Abstract
Introduction
Sensibility testing plays a role in the diagnosis of carpal tunnel syndrome (CTS). No single physical test has proven to be of critical value in the diagnosis especially when compared to electrodiagnostic studies (EDX). Correlations between individual tests and both symptoms and EDX have been elusive. Notably, previous literature has not documented differences between individual digits when examined with standard diagnostic tests and there is no suggestion that any digit should be systematically excluded from evaluation in CTS.
Methods
A prospective series of patients with EDX positive, isolated CTS patients were tested immediately preoperatively to evaluate individual digits with static two point discrimination (2PD) and abbreviated Semmes-Weinstein Monofilament (SWMF) tests. Detailed surveys of symptom density in the entire upper extremity were collected in addition to subjective perceptions of the most affected digit.
Results
Patients favored the middle finger over all others (51%) when asked which was the most affected by CTS. Objective 2PD results of each digit mirrored the subjective data, with higher values for the middle (mean 6.07mm, P<.0001). Values for the index failed to show a significant difference from the ulnar-innervated small. Subjective worst finger matched the worst digit 2PD in over two-thirds of patients. SWMF testing showed similar, statistically significant results (middle>thumb>index>small) but suffered from lack of continuously assessable sensibility. Correlations failed between EDX, symptoms, complaints, monofilament results or 2PD in the index. Positive correlation (P=.002, r=.42, weak) was found between EDX and 2PD of the middle in isolation.
Conclusions
The middle finger is the most likely to show changes in 2PD in patients with positive EDX findings. Middle finger 2PD is best able to correlate (weakly) with EDX when compared to 2PD of other fingers and SWMF testing. Monfilaments alone are capable of showing the middle as more sensitive but application of this result is hampered by unavailable monofilaments.
Introduction
Carpal tunnel syndrome is the most prevalent compression neuropathy and most commonly affects the radial three fingers of the hand (2, 3). The evaluation of patients with carpal tunnel syndrome (CTS) can include a vast array of physical tests (1, 2). Such signs elicited in the examination of CTS patients are often not perfectly sensitive or specific for the presence of compression of the median nerve (2). There is often little agreement between surgeons as to how to apply any given test.
Specifically, no test has been evaluated in individual digits to see if certain digits are better candidates for testing than others. It would stand to reason that, if present, systematic differences in sensibility between digits of the hand could play a role in altering the value of physical tests for CTS. Of the median innervated fingers of the hand, the use of digits less likely to show sensibility changes may have contributed to poor results and correlations in the evaluation of these tests (2, 4).
Two examples of tests which are used in the routine evaluation of CTS patients are static two point discrimination and Semmes-Weinstein Monofilament tests. Although these tests have been documented in the literature, no mention of the optimal digits for these tests has been made. We selected these tests to evaluate individual digits of patients with known CTS to note the presence or absence of systematic differences between the digits. We further correlated the results of these tests in individual fingers with EDX results.
Methods
Study patients were selected based on their diagnosis of carpal tunnel syndrome. Diagnostic evaluation included electrodiagnostic studies (EDX) with positive results in all cases. Thirty five patients (forty hands) were included in this study.
All patients underwent routine evaluation by a hand surgeon prior to inclusion into the study. All aspects of that evaluation, including the examination, the decision to pursue electrical studies and the decision to pursue surgery were independent of the data collection process. Inclusion criteria included EDX evidence of CTS in the absence of any other diagnosis. No patient with a competing electrical diagnosis (cervical radiculopathy, cubital tunnel syndrome, diffuse polyneuropathy) was encountered. No patient was encountered with a negative electrodiagnostic study.
Once selected for inclusion in the study, a separate evaluation of each patient was performed by a single independent hand surgeon blind to the extent of nerve compression present (other than the decision to pursue surgery) and having played no role prior to the day of surgery. This evaluation, performed on the day of surgery in the preoperative holding area, included sensibility testing in the form of static two point discrimination (2PD) and Semmes-Weinstein monofilament array (SWMF). Testing was completed in every finger but the ring finger in every operative hand.
Two point discrimination was assessed with a MacKinnon-Dellon Discriminator and was recorded as the smallest reliably detectable distance at which two points could be distinguished. Each assessment of each distance was termed a “trial”. Trials started with pressing discriminator into the skin in line with the path of a single digital nerve approximately five millimeters distal to the distal digital crease. Patients were coached to make a choice between “one” and “two” within one second of trial initiation (two alternative forced choices). A trial was disqualified if the patient: failed to answer in a timely fashion, was insensate to the discriminator, required excessive pressure/depth of the discriminator (more than three millimeters or blanching skin halo >3mm), indicated pain either verbally or by wincing. Correct trials were those where the above exclusion criteria were not met and the correct answer was encountered. Three correct trials at a given level combined with two incorrect assessments at the next smaller distance constituted a correct measurement of two point discrimination for a digit. Patients with unreliable responses or repetitive strings of answers (e.g. “one”, “two”, “one”, “two”, etc) were given a training period where visual input was allowed. Patients who failed to give reliable responses after two such training periods were excluded from the study (two patients).
Semmes-Weinstein Monofilament array testing was undertaken using the abbreviated array containing of six monofilaments (2.83, 3.31, 4.31, 4.56, and 6.65). Individual trials followed a similar structure as above consisting of a single contact of a monofilament with the finger tip skin pressured to a bend in the filament. Patients were not allowed to move their finger during trials and the monofilament was not struck against the finger in rapid succession. As with two-point discrimination, three correct trials at a given level combined with two incorrect assessments at the level below constituting a complete measurement.
For both tests, patients received no feedback regarding their performance during testing and were reassured only at the end of all objective testing.
Patients were also asked to choose a single most affected finger with regards to their condition. Patients answering two fingers were encouraged to chose a single most affected finger and were allowed to self assess their hands while answering this question. Additionally, patients answered questions about hand dominance and their most affected hand. Patients undergoing bilateral release were assessed twice (once for each hand).
Each patient was asked specifically about the presence of particular symptoms (pain, numbness, tingling, or strange sensations including burning and electrical shocks) in particular zones in the upper extremity. Zones were defined for the radial three fingers of the hand including the palm, the small finger with hypothenar eminence, anterior forearm, anterior arm, and dorsal arm with forearm. Each patient described the presence of absence of each symptom in each zone. Results were collected graphically in maps which were used for correlation analysis with other parameters of patient evaluation including objective 2PD and SWMF results in each finger in addition to electrodiagnostic study results.
Electrodiagnostic studies were reviewed for each patient. All studies were subjected to a grading system for severity. Patients with slowing of conduction (either sensory or motor) were grade 1 whereas slowing of both fiber types was assigned grade 2. Findings of motor weakness of any muscle in the presence of slowed conduction represented grade 3. Evidence of diffuse, but incomplete denervation was assigned to grade 4 and complete absence of response was given a grade of five. No patient was encountered with a normal electrodiagnostic examination.
Statistical analyses were performed using the standard functions available in Microsoft Excel (Redmond WA) software. Analyses were chosen based on the appropriateness of the data distribution (normally distributed vs. skewed) as well as the appropriateness of the given test for the hypothesis at hand. Data was found to be normally distributed across groups including 2PD, SWMF, and EDX findings. Comparisons of multiple groups were performed with ANOVA algorithms including post hoc analysis and pair wise comparisons. All statistically significant differences were also confirmed between individual groups with Student’s T test comparisons. Power analysis revealed greater than 80% was achieved for all statistically significant differences. This power analysis was used to determine the minimum number of patients to be included in the study for a given detectable difference in 2PD and SWMF array testing between fingers.
Results
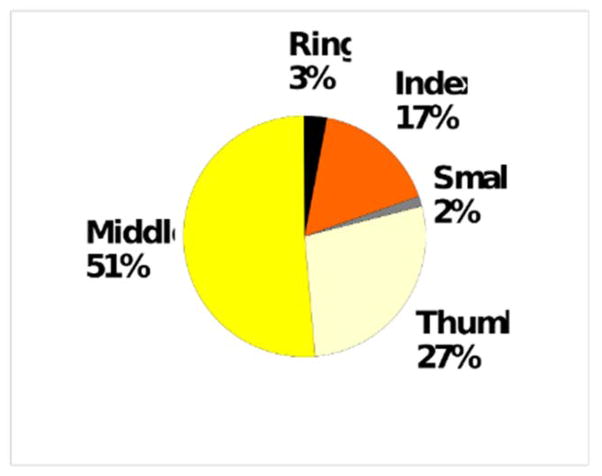
Subjective results of asking patients to identify their single most symptomatic digit revealed that patients clearly favored the middle finger over all others (Figure 1). They least often chose the small finger, and of the radial digits, the index was chosen least.
Figure 1.
Subjective response to most symptomatic digit. (n=40 hands)
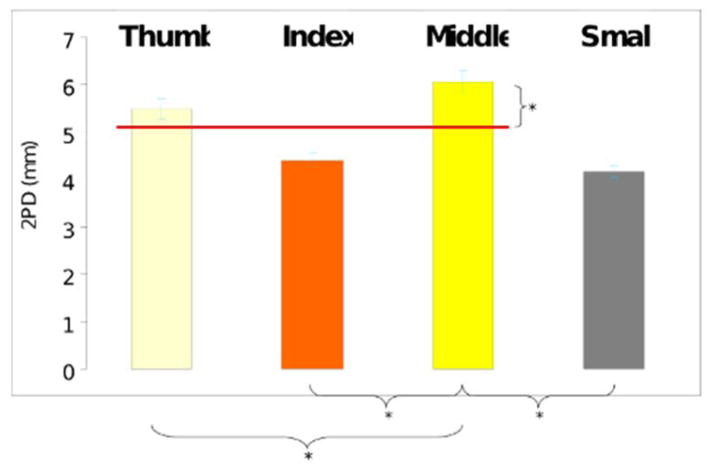
Objective 2PD results of each finger revealed the middle finger as more affected, with higher values for 2PD than found in the other digits (Figure 2). This difference was statistically significant when compared to any other tested finger. By comparison, the results for the index finger failed to be significantly different, on average, from the ulnar innervated small finger.
Figure 2.
Results of two-point discrimination by digit. Higher values represent greater 2PD measurement. Red line drawn at the average value of median innervated digits. Significant comparisons depicted below.
Data from individual fingers were also compared to the mean of 2PD found in the radial three fingers (thumb, index, middle). This mean was chosen as a possible undocumented standard for studies utilizing two point discrimination in the literature – as few studies document which of the median nerve innervated fingers is chosen when assessing 2PD. In this setting, the mean 2PD of median innervated digits represents an impartial measure which suffers from any less affected digit (e.g. index). When directly compared, the results for 2PD outperformed those for the average of the median innervated digits (thumb, index, and middle).
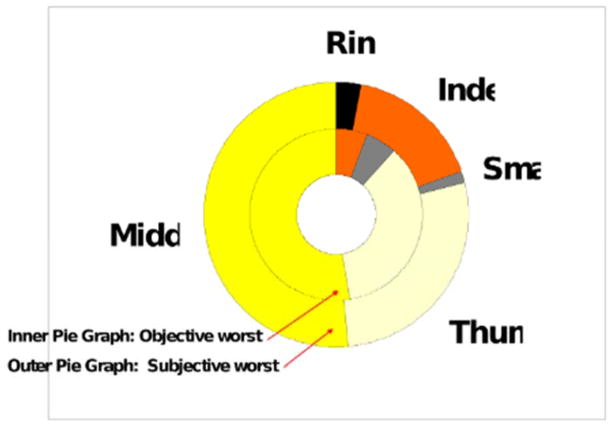
Data from the subjective patient’s assessment of their most affected finger and objective 2PD measurements in each finger were compared in the aggregate (Figure 3). The ratio of subjective reports reflected the most affected finger by 2PD despite the omission of the dual innervated ring finger in the objectively assessed group. Patients are correct in their assessment over two thirds of the time and most often strayed from the correct prediction when they chose a finger other than the middle finger (80% correct prediction when middle finger is chosen).
Figure 3.
Relationship between subjective and objective worst finger by 2PD. Comparison of subjective and objective (2PD) results for all patients.
Analysis of data collected using the abbreviated SWMF array was also performed (Figure 4). Each finger (except for ring) was tested with each monofilament and the smallest detectable monofilament was recorded (see methods). Data were then grouped by finger and averaged to reveal the average value for each digit across all patients. The value obtained for each digit corresponded to a theoretical monofilament strength which would be best suited to test that digit at its average sensibility in all patients. The monofilament, if it existed, would be detectable in 50% of that specific digit and undetectable in the rest.
Figure 4.
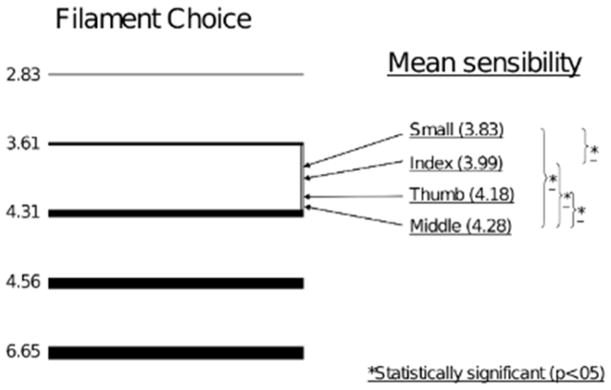
Position of optimal filament for testing of each digit on scale of SWMF (abbreviated) array. Left column: cartoon depicting available monofilaments. Right column: position of theoretical optimal filament strength to test each digit.
Our analysis revealed statistically significant differences in the theoretical strength of monofilaments best suited for each digit. Specifically, the best suited monofilament for one digit in our series was significantly different in theoretical caliber than the filament best suited for other tested digits. Such an analysis reveals that the most stout, digit-specific, monofilament would correspond to the middle finger – owing to the statistically significant sensibility loss present in middle fingers demonstrated with this test (monofilament strength 4.28, p<.0001). Although SWMF array testing was able to show a difference between the index and small finger (p=.04), the difference was smaller than the reliable resolution of any two filaments in any available testing array (optimal monofilament strength for index =3.99, small= 4.28, p=.04).
Regression analysis was performed to attempt to identify correlations between objective data from each finger and common parameters encountered in the evaluation of patients with carpal tunnel syndrome including electrodiagnostic study results (EDX). No correlation could be found between any combination of EDX results, symptoms, or patient complaints. Objective assessment of the middle finger using 2PD revealed the only positive, albeit weak correlation with EDX result grade (r=.42, r2=.18, p=.0003). Results from all other fingers failed to show any correlation with EDX results (p>.05).
Discussion
The tests available for the diagnosis of carpal tunnel syndrome have been extensively studied (2). Although the parameters, techniques, and utility of signs for CTS are known, little is known about the relative effect on these parameters of testing particular digits. The fact that patients with CTS often present with a variety of symptoms in the upper extremity may suggest that narrowing the use of any given test may increase the specificity of diagnosis (4).
When evaluating CTS patients objectively, we chose 2PD and SWMF testing because they represent two commonly used tests (2). Techniques for performing these tests have been described extensively (2, 5). However, there is a paucity of literature to suggest that these tests are either exceedingly sensitive or specific for the presence of CTS (7, 8). One key reason for this may be that studies which utilize these tests often fail to standardize the exact conditions and nature of the examination including the particular digit assessed.
Our data suggest that, when forced to choose, patients are able to single out one digit which is more symptomatic than the others. Also, as the testing was done on the day of and prior to surgery we felt we were able to obtain honest, unbiased answers which are relevant when dealing with worker’s compensation patients. This subjective evaluation revealed, on average, the middle finger, and to a slightly lesser extent the thumb as more symptomatic digits than the others in the hand.
Objective evaluation of the patient’s individual digits revealed small but definite differences in the results of objective tests when performed on each digit separately. In particular evaluation of 2PD and SWMF sensibility in each finger clearly supports the notion that fingers are affected differentially by CTS with the middle finger suffering the most severe sensibility loss. Strikingly, there is no significant objective difference in sensibility between the median innervated index finger and the ulnar innervated small finger with 2PD. With SWMF, the difference between index and small digits, though statistically significant, was small and only evident in the average of all patients. Taken together, the data available from both these tests reveal differences which favor the middle digit as the best choice if only one digit is to be tested. They also suggest that inclusion of the index digit in the evaluation may blur distinctions made more evident by evaluation of the middle digit alone.
When objective and subjective data are compared, a relationship was evident. Patient perceptions of the most symptomatic finger coincided with objective 2PD. Interestingly, the discrepancies in this relationship most often occurred when the patient’s most symptomatic digit was not the middle digit. If every patient in our study chose the middle finger as their most affected digit, then over 80% of patients would have chosen their most affected digit with 2PD.
If all studies utilized a strategy of measuring the index finger for the diagnosis of carpal tunnel syndrome, then our data suggests that the test would be little better than testing the small finger – a presumably uninvolved digit in CTS. This fact alone suggests that the index finger should be excluded from evaluation. Any other digit in the hand may offer diagnostic value which is diluted, or missed by the inclusion or selection of the index finger in the evaluation. Selecting out the digit which is most likely to show a loss of sensibility would be a better strategy for evaluation of carpal tunnel syndrome patients with known disease. Our data suggests that the middle finger is, on average, a better choice for sensibility testing if the goal is to detect the loss in sensibility.
There are perhaps some reasons why the middle finger might be more anatomically predisposed to sensibility loss in the setting of carpal tunnel syndrome. Fibers destined for the middle finger tip must travel a farther distance than those destined for other median innervated fingers – perhaps rendering them more susceptible to compressive pathology. Although anatomic studies suggest that the motor fibers lie directly beneath the transverse carpal ligament and on the radial aspect of the median nerve at the carpal tunnel (10), they often branch prior to the distal end of the transverse carpal ligament and have occupied a more radial position by the distal end of the carpal tunnel (9,10). The theoretical consequence of this is that sensory fibers be preferentially affected by compression at the distal end of the transverse carpal ligament. Given the functional anatomy of the median nerve at the carpal tunnel, it is unlikely long digit sensory fibers are more pressured by simple proximity to the transverse carpal ligament itself. These explanations notwithstanding, there is no available data to rule out the possibility that some fibers in a mixed nerve may be inherently more susceptible to pressure than others. Thus, fibers destined for the middle finger may have a susceptibility unrelated to their position relative to the ligament or distance from their target.
Signs and symptoms of carpal tunnel syndrome have been extensively characterized in the literature (4, 6). The combination of a thorough history and physical examination along with electrodiagnostic studies can be used to diagnose carpal tunnel syndrome (1, 5). The postoperative relief of symptoms can provide a gold standard for the presence or absence of pre-operative carpal tunnel disease. The evaluation of such patients has shed allowed determination of the sensitivity and specificity of many known diagnostic tests; typical studies compare tests to EDX (7, 8). It remains unclear why many of the elements of the preoperative evaluation of carpal tunnel syndrome patients remain uncorrelated with each other.
A notable weakness of our study is that we have not, and can not make any calculations of sensitivity and specificity of testing any of the digits for the presence or absence of carpal tunnel syndrome. Such an analysis would likely require more patients who represent a truly wide distribution of disease involvement from normal to severely affected. This is necessary because such an analysis requires true negatives (patients without the disease who fail to show sensory loss in the middle finger) and false positives (patients without the disease who indeed do show sensory loss in the middle finger). Such patients, if they exist, would not be uniformly treated with carpal tunnel release as in our series. They would also not be subjected to the gold standard test of surgery as some may not have the disease. Additionally, EDX would be required in each patient to define true positives and negatives. It is notable that, although the results of middle-finger 2PD correlate with EDX results, the correlation is weak – and so too must the validity of any finger-specific sensitivity and specificity measurements based on EDX as a gold-standard.
Particularly interesting is the lack of correlation in the literature between the results of electrodiagnostic studies and other pre-operative diagnostic tests for CTS (7, 11). Indeed the value of any given test can be linked to how well the results of that test correlate with EDX results as many surgeons use EDX as a critical part of the preoperative evaluation of carpal tunnel syndrome patients. EDX has become so important, in part because many of the available physical tests for CTS are strikingly not correlated to post operative relief.
References
- 1.D’Arcy CA, McGee S. The rational clinical examination. Does this patient have carpal tunnel syndrome? JAMA. 2000;283:3110–7. doi: 10.1001/jama.283.23.3110. [DOI] [PubMed] [Google Scholar]
- 2.Palumbo CF, Szabo RM. Examination of patients for carpal tunnel syndrome sensibility, provocative, and motor testing. Hand Clin. 2002;18:269–77. doi: 10.1016/s0749-0712(01)00007-5. [DOI] [PubMed] [Google Scholar]
- 3.Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosén I. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282:153–8. doi: 10.1001/jama.282.2.153. [DOI] [PubMed] [Google Scholar]
- 4.Stevens JC, Smith BE, Weaver AL, Bosch EP, Deen HG, Jr, Wilkens JA. Symptoms of 100 patients with electromyographically verified carpal tunnel syndrome. Muscle Nerve. 1999;22:1448–56. doi: 10.1002/(sici)1097-4598(199910)22:10<1448::aid-mus17>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 5.Gupta SK, Benstead TJ. Symptoms experienced by patients with carpal tunnel syndrome. Can J Neurol Sci. 1997;24:338–42. doi: 10.1017/s0317167100033023. [DOI] [PubMed] [Google Scholar]
- 6.Nora DB, Becker J, Ehlers JA, Gomes I. Clinical features of 1039 patients with neurophysiological diagnosis of carpal tunnel syndrome. Clin Neurol Neurosurg. 2004;107:64–9. doi: 10.1016/j.clineuro.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Massy-Westropp N, Grimmer K, Bain G. A systematic review of the clinical diagnostic tests for carpal tunnel syndrome. J Hand Surg [Am] 2000 Jan;25(1):120–7. doi: 10.1053/jhsu.2000.jhsu025a0120. [DOI] [PubMed] [Google Scholar]
- 8.Buch-Jaeger N, Foucher G. Correlation of clinical signs with nerve conduction tests in the diagnosis of carpal tunnel syndrome. J Hand Surg. 1994;19B:720–724. doi: 10.1016/0266-7681(94)90244-5. [DOI] [PubMed] [Google Scholar]
- 9.Lanz U. Anatomical variations of the median nerve in the carpal tunnel. J Hand Surg [Am] 1977 Jan;2(1):44–53. doi: 10.1016/s0363-5023(77)80009-9. [DOI] [PubMed] [Google Scholar]
- 10.Mackinnon SE, Dellon AL. Anatomic investigations of nerves at the wrist: I. Orientation of the motor fascicle of the median nerve in the carpal tunnel. Ann Plast Surg. 1988 Jul;21(1):32–5. doi: 10.1097/00000637-198807000-00006. Review. [DOI] [PubMed] [Google Scholar]
- 11.The American Academy of Electrodiagnostic Medicine The American Academy of Neurology, The American Academy of Physical Medicine and Rehabilitation. Practice parameter for electrodiagnosis in carpal tunnel syndrome: summary statement. Muscle Nerve. 1993;16:1390–1391. doi: 10.1002/mus.880161219. [DOI] [PubMed] [Google Scholar]