Abstract
M protein of spring viremia of carp virus (SVCV) was expressed in Escherichia coli and then used to immunize BALB/c mice. One monoclonal antibody (5A1) against M protein was generated by fusion of mouse myeloma cell line SP2/0 and spleen lymphocytes from immunized mice. The characterizations of this MAb were confirmed by ELISA (enzyme linked immunosorbent assay), IFA (immunofluorescent assay), and Western blot analysis. All results indicate that MAb 5A1 was specific to SVCV M protein.
Introduction
Spring viremia of carp virus (SVCV) is the pathogen of infectious hemorrhagic swimbladder inflammation in common carp, which could cause high mortality in juvenile carp (30–70%). In 2006, it was listed as noticeable in the International Aquatic Animal Health Code of the Office International des Epizooties (OIE).(1) The initial outbreak of this disease was in European countries. However, recently it has been reported in the United States, Middle East, China, Canada, and Brazil.(2)
SVCV, one of the rhabdoviruses, is a member of Rhabdoviridae, genus Vesiculovirus.(3) The genome of the virus is situated in an elongated nucleocapsid and surrounded by a lipoprotein layer with glycoprotein projections. It encodes five structural proteins: nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and viral RNA-dependent RNA polymerase (L).(4,5) The M protein is composed of 223 amino acids and has about 31.6% homology with the M protein of vesicular stomatitis virus (VSV).(6) Previous studies reported that the M protein could cause apoptosis and play an important role during the cytopathic effect.(7,8) Furthermore, M protein could also inhibit the transcription of host cells.(9)
In this study, we generated an MAb against the M protein of SVCV. This MAb can be a useful tool for the development of detection methods for SVCV and studying the function of M protein.
Materials and Methods
Cloning, expression, and purification of M protein
M gene was amplified from SVCV-infected epithelioma papulosum cyprini (EPC) cells by a one-step RT-PCR. The primers of this gene are listed in Table 1. The M gene fragment was then inserted into the bacterial expression vector pGEX-KG and named the recombinant plasmid KG-SVCV/M. KG-SVCV/M and control plasmid pKG were transformed into Escherichia coli BL21 cells and induced by isopropyl-β-thio-galactopyranoside (IPTG) at 37°C. Four hours later, bacterium was collected by centrifugation. Then, the bacterial pellet was resuspended and sonicated until clear lysate was obtained. The M protien was purified by dialysis and then stored at −80°C.(11)
Table 1.
Primer Sequences
| Gene | Primer orientation | Primer sequence (5′-3′) |
|---|---|---|
| KG-SVCV/M | Forward | AAAGGATCCATGTCTACTCTAAGAAAGCTC |
| Reverse | AAACTCGAGATCTCCCATGAACAGGGAA | |
| KG-SVCV/Ma | Forward | AAAGGATCCATGTCTACTCTAAGAAAGCT |
| Reverse | AAACTCGAGTTACGACATCGTTTTTAAGGGTGT | |
| KG-SVCV/Mb | Forward | AAAGGATCCATGCCCTTAAAAACGATGTCGGA |
| Reverse | AAACTCGAGTTAACTCCCTTGCACCTTGTTAGA | |
| KG-SVCV/Mc | Forward | AAAGGATCCATGAACAAGGTGCAAGGGAGTTGT |
| Reverse | AAACTCGAGTTACTGACACTCTTTCAACAAATTG | |
| KG-SVCV/Md | Forward | AAAGGATCCATGTTGTTGAAAGAGTGTCAGGTAG |
| Reverse | AAACTCGAGTTAATCTCCCATGAACAGGGAA |
Production of monoclonal antibody
Female BALB/c mice, about 4 weeks old, were immunized intramuscularly with 60 μL of a 1:2 purified M protein (30 μg per mouse) and Quickantibody adjuvant. After 2 weeks, the mice were immunized again with the same mixture. Two weeks later, before fusion, the mice were boosted with 60 μg of M protein, and 3 days later, mice splenocytes were harvested and fused with SP2/0 using 50% polyethylene glycol (PEG4000). The fused cells were cultured in HAT medium with fetal bovine serum. Ten days later, the aminopterin was omitted and fused cells were cultured in HT medium. The supernatants of hybridoma culture were screened by ELISA. Then, the positive hybridoma lines were chosen to subclone three times using limiting dilution method. The stable hybridoma clones were injected into BALB/c mice intraperitoneally.(12) Subsequently, seroperitoneum was collected from the immunized mice and the MAb was purified by the antibody purification kit according to the manufacturer's instructions.
Identification of MAb subtype
Subtype identification kit (Pierce Rapid ELISA Mouse MAb Isotyping Kit, Thermo Scientific, Boston, MA) was used to identify the subtype of the MAb according to the manufacturer's instructions.
Immunofluorescence assay
EPC cells were cultured in a 24-well plate (Costar Corning, Corning, NY) and infected with SVCV at one multiplicity of infection (MOI). Twenty-four hours post-infection, EPC cells were fixed with absolute methanol and processed for IFA using MAb 5A1, followed by the secondary antibody fluorescein isocyanate-conjugated goat anti-mouse IgG. Fluorescent images were examined under a fluorescent microscope.
Western blot analysis
Western blot assay was used to analyze the specificity of MAb 5A1. EPC cells were infected by SVCV for 36 h; then cells were lysed to collect the lysates. Samples were separated by 12% SDS-PAGE and transferred to the nitrocellulose membrane. The membrane was blocked overnight with 1% bovine serum albumin (BSA) in TBST buffer at 4°C, then incubated with MAb 5A1 (diluted 1:500) at 37°C for 1 h. After washing the membrane three times with TBST buffer, the membrane was incubated with the secondary antibody (horseradish peroxidase [HRP]-conjugated goat anti-mouse IgG; diluted 1:1000) at 37°C for 1 h. After washing three times, the membrane was visualized by enhanced chemiluminescence reagents.
Epitope mapping of MAbs
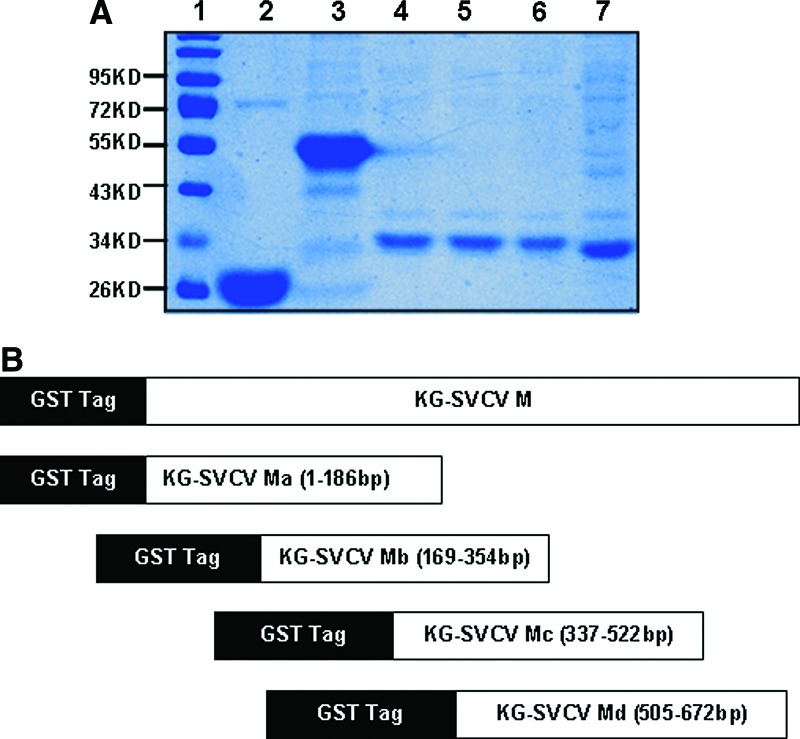
Four mutant proteins, KG-SVCV/Ma (1-186bp), KG-SVCV/Mb (169-354bp), KG-SVCV/Mc (337-522bp), and KG-SVCV/Md (505-672bp) (Fig. 1), were generated for basic epitope mapping.(11) The fragments were amplified from the EPC cells, which were infected by SVCV by a one-step RT-PCR. The primers for these fragments are listed in Table 1. Then the fragments were cloned into the vector pGEX-KG and transformed into competent E. coli BL21, respectively. After being induced by IPTG for 4 h, the bacterium was collected by centrifugation. The bacterial pellet was resuspended and sonicated to obtain the clear lysate. The four mutant proteins were purified and separated by 12% SDS-PAGE (Fig. 1) and transferred to the nitrocellulose membrane. Western blotting was used to analyze these four proteins according to the measures mentioned above.
FIG. 1.
SDS-PAGE analysis of recombinant proteins KG-SVCV/M, KG-SVCV/Ma (1-186bp), KG-SVCV/Mb (169-354bp), KG-SVCV/Mc (337-522bp), KG-SVCV/Md (505-672bp). (A) Lane 1, protein marker; lane 2, bacilli precipitation of pGEX-KG; lane 3, bacilli precipitation of KG-SVCV/M; lane 4, bacilli precipitation of KG-SVCV/Ma (1-186bp); lane 5, bacilli precipitation of KG-SVCV/Mb (169-354bp); lane 6, bacilli precipitation of KG-SVCV/Mc (337-522bp); lane 7, bacilli precipitation of KG-SVCV/Md (505-672bp). (B) Construction of recombinant plasmids expressing full length or truncated forms of M protein.
Results and Discussion
Expression of M protein
The recombinant plasmid KG-SVCV/M was transformed into competent E. coli BL21 cells. The cells were induced by IPTG (1 mmol/L) for 3 h at 37°C to expression M protein. The protein band (50 kDa) was detected by SDS-PAGE and Western blotting (Fig. 1).
Generation of MAb against M protein of SVCV
During the immunization, blood from mice were collected and monitored by indirect ELISA. The mouse serum that showed the highest binding affinity was chosen for the last booster and cell fusion. One MAb 5A1 was isolated for further use.
Subtype identification of MAb against M protein of SVCV
The subtype of MAb was identified by the rapid ELISA mouse MAb isotyping kit. The result showed that MAb 5A1 belonged to the subtype IgG2b. The light chain of the MAb was kappa.
MAb specifically recognize M protein of SVCV
IFA and Western blotting were used to analyze the specificity of MAb 5A1. In IFA, MAb 5A1 showed positive reaction to SVCV-infected EPC cells but there were no fluorescence signals in the negative control cells (Fig. 2). The 24 kDa protein band was also detected by Western blotting (Fig. 3). In conclusion, MAb 5A1 was highly specific to SVCV.
FIG. 2.
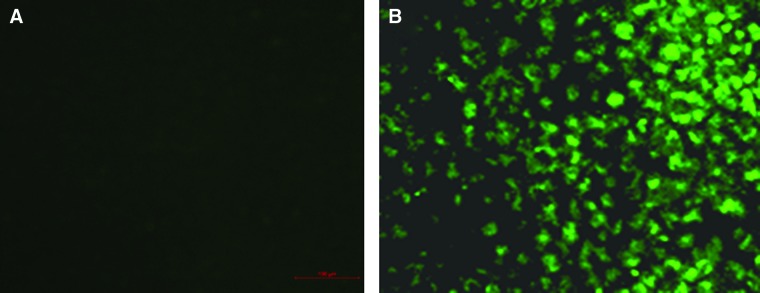
Immunofluorescence staining (IFA) to evaluate MAb 5A1. (A) EPC cells were infected with SVCV. 24 h post-infection, fluorescent images were examined with a fluorescent microscope using MAb 5A1 as primary antibody and Alexa Fluor 488 goat anti-mouse IgG as second antibody. (B) Uninfected EPC cells as control (400×).
FIG. 3.
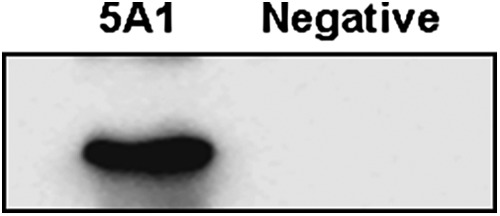
Western blot analysis to evaluate MAb 5A1. (A) EPC cells were infected with SVCV for 36 h and specificity of monoclonal antibody 5A1 against M protein was analyzed by Western blot. Uninfected EPC cells were treated as negative control.
Epitope mapping of MAb against SVCV
Recombinant M proteins KG-SVCV/Ma (1-186bp), KG-SVCV/Mb (169-354bp), KG-SVCV/Mc (337-522bp), and KG-SVCV/Md (505-672bp) were analyzed by Western blotting using 5A1. The results showed that the epitope that MAb 5A1 recognized is located in KG-SVCV/Mc (337-522bp) (Fig. 4).
FIG. 4.
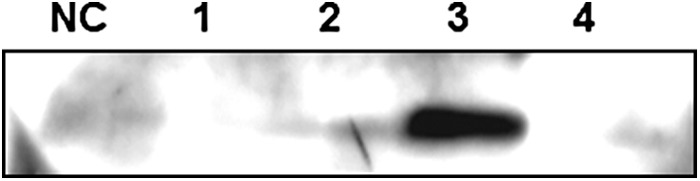
Epitope mapping of MAb 5A1. Recombinant proteins KG-SVCV/Ma (1-186bp), KG-SVCV/Mb (169-354bp), KG-SVCV/Mc (337-522bp), and KG-SVCV/Md (505-672bp) were analyzed by Western blot to detect the specificity of 5A1. Bacilli precipitation of pGEX-KG was used as negative control. NC, negative control; lane 1, KG-SVCV/Ma (1-186bp); lane 2, KG-SVCV/Mb (169-354bp); lane 3, KG-SVCV/Mc (337-522bp); lane 4, KG-SVCV/Md (505-672bp).
Conclusion
In this study, BALB/c mice were immunized with prokaryotic expressed SVCV M protein. Using the hybridoma technique, one clone of MAb (5A1) was generated. The subtype of MAb was IgG2b and light chain was kappa. The specificity of MAb was analyzed by IFA and Western blotting. These results provide the material for future study of the functions of SVCV M protien.
Acknowledgments
This work was supported by the National Natural Sciences Foundation of China (grant nos. 31172433, 30901118), the National Key Technology R&D Program of the Ministry of Science and Technology of China (2012BAD25B06), and the Fundamental Research Funds for the Central Universities (nos. 2011PY121, 2013PY071).
Author Disclosure Statement
The authors have no financial interests to disclose.
References
- 1.Liu Z, Teng Y, Xie X, Li H, Lv J, Gao L, Tian F, Jiang Y, Chu Z, Xie C, and Liu H: Development and evaluation of a one-step loop-mediated isothermal amplification for detection of spring viraemia of carp virus. J Appl Microbiol 2008;105(4):1220–1226 [DOI] [PubMed] [Google Scholar]
- 2.Basic A, Schachner O, Bilic I, and Hess M: Phylogenetic analysis of spring viraemia of carp virus isolates from Austria indicates the existence of at least two subgroups within genogroup Id. Dis Aquat Organ 2009;85(1):31–40 [DOI] [PubMed] [Google Scholar]
- 3.Zhang NZ, Zhang LF, Jiang YN, Zhang T, and Xia C: Molecular analysis of spring viraemia of carp virus in China: a fatal aquatic viral disease that might spread in East Asia. PLoS One 2009;4(7):e63371–e63379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann B, Schütze T, and Mettenleiter TC: Determination of the complete genomic sequence and analysis of the gene products of the virus of Spring Viremia of Carp, a fish rhabdovirus. Virus Res 2002;84(1):89–100 [DOI] [PubMed] [Google Scholar]
- 5.Koutna M, Vesely T, Psikal I, and Hulova J: Identification of spring viraemia of carp virus (SVCV) by combined RT-PCR and nested PCR. Dis Aquat Organ 2003;55(3):229–235 [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann B, Schutze H, and Mettenleiter TC: Determination of the complete genomic sequence and analysis of the gene products of the virus of spring viremia of carp, a fish rhabdovirus. Virus Res 2002;84(1–2):89–100 [DOI] [PubMed] [Google Scholar]
- 7.Melki R, Gaudin Y, and Blondel D: Interaction between tubulin and the viral matrix protein of vesicular stomatitis virus: possible implications in the viral cytopathic effect. Virology, 1994;202(1):339–347 [DOI] [PubMed] [Google Scholar]
- 8.Kopecky SA, and Lyles DS: The cell-rounding activity of the vesicular stomatitis virus matrix protein is due to the induction of cell death. J Virol 2003;77(9):5524–5528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan H, Puckett S, and Lyles DS: Inhibition of host transcription by vesicular stomatitis virus involves a novel mechanism that is independent of phosphorylation of TATA-binding protein (TBP) or association of TBP with TBP-associated factor subunits. J Virol 2001;75(9):4453–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjorklund HV, Higman KH, and Kurath G: The glycoprotein genes and gene junctions of the fish rhabdoviruses spring viremia of carp virus and hirame rhabdovirus: analysis of relationships with other rhabdoviruses. Virus Res 1996;42(1–2):65–80 [DOI] [PubMed] [Google Scholar]
- 11.Ruan X, Huang S, Shao L, Ye J, and Chen Z: Monoclonal antibodies against NS4B protein of Japanese encephalitis virus. Monoclon Antib Immunodiagn Immunother 2013;32(6):382–385 [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Shao L, Ye J, and Li Y: Monoclonal antibodies against NS3 and NS5 proteins of Japanese encephalitis virus. Hybridoma 2012;31:137–141 [DOI] [PMC free article] [PubMed] [Google Scholar]