Abstract
Background and Objective
The etiology and exact incidence of infantile hemangiomas (IH) are unknown. Prior studies have noted immunohistochemical and biologic characteristics shared by IH and placental tissue. We investigated the possible association between placental anomalies and the development of IH, as well as the demographic characteristics and other risk factors for IH.
Methods
578 pregnant women were prospectively enrolled and their offspring followed for 9 months. Placental evaluations were performed and demographic data collected on all mother-infant pairs.
Results
594 infants were evaluated: 32 hemangiomas (either IH or congenital (CH)) were identified in 27 infants, yielding an incidence of 4.5% for IH and 0.3% for CH. Placental anomalies were noted in almost 35% of hemangioma-related pregnancies, approximately twice the incidence noted in pregnancies with unaffected infants. (p = 0.025). Other risk factors for IH included prematurity (p = 0.016) and low birth weight (p = 0.028). All IH were present by 3 months of age, and cessation of growth had occurred in all by 9 months of age. Most occurred on the trunk. Of note, 20% of identified IH were abortive/telangiectatic in nature, small focal lesions that did not proliferate beyond 3 months of age. Only one IH required intervention.
Conclusions
This is the first prospective American study to document the incidence of IH in infants followed from birth to early infancy. The association with placental anomalies was statistically significant. The overall incidence mirrors prior estimates, but the need for treatment was lower than previously reported.
Keywords: infantile hemangioma, congenital hemangioma, abortive/telangiectatic hemangioma, placental anomalies
Introduction
Infantile hemangiomas (IH) are the most common vascular tumors of infancy. Their exact prevalence is unknown, but rates as high as 10% have been reported2,3. Although most resolve over time without major sequelae, a significant subset are either disfiguring or life threatening1. Effective, safer therapies are now available, making it important to diagnose lesions early and to intervene in a timely fashion in cases at risk for complications. Previous studies have identified prematurity, low birth weight, female gender, Caucasian race, twin births, and advanced maternal age as risk factors associated with IH development 2. The most commonly reported locations for these lesions include the head and neck2.
In the last decade, various pathogenic mechanisms have been proposed to explain the etiology of these tumors. IH share many characteristics with placental vascular tissue, suggesting that IH may be derived from or are related to placental tissue. In particular, various histochemical characteristics are virtually identical to placental blood vessels and a programmed life cycle of initial proliferation followed by subsequent apoptotic involution is similar to that of the placenta 4,5. This study investigated the possible association between placental abnormalities and the development of IH. The secondary objective was to define the demographics of affected infant-mother pairs and to test known and additional risk factors associated with the development of IH.
Patients and Methods
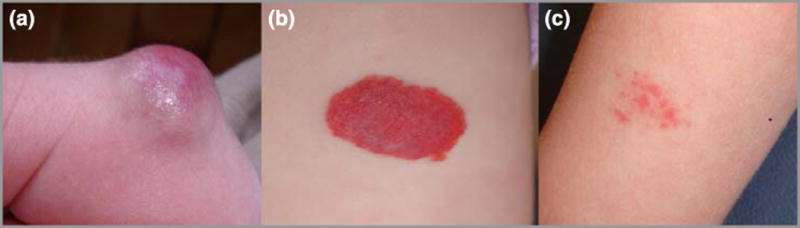
All pregnant women admitted to the Sharp Mary Birch Hospital (SMBH) for Women Labor and Delivery Suite were asked to participate in the trial, except those known to have intrauterine fetal demise. Approximately 8400 babies are born at SMBH each year, more than any other hospital in California. It serves as both a primary obstetrical care and referral center for the San Diego area. Subjects in our study were enrolled over a two year period. Investigators administered an Institutional Review Board approved questionnaire to obtain past and current medical and obstetrical history. Maternal serum, cord blood and placental tissue samples were collected at time of delivery. All neonates were examined by a pediatric dermatologist within 48 hours of delivery, who documented any congenital or post-natally acquired skin lesions and reviewed maternal and infant medical charts. Standard categorizations were utilized for IH: focal IH were well-circumscribed and of limited extent, while segmental IH were plaque-like and covered a larger surface area of the skin. The 2 CH) noted were rapidly involuting (RICH); fully formed at birth with rapid involution within the first 6 months of life (Fig. 1). Abortive lesions were focal, telangiectatic, and did not increase dramatically in size, with only a minority of the lesion showing a proliferative component6. Information regarding placental abnormalities and gross placental examination was obtained from the maternal medical history and as documented by the obstetrician. A pathologist specializing in placental pathology performed a histopathologic examination of the obtained placentas and umbilical cords.
Figure 1.
After hospital discharge, follow-up physical examinations were completed for infants with known IHs at 1, 3, 6, and 9 months of age by a pediatric dermatologist. All mothers were also contacted by telephone at the same time intervals and queried regarding “new red, brown or white skin spots” to document the status of their infants. Infants with a history of new cutaneous lesions were seen at the pediatric dermatology clinic for immediate examination.
Potential risk factors for the development of infantile hemangiomas were tested using Chi-square and binary logistical regression analysis. The database generated was utilized in a previous study to identify the incidence of all types of birthmarks in neonates.
Results
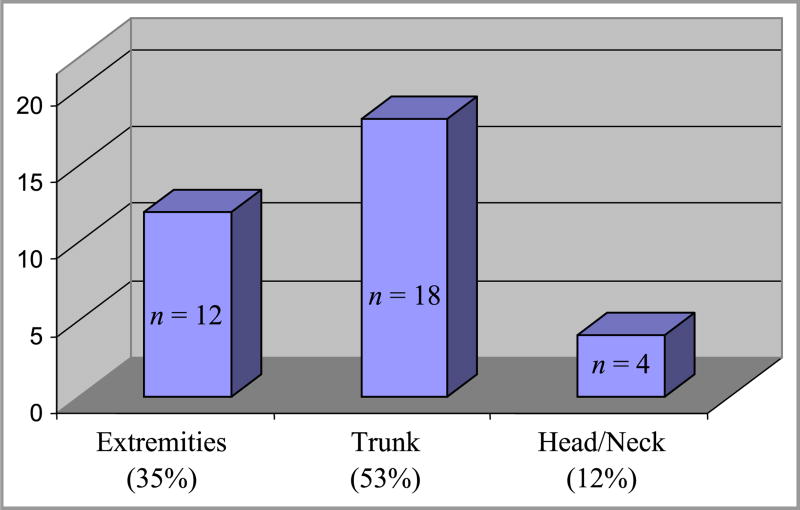
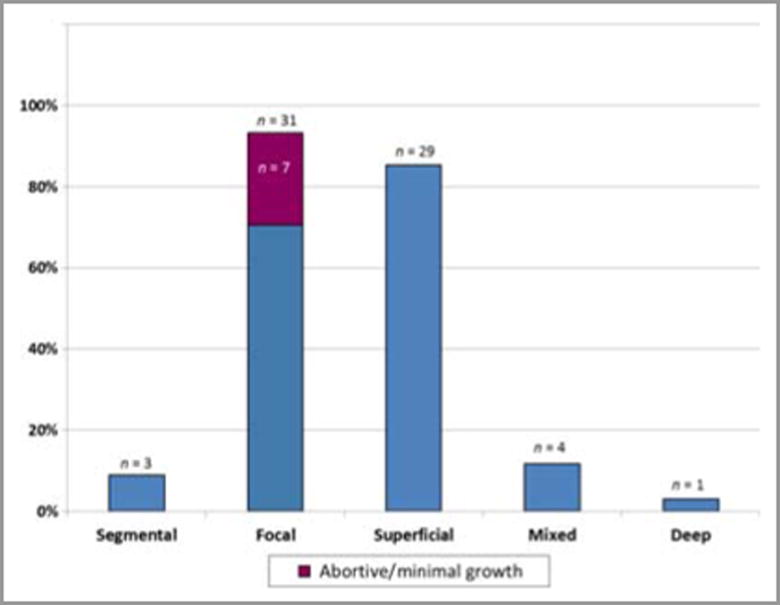
578 women were enrolled in the study and gave birth to 594 infants (Table 1). Infant ethnicity was determined by maternal reporting. Longitudinal data was collected on 88% of infants; 4 babies died before 1 month of age and had no follow-up information. Follow-up data at three months was available for 83% of infants. Thirty two IH and 2 CH were identified in 29 infants. Of the 29 infants with lesions, 3 had multiple lesions. Table 1 summarizes demographic characteristics of the study group. IH were over-represented in the 36-42 year-old age group, but a statistically significant difference between younger women and those of advanced maternal age was not detected. Sixteen lesions (47%) occurred in male infants, and eighteen (53%) in females. Three hemangiomas (9%) were noted on examination immediately after birth, 13 (38%) were present by 1 month and 18 (53%) were noted between 1 and 3 months of age. All IH presented within the first three months of life (Fig. 2) and cessation of growth was evident in all lesions by nine months of age. Two of three congenital lesions had completely involuted by nine months, but no post-natal lesions had completely resolved at that point. The third congenital lesion presented as an area of blanching (premonitory sign) which then evolved into a classic IH (erythematous, compressible plaque) by one month of life. Eighteen lesions (53%) were noted on the trunk and 12 (35%) on the extremities, while only 4 lesions (12%) were present on the head or neck (Fig. 3). Ninety-one percent of all lesions were focal. Abortive/minimal growth morphology comprised 23% of all focal lesions. These lesions were small and discrete but often non-confluent, similar to those described by Baselga et al.6, with early arrest of proliferation usually by 3 months of age. Two lesions were clinically worrisome; one large CH on the arm was of concern to the parents, but rapidly involuted by 9 months of age. Only 1 lesion required intervention: a segmental IH present on the left forearm and hand which required multiple pulsed dye laser treatments over the first 8 months of life, but which eventually involuted without sequelae. Fig. 4 provides morphologic characterization for all IH noted.
Table 1. Study population demographics (34 haemangiomas, either IH or CH, in 29 infants; 578 mothers and 594 infants in total).
| Mothers and infants, n (%) | Infants [n (%)] with haemangioma (IH + RICH) | |
|---|---|---|
| Race/ethnicity of mothers | ||
| White/non-hispanic | 267 (46.2) | 14 (48.3) |
| Hispanic | 145 (25.1) | 9 (31) |
| Asian | 56 (9.7) | 3 (10.3) |
| African American | 28 (4.8) | 1 (3.4) |
| Other | 82 (14.2) | 2 (6.8) |
| Maternal age, years | ||
| 15–21 | 50 (8.6) | 1 (3.4) |
| 22–28 | 207 (35.8) | 9 (31) |
| 29–35 | 238 (41.1) | 12 (41.4) |
| 36–42 | 79 (13.7) | 7 (24.1) |
| 43–49 | 4 (0.7) | 0 |
| Sex of infants | ||
| Male | 325 (54.7) | 13 (44.8) |
| Female | 267 (44.9) | 16 (55.2) |
| Unknown | 2 (0.03) | 0 |
CH, congenital haemangioma; IH, infantile haemangioma; RICH, rapidly involuting IH.
Figure 2.
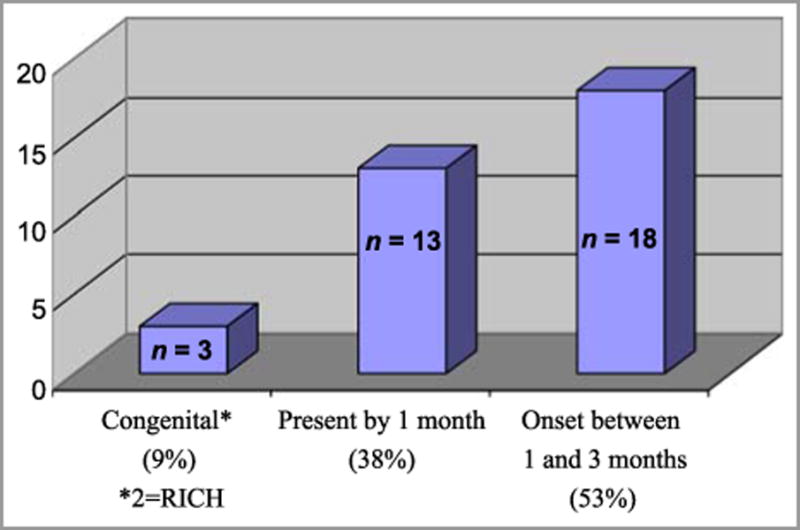
Figure 3.
Figure 4.
The overall incidence of IH was 4.5%. Seventy-four percent of all IH noted occurred in term infants (>36weeks), 14.8 % developed in premature infants (32+1d - 36 weeks) and 11% occurred in extremely premature infants (≤32wks). Only 3.9% of term infants were affected, while the incidence increased to 14.3% for extremely premature infants (Table 2), confirming the importance of gestational age as a risk factor for IH. Table 2 also provides the distribution of IH by birth weight. Risk factors for the development of IH included extreme prematurity (p=0.016) and very low birth weight (p=0.028). Although 11% of infants from twin gestations developed IH, statistical significance was not achieved due to confounding factors of prematurity and low birth weight in twins. We did not note a significant association with Caucasian race. No study patients underwent chorionic villus sampling.
Table 2. Incidence of infantile haemangioma (IH).
| Group | Total | Infants with IH | Incidence |
|---|---|---|---|
| All infants | 594 | 27 | 4.5% |
| Gestational agea | |||
| Very premature | 21 | 3 | 14.3 |
| Premature | 54 | 4 | 7.4 |
| Term | 513 | 20 | 3.9 |
| Birth weighta | |||
| Very low | 18 | 3 | 16.6 |
| Low | 52 | 3 | 5.7 |
| Normal | 521 | 21 | 4 |
Missing data among infants without IH.
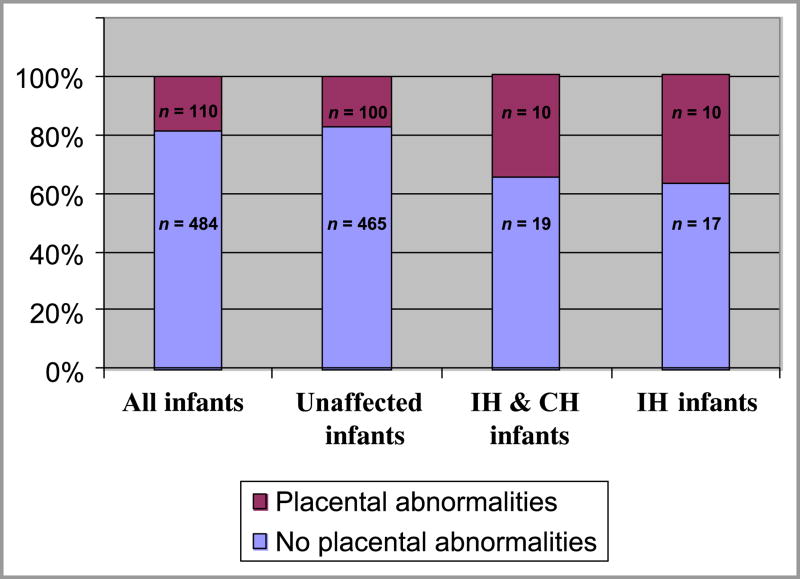
Ten of 29 placentas (34.5%) from pregnancies with affected infants exhibited a placental or cord abnormality associated with abnormal placental perfusion. In contrast, 18% of placentas from all deliveries showed an abnormality, and only 16.9% of non-affected pregnancies were associated with placental abnormalities (p=0.025) (Fig. 5). This data supports our hypothesis that placental anomalies are associated with the development of hemangioma-type lesions. This association was even stronger when lesions classified as NICH/RICH were subtracted, and only classic IH compared to unaffected infants (p=0.013). The two most common placental abnormalities were pre-eclampsia and placenta previa. The findings of histopathologic inspection are listed in Table 3; of note, no chorangiomas were detected.
Figure 5.
Table 3. Placental anomalies in mother–infant pairs associated with haemangiomas.
| Placental anomaly | n = 29 (%) |
|---|---|
| Pre-eclampsia | 2 (6.9) |
| Placenta previa | 2 (6.9) |
| Intrauterine growth restriction | 1 (3.4) |
| Subchorionic bleed | 1 (3.4) |
| Abruption | 1 (3.4) |
| Velamentous cord insertion | 1 (3.4) |
| Vasa previa | 1 (3.4) |
| Mother reported (unspecified placental problem) | 1 (3.4) |
Discussion
Many mechanisms have been proposed to explain the pathogenesis of infantile hemangiomas, but the exact etiology of these lesions remains unclear. Significant evidence suggests that they are derived from endothelial stem or progenitor cells7. In 2001 Boye and colleagues noted that endothelial cells isolated from IH were clonal in nature8. They subsequently isolated CD133+ positive endothelial progenitor cells from IH tissue. When injected into nude mice these cells formed tumors with histochemical and “life-cycle” characteristics of IH7. Other evidence points toward an aberration in vascular growth factor or receptor (VEGFR) expression. Jinnin et al identified a mutation in VEGFR-1 expression in IH cells which leads to constitutive upregulation of VEGFR2, resulting in vascular cell proliferation9.
The above findings, however, do not explain the distinct life cycle of IH, consisting of a proliferative phase followed by a morphologically “stable” period with subsequent involution. The placenta possesses a similar life cycle, and North et al10 suggested that IH may arise from ectopic placental tissue. IH and placentas share expression of the cell surface markers GLUT1, Lewis Y antigenic FcyR11 and merosin, proteins that are not typically expressed on tissues other than those of neural or placental origin10. Barnes et al subsequently performed transcriptome clustering analysis of placenta as well as vascular tumors, and found that microarray expression profiles of IH and placental tissue shared remarkable similarities, supporting a possible relationship11. Gutierrez et al evaluated placentas from 26 infants weighing <1500 grams, half of whom possessed IH. All placentas from IH-associated pregnancies possessed pathologic changes, while only 23% of the unaffected infant pairs manifested placental anomalies12. Recently, Itinteaga et al reported that endothelial cells of proliferating but not involuted IH express human chorionic gonadotropic (HCG) and human placental lactogen (HPL), suggesting that IH might be derived from placental chorionic villus tissue13. The risk of IH was noted to be 10 times higher in infants of women who have undergone chorionic-villus sampling, suggesting that placental trauma or embolization might play a role in the development of IH14. Chen et al noted a close relationship between maternal vaginal bleeding and progesterone therapy early in pregnancy with IH15. Pittman and colleagues were unable to find evidence of maternal-fetal chimerism in IH tissue, but the placenta is partially fetal in origin and therefore still a potential source of origin16.
Hypoxia may be a factor initiating or enhancing development of IH. Some investigators have noted that IH can first appear as an area of vasoconstrictive pallor, and that placentas from pregnancies with IH offspring are more likely to show signs of histopathological abnormalities which can be related to hypoxia17,18. In our study the development of IH was significantly associated with a history of disorders often related to placental hypoxia, such as pre-eclampsia and placenta previa (Table 3). Very low birth weight is known to be associated with placental insufficiency, and was also significantly associated with IH in the present study.
Central necrosis can occur in IH and suggests a local hypoxic environment that stimulates tissue response17,18. Recently Mihm et al suggested a “metastatic niche” theory for the development of IH, similar to that used to explain metastasis of malignant tumors19. In their model, the placenta elaborates humoral factors which prime or prepare susceptible tissue niches in the fetus. Progenitor cells, perhaps followed by the actual hemangioma cells of origin, then home to and proliferate at these ”nurturing” sites. This hypothesis is consistent with the observation that IH locations appear to favor facial placode fusion sites. These sites are covered by membranes and have a vascular “dead end” or “end artery” circulation, possibly facilitating concentration of both humoral factors and tumor cells19,20. Localized hypoxia at these sites might also favor the proliferation of IH. Kwon et al performed genome-wide association studies (GWAS) on 224 family trios with affected IH individuals, and identified over-represented single nucleotide polymorphisms related to NOSTRIN, a trafficking inducer protein involved in the eNOS /hypoxia pathway21. Thus, as Drolet and Frieden have suggested, there is provocative data to “connect the dots” between hypoxia and the development of IH18. Our findings, which link placental (and presumably hypoxia-inducing) anomalies with IH, are in congruence with their hypothesis.
The second study objective was to identify risk factors associated with the development of infantile hemangiomas. We chose to focus on those factors previously proposed by the Hemangioma Investigator Group, such as gender, race, prematurity, low birth weight, multiple gestations, and advanced maternal age1,2. Although most lesions occurred in Caucasian patients, the Caucasian population was also the most highly represented in our study. A statistically significant association between Caucasian race and IH risk was not documented in our study, in contrast to the findings of Drolet et al in which they compared 420 children with IH to 353 age-matched controls22. Several possibilities could explain this discrepancy. In our study, babies were categorized based on maternal self-identified racial data, not physician assessment. Careful attention was paid to cutaneous exam, and small, relatively inconspicuous lesions that might not otherwise be noted in children with more highly pigmented skin, (particularly on the trunk) might therefore have been identified. Alternatively, the relatively small sample size may have affected results.
A majority (53%) of lesions occurred in females, but not at the extreme F:M ratio of 4:1 that has been previously cited1,2 . Very low birth weight (p=0.028) and extreme prematurity (p=0.016) were found to be significant risk factors for the development of infantile hemangiomas. Multiple gestations and advanced maternal age were not found to predispose infants to the subsequent development of IH lesions. Positive associations have previously been reported by the Hemangioma Investigator Group1,2, but in a subsequent age-matched study by Drolet et al, neither maternal age nor multiple gestations were statistically significant risk factors for IH22.
In an extensive retrospective review of the literature, Kilcline et al sought to determine the true incidence of infantile hemangiomas as well as relevant risk factors associated with their development23. Due to methodologic limitations in the studies available for inclusion in their review, they were unable to definitively determine the incidence of IH, but estimated it at approximately 4 to 5%. Our prospective trial utilizing specialists in pediatric skin disease presumably allowed more precise differentiation of true IH from other newborn vascular lesions, and essentially confirmed their findings. A recent Dutch study utilizing case-control design cited a prevalence figure of 9.9%; methodologic differences or geographic variability may explain their differing findings24.
Unique aspects of our study are that: (1) it is truly prospective, identifying and enrolling unselected delivering women and their infants; and (2) only pediatric dermatologists performed skin assessments. Our catchment site is an urban, non-University hospital that delivers over 8,400 infants a year which services both an inner city and “suburban” community. Referral bias would be less likely in this setting that treats both indigent and fee-for service patients. The identified incidence of 4.5% is significantly lower than the upper range of 10% previously cited by experts. The only other prospective study focusing on identification of IH from birth noted an even lower incidence of 2.6%25. Dickison et al followed 1065 Australian infants from birth up to 6 weeks of age. While this important study had a large cohort size, their lower incidence may well reflect their limited duration of follow-up. In our study, the majority of lesions were not noted until the three-month evaluation.
Prior publications have reported that most IH occur on the head and neck. Investigators have been at a loss to explain this localization, but have noted that lesions predominantly occur along facial placode fusion lines20. This study, in contrast, found that the majority of lesions occur on the trunk, following the natural body surface area proportional distribution found in infants. These discordant findings may be explained by several considerations. Hemangiomas of the head and neck are more cosmetically concerning to families and, thus, more likely to lead to physician visits and recognition. In addition, many of the truncal lesions noted in our study were abortive and not as likely to raise notice or concern in parents or pediatricians. It may also be that large, troublesome lesions are more likely to occur on the head and neck. The finding of predominantly truncal IH which did not require intervention in a cohort without referral bias needs to be taken into account when educating primary care physicians. It is likely that patients with truncal lesions generally do not require referral, while facial lesions require more attention.
Cessation of growth was noted in all lesions by nine months of age. Two of three congenital lesions, but no post-natal lesions, were completely resolved by 9 months of age. Only 1 lesion, less than 4% of the overall IH population, required intervention, significantly lower than the 20% figure reported by others1. Over 90% of lesions observed were focal, 23% of which exhibited abortive/telangectatic morphology. Nine percent of total lesions were classified as segmental, a much lower percentage than the 18% reported by Chiller et al in 200226. The majority of the lesions noted in our study were superficial (85%), with only 12% showing mixed characteristics and only one deep lesion observed.
Limitations of this study include the relatively small number of IH identified, perhaps impacting our ability to identify risk factors. We were not able to definitively identify the incidence of a premonitory, or blanching sign. In addition, telephone follow-up might have led to an under-representation of lesions, but the repeated calls and excellent contact rate we achieved hopefully decreased that likelihood.
Conclusions
This is the first prospective American study to follow infants from birth through early infancy in order to specifically identify risk factors for the development of infantile hemangiomas, particularly in relationship to placental abnormalities. In addition to demonstrating a positive association with placental abnormalities and other known risk factors such as low birth weight and prematurity, some findings do differ from those previously reported. We observed a 4.5% incidence rate of IH overall, with risk of occurrence increasing with decreased gestational age and weight. While the incidence in term infants was only 3.9%, very premature infants had an incidence of 14.3%. The majority of lesions occurred on the trunk and extremities, a significant number of these were minimal, abortive lesions, and only a small percentage of IH required therapy. These findings may differ from previous studies because prior studies were not prospective from the time of birth, were possibly subject to referral bias, did not have lesional evaluations performed solely by pediatric dermatologists, and/or did not specifically search for vascular skin lesions, of particular importance in children with darkly pigmented skin, where it might be easier to overlook such lesions if they are small. Many of the IH noted were in “generally covered” truncal areas and some were abortive/minimal growth lesions which might well have been overlooked during routine pediatric newborn examinations. A recently published retrospective study by Suh and Frieden support our findings, in that their specific search for abortive-type lesions in infants found more than 60% present on the lower body27. Ongoing investigations evaluating genetic and environmental risk factors will hopefully lead to improved means of identifying at-risk mother-infant pairs and help optimize counseling, surveillance, and therapeutic intervention for these individuals.
What's known
Infantile hemangiomas, though often inconsequential, may cause significant disability or disfigurement. An association with aberrant placental or vascular stem cells has been hypothesized. The reported incidence varies widely, and complication rates as high as 20% have been documented.
What this study adds
This is the first prospective U.S. study to document the incidence and demographics of IH in infants evaluated from birth. The incidence and risk of complications appear lower than previously reported. A statistically significant association with placental anomalies was identified.
Acknowledgments
We would like to recognize the administrative support of Patti Oden, and the academic support of Ernie Beutler, MD, Ph.D., to whom this paper is dedicated.
Funding: Dr. Andrea Munden was the recipient of a summer research NRSA Short-term Institutional Training Grant (T35) NHLBI. Dr. Tom is funded by a Research Career Development Award, K23AR060274, NIAMS. Dr. Sheila Fallon Friedlander was supported by The Skaggs Clinical Scholars Program, Scripps Research Institute. Dr. Martin Friedlander received funding from the NEI (EY11254)
Abbreviations
- IH
Infantile Hemangioma
- RICH
Rapidly Involuting Childhood Hemangiomas
- CH
congenital hemangioma
- IRB
Institutional Review Board
- SMBH
Sharp Mary Birch Hospital
- NICH
Non-involuting congenital hemangioma
- HCG
Human chorionic gonadotropic
- HPL
Human placental lactogen
- VLBW
Very low birth weight
- GWAS
Genome-wide association studies
- VEGFR
Vascular Endothelial Growth Factor Receptor
Footnotes
Conflict of Interest: None
References
- 1.Haggstrom An, Drolet BA, Baselga E, Chamlin SL, et al. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics. 2006 Sep;118(3):882–7. doi: 10.1542/peds.2006-0413. [DOI] [PubMed] [Google Scholar]
- 2.Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. NEJM. 1999 Jul 15;31(3):173–181. doi: 10.1056/NEJM199907153410307. [DOI] [PubMed] [Google Scholar]
- 3.Hemangioma Investigator Group. Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW, Newell B, Nopper AJ, Frieden IJ. Prospective study of infantile hemangiomas: demographic, prenatal, and perinatal characteristics. J Pediatrics. 2007 Mar;150(3):291–4. doi: 10.1016/j.jpeds.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Ritter MR, Butschek RA, Friedlander M, Friedlander SF. Pathogenesis of infantile haemangioma: new molecular and cellular insights. Expert Rev Mol Med. 2007 Nov 29;9(32):1–19. doi: 10.1017/S146239940700052X. [DOI] [PubMed] [Google Scholar]
- 5.Boye E, Jinnin M, Olsen BR. Infantile hemangioma: challenges, new insights, and therapeutic promise. J Craniofac Surg. 2009 Mar;20(Suppl 1):678–84. doi: 10.1097/SCS.0b013e318193d6c1. [DOI] [PubMed] [Google Scholar]
- 6.Corella F, Garcia-Navarro X, Ribe A, Alomar A, Baselga E. Abortive or minimal-growth hemangiomas: Immunohistochemical evidence that they represent true infantile hemangiomas. J Am Acad Dermatol. 2008 Apr;58(4):685–90. doi: 10.1016/j.jaad.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Khan ZA, Boscolo E, Picard A, Psutka S, Melero-Martin JM, Bartch TC, Mulliken JB, Bischoff J. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. Nature Medicine. 2008;14:1236–1246. doi: 10.1172/JCI33493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boye E, Yu Y, Paranya G, Mulliken JB, Olsen BR, Bischoff J. Clonality and altered behavior of endothelial cells from hemangiomas. J Clin Invest. 2001 Mar;107(6):745–52. doi: 10.1172/JCI11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jinnin M, Medici D, Park L, Limaye N, Liu Y, Boscolo E, Bischoff J, Vikkula M, Boye E, Olsen BR. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangiomas. Nature. 2008 Nov;14(11):1236–46. doi: 10.1038/nm.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.North PE, Waner M, Mizeracki A, Mihm MC., Jr GLUT1: a newly discovered marker for juvenile hemangiomas. Human Pathol. 2000;31:11. doi: 10.1016/s0046-8177(00)80192-6. [DOI] [PubMed] [Google Scholar]
- 11.Barnés CM, Huang S, Kaipainen A, Sanoudou D, Chen EJ, Eichler GS, Guo Y, Yu Y, Ingber DE, Mulliken JB, Beggs AH, Folkman J, Fishman SJ. Evidence by molecular profiling for a placental origin of infantile hemangioma. Proc Natl Acad Sci U S A. 2005 Dec 27;102(52):19. doi: 10.1073/pnas.0509579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez Gutierrez JC, Avila LF, Sosa G, Patron M. Placental anomalies in children with infantile hemangioma. Pediatric Dermatology. 2007;24(4):353–5. doi: 10.1111/j.1525-1470.2007.00450.x. [DOI] [PubMed] [Google Scholar]
- 13.Intineaga T, Tan ST, Guthrie S, Tan CE, McIntyre BC, Brasch HD, Day DJ. A placental chorionic villus mesenchymal core cellular origin for infantile haemangioma. J Clin Pathol. 2011 Oct;64(10):870–4. doi: 10.1136/jclinpath-2011-200191. [DOI] [PubMed] [Google Scholar]
- 14.Burton BK, Schulz CJ, Angle B, Burd LI. An increased incidence of hemangiomas in infants born following chorionic villus sampling (CVS) Prenat Diagn. 1995;15:209–14. doi: 10.1002/pd.1970150302. [DOI] [PubMed] [Google Scholar]
- 15.Chen XD, MA G, Chen H, Ye XX, Jin YB, Lin XX. Maternal and perinatal risk factors for infantile hemangioma: a case-control study. Pediatr Dermatol. 2013 Jul-Au;30(4):457–61. doi: 10.1111/pde.12042. [DOI] [PubMed] [Google Scholar]
- 16.Pittman KM, Losken HW, Kleinman ME, Marcus JR, Blei F, Gurtner GC, Marchuk DA. No evidence for maternal-fetal microchimerism in infantile hemangioma: a molecular genetic investigation. J Invest Derm. 2006;126(11):2533–8. doi: 10.1038/sj.jid.5700516. [DOI] [PubMed] [Google Scholar]
- 17.Colonna V, Resta L, Napoli AE, Bonifazi E. Placental hypoxia and neonatal haemangioma clinical and histological observations. Br J Dermatol. 2010;162:208–234. doi: 10.1111/j.1365-2133.2009.09493.x. [DOI] [PubMed] [Google Scholar]
- 18.Drolet BA, Frieden IJ. Characteristics of Infantile Hemangiomas as Clues to Pathogenesis: Does Hypoxia Connect the Dots? Arch Dermtol. 2010 Nov;146:1295–98. doi: 10.1001/archdermatol.2010.1295. [DOI] [PubMed] [Google Scholar]
- 19.Mihm MC, Nelson JS. Hypothesis: the metastatic niche theory can elucidate infantile hemangioma development. J Cutan Pathol. 2010;37:83–87. doi: 10.1111/j.1600-0560.2010.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waner M, North PE, Schere KA, Frieden IJ, Waner A, Mihm MC., Jr The nonrandom distribution of facial hemangiomas. Arch Dermatol. 2003;139(7):869. doi: 10.1001/archderm.139.7.869. [DOI] [PubMed] [Google Scholar]
- 21.Kwon EM, Siegel Dh, Broeckel U, North PE, Worthey E, Chiu YE, Drolet BA. Functional analysis of candidate genes identified by genomewide association study (GWAS) of infantile hemangiomas (IH) Journal of Investigative Dermatology. 2012 May 1;393(1):S67. 2012 ; Abstract. [Google Scholar]
- 22.Drolet BA, Swanson EA, Frieden IJ. Infantile Hemangiomas: An Emeriging Health Issue Linked to an Increased Rate of Low Birth Weight Infants. J Pediatr. 2008 Nov;153(5):712–15. doi: 10.1016/j.jpeds.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 23.Kilcline C, Frieden IJ. Infantile hemangiomas: how common are they? A systematic review of the medical literature. Pediatr Dermatol. 2008 Mar-Apr;25(2):168–73. doi: 10.1111/j.1525-1470.2008.00626.x. Review. [DOI] [PubMed] [Google Scholar]
- 24.Hoornweg MJ, Smeulders MJ, Ubbink DT, van der Horst CM. The prevalence and risk factors of infantile haemanngiomas: a case-control study in the Dutch population. Paediatr Perinat Epidemiol. 2012 Mar;26(2):156–62. doi: 10.1111/j.1365-3016.2011.01214.x. [DOI] [PubMed] [Google Scholar]
- 25.Dickison P, Christou B, Wargon O. A Prospective Study of Infantile Hemangiomas with a Focus on Incidence and Risk Factors. Pediatr Dermatol. 2011 Nov-Dec;28(6):663–9. doi: 10.1111/j.1525-1470.2011.01568.x. [DOI] [PubMed] [Google Scholar]
- 26.Chiller KD, Passaro D, Frieden IJ. Hemangiomas of Infancy: clinical characteristics, morphologic subtypes and their relationship to race, sex and ethnicity. Arch Dermatol. 2002 Dec;138:1567–76. doi: 10.1001/archderm.138.12.1567. [DOI] [PubMed] [Google Scholar]
- 27.Suh KY, Frieden IJ. Infantile hemangiomas with minimal or arrested growth: a retrospective case series. Arch Dermatol. 2010 Sep;146(9):971–6. doi: 10.1001/archdermatol.2010.197. [DOI] [PubMed] [Google Scholar]