Abstract
Significance: Disruption of endothelial function is considered a key event in the development and progression of atherosclerosis. Endothelial nitric oxide synthase (eNOS) is a central regulator of cellular function that is important to maintain endothelial homeostasis. Recent Advances: Endothelial homeostasis encompasses acute responses such as adaption of flow to tissue's demand and more sustained responses to injury such as re-endothelialization and sprouting of endothelial cells (ECs) and attraction of circulating angiogenic cells (CAC), both of which support repair of damaged endothelium. The balance and the intensity of endothelial damage and repair might be reflected by changes in circulating endothelial microparticles (EMP) and CAC. Flow-mediated vasodilation (FMD) is a generally accepted clinical read-out of NO-dependent vasodilation, whereas EMP are upcoming prognostically validated markers of endothelial injury and CAC are reflective of the regenerative capacity with both expressing a functional eNOS. These markers can be integrated in a clinical endothelial phenotype, reflecting the net result between damage from risk factors and endogenous repair capacity with NO representing a central signaling molecule. Critical Issues: Improvements of reproducibility and observer independence of FMD measurements and definitions of relevant EMP and CAC subpopulations warrant further research. Future Directions: Endothelial homeostasis may be a clinical therapeutic target for cardiovascular health maintenance. Antioxid. Redox Signal. 22, 1230–1242.
Introduction
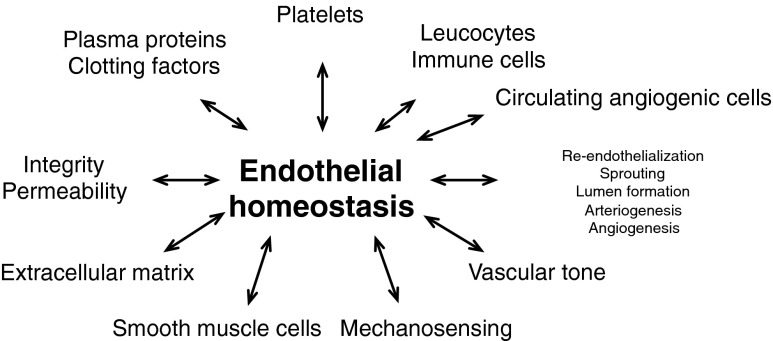
A disturbance of endothelial function is considered a key event in the development and progression of atherosclerosis (94). This article aims at capturing current knowledge on the role of endothelial homeostasis, maintenance of endothelial integrity, and functional competence throughout life. Endothelial homeostasis encompasses a number of acute responses such as adaption of flow to tissue's demand via mechanosensing and acute modulation of vascular tone via production of autocoids, including nitric oxide (NO), that are affected by sympathetic tone (Fig. 1). In the mid and long term, changes in endothelial homeostasis (i.e., endothelial injury) affect the vascular structure by interacting with extracellular matrix turnover and smooth muscle cells controlling intimal hyperplasia. Important determinants of endothelial homeostasis include not only endothelial membrane function and adhesiveness to proteins of the coagulation cascade and platelets but also membrane permeability and the integrity, that is, endovascular coverage by ECs (30). EC functions that support the repair of damaged endothelium and more sustained responses to injury include re-endothelialization, sprouting of ECs, and attraction of immune cells and circulating angiogenic cells (CAC).
FIG. 1.
Key aspects of endothelial homeostasis.
In this article, the physiological functions of the vascular endothelium will be reviewed with particular focus on the essential role of endothelial nitric oxide synthase (eNOS) in this context. Importantly, eNOS is intimately involved in coupling changes in blood flow to long-term remodeling of the vascular architecture, thereby having a long-term impact on vascular health and disease. The most important clinical and experimental methods to measure endothelial function, in particular flow-mediated vasodilation (FMD), will be outlined. FMD has emerged as the most important and most widely used non-invasive methodology to measure endothelial function and is now even available in rodents (41). We will discuss how genetic and environmental factors, in essence cardiovascular risk factors, induce endothelial dysfunction, promoting the initiation and progression of cardiovascular diseases (CVDs), and we will also critically review markers of endothelial injury and regenerative capacity. We will conclude with arguing on the emerging concept that endothelial homeostasis should serve as an integrated therapeutic target to maintain vascular health.
Physiological Roles of the Vascular Endothelium
The endothelium maintains vascular homeostasis through multiple complex interactions with cells in the vessel walls and lumen. It regulates the vascular tone by balancing the production of not only vasodilators, most importantly NO, but also prostaglandins, endothelium-derived hyperpolarizing factor, and vasoconstrictors, including Endothelin-1. Endothelium-derived NO also participates in systemic physiologic functions of the endothelium, such as the control of vascular tone, leucocyte immigration, and blood clotting. Furthermore, the endothelium controls blood fluidity and coagulation through the production of factors that regulate platelet activity, the clotting cascade, and the fibrinolytic system. Finally, the endothelium has the capacity to produce cytokines and adhesion molecules that regulate and direct inflammatory and regenerative processes, including leucocyte traffic. Therefore, the endothelium modulates the structure and physicomechanical properties of the vessel walls over time and profoundly affects vascular health.
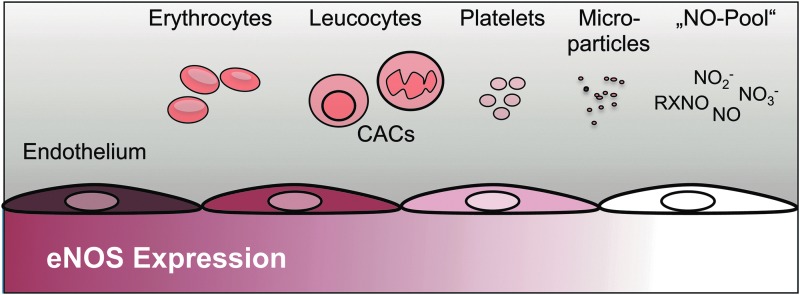
eNOS is a constitutively expressed enzyme that modulates most of the functions of the endothelium mentioned earlier. It is expressed mainly in the endothelium of large arteries with the expression decreasing in smaller arteries and not expressed in capillary ECs. As depicted in Figure 2, eNOS is also expressed in essentially all blood cells, including erythrocytes, leucocytes, platelets, and CAC (previously termed endothelial progenitor cells [EPCs]) (21, 40, 42). We have recently shown the expression of a functional eNOS enzyme in circulating microparticles (MP) (47). However, we were not able to link eNOS expression or activity to individual MP subpopulations. Given that MPs derived from other cells not classically known to express eNOS, including smooth muscle cells, have been described, it remains to be investigated whether or not eNOS is expressed only in certain subpopulations or all circulating MPs. Since the NO synthesized in the endothelium is a gas, it not only can act locally on the ECs and cells in the vessel wall but can also feed into the circulating NO pool, which, due to the short half life of NO itself, comprises more stable species, that is, nitrite, nitrate, and nitroso species. These species can also mediate NO-like bioactivities along the cardiovascular system, including vascular tone (74, 76, 78).
FIG. 2.
Expression of endothelial nitric oxide synthase (eNOS) in the blood vessels and blood cells. eNOS is expressed in vascular endothelial cells, and the expression decreases toward the microvasculature. Furthermore, eNOS is expressed in all blood cells and circulating microparticles (MPs).
The most widely studied physiological function of the endothelium is the regulation of vascular tone via responding to a variety of stimuli that are aimed at optimizing the blood flow to dependent tissues. This process involves a complex interplay between intracellular receptors, the synthesis, and the release of a variety of endothelium-derived relaxing and constricting substances. The endothelium is essential for the sensing of changes in blood flow and responds to it immediately by production of vasoactive substances, primarily NO (26, 68), rapidly by activation of extracellular-regulated kinases (ERK1/2; p44/p42 mitogen-activated protein [MAP] kinases), and also by tyrosine phosphorylation of focal adhesion kinase as a sustained response (48, 93). All these signals are linked to important aspects of vascular homeostasis: integrity, permeability, diameter regulation, and architecture.
The mechanical forces exerted by the circulating pulsatile blood flow are pressure, shear, and stretch, which are hard to distinguish but may account for different responses (15). It is long appreciated that acute increases in blood flow in conduit arteries such as the brachial artery and femoral artery due to dilation of downstream resistance vessels during reactive hyperemia of the forearm or lower leg lead to an increased synthesis of NO in the EC. The released NO will lead to relaxation of the underlying smooth muscle cells and decreased vascular tone in the conduit artery. This physiological flow-dependent vasodilator response of conduit arteries is also seen during exercise. For example, Gaenzer et al. observed that bicycle exercise leads to vasodilation of the femoral artery, which correlated with brachial artery FMD (27).
The mechanotransducing signaling events secondary to increased shear stress are not completely understood but involve deformation of the ECs and their glycocalix due to viscous drag of the flowing blood. This leads to activation of cell adhesion proteins, including integrins (57), platelet endothelial cell adhesion molecule (67), and cytoskeletal proteins (56). This, in turn, leads to PI3 kinase-dependent phosphorylation of protein kinase B (Akt), which phosphorylates eNOS, rendering it more active to produce NO (22). Another contributing mechanism is Ca2+ influx with Ca/calmodulin-mediated activation of eNOS activity. Freshly synthesized NO diffuses into the blood stream and vessel wall. In the vessel wall, it binds to the central iron atom of the guanylyl cyclase, displacing a histidine residue away from the active center. The resulting increased concentration of cGMP opens calcium channels, and calcium leads to relaxation of smooth muscle cells. NO reaching the blood may undergo transformation to other partly bioactive NO storage forms, including nitrosothiols or nitrite, which, in turn, may mediate NO bioactivities in the blood or vasculature distal to the site of production, including even systemic hemodynamics, that is, blood pressure (76–78). Longer exposure to shear stress increases eNOS expression in ECs via transcription factors and mRNA stability (9). Data suggest that eNOS expression is essential in coupling vascular architecture to blood flow demands (9). Illustrative of this, it was shown that eNOS knockout mice exhibit a pathological phenotype in response to experimentally altered blood flow in the common carotid artery (85). The authors occluded the external carotid artery to decrease blood flow in the ipsilateral common carotid. This led to a shrinking of the ipsilateral common carotid in the wild-type mice, whereas the common carotid artery of eNOS knockout mice did not remodel to shrink in order to accommodate the decreased blood flow but rather exhibited significant intimal hyperplasia.
Measurement of Endothelial Function
While atherosclerosis is associated with a broad alteration in endothelial phenotype, the assessment of endothelium-dependent vasodilator function of peripheral arteries has emerged as an accessible indicator of endothelial health (20). In particular, stimuli that increase production of endothelium-derived NO have proved useful in assessing endothelium-dependent vasodilation in humans. Such stimuli include increased shear stress resulting from augmented blood flow and receptor-dependent agonists, such as acetylcholine, bradykinin, or substance P. In healthy individuals, the endothelium responds to these stimuli by releasing vasodilator factors, particularly NO. Earlier studies demonstrated that patients with angiographically proven coronary artery disease (CAD) display impaired FMD (8) and a vasoconstrictor response to acetylcholine rather than the normal vasodilator response, likely reflecting loss of NO and unopposed constrictor effects of acetylcholine on vascular smooth muscle (59). Correlations exist between peripheral artery and coronary artery endothelial function (8). There has been considerable interest in noninvasive examination of endothelium-dependent FMD of the peripheral arteries using vascular ultrasound due to the ready accessibility of the brachial artery (20). Other more classical and invasive but also more recent yet less validated techniques to measure endothelial function, including epicardial coronary vasorectivity, venous occlusion plethysmography, or the Endo PAT system, respectively, were recently reviewed by Flammer et al. (25).
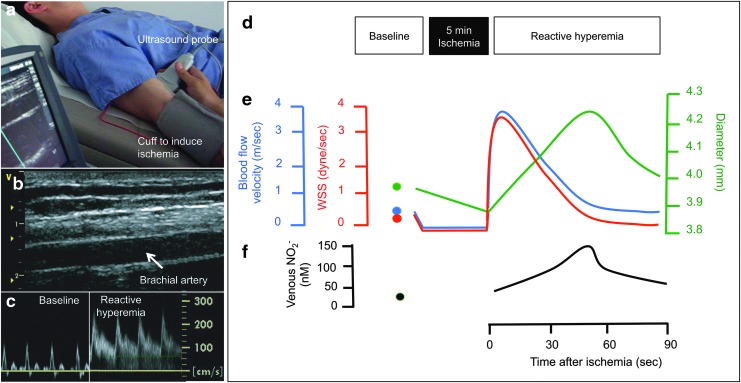
Celermajer et al. established a non-invasive method to measure FMD of the brachial artery using ultrasound (18). Later, the reproducibility was increased enormously by introduction of an automated edge-detection system to more observer-independently quantitate the diameter of the brachial artery (72). Briefly, FMD measures the percent diameter gain as calculated based on pre and postischemia (and after reactive hyperemia) diameter measurements of the brachial artery (20). In this context, ischemia is induced through vessel occlusion by means of inflating a blood pressure cuff around the forearm or upper arm (Fig. 3). Ischemic dilation of downstream resistance vessels leads to increased blood flow and shear stress in the upstream conduit brachial artery. Shear stress stimulates endothelial NO synthase and NO dilates underlying smooth muscle cells via activation of guanylyl cyclase. Under standard conditions, brachial artery FMD is largely mediated by NO synthesis and is, therefore, used as a functional NO readout (29). The absolute values may vary depending on various factors, including the position of the cuff, site of artery used to measure diameter, time of ischemia, and time point of measurement after ischemia. For instance, when the blood pressure cuff for the induction of brachial artery vasodilation is positioned around the upper arm, FMD is significantly greater and, to a larger degree, mediated by eNOS-independent factors as opposed to reactive hyperemia being induced by distal placement of the cuff at the forearm (29). Comparisons of FMD values should be made with caution and ideally refer to values of a control group measured with the identical setup. This technique can be safely applied to large and diverse groups of patients, including children (63). Repeated measurements can be made over time without side effects. As in the coronary circulation, endothelial function in the brachial circulation is impaired in the setting of traditional and novel risk factors and responds to interventions known to reduce cardiovascular risk. Studies suggesting that endothelial function detected non-invasively in the brachial artery correlates with function in other conduit arteries, including the coronaries demonstrating the systemic nature of endothelial dysfunction (100). Although the resolution of ultrasound and image analysis systems have greatly improved, one of the major limitations of FMD is the considerable dependence on the observer requiring well-trained personnel to perform and critically review ultrasound images (71). It was recently demonstrated that when applying rigorous standard operating procedures, training, and quality control, reproducible and comparable FMD measurements can be achieved for short- and medium-term evaluation even in a multicenter setting, justifying the use of FMD as an outcome measure for short- and medium-term assessment of pharmacological interventions (19). While several ex vivo and in vivo experimental models are available to measure vasodilator functions, including aortic rings, cremaster, or mesenteric artery arteriography (57), only a few can be easily translated to human findings. We have published a rodent model allowing FMD measurements of the femoral artery in living rats (41) and mice (unpublished). Although it is believed that the measurement of endothelium-dependent vasodilation can be used as a marker of endothelial function per se, this has not been proved and it is likely that a more diversified definition of endothelial function will arise in the future. Besides measurements of endothelium-dependent vasodilation, a plethora of models that reflect other aspects of endothelial function, including barrier function (66), arteriogenesis, and angiogenesis (55) models, are available, but will not be discussed in this review.
FIG. 3.
Measurement of endothelial function in humans as flow-mediated vasodilation (FMD). (a) Setup: FMD is measured by ultrasound of the brachial artery, und reactive hyperemia is induced by 5 min inflation of a forearm blood pressure cuff. (b) Original B-mode ultrasound image of brachial artery. (c) Pulsed-wave Doppler measurements of blood flow velocity in the brachial artery at baseline and at the onset of reactive hyperemia after 5 min of lower arm ischemia. (d) Measurements for FMD are taken after 5 min of supine rest and during reactive hyperemia after 5 min of forearm ischemia. (e) Ischemia leads to vasodilation of resistance arteries in the forearm. At deflation of the forearm blood pressure cuff, the blood flow velocity increases and this leads to increased shear stress (WSS=wall shear stress). This leads to a vasodilation of the brachial artery with a maximum at ∼60 s. (f) The brachial artery vasodilation is caused by an increased activity of eNOS, which is reflected by increased concentrations of the NO metabolite nitrite in the draining vein (74).
Pathophysiology of Endothelial Dysfunction, Cardiovascular Health, and Disease
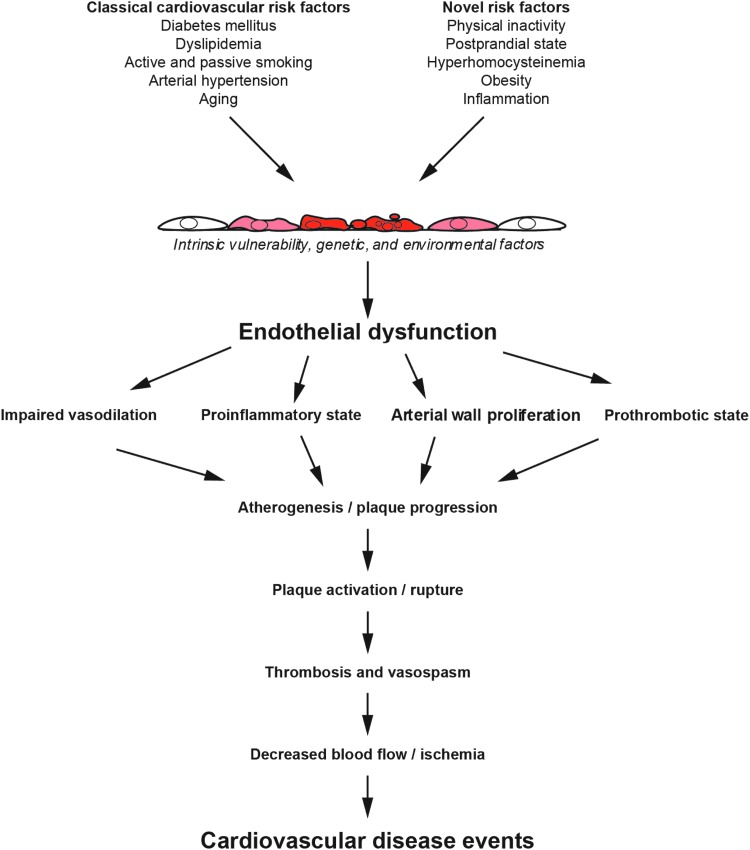
In the presence of risk factors for CVD, concerted with a genetic disposition and environmental factors, the arterial endothelium loses its normal regulatory function for vessel wall homeostasis, a concept termed “endothelial dysfunction” (100) (Fig. 4). The development and clinical manifestations of atherosclerosis include stable and unstable angina, acute myocardial infarction, claudication, and stroke. These outcomes correlate with and are preceded by a loss of endothelial control of vascular tone, thrombosis, and the composition of the vascular wall. The severity of endothelial dysfunction relates to a patient's risk for experiencing an initial or recurrent cardiovascular event (70, 73).
FIG. 4.
Pathophysiology of endothelial dysfunction. Adapted from Widlansky et al. (100).
Both traditional and novel CVD risk factors initiate a chronic inflammatory process that is accompanied by a loss of vasodilator and anti-thrombotic factors and an increase in vasoconstrictor and pro-thrombotic products (84). Risk factors as diverse as smoking (active and passive) (32), aging (36), hypercholesterolemia, hypertension, hyperglycemia, and a family history of premature atherosclerotic disease are associated with an attenuation or loss of endothelium-dependent vasodilation in both adults and children (18). More recently, recognized risk factors such as obesity, elevated C-reactive protein, postprandial state, hyperhomocysteinemia, and chronic systemic infection are also associated with endothelial dysfunction (100). A growing number of interventions known to decrease cardiovascular risk, including diets rich in fruit and vegetables, exercise, smoking cessation, weight reduction, or medication with angiotensin converting enzyme (ACE) inhibitors (7) and statin (81) administration, will also improve endothelial function (100). Therefore, endothelial function is viewed as a “barometer” for cardiovascular health that can be used for evaluation of novel therapeutic strategies aimed at improving vascular function (98).
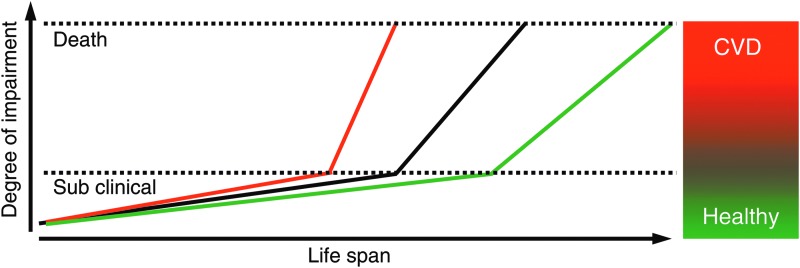
The clinical relevance of endothelial homeostasis lies in the fact that it may be the central biological switch at the intersection of health and disease. The understanding of health has undergone a paradigm shift. The broad term of health is classically defined by the World Health Organization (WHO) as “a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity.” Recently, the American Heart Association (AHA) has introduced the concept of cardiovascular health (102). According to AHA, cardiovascular health is defined as the absence of clinical CVDs, including CAD, cerebrovascular or peripheral artery disease, and a specific metrics allows grading of cardiovascular health into the categories ideal, intermediate, and poor cardiovascular health according to the seven most important key health factors and behaviors: blood pressure, cholesterol, smoking status, healthy diet, physical activity, healthy weight, and blood glucose. This is based on the fact that these factors are the key drivers of CVD development long before clinical symptoms occur which promote intimal hyperplasia and plaque development. These associated changes usually only cause little or no impairment of life quality or physical performance (Fig. 5). In most cases, CVD becomes clinically apparent when a major adverse cardiovascular event (MACE), that is, acute coronary syndrome or stroke, occurs. After the first MACE, the impairment usually more rapidly progresses and risk to die from cardiovascular cause becomes very high, that is, >20% older than 10 years (69). The factors that promote progression of disease, including further plaque development, destabilization, and rupture, remain the same. Due to the fact that the processes that initiate and promote the progression of CVD are very similar, professional societies, including the European Society of Cardiology, have published prevention guidelines which no longer strictly distinguish between primary and secondary prevention (69). Therapeutically targeting the factors mentioned earlier along with medical therapy is the main stake of primary and secondary prevention aiming at increasing not only lifespan, but also, more importantly, healthy life span (“health span”). Taken together, these factors appear to converge both in positive and in negative ways, on the vascular endothelium, with profound effects on both health span and total life span, rendering endothelial function the key target of CVD prevention.
FIG. 5.
Concept of health and disease. The course of life is accompanied by a gradually increasing degree of physical impairment (black line). For a long time, this remains clinically silent despite subtle changes in the vascular system, including endothelial dysfunction, vascular stiffening, or plaque build-up in arteries. However, humans are “healthy” until a major adverse cardiovascular event, mostly a heart attack, occurs. From this point on, cardiovascular disease (CVD) progresses until death. This is promoted by cardiovascular risk factors (red line). It is one of the major concepts of cardiovascular medicine to prevent disease and death by interfering with the underlying processes, that is, endothelial dysfunction (green line).
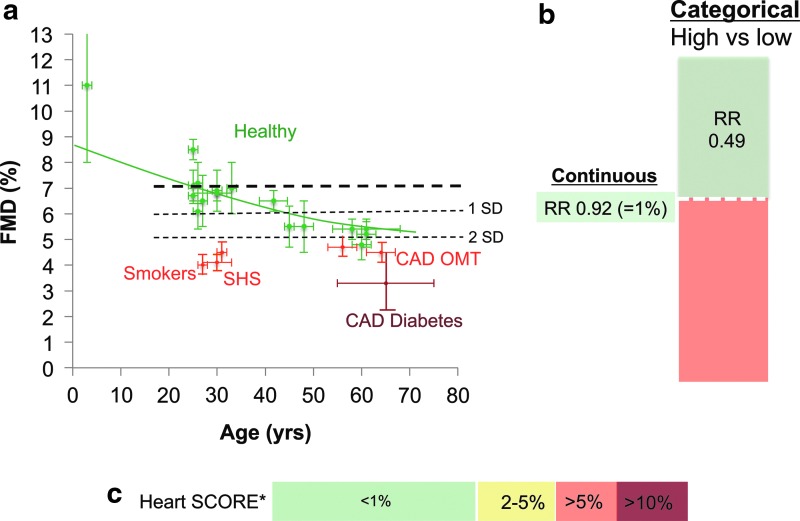
The prognostic value of endothelial functional measurements was analyzed in a meta-analysis, including a total of 23 studies including 14,753 subjects that have addressed the predictive value of endothelial function as measured by brachial artery FMD (73). For studies reporting continuous risk estimates, the pooled overall CVD risk was 0.92 (95% CI: 0.88; 0.95) per 1% higher FMD (Fig. 6). The observed association seemed stronger (p<0.01) in diseased populations than in asymptomatic populations (0.87 [95% CI: 0.83; 0.92] and 0.96 [95% CI: 0.92; 1.00] per 1% higher FMD, respectively). For studies reporting categorical risk estimates, the pooled overall CVD risk for high versus low FMD was on average 0.49 (95% CI: 0.39; 0.62). However, the incremental value of FMD measurements in addition to classical cardiovascular risk prediction models, including the Framingham Risk Score or Heart SCORE, has only been evaluated in a limited number of studies (70). In one study in male subjects at intermediate risk, the addition of FMD using 4.75% as a cut-off value increased the AUC (c-statistic) from 0.68 to 0.76. In the Cardiovascular Health Study and Multi-Ethnic Study of Atherosclerosis (MESA), no changes in the c-statistic were reported after the addition of FMD to the basic prediction model. The MESA Study showed that the addition of FMD to the model reclassified 29% of the individuals into appropriate risk categories (p<0.0001), and was most apparent in those at intermediate risk (Net reclassification improvement in intermediate risk group: 28%, p<0.0001). The authors of the meta-analysis concluded that these results need to be replicated in other cohorts and that the interobserver and intraobserver variability of FMD measurements should decrease before implementation of FMD as a formal screening tool for CVD risk could be justified (70).
FIG. 6.
Age-dependent decline in endothelial function and cardiovascular risk.(a) Age-dependent decline in FMD in healthy male volunteers (32, 36, 38–40, 46, 52, 63, 65, 74, 75, 82, 88) (green symbols) studied in our institution using the identical standardized methodology. Decreased values in smokers (34, 37) and non-smokers exposed to second-hand smoke (32) and patients with coronary artery disease (CAD) (40, 47, 75), with diabetes mellitus (10, 50) and on optimal medical therapy (OMT) (35, 40). (b) Relative cardiovascular risk (RR) associated with continuous (e.g., 1% absolute difference) and categorical differences (“high vs. low” populations) in FMD values according to a recent meta-analysis (73). (c) Cardiovascular risk (10 year cardiovascular mortality) associated with age according to heart SCORE in male non-smokers with optimal blood pressure and cholesterol in a low-risk European country (69).
Endothelial Injury, Circulating Endothelial MPs and Cells
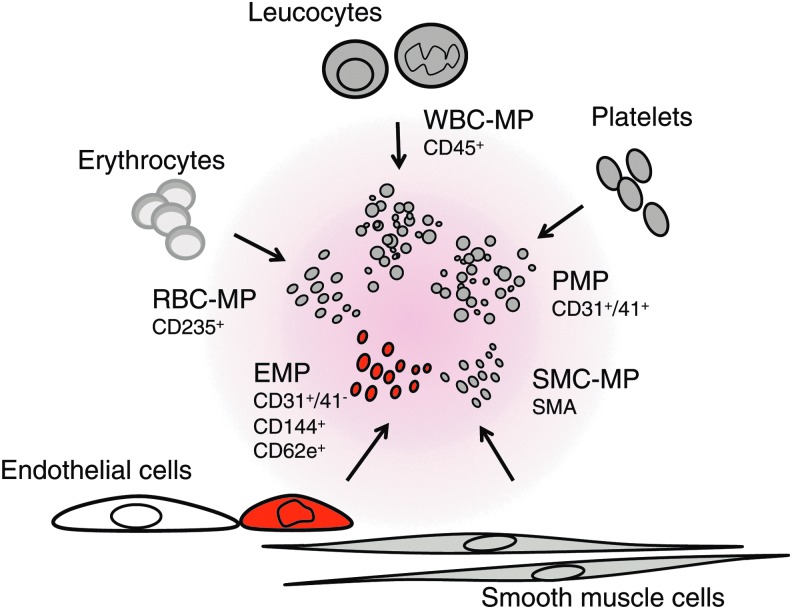
Endothelial microparticles (EMPs) are emerging markers of endothelial injury and may contribute to the progression of CVD as mediators of endothelial dysfunction. EMPs are membrane particles of less than a micrometer in diameter, carry endothelial surface markers (14, 62, 79) and enzymes, including eNOS (47). Besides EMPs, MPs exist in human plasma that stem from platelets, erythrocytes, leucocytes, and smooth muscle cells and can be distinguished due to their surface marker expression reminiscent of their origin (Fig. 7). Typical surface markers used to identify EMPs include CD31, CD62e, CD144, CD105, CD106, and CD146. The current knowledge on MP formation derives mainly from experiments on isolated or cultured cells showing that both, cell activation and cell apoptosis, can lead to MP release by increasing intracellular calcium, loss in membrane lipid asymmetry, and cytoskeleton protein reorganization (91). Vion et al. have recently identified endothelial shear stress as a physiological regulator of MP release (97). They demonstrated that sustained atheroprone low shear stress stimulates EMP release through activation of Rho kinases and ERK1/2 pathways, whereas atheroprotective high shear stress limits EMP release in an NO-dependent regulation of ABCA1 expression and of cytoskeletal reorganization. A different mode of mobilization due to cell activation, for example, by tumor necrosis factor-α rather involved p38-MAP kinase and nuclear factor κB activation. Some authors argue that phosphatidyl serine exposure mediating annexin V binding can be seen as an indicator of apoptosis being involved in their release (86).
FIG. 7.
Cellular origin of circulating microparticles.MPs are shed from a variety of cells and retain surface markers from their parent cells, enabling their identification.
Circulating levels of EMPs increase in plasma early in atherosclerotic processes, correlate with the degree of endothelial dysfunction (100), and have been established as prognostic biomarkers that predict adverse cardiovascular outcome (5, 6, 54, 90). Cardiovascular risk factors may trigger endothelial MP release (3, 79, 92). For instance, smoking, as well as second-hand smoking, forced physical inactivity, and increased blood pressure were associated with increases in circulating EMPs in healthy subjects (28, 32, 64). Increases in plasma endothelial MPs were also observed after high-fat meals with augmented circulating levels of modified low density lipoprotein and triglycerides (24). Conversely, Mediterranean diet was shown to lower levels of circulating EMPs in healthy elderly subjects, indicating that diet can improve endothelial integrity or prevent endothelial damage (31, 61, 95).
However, in addition to being a marker of endothelial integrity, EMPs were shown to play an active role in the induction of endothelial dysfunction. Both in vivo–and in vitro–generated isolated EMPs were shown to impair endothelial function by decreasing the production of NO when incubated with rat aortic rings ex vivo (4, 11, 13). Therefore, the decreased presence of EMPs in circulation as observed in some studies might contribute to the improvement of endothelial dysfunction (46).
During their formation, MPs retain surface molecules from parent cells, as well as a part of their cytosolic content (proteins, RNA, and microRNA). We have recently shown that functional eNOS exists in MPs from subjects with normal endothelial function which was significantly lower in patients with CAD and endothelial dysfunction (46, 47). Whether eNOS is only present in circulating EMPs or all MP subpopulations and has a physiological and potentially beneficial role in healthy subjects remains to be shown. Besides this, MPs have been proposed to be potential carriers of cellular information via their protein, lipid, or nucleic acid content that can be transported into other cells and induce effects, including transfer of miRNA from endothelial to smooth muscle cells (58, 79).
Regenerative Capacity of the Endothelium and CACs
The regenerative capacity of the endothelium is key to the maintenance of its function. Successful endothelial regeneration is crucial for the prevention of atherosclerotic lesion formation and vascular regeneration (23, 83). CACs, previously referred to as early EPCs (45), are key players in vascular repair, and represent a key contributing factor with regard to the maintenance of endothelial function (23). Almost two decades ago, it was assumed that endothelial repair mechanisms mainly comprised proliferation and migration processes that involve mature ECs. However, more current studies demonstrated that bone marrow-derived cells, including CACs, essentially contribute to the regeneration and maintenance of endothelial function. CACs have the ability to home to sites of ischemic damage and endothelial injury, and may promote angiogenesis and endothelial repair without differentiating into mature ECs themselves (80). It is currently believed that these cells contribute at the site of regeneration to the endothelial maintenance and healing via production of factors, including not only VEGF, SDF-1α, and MCP-1 but also NO. We and others have previously shown that the presence of cardiovascular risk factors is associated with a reduced number and function of CACs, as well as with endothelial dysfunction (36, 44, 96). In patients with CAD, a low number of CACs has been shown to predict an increased risk of experiencing cardiovascular events (99). Such learning has led to the successful therapeutic use of CACs and different types of circulating progenitor cells in animal models of CVDs (60, 89) as well as in a human context (12). Primary and secondary preventive life-style intervention therapy, including smoking cessation, exercise training, and a dietary flavanol or nitrate intervention, as well as statin therapy have been shown to enhance endogenous endothelial repair mechanisms, including CACs mobilization (35, 51, 53). Despite these promising results, the field of CAC research suffers from the fact that no gold standard exists to quantify CACs in vivo and to determine their proangiogenic capacity (103).
Interestingly, eNOS appears to be an important modulator of CAC function similar to ECs (40) and mobilization from the bone marrow (2). We have shown that eNOS activity is required for CAC chemotaxis (40). Furthermore, in CAD patients, impairment of NOS expression and NO bioavailability, rather than response to NO, may contribute to dysfunction of CACs and limit their regenerative capacity. Furthermore, eNOS appears to play an essential role in the release of these cells from the bone marrow via activation of MMP-2 and 9 (1). Therefore, therapeutic approaches that improve endothelial function or factors which induce endothelial dysfunction may also affect CAC function.
Endothelial Homeostasis as a Therapeutic Target
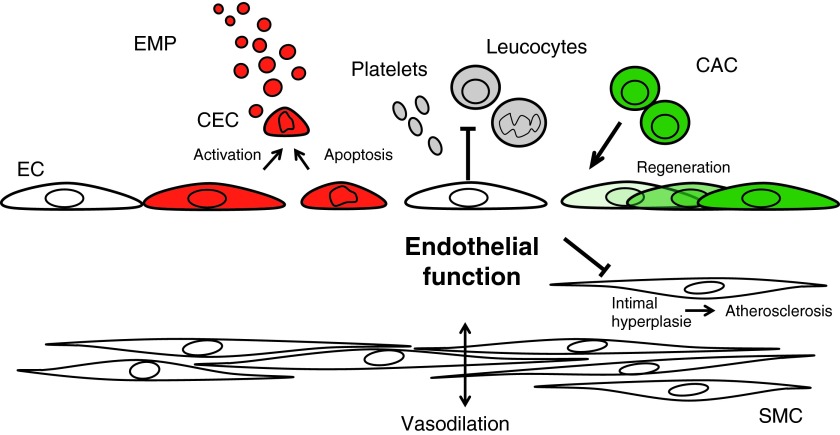
The vascular endothelium has emerged as a dynamic organ that responds to environmental factors (Fig. 8). According to the response to injury theory, mechanical injury and exposure to cardiovascular risk factors impairs the regulatory functions of the endothelium and progresses toward a pro-inflammatory phenotype, senescence, and apoptosis (83). Along with endothelial functional impairment, the endothelial integrity can be disrupted with ECs or a part of these cells being detached and released into the circulation (101). Therefore, EMPs can be viewed as circulating markers of a compromised endothelial integrity that are released from activated and apoptotic ECs (14, 62). In order to maintain endothelial integrity and functionality, the endothelium constantly regenerates by proliferating, migrating, and with the support of circulating cells, for example, CACs. Dignat-George and coworkers have proposed the concept of “vascular competence” (86). The authors suggest that regenerative versus degenerative endothelial responses can be integrated in a clinical endothelial phenotype, reflecting the net result between damage from risk factors and endogenous repair capacity (i.e., EMPs and CACs, respectively). This endothelial phenotype characterizes a vascular status that could define the “vascular competence” of each individual. As important examples, we will discuss this concept in the context of modifiable environmental cardiovascular risk factors such as smoking and diet. However, similar arguments could be made for the treatment with statins or ACE inhibitors in the context of primary and secondary prevention.
FIG. 8.
Concept of endothelial functional integrity. Endothelial homeostasis can be conceptualized as the successful balance between endothelial injury and regeneration enabling optimal endothelial function. It is one of the primary functions of the endothelium to inhibit intimal hyperplasia and atherosclerosis. Endothelial function can be monitored as FMD.
Smoking
It is known for a long time that not only cigarette smoking but also second-hand smoke exposure leads to accelerated development of CVD and mortality (49). Cigarette smoking and exposure to environmental cigarette smoke are associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults (16, 17). A part of these detrimental effects of cigarette smoke were ascribed to uncoupling of eNOS due to decreased availability of tetrahydrobiopterin, an important cofactor of eNOS (43). In order to investigate the effects of second-hand smoke, we exposed healthy young non-smokers to 30 min of environmental cigarette smoke (32). In this study, second-hand smoke acutely increased EMPs and decreased FMD, suggesting a significant endothelial activation and injury with functional impairment of the vascular endothelium. CAC and plasma vascular endothelial growth factor also increased acutely. This could be interpreted as an endogenous regenerative response. However, the mobilized CACs were functionally severely impaired and chemotaxis was completely abolished in these cells during 24 h after exposure. Exposure to smoke-free air had no effect. Incubation of CACs from non-exposed subjects with plasma isolated from subjects exposed to second-hand smokers decreased chemotaxis in vitro by blockade of vascular endothelial growth factor-stimulated NO production (43). We have recently confirmed this in subjects undergoing radial artery injury due to transradial coronary catheterization. In smokers, the recovery of local endothelium-dependent vasodilation after radial injury was significantly slower (33). Active cigarette smokers had functionally impaired CACs and exhibited significantly greater intimal hyperplasia at 6–10 months at the site of injury than age-matched non-smokers (87). Taken together, cigarette smoke appears to not only acutely injure the vascular endothelium and block eNOS activity, but also interfere with the vascular regenerative capacity.
Healthy diet
It is believed that diets, that is, Mediterranean diet, and dietary compounds such as flavonoids can have positive cardiovascular health effects. One study suggests that diet may be capable of improving endothelial phenotype in humans, thus re-establishing “vascular competence” (61). In this randomized, cross-over dietary intervention study, the authors show that a 1 month Mediterranean diet, that is, a diet rich in fruits and vegetables (which, in turn, is high in flavonoids) can improve ischemic reactive hyperemia and that this was associated with a decrease in the levels of circulating EMPs and increase in CACs in healthy elderly subjects (61). A more recent study of our group yielded similar responses in CAD patients on optimal medical therapy after 1 month of cocoa flavanol (CF) intervention. In this study, we observed a significant improvement in endothelial function as measured by FMD, increase in plasma nitrite indicative of increased NO availability, and decrease in systolic blood pressure (35). These positive effects were associated with mobilization of CACs (35) and a decrease in EMPs (46), suggesting that CF are capable of improving vascular functional competence by re-establishing endothelial homeostasis.
Conclusion
Both cardiovascular risk and protective factors converge on the vascular endothelium via interaction with injurious effects and regenerative capacity. eNOS expression and activity is intimately involved in the these processes. Given that endothelial phenotypes vary according to time and location in the vascular tree, in both health and disease states, it is essential to modulate therapy to specific vascular beds. Further challenges include reproducibility of endothelial functional measurements and agreement on markers in the context of EMPs and CACs. Conceptually, endothelial homeostasis appears to be the central biological balance, enabling to maintain functional integrity in the short term and prevent CVD in the future.
Abbreviations Used
- ACE
angiotensin converting enzyme
- AHA
American Heart Association
- CAC
circulating angiogenic cell
- CAD
coronary artery disease
- CEC
circulating endothelial cell
- CF
cocoa flavanol
- CVD
cardiovascular disease
- EC
endothelial cells
- EMP
endothelial microparticle
- eNOS
endothelial nitric oxide synthase
- EPC
endothelial progenitor cell
- FMD
flow-mediated vasodilation
- MACE
major adverse cardiovascular event
- MAP
mitogen-activated protein
- MESA
Multi-Ethnic Study of Atherosclerosis
- MP
microparticle
- RBC
red blood cell
- RR
relative risk
- SMC
smooth muscle cell
- WBC
white blood cells
Acknowledgments
The authors are senior investigators in the FLAVIOLA research consortium of the European Union (FP7-KBBE-2008-2B). Funding was provided by the Deutsche Forschungsgemeinschaft (KE405/5-1 to M.K. and IRTG1902 to M.K. and C.H.), and the Forschungskommission of the Medical Faculty, Heinrich-Heine University Duesseldorf, to C.H. and A.R.M. M.K., A.R.M. and C.H. have received unrestricted research grants from Mars, Inc.
References
- 1.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, and Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med 9: 1370–1376, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Aicher A, Zeiher AM, and Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension 45: 321–325, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Amabile N, Cheng S, Renard JM, Larson MG, Ghorbani A, McCabe E, Griffin G, Guerin C, Ho JE, Shaw SY, Cohen KS, Vasan RS, Tedgui A, Boulanger CM, and Wang TJ. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. Eur Heart J 35: 2972–2979, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, London GM, Tedgui A, and Boulanger CM. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol 16: 3381–3388, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Amabile N, Guerin AP, Tedgui A, Boulanger CM, and London GM. Predictive value of circulating endothelial microparticles for cardiovascular mortality in end-stage renal failure: a pilot study. Nephrol Dial Transplant 27: 1873–1880, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Amabile N, Heiss C, Chang V, Angeli FS, Damon L, Rame EJ, McGlothlin D, Grossman W, De Marco T, and Yeghiazarians Y. Increased CD62e(+) endothelial microparticle levels predict poor outcome in pulmonary hypertension patients. J Heart Lung Transplant 28: 1081–1086, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Anderson TJ, Elstein E, Haber H, and Charbonneau F. Comparative study of ACE-inhibition, angiotensin II antagonism, and calcium channel blockade on flow-mediated vasodilation in patients with coronary disease (BANFF study). J Am Coll Cardiol 35: 60–66, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delegrange D, Lieberman EH, Ganz P, Creager A, Yeung AC, and Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulation. J Am Coll Cardiol 26: 1235–1241, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Balligand JL, Feron O, and Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev 89: 481–534, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Balzer J, Rassaf T, Heiss C, Kleinbongard P, Lauer T, Merx M, Heussen N, Gross HB, Keen CL, Schroeter H, and Kelm M. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. J Am Coll Cardiol 51: 2141–2149, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Boulanger CM, Scoazec A, Ebrahimian T, Henry P, Mathieu E, Tedgui A, and Mallat Z. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation 104: 2649–2652, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, Vogl TJ, Martin H, Schachinger V, Dimmeler S, and Zeiher AM. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation 108: 2212–2218, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Brodsky SV, Zhang F, Nasjletti A, and Goligorsky MS. Endothelium-derived microparticles impair endothelial function in vitro. Am J Physiol Heart Circ Physiol 286: H1910–H1915, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Burnier L, Fontana P, Kwak BR, and Angelillo-Scherrer A. Cell-derived microparticles in haemostasis and vascular medicine. Thromb Haemost 101: 439–451, 2009 [PubMed] [Google Scholar]
- 15.Busse R. and Fleming I. Pulsatile stretch and shear stress: physical stimuli determining the production of endothelium-derived relaxing factors. J Vasc Res 35: 73–84, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, and Deanfield JE. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med 334: 150–154, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, and Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 88: 2149–2155, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Celermajer DS, Sorensen KE, Gooch VM, Spiegelthaler DJ, Miller OI, Sullivan ID, Lloyd JK, and Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Charakida M, de Groot E, Loukogeorgakis SP, Khan T, Luscher T, Kastelein JJ, Gasser T, and Deanfield JE. Variability and reproducibility of flow-mediated dilatation in a multicentre clinical trial. Eur Heart J 34: 3501–3507, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Charakida M, Masi S, Luscher TF, Kastelein JJ, and Deanfield JE. Assessment of atherosclerosis: the role of flow-mediated dilatation. Eur Heart J 31: 2854–2861, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Cortese-Krott MM, Rodriguez-Mateos A, Sansone R, Kuhnle GG, Thasian-Sivarajah S, Krenz T, Horn P, Krisp C, Wolters D, Heiss C, Kroncke KD, Hogg N, Feelisch M, and Kelm M. Human red blood cells at work: identification and visualization of erythrocytic eNOS activity in health and disease. Blood 120: 4229–4237, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, and Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Dimmeler S. and Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med 82: 671–677, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Ferreira AC, Peter AA, Mendez AJ, Jimenez JJ, Mauro LM, Chirinos JA, Ghany R, Virani S, Garcia S, Horstman LL, Purow J, Jy W, Ahn YS, and de Marchena E. Postprandial hypertriglyceridemia increases circulating levels of endothelial cell microparticles. Circulation 110: 3599–3603, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, and Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation 126: 753–767, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furchgott RF. and Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980 [DOI] [PubMed] [Google Scholar]
- 27.Gaenzer H, Neumayr G, Marschang P, Sturm W, Kirchmair R, and Patsch JR. Flow-mediated vasodilation of the femoral and brachial artery induced by exercise in healthy nonsmoking and smoking men. J Am Coll Cardiol 38: 1313–1319, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Gordon C, Gudi K, Krause A, Sackrowitz R, Harvey BG, Strulovici-Barel Y, Mezey JG, and Crystal RG. Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am J Respir Crit Care Med 184: 224–232, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green DJ, Jones H, Thijssen D, Cable NT, and Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension 57: 363–369, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Gulino-Debrac D. Mechanotransduction at the basis of endothelial barrier function. Tissue Barriers 1: e24180, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison M, Murphy RP, O'Connor PL, O'Gorman DJ, McCaffrey N, Cummins PM, and Moyna NM. The endothelial microparticle response to a high fat meal is not attenuated by prior exercise. Eur J Appl Physiol 106: 555–562, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Heiss C, Amabile N, Lee AC, Real WM, Schick SF, Lao D, Wong ML, Jahn S, Angeli FS, Minasi P, Springer ML, Hammond SK, Glantz SA, Grossman W, Balmes JR, and Yeghiazarians Y. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. J Am Coll Cardiol 51: 1760–1771, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Heiss C, Balzer J, Hauffe T, Hamada S, Stegemann E, Koeppel TA, Merx M, Rassaf T, Kelm M, and Lauer T. Vascular dysfunction of brachial artery following transradial access for coronary catheterization: impact of smoking and catheter changes. JACC Cardiovasc Interv 2: 1067–1073, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Heiss C, Finis D, Kleinbongard P, Hoffmann A, Rassaf T, Kelm M, and Sies H. Sustained increase in flow-mediated dilation after daily intake of high-flavanol cocoa drink over 1 week. J Cardiovasc Pharmacol 49: 74–80, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Heiss C, Jahn S, Taylor M, Real WM, Angeli FS, Wong ML, Amabile N, Prasad M, Rassaf T, Ottaviani JI, Mihardja S, Keen CL, Springer ML, Boyle A, Grossman W, Glantz SA, Schroeter H, and Yeghiazarians Y. Improvement of endothelial function with dietary flavanols is associated with mobilization of circulating angiogenic cells in patients with coronary artery disease. J Am Coll Cardiol 56: 218–224, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, and Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol 45: 1441–1448, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Heiss C, Kleinbongard P, Dejam A, Perre S, Schroeter H, Sies H, and Kelm M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol 46: 1276–1283, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Heiss C, Lauer T, Dejam A, Kleinbongard P, Hamada S, Rassaf T, Matern S, Feelisch M, and Kelm M. Plasma nitroso compounds are decreased in patients with endothelial dysfunction. J Am Coll Cardiol 47: 573–579, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Heiss C, Meyer C, Totzeck M, Hendgen-Cotta UB, Heinen Y, Luedike P, Keymel S, Ayoub N, Lundberg JO, Weitzberg E, Kelm M, and Rassaf T. Dietary inorganic nitrate mobilizes circulating angiogenic cells. Free Radic Biol Med 52: 1767–1772, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Heiss C, Schanz A, Amabile N, Jahn S, Chen Q, Wong ML, Rassaf T, Heinen Y, Cortese-Krott M, Grossman W, Yeghiazarians Y, and Springer ML. Nitric oxide synthase expression and functional response to nitric oxide are both important modulators of circulating angiogenic cell response to angiogenic stimuli. Arterioscler Thromb Vasc Biol 30: 2212–2218, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heiss C, Sievers RE, Amabile N, Momma TY, Chen Q, Natarajan S, Yeghiazarians Y, and Springer ML. In vivo measurement of flow-mediated vasodilation in living rats using high-resolution ultrasound. Am J Physiol Heart Circ Physiol 294: H1086–H1093, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Heiss C, Wong ML, Block VI, Lao D, Real WM, Yeghiazarians Y, Lee RJ, and Springer ML. Pleiotrophin induces nitric oxide dependent migration of endothelial progenitor cells. J Cell Physiol 215: 366–373, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, Meinertz T, and Munzel T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ Res 86: E36–E41, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, and Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348: 593–600, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Hirschi KK, Ingram DA, and Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 28: 1584–1595, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horn P, Amabile N, Angeli FS, Sansone R, Stegemann B, Kelm M, Springer ML, Yeghiazarians Y, Schroeter H, and Heiss C. Dietary flavanol intervention lowers the levels of endothelial microparticles in coronary artery disease patients. Br J Nutr 117: 1245–1252, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Horn P, Cortese-Krott MM, Amabile N, Hundsdorfer C, Kroncke KD, Kelm M, and Heiss C. Circulating microparticles carry a functional endothelial nitric oxide synthase that is decreased in patients with endothelial dysfunction. J Am Heart Assoc 2: e003764, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishida T, Takahashi M, Corson MA, and Berk BC. Fluid shear stress-mediated signal transduction: how do endothelial cells transduce mechanical force into biological responses? Ann N Y Acad Sci 811: 12–23; discussion 23–24, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Kannel WB, D'Agostino RB, and Belanger AJ. Fibrinogen, cigarette smoking, and risk of cardiovascular disease: insights from the Framingham study. Am Heart J 113: 1006–1010, 1987 [DOI] [PubMed] [Google Scholar]
- 50.Keymel S, Heiss C, Kleinbongard P, Kelm M, and Lauer T. Impaired red blood cell deformability in patients with coronary artery disease and diabetes mellitus. Horm Metab Res 43: 760–765, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, Inden Y, and Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol 24: 1442–1447, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Lauer T, Heiss C, Balzer J, Kehmeier E, Mangold S, Leyendecker T, Rottler J, Meyer C, Merx MW, Kelm M, and Rassaf T. Age-dependent endothelial dysfunction is associated with failure to increase plasma nitrite in response to exercise. Basic Res Cardiol 103: 291–297, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jurgens K, Miche E, Bohm M, and Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 109: 220–226, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Lee ST, Chu K, Jung KH, Kim JM, Moon HJ, Bahn JJ, Im WS, Sunwoo J, Moon J, Kim M, Lee SK, and Roh JK. Circulating CD62E+ microparticles and cardiovascular outcomes. PLoS One 7: e35713, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Limbourg A, Korff T, Napp LC, Schaper W, Drexler H, and Limbourg FP. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat Protoc 4: 1737–1746, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Loufrani L. and Henrion D. Role of the cytoskeleton in flow (shear stress)-induced dilation and remodeling in resistance arteries. Med Biol Eng Comput 46: 451–460, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loufrani L, Retailleau K, Bocquet A, Dumont O, Danker K, Louis H, Lacolley P, and Henrion D. Key role of alpha(1)beta(1)-integrin in the activation of PI3-kinase-Akt by flow (shear stress) in resistance arteries. Am J Physiol Heart Circ Physiol 294: H1906–H1913, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Loyer X, Vion AC, Tedgui A, and Boulanger CM. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ Res 114: 345–353, 2014 [DOI] [PubMed] [Google Scholar]
- 59.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, and Alexander RW. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med 315: 1046–1051, 1986 [DOI] [PubMed] [Google Scholar]
- 60.Lyngbaek S, Schneider M, Hansen JL, and Sheikh SP. Cardiac regeneration by resident stem and progenitor cells in the adult heart. Basic Res Cardiol 102: 101–114, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Marin C, Ramirez R, Delgado-Lista J, Yubero-Serrano EM, Perez-Martinez P, Carracedo J, Garcia-Rios A, Rodriguez F, Gutierrez-Mariscal FM, Gomez P, Perez-Jimenez F, and Lopez-Miranda J. Mediterranean diet reduces endothelial damage and improves the regenerative capacity of endothelium. Am J Clin Nutr 93: 267–274, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Martinez MC, Tesse A, Zobairi F, and Andriantsitohaina R. Shed membrane microparticles from circulating and vascular cells in regulating vascular function. Am J Physiol Heart Circ Physiol 288: H1004–H1009, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Natarajan S, Heiss C, Yeghiazarians Y, Fineman JR, Teitel DF, and Tacy TA. Peripheral arterial function in infants and young children with one-ventricle physiology and hypoxemia. Am J Cardiol 103: 862–866, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Navasiolava NM, Dignat-George F, Sabatier F, Larina IM, Demiot C, Fortrat JO, Gauquelin-Koch G, Kozlovskaya IB, and Custaud MA. Enforced physical inactivity increases endothelial microparticle levels in healthy volunteers. Am J Physiol Heart Circ Physiol 299: H248–H256, 2010 [DOI] [PubMed] [Google Scholar]
- 65.Oplander C, Volkmar CM, Paunel-Gorgulu A, van Faassen EE, Heiss C, Kelm M, Halmer D, Murtz M, Pallua N, and Suschek CV. Whole body UVA irradiation lowers systemic blood pressure by release of nitric oxide from intracutaneous photolabile nitric oxide derivates. Circ Res 105: 1031–1040, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Oschatz C, Maas C, Lecher B, Jansen T, Bjorkqvist J, Tradler T, Sedlmeier R, Burfeind P, Cichon S, Hammerschmidt S, Muller-Esterl W, Wuillemin WA, Nilsson G, and Renne T. Mast cells increase vascular permeability by heparin-initiated bradykinin formation in vivo. Immunity 34: 258–268, 2011 [DOI] [PubMed] [Google Scholar]
- 67.Otte LA, Bell KS, Loufrani L, Yeh JC, Melchior B, Dao DN, Stevens HY, White CR, and Frangos JA. Rapid changes in shear stress induce dissociation of a G alpha(q/11)-platelet endothelial cell adhesion molecule-1 complex. J Physiol 587: 2365–2373, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palmer RM, Ferrige AG, and Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526, 1987 [DOI] [PubMed] [Google Scholar]
- 69.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, and Zannad F. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 33: 1635–1701, 2012 [DOI] [PubMed] [Google Scholar]
- 70.Peters SA, den Ruijter HM, and Bots ML. The incremental value of brachial flow-mediated dilation measurements in risk stratification for incident cardiovascular events: a systematic review. Ann Med 44: 305–312, 2012 [DOI] [PubMed] [Google Scholar]
- 71.Preik M, Lauer T, Heiss C, Sonka M, and Kelm M. Automated analysis of ultrasound images to quantify endothelial function of peripheral arteries. Z Kardiol 90 (Suppl. 2): II/226, 2001 [Google Scholar]
- 72.Preik M, Lauer T, Heiss C, Tabery S, Strauer BE, and Kelm M. Automated ultrasonic measurement of human arteries for the determination of endothelial function. Ultraschall Med 21: 195–198, 2000 [DOI] [PubMed] [Google Scholar]
- 73.Ras RT, Streppel MT, Draijer R, and Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol 168: 344–351, 2012 [DOI] [PubMed] [Google Scholar]
- 74.Rassaf T, Heiss C, Hendgen-Cotta U, Balzer J, Matern S, Kleinbongard P, Lee A, Lauer T, and Kelm M. Plasma nitrite reserve and endothelial function in the human forearm circulation. Free Radic Biol Med 41: 295–301, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Rassaf T, Heiss C, Mangold S, Leyendecker T, Kehmeier ES, Kelm M, and Lauer T. Vascular formation of nitrite after exercise is abolished in patients with cardiovascular risk factors and coronary artery disease. J Am Coll Cardiol 55: 1502–1503, 2010 [DOI] [PubMed] [Google Scholar]
- 76.Rassaf T, Kleinbongard P, Preik M, Dejam A, Gharini P, Lauer T, Erckenbrecht J, Duschin A, Schulz R, Heusch G, Feelisch M, and Kelm M. Plasma nitrosothiols contribute to the systemic vasodilator effects of intravenously applied NO: experimental and clinical study on the fate of NO in human blood. Circ Res 91: 470–477, 2002 [DOI] [PubMed] [Google Scholar]
- 77.This reference has been deleted
- 78.Rassaf T, Preik M, Kleinbongard P, Lauer T, Heiss C, Strauer BE, Feelisch M, and Kelm M. Evidence for in vivo transport of bioactive nitric oxide in human plasma. J Clin Invest 109: 1241–1248, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rautou PE, Vion AC, Amabile N, Chironi G, Simon A, Tedgui A, and Boulanger CM. Microparticles, vascular function, and atherothrombosis. Circ Res 109: 593–606, 2011 [DOI] [PubMed] [Google Scholar]
- 80.Rehman J, Li J, Orschell CM, and March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107: 1164–1169, 2003 [DOI] [PubMed] [Google Scholar]
- 81.Reriani MK, Dunlay SM, Gupta B, West CP, Rihal CS, Lerman LO, and Lerman A. Effects of statins on coronary and peripheral endothelial function in humans: a systematic review and meta-analysis of randomized controlled trials. Eur J Cardiovasc Prev Rehabil 18: 704–716, 2011 [DOI] [PubMed] [Google Scholar]
- 82.Rodriguez-Mateos A, Rendeiro C, Bergillos-Meca T, Tabatabaee S, George TW, Heiss C, and Spencer JP. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: a randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am J Clin Nutr 98: 1179–1191, 2013 [DOI] [PubMed] [Google Scholar]
- 83.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362: 801–809, 1993 [DOI] [PubMed] [Google Scholar]
- 84.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 85.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, and Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest 101: 731–736, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sabatier F, Camoin-Jau L, Anfosso F, Sampol J, and Dignat-George F. Circulating endothelial cells, microparticles and progenitors: key players towards the definition of vascular competence. J Cell Mol Med 13: 454–471, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sansone R, Stegemann E, Özaslan G, Schuler D, Lukosz M, Rodriguez-Mateos A, Lauer T, Westenfeld R, Kelm M, and Heiss C. Early and late response-to-injury in patients undergoing transradial coronary angiography: arterial remodeling in smokers. Am J Cardiovasc Dis 4: 47–57, 2014 [PMC free article] [PubMed] [Google Scholar]
- 88.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, and Kelm M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A 103: 1024–1029, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schuh A, Liehn EA, Sasse A, Hristov M, Sobota R, Kelm M, Merx MW, and Weber C. Transplantation of endothelial progenitor cells improves neovascularization and left ventricular function after myocardial infarction in a rat model. Basic Res Cardiol 103: 69–77, 2008 [DOI] [PubMed] [Google Scholar]
- 90.Sinning JM, Losch J, Walenta K, Bohm M, Nickenig G, and Werner N. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur Heart J 32: 2034–2041, 2011 [DOI] [PubMed] [Google Scholar]
- 91.Skeppholm M, Mobarrez F, Malmqvist K, and Wallen H. Platelet-derived microparticles during and after acute coronary syndrome. Thromb Haemost 107: 1122–1129, 2012 [DOI] [PubMed] [Google Scholar]
- 92.Stepien E, Stankiewicz E, Zalewski J, Godlewski J, Zmudka K, and Wybranska I. Number of microparticles generated during acute myocardial infarction and stable angina correlates with platelet activation. Arch Med Res 43: 31–35, 2012 [DOI] [PubMed] [Google Scholar]
- 93.Takahashi M, Ishida T, Traub O, Corson MA, and Berk BC. Mechanotransduction in endothelial cells: temporal signaling events in response to shear stress. J Vasc Res 34: 212–219, 1997 [DOI] [PubMed] [Google Scholar]
- 94.Toborek M. and Kaiser S. Endothelial cell functions. Relationship to atherogenesis. Basic Res Cardiol 94: 295–314, 1999 [DOI] [PubMed] [Google Scholar]
- 95.Tushuizen ME, Nieuwland R, Rustemeijer C, Hensgens BE, Sturk A, Heine RJ, and Diamant M. Elevated endothelial microparticles following consecutive meals are associated with vascular endothelial dysfunction in type 2 diabetes. Diabetes Care 30: 728–730, 2007 [DOI] [PubMed] [Google Scholar]
- 96.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, and Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89: E1–E7, 2001 [DOI] [PubMed] [Google Scholar]
- 97.Vion AC, Ramkhelawon B, Loyer X, Chironi G, Devue C, Loirand G, Tedgui A, Lehoux S, and Boulanger CM. Shear stress regulates endothelial microparticle release. Circ Res 112: 1323–1333, 2013 [DOI] [PubMed] [Google Scholar]
- 98.Vita JA. and Keaney JF., Jr.Endothelial function: a barometer for cardiovascular risk? Circulation 106: 640–642, 2002 [DOI] [PubMed] [Google Scholar]
- 99.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, and Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353: 999–1007, 2005 [DOI] [PubMed] [Google Scholar]
- 100.Widlansky ME, Gokce N, Keaney JF, Jr., and Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 42: 1149–1160, 2003 [DOI] [PubMed] [Google Scholar]
- 101.Woywodt A, Bahlmann FH, De Groot K, Haller H, and Haubitz M. Circulating endothelial cells: life, death, detachment and repair of the endothelial cell layer. Nephrol Dial Transplant 17: 1728–1730, 2002 [DOI] [PubMed] [Google Scholar]
- 102.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, and Hu FB. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA 307: 1273–1283, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yoder MC. Endothelial progenitor cell: a blood cell by many other names may serve similar functions. J Mol Med (Berl) 91: 285–295, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]