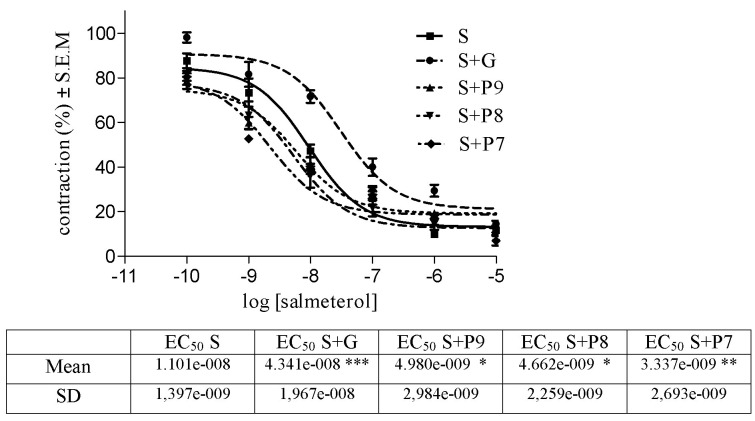
Figure 1.
The tocolytic effect of β2-AR agonist salmeterol alone (10−10-10−5 M) (S) and in the presence of glibenclamide (S+G) and pinacidil (10−9 M: S+P9, 10−8 M: S+P8 and 10−7 M: S+P7) in the myometrium of 6-day-pregnant rat in vitro (mean± SE). The table shows EC50 data (mean ± SD, n = 6). Abbreviations: P9: pinacidil 10−9M, P8: pinacidil 10−8M, P7: pinacidil 10−7M, EC50: the concentrations eliciting 50% of the maximum inhibitions of uterine contraction, SE: standard error, SD: standard deviation.
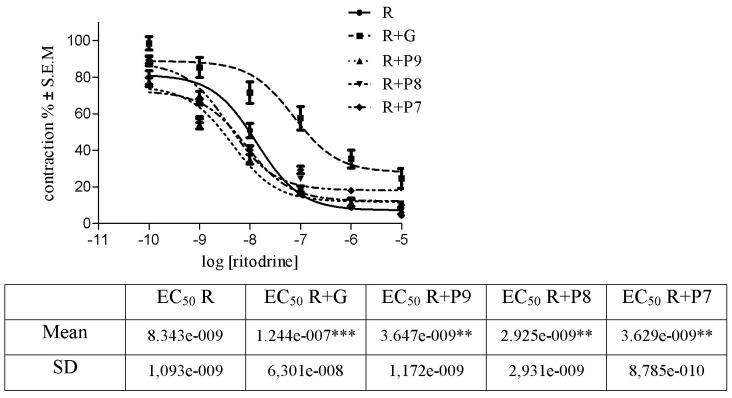
Figure 2.
The tocolytic effect of β2-AR agonist ritodrine alone (10−10-10−5 M) (R) and in the presence of glibenclamide (R+G) and pinacidil (10−9 M: R+P9, 10−8 M: R+P8 and 10−7 M: R+P7) in the myometrium of 6-day-pregnant rat in vitro (mean± SE). The table shows EC50 data (mean ± SD, n = 6). See Figure 1 for abbreviations.
Neither glibenclamide nor pinacidil influenced the effects of the β2-AR agonists on the 22 day pregnant uterus
The uterus-relaxant effects of ritodrine and salmeterol (10−10-10−5 M) on the 22-day-pregnant rat uterus were investigated in the presence of glibenclamide (10−6 M) or different doses of pinacidil (10−9, 10−8 and 10−7 M) (Figure 3 and 4).
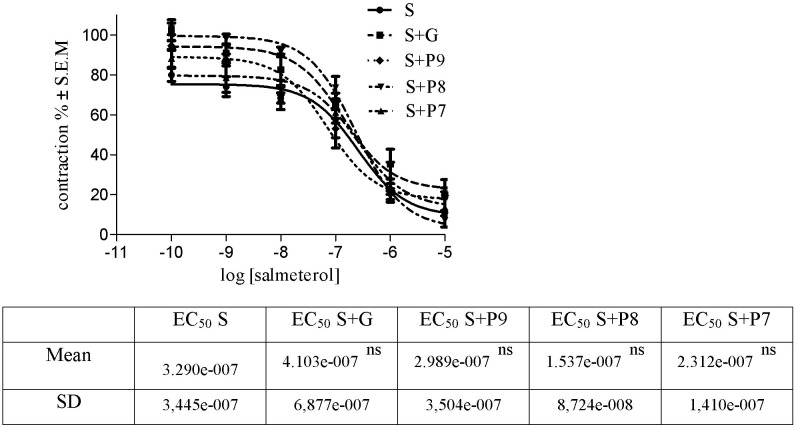
Figure 3.
The tocolytic effect of β2-AR agonist salmeterol alone (10−10-10−5 M) (S), in the presence of glibenclamide (S+G), and pinacidil (10−9 M: S+P9, 10−8 M: S+P8 and 10−7 M: S+P7) in the myometrium of 22-day-pregnant rat in vitro (mean± SE). The table shows EC50 data (mean ±SD, n = 6). See Figure 1 for abbreviations.
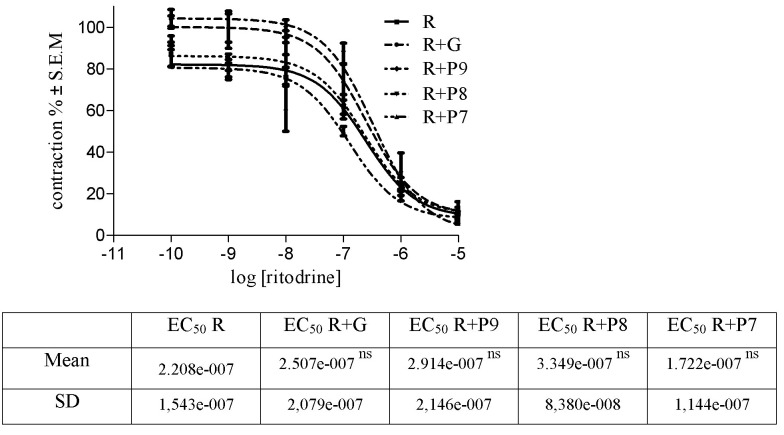
Figure 4.
The tocolytic effect of β2-AR agonist ritodrine (10−10-10−5 M) alone (R) in the presence of glibenclamide (R+G) and pinacidil (10−9 M: R+P9, 10−8 M: R+P8 and 10−7 M: R+P7) in the myometrium of 22-day-pregnant rat in vitro (mean± SE). The table shows EC50 data (mean ± SD, n = 6). See Figure 1 for abbreviations.
Discussion
Preterm delivery is one of the greatest challenges in obstetrical practice. The factors regulating myometrial function during pregnancy and labor are poorly understood. Understanding of these processes at cellular and molecular levels is essential for development of new therapeutic strategies. β2-ARs affect the contractility of the pregnant uterus which is why they are used for the treatment of premature labor.
KATP channels are large hetero-octameric complexes containing four subunits from the inwardly rectifying K+ channel family (Kir6.x: either Kir6.1 or Kir6.2) and four SUR subunits from the ABC transporter family: ABCC8 (SUR1) and ABCC9 (SUR2). SUR2 has two different isoforms, SUR2A and SUR2B, which are splicing variants. Both types of subunits, SURs and Kir6.x are necessary for the channel function. Kir6.x comprises the channel component of the KATP, while the SURs are responsible for the ATP sensitivity, pharmacological properties, and trafficking of this channel (14-18). KATP channels have different molecular structure, due to the heterologous expression of the Kir6.x and SUR subunits. This leads to different combinations and creates different types of KATP channels with distinct electrophysiological properties and pharmacological sensitivities.
We found earlier (13) that both SUR1 and SUR2 subunits were expressed in the rat uterus during gestation: SUR1 was markedly increased on day 6 and dramatically decreased from day 8 to term, while the level SUR2 subunit remained low during the entire gestation. The present study showed that KATP channels modulated the tocolytic effect of β2-AR agonists in the rat on day 6 of gestation. We clearly demonstrated that in the early gestation, when SUR1 level was elevated, tocolytic effect of β2-AR agonist was inhibited by glibenclamide and potentiated by pinacidil, while at the end of gestation, when SUR1 level was decreased, it was influenced by neither glibenclamide nor pinacidil. It can be concluded that the mediation effect of the KATP channels on the efficacy of the β2-AR agonist depends on the expression of the SUR1 subunit of the KATP channels. We had earlier demonstrated that the tocolytic effects of the β2-AR agonists in the rat significantly decreased in late (days 15, 18, 20, and 22 of gestation) compared to early gestation (19). This phenomenon could be explained by a decrease in the β2-AR function, which is partially controlled by β-adrenergic kinase, the estrogen/progesterone levels, and G-protein-coupled receptor kinases (20-22). Our results indicate that there are other mechanisms that decrease the tocolytic effect of β2-AR agonists at the end of gestation. The low levels of KATP channels at the end of gestation may facilitate the enhanced excitability and contractility of the myometrium, which is one of the reasons for the decreased efficacy of the betamimetics. It was shown (23) that SUR1 expression in the human myometrium was decreased in late pregnancy compared with non-pregnant women. Moreover, low levels of Kir6.1 and Kir6.2 subunits were determined at the end of gestation. Since open KATP channels draw the cell membrane potential closer to the K+ equilibrium potential, KATP channels are closely involved in reducing cellular excitability and contractility. The combination of betamimetics with a KATP channel opener will therefore not have any therapeutic relevance in the treatment of preterm birth. However, we can hypothesize that this combination may be used as a uterus relaxant in the early gestation (eg, habitual abortion).
Acknowledgments
Funding This work was supported by the New Hungary Development Plan (TÁMOP-4.2.2.A-11/1/KONV-2012-0035).
Ethical approval received from Hungarian Ethical Committee for Animal Research (registration number: IV/01758-2/2008).
Declaration of authorship NL, PhD student, performed investigation of uterine contractility in an in vitro tissue bath system in part, calculation of results. AK, pharmacy student, performed determination of oestrus period, mating of the animals, identification of pregnancy. DD, PhD student, performed investigation of uterine contractility and pharmacological reactivity in an in vitro tissue bath experiments in part. RG, head of the laboratory of the in vitro tissue bath system, responsible for standardization and quality control. GF supervisor of PhD students, responsible for management of the experiments, writing of the paper, and is corresponding author.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Kim A, Shim JY. Emerging tocolytics for maintenance therapy of preterm labour: oxytocin antagonists and calcium channel blockers. BJOG. 2006;113(Suppl 3):113–5. doi: 10.1111/j.1471-0528.2006.01135.x. [DOI] [PubMed] [Google Scholar]
- 2.Husslein P, Quartarolo JP. Review of clinical experience with atosiban and the Tractocile Efficacy Assessment Survey in Europe (TREASURE) study protocol. Int J Clin Pract. 2003;57:121–7. [PubMed] [Google Scholar]
- 3.Pryde PG, Besinger RE, Gianopoulos JG, Mittendorf R. Adverse and beneficial effects of tocolytic therapy. Semin Perinatol. 2001;25:316–40. doi: 10.1053/sper.2001.27547. [DOI] [PubMed] [Google Scholar]
- 4.Oei SG. Calcium channel blockers for tocolysis: a review of their role and safety following reports of serious adverse events. Eur J Obstet Gynecol Reprod Biol. 2006;126:137–45. doi: 10.1016/j.ejogrb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Galik M, Gaspar R, Kolarovszki-Sipiczki Z, Falkay G. Gestagen treatment enhances the tocolytic effect of salmeterol in hormone-induced preterm labor in the rat in vivo. Am J Obstet Gynecol. 2008;198:319–5. doi: 10.1016/j.ajog.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Hajagos-Toth J, Falkay G, Gaspar R. Modification of the effect of nifedipine in the pregnant rat myometrium: the influence of progesterone and terbutaline. Life Sci. 2009;85:568–72. doi: 10.1016/j.lfs.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Sheridan BC, McIntyre RC, Jr, Meldrum DR, Fullerton DA. KATP channels contribute to beta- and adenosine receptor-mediated pulmonary vasorelaxation. Am J Physiol. 1997;273:950–6. doi: 10.1152/ajplung.1997.273.5.L950. [DOI] [PubMed] [Google Scholar]
- 8.Chang HY. The involvement of ATP-sensitive potassium channels in beta 2-adrenoceptor agonist-induced vasodilatation on rat diaphragmatic microcirculation. Br J Pharmacol. 1997;121:1024–30. doi: 10.1038/sj.bjp.0701192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Randall MD, McCulloch AI. The involvement of ATP-sensitive potassium channels in beta-adrenoceptor-mediated vasorelaxation in the rat isolated mesenteric arterial bed. Br J Pharmacol. 1995;115:607–12. doi: 10.1111/j.1476-5381.1995.tb14975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hudman D, Elliott RA, Norman RI. KATP channels mediate the beta(2)-adrenoceptor agonist-induced relaxation of rat detrusor muscle. Eur J Pharmacol. 2000;397:169–76. doi: 10.1016/S0014-2999(00)00229-6. [DOI] [PubMed] [Google Scholar]
- 11.Choudhury S, Garg SK, Singh TU, Mishra SK. Cellular coupling of potassium channels with beta2 adrenoceptors in mediating myometrial relaxation in buffaloes (Bubalus bubalis). J Vet Pharmacol Ther. 2010;33:22–7. doi: 10.1111/j.1365-2885.2009.01084.x. [DOI] [PubMed] [Google Scholar]
- 12.Shorter K, Farjo NP, Picksley SM, Randall VA. Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. FASEB J. 2008;22:1725–36. doi: 10.1096/fj.07-099424. [DOI] [PubMed] [Google Scholar]
- 13.Lovasz N, Ducza E, Gaspar R, Falkay G. Ontogeny of sulfonylurea-binding regulatory subunits of K(ATP) channels in the pregnant rat myometrium. Reproduction. 2011;142:175–81. doi: 10.1530/REP-10-0492. [DOI] [PubMed] [Google Scholar]
- 14.Aguilar-Bryan L. Clement JPt, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. Toward understanding the assembly and structure of KATP channels. Physiol Rev. 1998;78:227–45. doi: 10.1152/physrev.1998.78.1.227. [DOI] [PubMed] [Google Scholar]
- 15.Gross I, Toman A, Uhde I, Schwanstecher C, Schwanstecher M. Stoichiometry of potassium channel opener action. Mol Pharmacol. 1999;56:1370–3. doi: 10.1124/mol.56.6.1370. [DOI] [PubMed] [Google Scholar]
- 16.Bryan J, Vila-Carriles WH, Zhao G, Babenko AP, Aguilar-Bryan L. Toward linking structure with function in ATP-sensitive K+ channels. Diabetes. 2004;53(Suppl 3):104–12. doi: 10.2337/diabetes.53.suppl_3.S104. [DOI] [PubMed] [Google Scholar]
- 17.Teramoto N. Physiological roles of ATP-sensitive K+ channels in smooth muscle. J Physiol. 2006;572:617–24. doi: 10.1113/jphysiol.2006.105973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res. 2008;44:65–81. doi: 10.1540/jsmr.44.65. [DOI] [PubMed] [Google Scholar]
- 19.Gaspar R, Ducza E, Mihalyi A, Marki A, Kolarovszki-Sipiczki Z, et al. Pregnancy-induced decrease in the relaxant effect of terbutaline in the late-pregnant rat myometrium: role of G-protein activation and progesterone. Reproduction. 2005;130:113–22. doi: 10.1530/rep.1.00490. [DOI] [PubMed] [Google Scholar]
- 20.Ruzycky AL, DeLoia JA. Expression of beta-adrenergic receptor kinase subtypes in the pregnant rat myometrium. Am J Obstet Gynecol. 1997;176:1077–83. doi: 10.1016/S0002-9378(97)70405-8. [DOI] [PubMed] [Google Scholar]
- 21.Simon V, Mhaouty-Kodja S, Legrand C, Cohen-Tannoudji J. Concomitant increase of G protein-coupled receptor kinase activity and uncoupling of beta-adrenergic receptors in rat myometrium at parturition. Endocrinology. 2001;142:1899–905. doi: 10.1210/endo.142.5.8127. [DOI] [PubMed] [Google Scholar]
- 22.Simon V, Robin MT, Legrand C, Cohen-Tannoudji J. Endogenous G protein-coupled receptor kinase 6 triggers homologous beta-adrenergic receptor desensitization in primary uterine smooth muscle cells. Endocrinology. 2003;144:3058–66. doi: 10.1210/en.2002-0138. [DOI] [PubMed] [Google Scholar]
- 23.Curley M, Cairns M, Friel A, McMeel O, Morrison J, Smith T. Expression of mRNA transcripts for ATP-sensitive potassium channels in human myometrium. Mol Hum Reprod. 2002;8:941–5. doi: 10.1093/molehr/8.10.941. [DOI] [PubMed] [Google Scholar]