Abstract
Measurement of C-reactive protein, a marker of inflammation, in dried blood spots has been increasingly incorporated in community-based social surveys internationally. Although the dried blood spot based CRP assay protocol has been validated in the United States, it remains unclear whether laboratories in other less developed countries can generate C-reactive protein results of similar quality. We therefore conducted external quality monitoring for dried blood spot based C-reactive protein measurement for the Indonesia Family Life Survey and the Longitudinal Aging Study in India. Our results show that dried blood spot based C-reactive protein results in these two countries have excellent and consistent correlations with serum-based values and dried blood spot based results from the reference laboratory in the United States. Even though the results from duplicate samples may have fluctuations in absolute values over time, the relative order of C-reactive protein levels remains similar and the estimates are reasonably precise for population-based studies that investigate the association between socioeconomic factors and health.
Introduction
Social, economic, and psychological factors are known determinants of health (Marmot et al. 2012, Kawachi and Berkman 2001). In the last decade, an increasing number of community-based social surveys have included molecular biomarkers, either as objective measurements of health or as part of the effort to understand the biological pathways through which socioeconomic factors influence population health. One of the biomarkers that has been tested for in many surveys is C-reactive protein (CRP), an acute phase protein produced by the liver and a commonly-used marker of general systemic inflammation (Ridker et al. 1997).
Inflammation plays an important role in the process of aging and age-related diseases (Singh and Newman 2011). Chronic inflammation, often indicated by persistently elevated CRP level, may lead to more cardiovascular events, increased risk of diabetes mellitus, functional impairment, frailty, and higher mortality in older adults (Ridker et al. 1997; Lindahl et al. 2000; Figaro et al. 2006; Pradhan et al. 2001; Kuo et al. 2006; Garrod et al. 2007; Fulop et al. 2010). Measurement of CRP in community settings has been greatly facilitated by the development of a high sensitivity CRP (hsCRP) assay for dried blood spot (DBS) specimens (McDade, Burshop, and Dohnal 2004). Compared to venous samples, DBS are easier to collect, and do not need to be processed and frozen immediately. Consequently, the DBS-based CRP assay has been incorporated into a number of major studies in the United States, such as the Health and Retirement Study (HRS) and the National Social Life, Health, and Aging Study (NSHAP), and is also being adopted by other HRS-type studies internationally.
Although the DBS-based CRP assay protocol has been validated in the United States, it remains unclear whether laboratories in less developed countries can generate CRP results of similar quality. Potential errors and between-laboratory variation in assay results may result from inadequate training of the laboratory personnel on the assay protocol, variation in the types of equipment used for the assay, and the quality of test reagents and other supplies. Assay quality control is important everywhere, but many countries may make comparisons difficult because of rules and regulations that restrict the shipment of biological specimens out of the country. In this paper, we report our experience in external quality control for DBS-based CRP assay in Indonesia and India, where specimens collected as part of studies cannot generally leave the country. This ability to accurately measure CRP levels on DBS specimens and generate external quality control data in developing countries is essential for harmonizing bioassay data from these two population-level studies with similar studies in other countries. It also has the potential to provide valuable information on natural variation in CRP in response to lifestyle factors and infectious disease burden (Mcdade et al. 2012)
Methods
Description of the studies
The Indonesia Family Life Survey (IFLS) is an on-going longitudinal study of economic and social behaviors, and health. Detailed descriptions of the study, documentation, and data for public use are available on the IFLS homepage (http://www.rand.org/FLS/IFLS). Briefly, this panel survey collects individual and household level information on household consumption; assets; income; work and retirement experiences; intergenerational transfers of time, goods, and money; living arrangements; individual health measures; and health care utilization. It also collects community data, including health facilities serving the local elderly population over time. The fieldwork of the fourth wave of ILFS (IFLS4) took place between November of 2007 and July of 2008, approximately 15 years after its first wave in 1993. IFLS4 collected DBS specimens during the interviews, which were assayed for CRP in 2012.
The Longitudinal Aging Study in India (LASI) is a panel survey representative of persons at least 45 years of age in India. The survey instrument has been designed to collect information that is conceptually comparable to the HRS, and includes variables on demographics, family structure and social network, housing and environment, health and health behaviors, health care utilization, work and pension, income, assets, debts, and consumption. The LASI pilot study, which was fielded in 2010, also measured anthropometric parameters and physical performance, and collected DBS specimens (https://mmicdata.rand.org/meta/?section=study&studyid=36). The DBS-based bioassays, including CRP, hemoglobin, and Epstein-Barr virus (EBV) antibody titer, were conducted from October of 2012 to April of 2013.
Preparation for DBS-based CRP assay
For the DBS-based CRP assay, the IFLS collaborated with the laboratory at the Clinical Pathology Department at Gadjah Mada University in Yogyakarta, Indonesia. The LASI’s collaborating institution was the National AIDS Research Institute (NARI) in Pune, India. These two institutions are part of the laboratory network that also performs DBS-based assays for the World Health Organization (WHO)’s Study on Global AGEing and Adult Health (SAGE) project. As a result, the personnel from both laboratories were trained for DBS-based assays at NARI in March of 2011. This training course, organized by the SAGE and conducted by Dr. Sharon Williams of Purdue University, lasted for five days and provided training on four assays: CRP, hemoglobin, EBV antibody titer, and glycosylated hemoglobin (Hba1c).
For the CRP assay, both IFLS and LASI modified the ELISA-based protocol, which was originally developed and validated by Thomas McDade (McDade, Burshop, and Dohnal 2004), by substituting Dako CRP polycolonal antibodies with antibodies manufactured by Meridian Life Science (https://meridianlifescience.com/). This high-sensitivity assay protocol requires individual ordering of components reagents as well as the creation of DBS-based CRP standards. The lower limit of detection is 0.148 mg/L. In Indonesia, some of the reagents were not available locally. Consequently, IFLS received assistance from the WHO (SAGE project) to purchase and ship these reagents into Indonesia, under shipping conditions specified by the manufacturers. For LASI, all assay reagents were purchased through local vendors.
Pre-test for CRP assay
Before testing study specimens, a pre-test for the DBS-based CRP assay was conducted at each laboratory to satisfy several aims: 1) to evaluate the technical skills of trained laboratory personnel, including transfer of knowledge and skills to those technicians in these laboratories who did not participate in the original training organized by WHO; 2) to verify that correct equipment was being used for the planned assays; 3) to verify that correct test reagents and supplies were being used; 4) to evaluate general conditions of the laboratory (e.g., adequate work space, proper temperature control); and 5) to evaluate the reliability and validity of the assay results, using both study samples and samples for external quality control.
The USC/UCLA Center on Biodemography and Population Health (CBPH) prepared quality control samples for both IFLS and LASI. Researchers at the Biodemography Center collected venous and DBS specimens from 67 volunteers for the IFLS pre-test. Serum samples were sent to the laboratory at the Department of Laboratory Medicine, University of Washington (UW), for a “gold standard” hsCRP assay (laboratory director: Alan Potter, PhD). Two DBS cards were also collected from each volunteer via direct finger prick, with two blood spots on each card. One set of DBS cards was sent to UW for DBS-based hsCRP assay, which uses reagents from Percipio Biosciences, (http://www.percipiobio.com/product_details.php?ProductSelector=11190), while the other set was sent to the laboratory at Gadjah Mada University in Indonesia. Quality control samples for both laboratories were shipped on dry ice. Duration of time for shipment to Indonesia was approximately 48 hours.
For the LASI pre-test, researchers at the Biodemography Center collected venous specimens from 50 volunteers. Based on the previous validation work done by the Center, the DBS cards were created from venous blood, with five blood spots on each card. Serum samples were sent to the UCLA Clinical Laboratory for hsCRP assay, using Siemens Vista platform. One set of the DBS cards was sent to UW for DBS-based hsCRP assay, the same reference laboratory used for IFLS. Two sets of DBS cards were sent to the NARI in Pune, India through World Courier, a commercial shipping company. A temperature monitor was included in the package containing the DBS quality control samples, which recorded the temperature inside the shipping box once every two hours. The subsequent analysis of the data from the temperature monitor showed that the duration of shipment (including customs clearance) was approximately 162 hours and temperature was maintained at less than −40°C during the shipment from Los Angeles to Pune, India.
The pre-tests at the IFLS and LASI laboratories were conducted in December of 2011 and August of 2012, respectively. Both pre-tests lasted for five days. The detailed schedule for each pre-test is shown in Table 1. Because LASI had more blood spot material than IFLS, it measured more quality control samples during the course of the pre-test.
Table 1.
C-reactive protein (CRP) pre-test schedule for the Indonesia Family Life Survey (IFLS) and the Longitudinal Aging Study in India (LASI)
| IFLS | LASI | |
|---|---|---|
| Day 1 | Inspection of general laboratory working environment, equipment, and reagents and other supplies. Sample preparation for the next day. | |
| Day 2 | Measured CRP levels on 20 IFLS study samples | Measured CRP levels on 22 validation samples and 5 additional samples from local volunteers |
| Day 3 | Measured CRP levels on 32 IFLS study samples and 5 validation samples | Measured CRP levels on 20 validation samples and 5 samples from the same local volunteers |
| Day 4 | Measured CRP levels on 27 IFLS study samples and 10 validation samples | Measured CRP levels on 7 validation samples and 4 samples from the same local volunteers |
| Day 5 | Review of pre-test results with local laboratory personnel and plan for subsequent quality control during testing of study samples | |
Ongoing assay quality control
The IFLS and LASI research teams were in regular communication with the laboratories throughout the period of assay and reviewed the CRP results from the study samples on a biweekly basis. Both studies also tested quality control samples periodically to monitor for possible laboratory drift in CRP results over time. For IFLS, eight quality control samples were inserted after approximately every 1,500 IFLS study samples, due to the large number of study specimens and limited blood spots for quality control. LASI included five quality control samples on each microplate for the first 10 microplates that measured study samples, followed by eight quality control samples for every 150 study samples. Moreover, because of the greater availability of blood spots generated from venous blood, LASI was able to use quality control samples from selected volunteers on multiple assay runs, so that it could track changes in absolute CRP values for these samples over time.
Statistical Methods
The minimal detection limit of the DBS-based CRP assay is 0.148 mg/. For calculation purpose, two specimens with undetectable CRP level at IFLS laboratory were assigned a value of 0.001 mg/L (the corresponding values from UW laboratory were 0.25 mg/L and 0.38 mg/L). Pearson’s correlation coefficients were calculated to examine the relationship between the results from different laboratories. We also performed Bland-Altman analysis and reported coefficient of variation (CV) and summary statistics for the absolute value differences of duplicate quality control samples between the laboratories.
Results
Pre-test Results
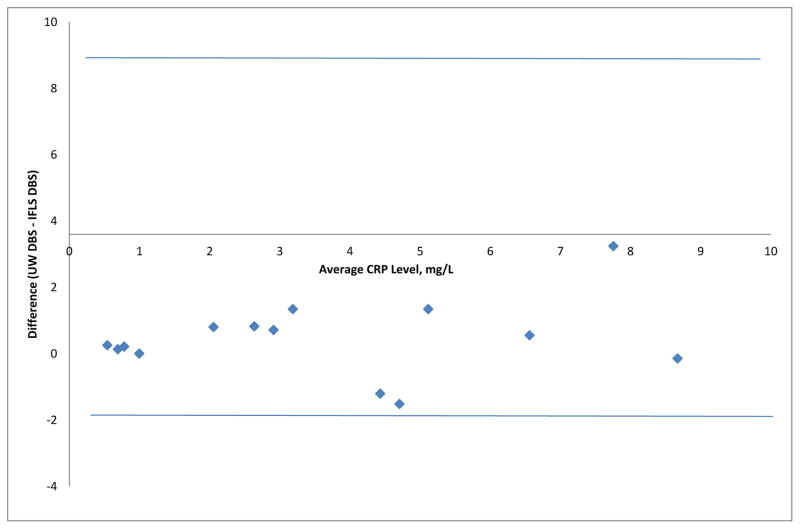
For the ILFS pre-test, 14 quality control samples had DBS-based CRP values from the ILFS laboratory as well as both DBS-based and serum-based values from UW. The mean CRP concentration for these quality control samples was 3.41 mg/L (standard deviation: 2.60 mg/L) at the IFLS laboratory. For UW, the mean concentrations were 3.88 mg/L (standard deviation: 2.85mg/L) from DBS-based assay and 3.28 mg/L (standard deviation: 2.70 mg/L) from serum-based assay. The pair-wise correlation coefficients and average absolute difference in CRP values are summarized in Table 2. Figure 1 shows the Bland-Altman plot comparing DBS-based CRP results from IFLS and UW.
Table 2.
Comparison of dried blood spot (DBS) based values from the Indonesia Family Life Survey (IFLS), and DBS and serum-based results from University of Washington (UW)
| UW DBS vs. ILFS DBS * | IFLS DBS vs. serum | UW DBS vs. serum | |
|---|---|---|---|
| Correlation coefficients | 0.98 | 0.97 | 0.97 |
| Mean absolute difference, mg/L | 0.11 | 0.14 | 0.21 |
| Standard deviation of the difference, mg/L | 0.54 | 0.60 | 0.61 |
means C-reactive protein concentrations from IFLS DBS, UW DBS, and UW serum were 3.41 mg/L, 3.88 mg/L, and 3.28 mg/L, respectively.
Figure 1.
Bland-Altman Plot of the Dried Blood Spot (DBS) Based C-reactive Protein (CRP) Results from Indonesia Family Life Survey (IFLS) and University of Washington (UW)
During the LASI pre-test, LASI’s CRP results on 32 quality control samples were compared with DBS-based values from UW. The correlation coefficient was 0.95. The average difference was 1.75 mg/L (standard deviation: 1.81 mg/L). The Bland-Altman analysis indicated no significant difference between LASI laboratory and UW. LASI also measured 10 quality control samples twice during the pre-test. The correlation coefficient between the test-retest values was 0.998. The average difference in absolute values was 0.62 mg/L (standard deviation: 0.64 mg/L). The coefficients of variation (CVs) for the repeated measurements of all specimens were less than 15%, except one, which had a CV of 22%.
Results for Ongoing Quality Control
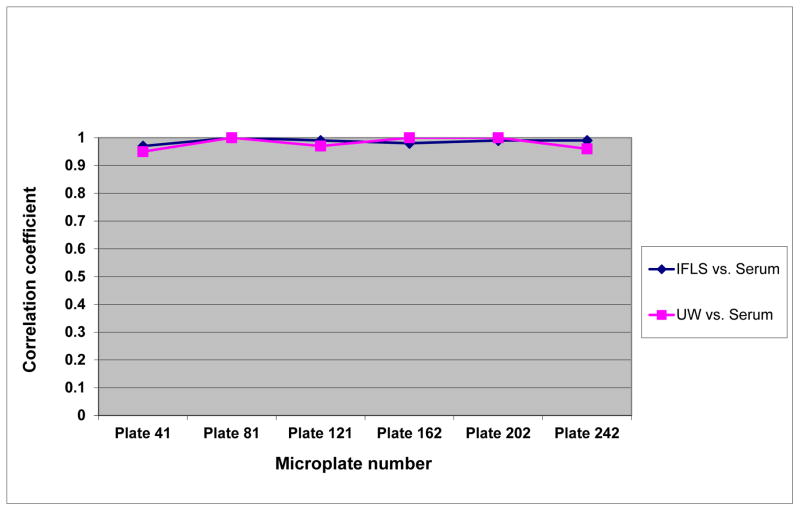
During the testing of study samples, IFLS included eight quality controls on every fortieth microplate, or approximately once in every 1,500 study samples: According, a total of 48 quality control samples were measured over 6 months. Figure 2 shows the correlation coefficients of CRP values between IFLS DBS and serum, and between UW DBS and serum over that period. Using serum-based CRP levels as the gold standard, the results from IFLS and UW were comparable. All correlation coefficients were above 0.95
Figure 2.
Correlations between the Indonesia Family Life Survey (IFLS), University of Washington (UW), and serum C-reactive protein values by microplate numbers
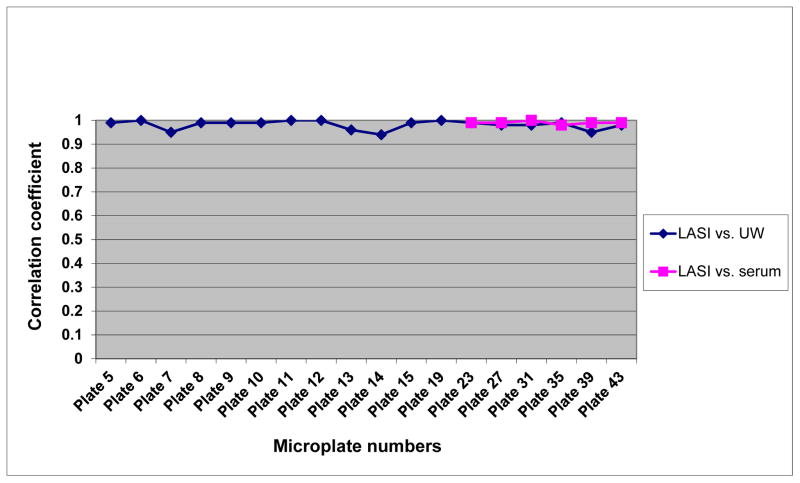
Figure 3 summarizes correlations of DBS-based CRP results between LASI and UW as well as the correlations between LASI and serum-based values, when quality control samples with corresponding serum hsCRP results became available during the second half of study sample measurement. The correlation coefficients between LASI and UW DBS ranged from 0.94 to 1.00, and the coefficients between LASI and serum values were from 0.98 to 1.00.
Figure 3.
Correlations between the Longitudinal Aging Study in India (LASI), University of Washington (UW), and serum C-reactive protein values by microplate numbers
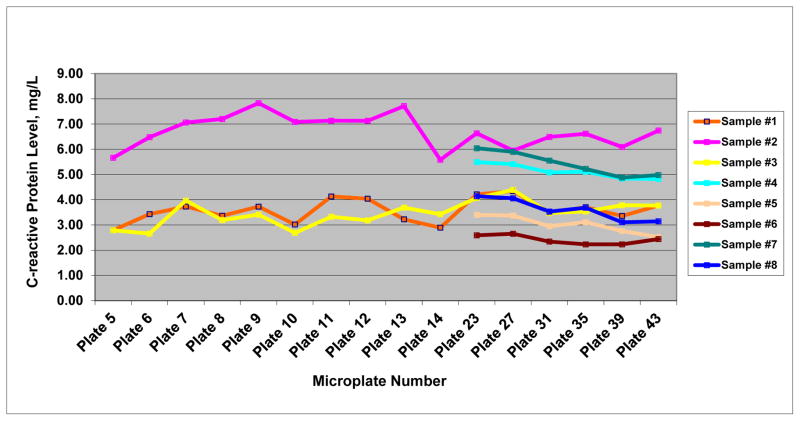
Compared with IFLS, LASI had significantly more blood spot material available for quality control purposes, because LASI laboratory received two Whatman cards per volunteer, each with five high-quality blood spots. As a result, LASI was able to track changes in absolute CRP values from eight quality control samples over time (Figure 4). The CVs for the repeated measurements of these 8 specimens ranged from 5.46% to 14.20% (median was approximately 10%). For the quality control sample with the highest CRP value (sample #2), the DBS-based value from the UW reference laboratory was 6.96 mg/L. The absolute values from 16 repeated measurements at LASI laboratory varied from 5.58 mg/L to 7.83 mg/L, ranging from 20 percent under-estimation to 13 percent over-estimation. Although the results from duplicate samples fluctuated in absolute values over time, the relative order of CRP concentrations remained similar (Figure 4).
Figure 4.
Changes in absolute C-reactive protein values for selective validation samples over time
Discussion
The results from the testing of quality control samples show that DBS-based CRP values from IFLS and LASI had excellent correlations with serum-based values and with DBS-based results from the reference laboratory in the United States. Both laboratories were able to maintain their assay quality during the testing of actual study samples. Repeated measurements of quality control samples from the same individuals demonstrated some fluctuations in absolute CRP values over time. However, the relative order of CRP levels was preserved.
To our knowledge, this is the first study that has systematically evaluated the quality of DBS-based CRP assays that were conducted in the laboratories outside the United States. Comparing DBS-based results, both IFLS and LASI have consistently high correlation coefficients with UW during the pre-test and subsequent testing of the study sample (all correlation coefficients are above 0.95). Results from both laboratories also correlated well with venous-based CRP values. These coefficients were very similar to those reported by previous validation studies in the United States (McDade, Burshop, and Dohnal 2004; Brindle et al. 2010). These findings suggested that, with proper personnel training, close adherence to assay protocol, ongoing technical support, and quality assessment, a DBS-based CRP assay can have a validity that approximates venous-based testing and may be successfully implemented in community-based surveys in other less developed countries.
Compared to venous-based assay, the DBS approach offers several important advantages (McDade, Williams, Snodgrass 2007). First, the collection of DBS specimens is less invasive and, for both IFLS and LASI, was conducted in the field by interviewers without formal medical training. Second, DBS samples do not need to be processed and frozen immediately, nor do they require cold chain for shipment from collection site to a central storage facility. Third, DBS specimens may remain stable in laboratory freezers for a long period of time and can be analyzed in the future when DBS-based methods for new biomarkers become available. Therefore, the DBS approach allows collection of valid and reliable blood-based biological information in large-scale population-based surveys for which collection of venous specimens may not always be possible.
One unique feature of the quality control effort for LASI was the availability of a large number of blood spots from same individuals and the ability to measure quality control samples from these individuals over time. The findings have showed that there is a fluctuation in the absolute CRP values over time, which could be up to approximately 20 percent. Some variations in test results are not unexpected. For DBS-based CRP assay, the absolute values may be influenced by many factors such as temperature variation in laboratories, slow degradation of test reagents over time, and volume of reagents added to different microwells. Despite this fluctuation in absolute CRP values over time, our results suggest that the relative order of CRP concentrations from different individuals remained similar. Therefore, DBS-based CRP measurement may be reasonably precise for population-based studies that explore the associations between socioeconomic factors and health outcomes.
Our experience with IFLS and LASI also indicates that pre-testing and ongoing quality controls for the DBS-based assay depend on availability of adequate blood spots in quality control samples. Preparation of DBS quality control samples from venous blood seems to be a better approach for CRP assay, because it will ensure a large number of blood spots, maintain good spot quality, and minimize volunteer discomfort. This approach is even more valuable for between-laboratory comparisons across multiple countries and multiple waves of the study, which may require large amount of blood spot material from same individuals. However, a previous validation project conducted by USC/UCLA CBPH shows that the comparability of DBS results based on standard “finger-prick” DBS collection versus DBS created by drops from venous blood samples may vary by the type of assays, ranging from virtually indistinguishable results for HbA1c; to very similar for CRP; to only a modest relationship for cholesterol (personal communication with USC/UCLA CBPH). Thus, comparability for other analytes will require carefully planned tests in the future.
Several limitations should be noted about our quality control work for ILFS and LASI. First, given the budget constraints, our sample size for quality control specimens was relatively small, particularly for those with very high CRP levels. While this may not affect overall cross-laboratory correlations in a significant way, we could have under-estimated the magnitude of the fluctuation in the absolute values, when CRP levels are very high (e.g. above 10 mg/L). Second, DBS quality control samples were collected in the U.S., frozen at −70 Celcius, and then shipped to various laboratories and assayed at different times. Although frozen DBS samples are generally considered very stable for CRP assay, it is still possible to have some low level sample degradation during the storage and shipment. Third, although this study has compared the quality of DBS-based CRP assay in other countries against a reference laboratory in the United States, it was not designed to harmonize CRP data across multiple studies, due to possible differences in test reagents, equipment and personnel.
Despite these limitations, our study has shown that it is possible to conduct well-designed pre-test and ongoing monitoring to ensure data quality for DBS-based CRP assay. Our data indicate that DBS-based CRP results from less developed countries can have excellent and consistent correlations with serum-based values and DBS-based results from the reference laboratory in the United States. Even though there may be fluctuations in absolute CRP values over time, the relative order of CRP concentrations remains similar and the CRP estimates may be reasonably precise for population-based studies that examine the associations between socioeconomic factors and health. New effort is needed to further improve the precision of DBS-based CRP assay, to establish specific criteria to define acceptable quality and between-laboratory variability of the assay, and to develop calibration algorithms for laboratories in multiple countries, which is essential for biomarker data harmonization from different studies.
Acknowledgments
Funding
Funding for the LASI pilot study was provided by the National Institute on Aging (NIA), grant 1R21 AG034443. Funding for the IFLS4 was provided by the NIA, grant 1R01 AG026676, the National Institute for Child Health and Human Development (NICHD), grant 1R01 HD050764, and grants from the World Bank, Indonesia Office, and the Australian Aid Agency (AUSAID). Funding for the USC/UCLA Center on Biodemography and Population Health (CBPH) was provided by the NIA, grant P30 AG017265.
References
- Brindle E, Fujita M, Shofer J, O’Connor KA. Serum, plasma, and dried blood spot high-sensitivity C-reactive protein enzyme immunoassay for population research. J Immunol Methods. 2010;362:112–120. doi: 10.1016/j.jim.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figaro MK, Kritchevsky SB, Resnick HE, Shorr RI, Butler J, Shintani A, Penninx BW, Simonsick EM, Goodpaster BH, Newman AB, Schwartz AV, Harris TB. Diabetes, inflammation, and functional decline in older adults: findings from the Health, Aging and Body Composition (ABC) study. Diabetes Care. 2006;29:2039–2045. doi: 10.2337/dc06-0245. [DOI] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Witkowski JM, McElhaney J, Loeb M, Mitnitski A, Pawelec G. Aging frailty and age-related diseases. Biogerontology. 2010;11:547–563. doi: 10.1007/s10522-010-9287-2. [DOI] [PubMed] [Google Scholar]
- Garrod R, Marshall J, Barley E, Fredericks S, Hagan G. The relationship between inflammatory markers and disability in chronic obstructive pulmonary disease (COPD) Prim Care Respir J. 2007;16:236–240. doi: 10.3132/pcrj.2007.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi I, Berkman LF. Social ties and mental health. J Urban Health. 2001;78:458–467. doi: 10.1093/jurban/78.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo HK, Bean JF, Yen CJ, Leveille SG. Linking C-reactive protein to late-life disability in the National Health and Nutrition Examination Survey (NHANES) 1999–2002. J Gerontol A Biol Sci Med Sci. 2006;61:380–387. doi: 10.1093/gerona/61.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl B, Toss H, Siegbahn A, et al. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. N Engl J Med. 2000;343:1139–1147. doi: 10.1056/NEJM200010193431602. [DOI] [PubMed] [Google Scholar]
- Marmot M, Allen J, Bell R, Bloomer E, Goldblatt P. Consortium for the European Review of Social Determinants of Health and the Health Divide. WHO European review of social determinants of health and the health divide. Lancet. 2012;380:1011–1029. doi: 10.1016/S0140-6736(12)61228-8. [DOI] [PubMed] [Google Scholar]
- McDade TW, Burhop J, Dohnal J. High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin Chem. 2004;50:652–654. doi: 10.1373/clinchem.2003.029488. [DOI] [PubMed] [Google Scholar]
- McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;44:899–925. doi: 10.1353/dem.2007.0038. [DOI] [PubMed] [Google Scholar]
- McDade TW, Tallman PS, Madimenos FC, et al. Analysis of variability of high sensitivity C-reactive protein in lowland Ecuador reveals no evidence of chronic low-grade inflammation. Am J Hum Biol. 2012;24:675–681. doi: 10.1002/ajhb.22296. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]