Abstract
Coxiella burnetii is the etiological agent of Q fever, and outbreaks of Q fever have been reported in different parts of Europe both in animals and humans. Human infections are mostly associated with infections in ruminants, e.g., sheep, goats, and cows. Various professional groups are occupationally exposed to infection with C. burnetii. The aim of this study was investigate the prevalence of C. burnetii in farm workers. Serum samples were collected from 151 persons from six different regions of Poland. The serum samples were tested using three serological methods—complement fixation test (CFT), enzyme-linked immunosorbent assay (ELISA), and indirect fluorescent antibody (IFA). A total of 71 samples of blood were also tested by real-time PCR. The results showed that antibodies against C. burnetii were present in the tested sera. Average percentages of seropositive samples in IFA, ELISA, and CFT were 31.12%, 39.07%, and 15.23%, respectively. Positive results were noted in each testing center. Of the three test types, IFA results were considered the most sensitive. Real-time PCR confirmed the presence of DNA specific for C. burnetii in 10 patients. The farming workforce constitutes an occupational risk group with an increased risk for C. burnetii infection, presumably because of their contact with infected livestock.
Key Words: : Coxiella burnetii, Q fever, Prevalence, Humans, Serological testing
Introduction
Coxiella burnetii is the etiological agent of Q fever. This pathogen is zoonotic and occurs in ticks, birds, and mammals. Human infections are mostly related to infected ruminants, e.g., sheep, goats, and cows (Gilsdorf et al. 2008). C. burnetii is extremely infectious and it may survive environmental stresses for several weeks (Maurin and Raoult 1999). Transmission of this pathogen is generally associated with abortion in domestic ruminants, particularly sheep. The infection may be acquired by the respiratory or alimentary route or the bite of an arthropod (Maurin and Raoult 1999). The infection of humans occurs most often through direct contact with infected animals, e.g., in slaughterhouses, tanneries, fur, meat, leather, and timber industries, on agricultural farms, where veterinarians work in the field, etc. Infection with C. burnetii has been reported both in humans and animals from many countries, including Poland (Cisak et al. 2003, Niemczuk et al. 2011, Brom et al. 2013, Georgiev et al. 2013, Szymańska-Czerwińska et al. 2013, 2014). The southeastern region of Poland is considered to be an endemic area for the occurrence of C. burnetii (Cisak et al. 2003, Galińska et al. 2011).
Q fever diagnosis based on clinical symptoms or postmortem pictures is almost impossible because signs and symptoms of the disease are nonspecific. It is in the clinical symptoms in particular that nonspecificity poses a great problem. Moreover, these symptoms very often do not occur at all in infected animals or humans. The reliable diagnosis of Q fever should be based on laboratory tests, including serological and molecular assays. The most common diagnostic methods are serological tests, e.g., indirect fluorescent antibody (IFA), enzyme-linked immunosorbent assay (ELISA), and complement fixation test (CFT) (Maurin and Raoult 1999, Herremans et. al 2013). IFA is considered the gold standard for detection of antibodies against C. burnetii in humans (Maurin and Raoult 1999, Herremans et. al 2013). Molecular methods, e.g., PCR and real-time PCR, are used increasingly, and PCR is particularly useful in the early stage of infection when antibodies are not detectable (Schneeberger et. al 2010). The aim of the studies reported here was investigation of the prevalence of C. burnetii in humans exposed to animals in Poland.
Methods
Investigations in animals
The samples were collected from animals by authorized veterinarians during clinical studies following standard procedures. They were collected specifically for this study with the agreement of the farmers. According to the Local Ethical Committee on Animal Testing at the University of Life Sciences in Lublin (Poland), formal ethical approval is not required for this kind of study. We used guidelines published by the National Ethics Committee for Animal Experimentation (Resolution No. 22/2006, November 7, 2006), which confirm that this work is acceptable without specific ethical approval.
The samples of sera from cattle and small ruminants were taken from clinically affected farms and tested by using CFT, a diagnostic technique recommended by the World Organisation for Animal Health (OIE). Additionally, the placentas, aborted fetuses or blood, and bulk tank milk were tested by real-time PCR. The kind of tested biological material was dependent on availability. Detailed information about number and species of tested animals are presented in Table 2, below. Our studies were performed by the National Veterinary Reference Laboratories for Q fever.
Table 2.
Results for Animals Tested
| Place of sampling | Species of tested animals | Number of tested animals | Number of seropositives animals | Detection of C. burnetii by real-time PCR in biological material |
|---|---|---|---|---|
| Dębno | Cattle | 213 | 74 | Positive results (blood) |
| Tarnogród | Cattle | 199 | 120 | Positive results (placenta) |
| Krosno | Cattle | 60 | 19 | Positive result (blood) |
| Gliwice | Cattle | 21 | 17 | Positives results (placenta) |
| Chodzież | Cattle and small ruminants | 2104 | 249 | Positive results (blood, BTM) |
| Ciechanów | Cattle | 29 | 10 | Positive results (placenta, BTM) |
BTM, bulk tank milk.
Examined populations of humans
The samples from humans were taken during routine diagnostic tests following standard procedure and with their agreement, and additional ethical approval was not required.
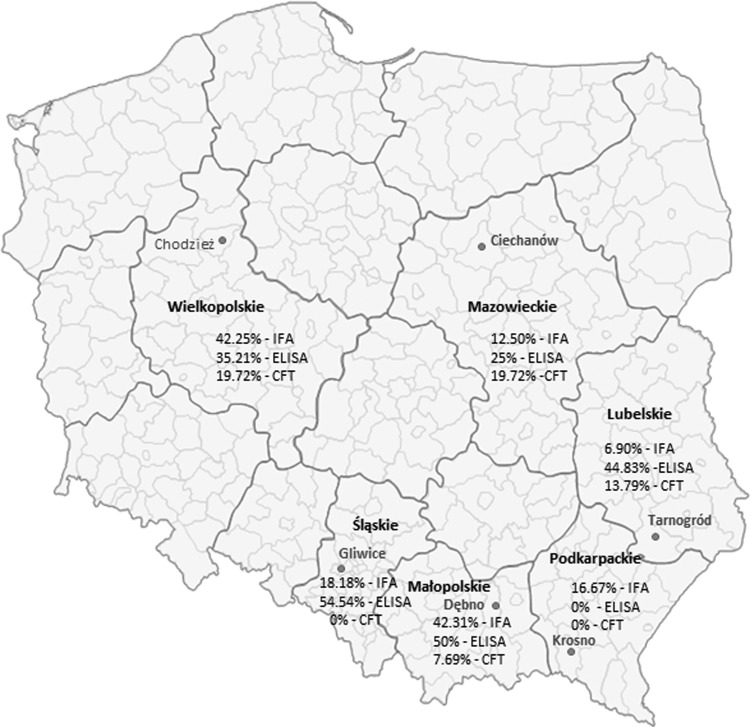
A total of 151 farm-employed persons occupationally exposed to infection via animals were examined. The average age of the tested subjects was 47.11±8.41; 76 women and 75 men were tested. Detailed information about tested humans (gender, age) is included in Table 1. The farm workers were employed in the breeding of cattle and small ruminants and had contact during routine service, e.g., milking, veterinary service, or housekeeping tasks. The populations under investigation lived and worked in six different regions of Poland, including the endemic southeastern regions, and correspondingly comprised six groups (Fig. 1). Blood samples were taken by venipuncture, and sera were separated by centrifugation. Whole blood for real-time PCR assays was taken only from one farm's workers (Chodzież), and it took place twice, with the second sampling following after 3 weeks. Human whole blood samples from other farms were not available. The majority of tested men and women did not have clinical signs of disease, except for people from Dębno and Tarnogród, in whom clinical signs of Q fever were partly described by Galińska et al. (2011).
Table 1.
Results of Serological Investigations in Humans
| IFA Cut-off for chronic form of disease, IgG phase I antibody titer ≥1:1024 Cut-off for acute form, IgG or IgM II titer ≥1:32 | ELISAa | CFT Cut-off titer ≥1:32 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of seropositive for phase II | |||||||||||||
| Examined group localities-voivodship | Number of examined (gender)/average age±standard deviation | Total number of seropositive | Number of seropositive for IgG phase II | Number of seropositive for IgG phase Ib | Ttotal number of seropositive | IgM | IgG | Seropositive for both IgM and IgG | totally | Number of seropositive for IgG phase I | Number of seropositive (phase I and II) | Seropositive phase I | Seropositive phase II |
| Dębno Małopolskie |
26 (18 women, 8 men) 44.1±15.25 |
11 (42.31%) | 3 (11.53%) | 8 (30.76%) | 13 (50%) | 1 (3.84%) | 7 (26.92%) | 3 (11.54%) | 11 (42.31%) | 2 (7.69%) | 6 (23.08%) | 0 | 2 (7.69%) |
| Tarnogród Lubelskie |
29 (20 women, 9 men) 48.4±8.24 |
2 (6.90%) | 2 (6.90%) | 0 | 13 (44.83%) | 4 (13.79%) | 5 (17.24%) | 1 (3.45%) | 10 (34.48%) | 3 (10.34%) | 2 (6.90%) | 0 | 4 (13.79%) |
| Krosno Podkarpackie |
6 (6 men) 51.33±4.22 |
1 (16.67%) | 0 | 1 (16.67%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gliwice Śląskie |
11 (9 women, 2 men) 45.36±9.18 |
2 (18.18%) | 1 (9.09%) | 1 (9.09%) | 6 (54.54%) | 0 | 2 (18.18%) | 0 | 2 (18.18%) | 4 (36.36%) | 1 (9.09%) | 0 | 0 |
| Chodzież Wielkopolskie |
71 (25 women, 46 men) 46.7±3.48 |
30 (42.25%) | 6 (8.45%) | 24 (33.80%) | 25 (35.21%) | 4 (5.63%) | 9 (12,67%) | 3 (4.22%) | 16 (22.53%) | 9 (12.68%) | 8 (11.27%) | 0 | 14 (19.72%) |
| Ciechanów Mazowieckie |
8 (4 women, 4 men) 51.3±5.43 |
1 (12.50%) | 1 (12.50%) | 0 | 2 (25%) | 1 (12.50%) | 1 (12.50%) | 0 | 2 (25%) | 0 | 1 (12.50%) | 0 | 3 (37.50%) |
| Total | 151 (76 women, 75 men) 47.11±8.41 |
46 (31.12%) | 13 (8.60%) | 34 (22.52%) | 59 (39.07%) | 10 (6.62%) | 24 (15.89%) | 7 (4.63%) | 41 (27.15%) | 18 (11.92%) | 18 (11.92%) | 0 | 23 (15.23%) |
Presence of antibodies IgM and IgG in phase II=possible acute phase of Q fever.
Presence of antibodies IgG in phase I possible chronic infection (the reference methods for confirmation chronic form of Q fever is IFA).
IFA, indirect fluorescent antibody; IgG, immunoglobulin G; ELISA, enzyme-linked immunosorbent assay; CFT, complement fixation test.
FIG. 1.
Map of Poland showing the places of sampling and the serological results in humans.
Serological tests
ELISA was used for the qualitative determination of immunoglobulin G (IgG) class antibodies against C. burnetii phases 1 and 2 and IgM class antibodies against C. burnetii phase 2. The kit selected was NovaLisa (NovaTec Immundiagnostica GmbH, Germany), and was used according to the manufacturer's instructions. The presence of IgM class antibodies in phase 1 (2–3 weeks after infection) and IgG class in phase 2 (2 months after infection) confirmed the acute phase of Q fever; by contrast, in chronic infection, IgG antibodies are detected in phase 1. The cutoff is the mean absorbance value of the cutoff control determinations. Samples are considered positive if the absorbance value is higher than 10% over cutoff and negative if the absorbance values is lower than 10% below the cutoff. Interpretation of results was based on the value of nephelometric turbidity units (NTU). Sera were considered to be ELISA negative if NTU<9, dubious if 9≥NTU ≤ 11, and positive if NTU>11.
The IFA was performed by using the Q Fever IFA IgG Kit (Focus Diagnostic, USA). Results were interpreted by the class of antibodies present. If the reactivity of antibodies to both phase I and II antigens (titer ≥16) was observed, it strongly indicated C. burnetii infection. Phase I antibody titers greater than or equal to phase II antibody titers were consistent with chronic infection or the convalescent phase of Q fever. If the reactivity of antibodies to both phase I and II antigens at a titer <16 was noted, the result was negative. Titers of phase II antibodies greater than phase I characterized the acute form of Q fever. Reactivity to the phase II antigen only (titers <256) argued against C. burnetii infection. An IgG phase I titer ≥1:1024 was used as a cutoff value, above which chronic Q fever is conceivable.
For CFT, Institut Virion/Serion GmbH (Germany) and Institute of Biotechnology, Sera and Vaccines/Biomed S.A (France) reagents were used. The initial dilution of the sample was 1:5 and the final dilution was 1:80. Serum was considered positive when a partial inhibition of hemolysis (++) was observed in the dilution 1:10. Two different antigens of C. burnetii specific for phase I and II were used.
Real-time PCR
DNA extraction from blood was performed using the QIAamp DNA Mini Kit (Qiagen, USA) following the manufacturer's instructions. A 1-μL aliquot of extracted DNA was subjected to C. burnetii–specific real-time PCR (IS1111), as described previously (Niemczuk et al. 2011). The positive control was template DNA extracted from reference strain of C. burnetii (Nine Mile phase II). Samples values falling below a cycle threshold (Ct) of 38 were considered positive.
Statistical analyses
Chi-squared and Fisher tests were used to compare results obtained for the group of women and men and the between-individual group divided based on localization. The Mann–Whitney test was used to compare the results obtained between age groups. The statistical tests were used at the level of significance α=0.05
Results
The serological and molecular investigations performed in ruminant herds showed that Q fever was present in the six herds tested. The presence of both antibodies and DNA specific for C. burnetii (IS1111) was detected in all herds tested. Detailed results are presented in Table 2. Antibodies against C. burnetii were found among 151 persons occupationally exposed to animal-borne infection, with the percentage of positive samples differing depending on the serological method. The results obtained by three different serological methods (IFA, ELISA, and CFT) are presented in Table 1. The average percentages of seropositive samples in IFA, ELISA, and CFT were 31.12%, 39.07%, and 15.23%, respectively. The most positive results were obtained for the group from Dębno. Differences in the percentage of seropositive samples were also observed between the examined groups, but statistically significant (mainly for IFA results) differences were noted between people from Dębno and other groups (excluding Krosno) and between the Tarnogród and Chodzież groups. The result of statistical analysis (p values) between individual groups are presented in Table 3.
Table 3.
Results of Statistical Analysis for Individual Groups
| p value | ||||||||
|---|---|---|---|---|---|---|---|---|
| Groups compared | IFA phase I | IFA II phase II | IFA phase I or II | ELISA IgM phase II | ELISA IgG phase II | ELISA IgG phase I | ELISA phase I or II | CFT phase II |
| Dębno vs. Tarnogród | 0.0014* | 0.00056* | 0.00007* | 1 | 0.23421 | 1 | 0.78809 | 0.67228 |
| Dębno vs. Krosno | 0.36055 | 0.19594 | 0.17192 | 0.56618 | 0.14222 | 1 | 0.0711 | 1 |
| Dębno vs. Gliwice | 0.05735 | 0.02715* | 0.03646* | 0.29631 | 0.27934 | 0.0515 | 1 | 1 |
| Dębno vs. Chodzież | 0.34512 | 0.00002 | 0.25041 | 0.47857 | 0.03149* | 0.7219 | 0.32943 | 0.22174 |
| Dębno vs. Ciechanów | 0.03036* | 0.10208 | 0.04251* | 1 | 0.22746 | 1 | 0.21025 | 0.07215 |
| Tarnogród vs. Krosno | 0.44171 | 0.44171 | 0.44171 | 0.56102 | 0.56102 | 1 | 0.14615 | 1 |
| Tarnogród vs. Gliwice | 1 | 1 | 0.30031 | 0.29753 | 1 | 0.07547 | 0.72752 | 0.56019 |
| Tarnogród vs. Chodzież | 0.00511* | 1 | 0.00037* | 0.32191 | 0.77498 | 1 | 0.48113 | 0.57665 |
| Tarnogród vs. Ciechanów | 1 | 0.52973 | 0.52973 | 1 | 1 | 1 | 0.23231 | 0.15632 |
| Krosno vs. Gliwice | 1 | 1 | 1 | — | 0.51471 | 0.23739 | 0.10229 | — |
| Krosno vs. Chodzież | 0.65707 | 0.44699 | 0.39216 | 1 | 0.58194 | 1 | 0.18085 | 0.58496 |
| Krosno vs. Ciechanów | 0.42857 | 1 | 1 | 1 | 1 | — | 1 | 0.20879 |
| Gliwice vs. Chodzież | 0.15881 | 1 | 0.18767 | 0.58561 | 1 | 0.0676 | 0.31279 | 0.19688 |
| Gliwice vs. Ciechanów | 1 | 1 | 1 | 0.42105 | 1 | 0.1032 | 0.17699 | 0.05779 |
| Chodzież vs. Ciechanów | 0.05342 | 0.54122 | 0.13893 | 1 | 1 | 0.58645 | 0.43153 | 0.35907 |
The differences are statistically significant at the level α=0.05.
IFA, indirect fluorescent antibody; ELISA, enzyme-linked immunosorbent assy; CFT, complement fixation test.
It should be noted that CFT did not detect some positive samples with antibodies to phase I antigen, whereas the IFA and ELISA tests confirmed the presence of antibodies to phase I antigen in 22.52% and 11.92%, respectively. The ELISA results showed that 6.62% of tested individuals had IgM class antibodies specific to the phase II antigen, whereas the percentage of sera containing the IgG class of antibodies for the phase II of of C. burnetii antigen was higher (15.89%). Moreover, both classes of antibodies (IgG and IgM) were detected by ELISA in 4.63% of tested samples.
The results of the first real-time PCR assay in human whole blood samples from Chodzież showed the presence of specific sequences of C. burnetii DNA in 10 tested samples. Ct values in PCR-positive blood varied from 29.1 to 33.4. C. burnetii DNA was detected in four seropositive human samples that had IgM antibodies to phase II antigen in ELISA. In nine seropositive patients who had IgG antibodies (phase II) in ELISA, the presence of IS1111 elements was not confirmed. In one of three patients who had antibodies IgG and IgM (phase II), C. burnetii DNA was present in blood. However, the second examination of human samples after 3 weeks did not confirm these results and all tested samples were negative. We did not detect C. burnetii DNA in seropositive samples from patients with serologically proven chronic Q fever. Moreover, the positive results in real-time PCR were obtained in five seronegative humans.
The clinical signs of Q fever were noted only in patients from Dębno and Tarnogród. One person from Dębno had signs typical for chronic form of diseases. In two-dimensional echocardiography, lesions in the heart valves were observed. The second person had fever with high titers of antibodies in IFA (dynamics of antibodies titer during convalescence in range from 1:1024 to 1:256 in IFA). Other seropositive patients from Dębno had flu-like symptoms. Flu like-symptoms were noted in seropositve persons from Tarnogród. No significant differences were observed between genders (women and men). When age groups were compared, only one significant difference was noted for ELISA results (IgG phase II). The differences in the percentage of positive samples between ages 45 and 48 with ELISA were significantly higher (48 vs. 45, p=0.04) for negative patients than for positive patients.
Discussion
The serological assays were performed by using three different techniques. The IFA results showed that the percentage of samples with the presence of antibodies to phase I antigen of C. burnetii was higher than for the phase II of the antigen. Similar results were described previously in foresters tested during the same research project (Szymańska-Czerwińska et al. 2013). The contradictory results were obtained when ELISA was used and then the antibodies to phase II of the antigen were dominant. The ELISA test discriminated between two classes of antibodies (IgG and IgM) to phase II antigen. IgM phase II is the first antibody to be detected in blood, followed by IgG phase II. It should be noted that IgM phase II and IgG phase II can persist for a year and longer. Moreover, the decline of IgG phase II antibody titers is slower than that of IgG phase I titers, and IgG phase II antibodies are detected more often with IFA after a year (Wegdam-Blans et al. 2012b).
Definitive serological evidence of acute Q fever has to be based on the detection of both IgG and IgM antibodies. Our ELISA results showed that IgM and IgG phase II antibodies were present in the tested sera. The presence of the IgM phase II type indicated an early infection, but the percentage of sera positive for IgM antibodies was lower (6.62%) than the percentage of samples with IgG (15.89%). The level of seropositive samples was the lowest (15.23%) for the antibodies to phase II antigen in CFT, and this diagnostic method did not detect sera containing phase I antibodies. Similar results were obtained by Wegdam-Blans et al. (2014), who suggested that CFT-based phase I antibody detection is not recommended for serological diagnosis of chronic Q fever. Generally, IFA detected a lower percentage of positive samples than ELISA. The highest percentage of seropositive samples for phase II of Q fever antigen could be attributed to the detection of IgM antibodies. IgM were not demonstrated by IFA because only IgG antibodies were detected.
It should be noted that serological diagnosis based only on the presence of IgM phase II antibodies can be inaccurate because solitary IgM can be a false positive. Schneeberger et al. (2010) reported cases with isolated IgM-II antibodies in an acute-phase serum sample that did not progress to a serological profile with any of the other three antibodies (IgG-II, IgM-I, or IgG-I) (Schneeberger et al. 2010). Moreover, the results of PCR for these samples were negative. Similar results were obtained in our investigation. The 71 blood samples from humans from Chodzież were tested twice by PCR. The first test detected the presence of the IS1111 gene specific for C. burnetii in 10 tested samples, but the second investigation after 3 weeks did not confirm these results. According to the literature data and our results, C. burnetii DNA is undetectable in blood or serum as the serological response develops (Schneeberger et al. 2010).
Recent papers (Van der Hoek et al. 2011, Wegdam-Blans et al. 2012a) define the chronic form of Q fever as the presence of least two of the following three criteria: (1) IgG phase I antibody titer ≥1:1024; (2) positive PCR results 3 or more months after the acute episode; and (3) clinical or radiological signs. On the other hand, Raoult (2012) proposed that definition of chronic Q fever should be avoided and suggest a new score-based diagnosis for Q fever endocarditis and vascular infection. In our studies, only in two patients from Dębno infection could be classified as a probable chronic Q fever. In a group of humans from Chodzież, the presence of DNA C. burnetii was detected, but clinical signs were not observed and the titers of IgG phase I antibodies in IFA were ≤1:1024, thus we cannot conclude that it could be a chronic form of disease. The most positive group was from Dębno, which was confirmed by results of statistical analysis showing that there are statistically significant differences between people from Dębno and other localizations. It should be noted that only in this group was the probable chronic form of Q fever with clinical symptoms observed. The differences statistically significant noted only for IFA results could be considered as evidence that IFA is a gold standard in serological diagnosis of Q fever in humans and gives the most reliable results. A relationship between results obtained for different genders was not observed. One significant difference was noted (p=0.04) for ELISA results (IgG phase II) between ages 45 and 48, but on the basis of this one difference is impossible to state that the age can be related with prevalence of C. burnetii infection in tested humans.
In Poland, both human and animal cases of Q fever are notifiable. Cases of Q fever in animals are confirmed by the National Reference Laboratories for Q fever. The information on animal cases is sent by the regional state veterinary officer to the National Sanitary Inspectorate. Elimination of the source of infection is achieved through established cooperation between veterinary and health services. Moreover, there is a monitoring program for Q fever in Poland for cattle and small ruminants.
Outbreaks of Q fever in both humans and animals have been noted in Poland since 1956 (Lutyński 1956). The largest epidemic of Q fever among humans and animals was recognized near Zamość (in the Lublin voivodeship in eastern Poland) in 1983 (Cygan et al. 1983, Mikołajczyk et al. 1986). More than 1300 people fell ill in this epidemic centered around the area of Hrubieszów and Tomaszów Lubelski. Until 2007 when the large Q fever epidemic in The Netherlands broke out, it had been considered the biggest Q fever epidemic in humans in the world. According to literature data from this time, anti–C. burnetii antibodies were found in cattle from this area (Cisak et al. 2003, Galińska et al. 2011, Niemczuk et al. 2011). Moreover, the serological studies performed by Cisak et al. (2003) among the farmers living in villages located in the Lublin voivodeship showed the presence of specific antibodies to C. burnetii in 17.8% of 90 tested subjects for comparison in our studies; the percentage of seroprevalence in the tested farming population was higher in IFA (31.12%) and ELISA (39.07%).
Generally, the small number of human cases recognized in Poland in comparison with the other European countries may indicate that in this country the disease is underdiagnosed and morbidity is underestimated. For comparison, in France 1326 acute cases and 1083 chronic cases of Q fever were reported between 1985 and 2009 (Frankel et al. 2011). Outbreaks of Q fever are also noted in neighboring countries (Germany, Ukraine, the Czech Republic and the Slovak Republic) (Literak 1995, Maksimovich et al. 1995, Literak and Rehacek 1996, Hilbert et al. 2011).
Conclusions
The results of the present study, obtained with the use of serological methods and real-time PCR, seem to indicate that C. burnetii is present in the farm workforce in Poland, including in the southeastern region where Q fever is considered endemic. Moreover, outbreaks of Q fever are present in other parts of the country (Chodzież and Gliwice). The endemic region should be the location of further studies in humans, animals, and the vectors of C. burnetii. Since 2013, vaccination of cattle and sheep has been available in Poland, and it can reduce the number of both animal and human cases of Q fever. On the other hand, the vaccine for humans is not available for humans in Poland, but taking into account the increasing percentage of infected ruminants, the Sanitary Inspection should consider the possibility of its introduction, particularly for humans who are exposed to domestic ruminants. Additionally, on the basis of this data and previously published data, testing of persons in contact occupations around cattle or small ruminants for the presence of C. burnetii infection should be considered. However, the most important element is collaboration between veterinary and sanitary services aimed at detection and elimination the sources of infection both in humans and animals.
Acknowledgment
The study was supported by the Ministry of Science and Higher Education of the Republic of Poland from resources for science in the years 2009–2013 under Commissioned Research Project no. N N 404 204336.
Author Disclosure Statement
No competing financial interests exist.
References
- Brom RV, Schimmer B, Swart WA, Hoek W. Seroepidemiological survey for Coxiella burnetii antibodies and associated risk factors in Dutch livestock veterinarians. PLoS One 2013; 8:e54021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisak E, Chmielewska-Badora J, Mackiewicz B, Dutkiewicz J. Prevalence of antibodies to Coxiella burnetii among farming population in eastern Poland. Ann Agric Environ Med 2003; 10:265–267 [PubMed] [Google Scholar]
- Cygan Z, Buczek J, Modzelewska A, Guzik Z. Ognisko gorączki Q rozpoznane serologicznie w stadzie krów mlecznych. Med Weter 1983; 9:536–538 [Google Scholar]
- Frankel D, Richtel H, Renvoisé A, Raoult D. Q fever in France, 1985–2009. Emerg Infect Dis 2011; 17:350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galińska EM, Knap JP, Chmielewska-Badora J, Wstępne wyniki badań seroepidemiologicznych i klinicznych w kierunku gorączki Q u osób zawodowo narażonych. Med Ogól 2011; 17:001–006 [Google Scholar]
- Georgiev M, Afonso A, Neubauer H, Needham H, et al. Q fever in humans and farm animals in four European countries, 1982 to 2010. Eurosurveillance 2013; 18:1–13 [PubMed] [Google Scholar]
- Gilsdorf A, Kroh C, Grimm S, Jensen , et al. Large Q fever outbreak due to sheep farming near residential areas, Germany, 2005. Epidemiol Infect 2008; 136:1084–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herremans T, Hogema BM, Nabuurs M, Peeters M, et al. Comparison of performance of IFA, CFA and ELISA assys for the serodiagnosis of acute Q fever by quality assessment. Diagn Micr Infec Dis 2013; 75:16–21 [DOI] [PubMed] [Google Scholar]
- Hilbert A, Reih P, Brockmann SO, Tyczka J, et al. Epidemiological enquires in two Q fever outbreaks in a community of Baden-Württemberg during 2008 and 2009. Berliner und Münchener Tierärztliche Wochenschrift 2011; 124:295–302 [PubMed] [Google Scholar]
- Literak I. Occurrence of Coxiella burnetii in cattle, sheep and small terrestrial mammals in the western region of Bohemia. Vet Med (Praha) 1995; 40:77–80 [PubMed] [Google Scholar]
- Literak I, Rehacek J. Q fever occurrence and significance of this disease in the Czech Republic and Slovak Republic. Vet Med (Praha) 1996; 41:45–63 [PubMed] [Google Scholar]
- Lutyński R. First focus of Q fever on the territory of Poland. Przegląd Lekarski 1956; 12:187–188 [Google Scholar]
- Maksimovich MB, Kushnir ZG, Zastavnyi IV, et al. Q fever in the western region of Ukraine. Zh Mikrobiol Epidemiol Immunobiol 1995; 1:30–33 [PubMed] [Google Scholar]
- Maurin M, Raoult D. Q fever. Clin Microbiol Rev 1999; 12:518–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikołajczyk EZ, Lewińska R, Lojewska R, Rumin W, et al. Serologic reaction in humans during the outbreaks of Q fever. Przegląd Epidemiologiczny 1986; 40:342–348 [PubMed] [Google Scholar]
- Niemczuk K, Szymańska-Czerwińska M, Zarzecka A, Konarska H. Q fever in a cattle herd and humans in the south-eastern Poland. Laboratory diagnosis of the diseases using serological and molecular methods. Bull Vet Inst Puławy 2011; 55:593–598 [Google Scholar]
- Raoult D. Chronic Q fever. J Infect 2012; 65:102–108 [DOI] [PubMed] [Google Scholar]
- Schneeberger PM, Hermans MH, Hannen EJ, Schellekens JJA, Leenders ACAP. Real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever. Clin Vaccine Immunol 2010; 17:286–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymańska-Czerwińska M, Galińska EM, Niemczuk K, Zasępa M, et al. Prevalence of Coxiella burnetii infection in foresters and ticks in the south-eastern Poland and comparison of diagnostics methods. 2013. Ann Agric Environ Med 2013; 20:699–704 [PubMed] [Google Scholar]
- Szymańska-Czerwińska M, Niemczuk K, Mitura A. Prevalence of Coxiella burnetii in dairy herds—diagnostic methods and risk to humans—a review. Bull Vet Inst Pulawy 2014; 58:337–340 [Google Scholar]
- Van der Hoek W, Versteeg B, Meekelenkamp JCE, Renders NHM, et al. Follow-up of 686 patients with acute Q fever and detection of chronic infection CID 2011; 52:1431–1436 [DOI] [PubMed] [Google Scholar]
- Wegdam-Blans MCA, Kampschreur LM, Delsing CE, Bleeker-Rovers CP. Chronic Q fever: Review of the literature and a proposal of new diagnostic criteria. J Infect 2012a; 64:247–259 [DOI] [PubMed] [Google Scholar]
- Wegdam-Blans MCA, Wielders CCH, Meekelenkamp J, Korbeeck JM, et al. Evaluation of commonly used serological tests for detection of Coxiella burnetii antibodies in well-defined acute and follow-up sera. Clin Vaccine Immunol 2012b; 19:1110–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegdan-Blans MCA, Tjhie HT, Korbeeck JM, Nabuurs-Franssen , et al. Serology in chronic Q fever is still surrounded by question marks. Eur J Clin Microbiol Infect Dis 2014; 33:1089–1090 [DOI] [PubMed] [Google Scholar]