Abstract
CXCL4 plays important roles in numerous disease processes, which makes the CXCL4 signaling pathway a potential therapeutic target. In this study, we aimed to develop a neutralizing antibody against both human and mouse CXCL4. Rats were immunized with recombinant human CXCL4 (rhCXCL4). Hybridoma clones were created by fusion of the immunized rat spleen cells with mouse myeloma SP2/0 cells and screened using recombinant mouse CXCL4 (rmCXCL4) and rhCXCL4. The CXCL4 monoclonal antibody (CXCL4 MAb) produced by the 16D6-3 hybridoma clone was sequenced and characterized by Western blot and Biacore assays. It recognized both human and mouse CXCL4 with high affinity and neutralized the effect of rhCXCL4 in vitro. Thus, the antibody may be used in the studies of CXCL4 in murine disease models and as a template in the antibody humanization for clinical developments.
Introduction
CXCL4/PF4 chemokine is a platelet protein with anti-heparin activity containing 70 amino acids.(1) It shares 30–95% amino acid identity and hence shows great overall structural similarity with other members of the CXC chemokine family.(2) During the past decade, CXCL4 has been reported to be involved in numerous long-term regulatory biological processes, such as cell proliferation, differentiation, survival, and apoptosis, by acting on a broad spectrum of different cell types, including monocytes,(3–5) T cells,(6–8) neutrophilic granulocytes,(9,10) and endothelial cells.(11,12) CXCL4 has also been clearly implicated in the inhibition of three classes of megakaryocyte progenitor cells (CFU-MK, mCFU-MK, and BFU-MK)(13,14) and plays a role in the regulation of hematopoiesis.(15)
As one of the most abundant proteins released during platelet activation, CXCL4 has been shown to modulate the inflammatory response in many disease models such as inflammatory bowel disease,(16) malaria,(17) rheumatoid arthritis,(18) and, in particular, early tumor growth of human liposarcoma, mammary adenocarcinoma, and osteosarcoma.(19) Recently, CXCL4 mRNA levels are reported significantly upregulated in Henoch-Schönlein purpura (HSP) peripheral blood mononuclear cells (PBMCs) of patients with kidney damage.(20) The low basal expression of CXCL4 mRNA and protein in intestinal epithelial cells (IEC) are also dramatically increased after 120 min of reperfusion of mice.(21) The mRNA and protein expression of CXCL4 in kidney and gut may be important for mediating tissue homeostasis. Upregulation of CXCL4 in the vessel wall within minutes of platelet attachment to the site of endothelial injury(22) and introduction of a CXCL4 null locus into the ApoE-/- mouse(23) suggest that CXCL4 promotes the development of atherosclerotic lesion. In summary, identification of CXCL4 in different pathological conditions suggests that it may become an interesting target for disease diagnosis or therapy.
Here we describe the development of the CXCL4-specific neutralizing antibody. We have successfully produced and characterized a rat monoclonal anti-mouse and human CXCL4 antibody based on Western blot analysis, Biacore, and functional in vitro assays. It provides a useful tool to evaluate the effect and to measure the level of CXCL4 in numerous biological processes.
Materials and Methods
Materials
Pathogen-free, male BALB/c, nude mice and Sprague-Dawley (SD) rats (8–10 weeks old, SLACCAS, Shanghai, China) were maintained in air-filtered units at 23°C±5°C with 50±15% relative humidity throughout the experimental period. SP2/0 myeloma cell line was purchased from Chinese Academy of Sciences (Shanghai, China).
Establishment of monoclonal antibodies against CXCL4
Rat monoclonal antibodies against human CXCL4 were generated by immunizing SD rats at five sites with 200 μg recombinant human CXCL4 (rhCXCL4) in Freund's complete adjuvant at the ratio (1:1). Reimmunization was accomplished using the same protocol but with the antigen in Freund's incomplete adjuvant once a week for 3 weeks. Testing bleed was performed until serum became positive to the antigen in enzyme-linked immunosorbent assays (ELISAs) against rhCXCL4. Three days after the last injection of the antigen, lymphocytes were isolated from the spleen of the immunized rat and fused with the mouse myeloma cell line SP2/0 in tissue culture. Several hybridoma clones were isolated and established with ELISA against both human and mouse recombinant CXCL4 (4 μg/well). The positive clones were subcloned at least three times using the limiting dilution method. Furthermore, we excluded the His-tag provoked immunogenicity by re-screening the clones that were not recognizing recombinant mouse CXCL14 protein (rmCXCL14) with His-tag. rmCXCL4 also shares 39% amino acid identity with rmCXCL14, which provided additional high specificity to the positive clones. We calculated the ratio of the absorbance of samples and the negative control (P/N), and chose the P/N value of 2 for our cutoff base line.
Antibody production
To produce ascitic fluid, hybridoma cells were injected into the peritoneum of paraffin liquid-primed nude mice. Ascitic fluid was then drained from the peritoneum by using an 18-gauge needle, and the monoclonal antibody (MAb) was purified by protein G affinity chromatography (HiTrap protein G HPcolumn, GE Healthcare, Buckinghamshire, United Kingdom). The MAb concentration was detected according to BCA kit (Beyotime Biotechnology, Haimen, China). The properties of the antibody were analyzed by sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie brilliant blue.
Western blot analysis
rhCXCL4 and rmCXCL4 was loaded in equal amounts and separated by SDS-PAGE, followed by immunoblotting with MAb produced by hybridoma clones for CXCL4. Briefly, samples were mixed with Laemmli buffer, boiled at 95°C for 10 min and loaded onto SDS-PAGE. Proteins were separated by electrophoresis and blotted onto nitrocellulose (Pierce, Rockford, IL). Non-specific binding was reduced by blocking the membrane in 5% non-fat dry milk. The purified antibody (diluted 1:100 in TBS) was applied at 4°C overnight. After washing, the membranes were incubated in peroxidase-coupled goat anti-rat IgG (Beyotime Biotechnology) and were diluted 1:1000 in 5% non-fat dry milk for 1 h at room temperature. After four washes, enhanced chemiluminescence (ECL, Pierce) was applied to the membranes, which were then exposed to an X-ray film (Kodak, Rochester, NY).
Amplification of VL and VH gene fragments and nucleotide sequencing
The total RNA was extracted from 107 cells of hybridoma 16D6-3 with TRIzol reagent (Invitrogen, Carlsbad, CA) and retro-transcripted into cDNA with a retro-transcriptase kit (Toyobo, Osaka, Japan) according to the manufacturer's protocol. The resulting cDNA was split into six tubes (3 for VH and 3 for VL PCR) in equal amount and subjected to amplification: one step of denaturation (95°C, 5 min), 30 cycles (95°C, 30 s; 60°C, 30 s; 72°C, 30 s), and a finishing step (72°C, 10 min). PCR reactions were performed by ExTaq DNA polymerase (Takara Biotechnology, Dalian, China) using the degenerated primers at a concentration of 1 μM each. All forward primers were used separately with a mix of the corresponding backward primers as described previously.(24) The amplified VH and VL genes were cloned into pMD19-T Vectors (Takara Biotechnology), and sequenced using M13 primers (Jie Li Bio., Shanghai, China).
Measurement of affinity and binding kinetics
The Kd of CXCL4 MAb was determined using Biacore 3000, and the data were analyzed using Biaevaluation software, v. 4.1 (Biacore, Piscataway, NJ). Standard EDC/NHS coupling was used to covalently immobilize CXCL4 MAb to CM5 sensor chips. Flow cell 1 was left blank as a negative control. Association rates were measured under continuous flow of 10 μL/min using rhCXCL4 concentrations ranging from 3×10−7 M to 2×10−6 M, plus zero concentration, and the data were fitted using a 1:1 Langmuir binding with no bulk refractive shift.
Cell culture
Human renal cell carcinoma cell line (ACHN) were routinely grown in culture flaks at 37°C in a 95% humidified atmosphere of 5% v/v CO2 in MEM medium supplemented with 5% fetal bovine serum (Hyclone, Logan, UT), 100 IU penicillin, and 100 μg/mL streptomycin (Invitrogen).
Cell inhibition assay
About 3000 ACHN cells were plated per well of a 96-well plate and left overnight for attachment. Cells were incubated with both rhCXCL4 and CXCL4 MAbs at indicated series concentrations. Cells were subjected to MTT survival assay 48 h later.
Statistical analysis
All experiments were performed at least in triplicates. A two-tailed Student's t test was used to establish statistically significant differences between the treatment groups; where applicable, mean±SD of multiple measurements were reported, as indicated.
Results
Production and analysis of monoclonal antibodies against CXCL4
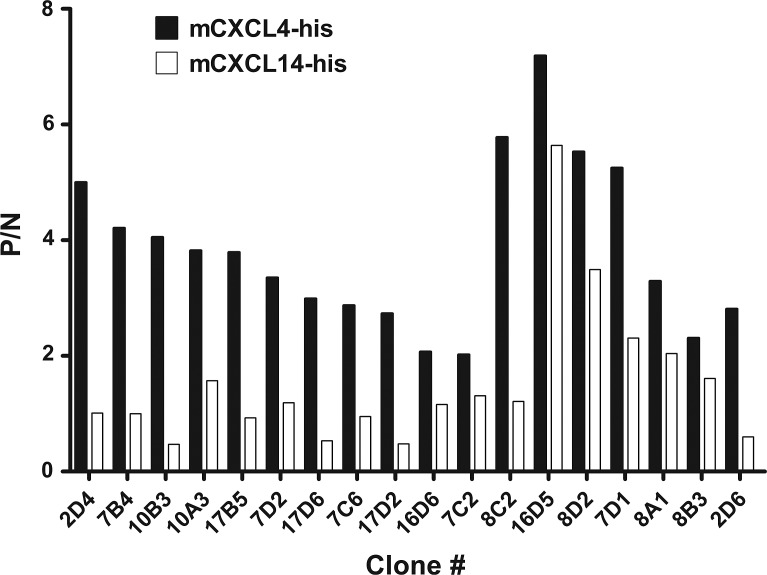
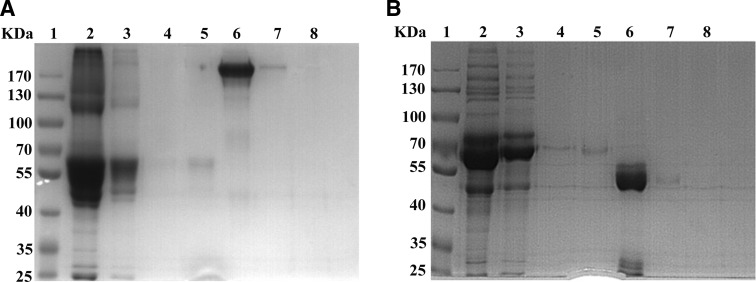
The monoclonal antibody against CXCL4 was obtained by the fusion of SP2/0 myeloma cells and the spleen cells from rhCXCL4 immunized SD rat. From the fusion experiments, hybridomas positive in ELISA against both human and mouse CXCL4 were identified; these mostly produced IgG. Thirty-five clones with the highest OD values in capture ELISA were picked and expanded. Futhermore, we identified several candidate clones that exhibited rmCXCL4 positive but rmCXCL14 negative for excluding the His-tag provoked immunogenicity (Fig. 1). Thus, hybridoma clone 16D6 (i.e., its subclone 16D6-3) and the corresponding antibody 16D6-3 were assigned. The antibody produced in the ascitic fluid was purified by the chromatography on HiTrap protein G HP column and analyzed with SDS-PAGE (Fig. 2).
FIG. 1.
Analysis of anti-CXCL4 antibodies by ELISA. Hybridomas were tested in ELISA using rmCXCL4 (His-tag) and rmCXCL14 (His-tag). Clones exhibiting P/N values (ratio of OD450 of a sample to that of the negative control) >2 were considered positive. Data are from a representative experiment repeated two times independently with similar results.
FIG. 2.
SDS-PAGE analysis of purified anti-CXCL4 monoclonal antibody. Eluted solutions from the protein G affinity column were estimated by Coomassie staining after 8% SDS-PAGE. (A) Non-reducing SDS-PAGE electrophoresis. Lane 2, flow-through fraction; lane 3, wash fraction; lanes 4–8, elution fractions. (B) Reducing SDS-PAGE electrophoresis. Lane 2, flow-through fraction; lane 3, wash fraction; lanes 4–8, elution fractions.
Cloning VH and VL gene fragments of 16D6-3 antibody
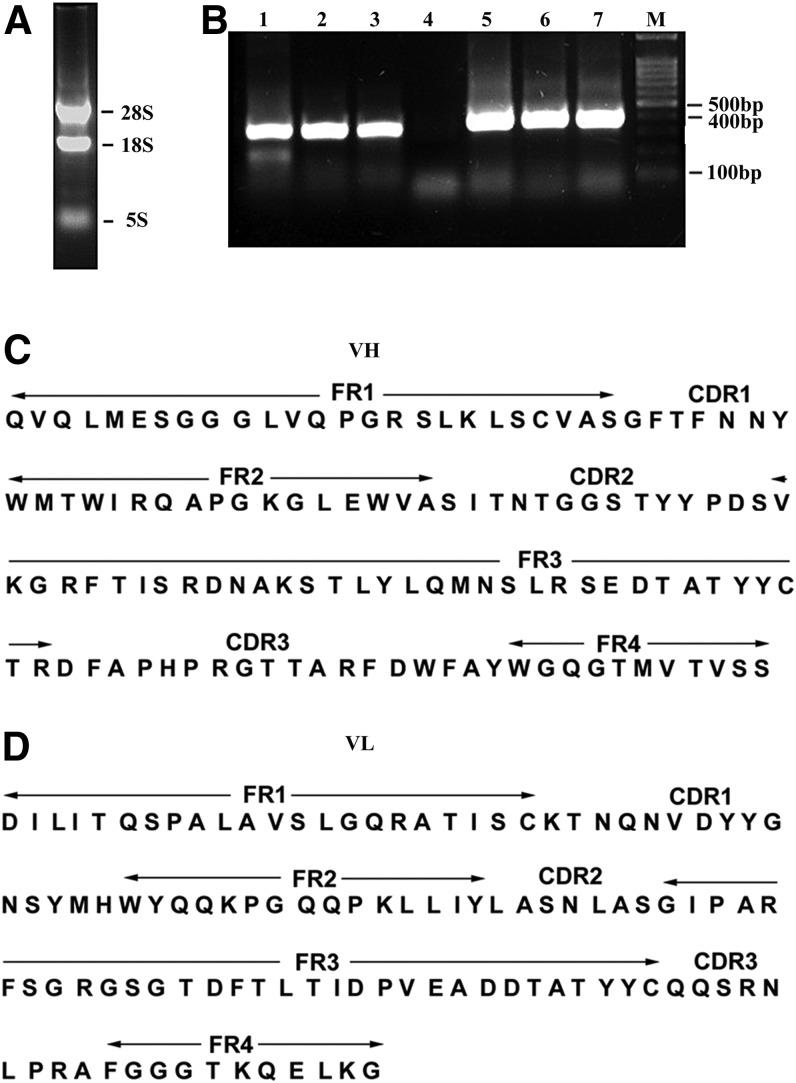
Hybridoma 16D6-3, which secretes a CXCL4 antibody, was established and maintained in our laboratory. The gene encoding 16D6-3 antibody was sequenced. Electrophoresis of the total RNA from hybridoma 16D6-3 showed that there were three bands demonstrating the integrity of the extracted RNA (Fig. 3A). The results of electrophoresis of the PCR products, obtained using the primers VH backward and VH forward to amplify the VH gene fragment from retrotranscripted cDNA and using primers VL backward and VL forward to amplify the VL gene fragment, are shown in Figure 3. The VH and VL gene fragments had the expected molecular sizes of 360 bp and 320 bp, respectively (Fig. 3B). Alignment of the amino acid and DNA sequences with the IMGT databank revealed that the cloned VH and VL genes closely matched the sequence of the variable regions of the rat antibody (Fig. 3C).
FIG. 3.
Cloning and sequence analysis of 16D6-3 antibody. (A) Agarose gel electrophoresis of hybridoma 16D6-3 RNA. (B) Agarose gel electrophoresis of 16D6-3 variable region genes. Lane M, PCR marker; lanes 1–3, VL gene fragment; lane 4, negative control; lanes 5–7, VH gene fragment. (C, D) Amino acid and DNA sequences of 16D6-3 antibody. Rat VH (C) and VL (D) domains contained within the antibody are shown by overlining.
Characterization of CXCL4 monoclonal antibody
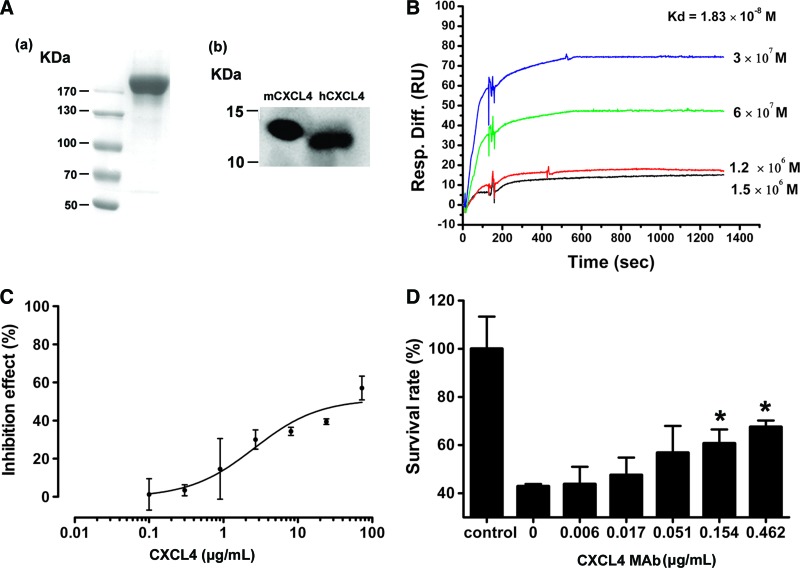
In Western blot analysis, protein G purified 16D6-3 antibody (5.2 mg/mL) strongly reacted against both mouse and human CXCL4 proteins resulting in bands corresponding to a relative molecular weight between 10 and 15 kDa. Furthermore, the binding affinity was analyzed by Biacore. The values for Ka and Kd were calculated from sensorgrams using five concentrations. Fitting the data to a 1:1 binding model yielded an apparent binding affinity of Kd=1.83×10−8 M, obtained from the ratio of the rate constants koff/kon (Fig. 4B).
FIG. 4.
Characterization of CXCL4 MAb. (A) Purified CXCL4 MAb was analyzed by SDS/PAGE (a) and Western blot for reactivity with both rmCXCL4 (left) and rhCXCL4 (right) (b), respectively. 16D6-3 recognized CXCL4 with the molecular weight between 10 and 15 KDa. (B) Affinity measurement of CXCL4 MAb using Biacore. The response signals were obtained during the injection of gradient concentrations of rhCXCL4 (3×10−7 to 2×10−6 M, plus zero concentration) through flow cells immobilized with CXCL4 MAb. The kinetic profiles are shown. Association rates (kon) and dissociation rates (koff) are calculated using the one-to-one Langmuir binding model. The equilibrium dissociation constant (Kd) is derived as the kon/koff ratio. (C) rhCXCL4 inhibited growth of ACHN cells. (D) CXCL4 MAb neutralized effect of CXCL4 in the culture of ACHN cells. ACHN cells were cultured with rhCXCL4 at its IC50 (72.6 μg/mL). CXCL4 MAb inhibited the growth arrest of rhCXCL4 on ACHN in a dose-dependent manner. Data are presented as the mean±SD. **p<0.01 versus CXCL4 MAb of 0. Control refers to ACHN cells without treatment of rhCXCL4 and CXCL4 MAbs.
Because CXCL4 reportedly inhibits the proliferation of ACHN cells by binding CXCR3,(25) CXCL4 MAb was tested for its role in neutralizing rhCXCL4 in the culture of ACHN cell line. As shown in Fig. 4C, CXCL4 (IC50=72.6 μg/mL) induced growth inhibition of the ACHN cell line with a peak response occurring at 72 h at concentrations of 10–100 μg/mL, which was in agreement with the previous report.(25) The inhibition effect of CXCL4 (72.6 μg/mL) was significantly blocked (p<0.01) by CXCL4 MAb on a dose-dependent manner.
Discussion
Our study describes the generation and characterization of a highly specific rat monoclonal antibody against both human and mouse CXCL4. This development is important for the further study of the role of CXCL4 in many biological processes.
In this work, we produced an antibody against CXCL4 by fusing the rat spleen cells with the mouse SP2/0 cells. The 16D6-3 antibody was produced in the ascites and purified by protein-G affinity chromatography. Next, we sequenced the 16D6-3 antibody gene. Blast analysis of the Genebank showed that it matched with a rat immunoglobin protein domain. The complementarity determining regions (CDRs) were identified by examining the sequence in IMGT database. In typical characterizations of rat CDRs, variable fragments containing four frame regions (FRs), three CDRs, and two cysteine residues were identified. The binding affinity of CXCL4 MAb to rhCXCL4 with Kd of 1.83×10−8 M was determined.
CXCL4 is a growth inhibitor of the tumor cell line ACHN.(25) We have chosen this activity as a functional assay to test the antibody for its neutralization property. The activity is believed to be mediated by the chemokine receptor activation. Neutralizing antibodies against CXCR3 blocked CXCL4-induced growth inhibition of the ACHN cell.(25) Our CXCL4 antibody was also shown to be effective in the inhibition of CXCL4-induced growth arrest of ACHN cells.
Increasing data support a central role for platelets and their products in the pathogenesis of many inflammatory diseases.(26–29) Platelet-derived PF4 plays important roles in endothelial cell proliferation and apoptosis, angiogenesis,(11) tumor development,(19) and atherosclerosis.(23,30–33) Recently, it has been reported that PF4 participates in tissue damage, especially as an important mediator of intestine tissue damage.(34) Thus, the monoclonal antibody should be a useful tool for further studies of CXCL4 in murine disease models.
In conclusion, we demonstrated that the antibody produced from the monoclonal hybridoma line 16D6-3 recognized both human and mouse CXCL4. CXCL4 MAb may be used for studies of CXCL4 in vitro and in vivo. In particular, it will be helpful for efficacy studies using murine disease models.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grants 81373467/H3108, 81173113/H3109, and 81273573/H3108).
Author Disclosure Statement
The authors have no financial interests to disclose.
References
- 1.Deutsch E, Johnson SA, and Seegers WH: Differentiation of certain platelet factors related to blood coagulation. Circ Res 1955;3:110–115 [DOI] [PubMed] [Google Scholar]
- 2.Deuel TF, Keim PS, Farmer M, and Heinrikson RL: Amino acid sequence of human platelet factor 4. Proc Natl Acad Sci USA 1977;74:2256–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pervushina O, Scheuerer B, Reiling N, Behnke L, Schroder JM, Kasper B, Brandt E, Bulfone-Paus S, and Petersen F: Platelet factor 4/CXCL4 induces phagocytosis and the generation of reactive oxygen metabolites in mononuclear phagocytes independently of Gi protein activation or intracellular calcium transients. J Immunol 2004;173:2060–2067 [DOI] [PubMed] [Google Scholar]
- 4.Scheuerer B, Ernst M, Durrbaum-Landmann I, Fleischer J, Grage-Griebenow E, Brandt E, Flad HD, and Petersen F: The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood 2000;95:1158–1166 [PubMed] [Google Scholar]
- 5.Fricke I, Mitchell D, Petersen F, Bohle A, Bulfone-Paus S, and Brandau S: Platelet factor 4 in conjunction with IL-4 directs differentiation of human monocytes into specialized antigen-presenting cells. FASEB J 2004;18:1588–1590 [DOI] [PubMed] [Google Scholar]
- 6.Fleischer J, Grage-Griebenow E, Kasper B, Heine H, Ernst M, Brandt E, Flad HD, and Petersen F: Platelet factor 4 inhibits proliferation and cytokine release of activated human T cells. J Immunol 2002;169:770–777 [DOI] [PubMed] [Google Scholar]
- 7.Liu CY, Battaglia M, Lee SH, Sun QH, Aster RH, and Visentin GP: Platelet factor 4 differentially modulates CD4+CD25+ (regulatory) versus CD4+CD25- (nonregulatory) T cells. J Immunol 2005;174:2680–2686 [DOI] [PubMed] [Google Scholar]
- 8.Romagnani P, Maggi L, Mazzinghi B, Cosmi L, Lasagni L, Liotta F, Lazzeri E, Angeli R, Rotondi M, Fili L, Parronchi P, Serio M, Maggi E, Romagnani S, and Annunziato F: CXCR3-mediated opposite effects of CXCL10 and CXCL4 on TH1 or TH2 cytokine production. J Allergy Clin Immunol 2005;116:1372–1379 [DOI] [PubMed] [Google Scholar]
- 9.Petersen F, Bock L, Flad HD, and Brandt E: Platelet factor 4-induced neutrophil-endothelial cell interaction: involvement of mechanisms and functional consequences different from those elicited by interleukin-8. Blood 1999;94:4020–4028 [PubMed] [Google Scholar]
- 10.Petersen F, Ludwig A, Flad HD, and Brandt E: TNF-alpha renders human neutrophils responsive to platelet factor 4. Comparison of PF-4 and IL-8 reveals different activity profiles of the two chemokines. J Immunol 1996;156:1954–1962 [PubMed] [Google Scholar]
- 11.Maione TE, Gray GS, Petro J, Hunt AJ, Donner AL, Bauer SI, Carson HF, and Sharpe RJ: Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science 1990;247:77–79 [DOI] [PubMed] [Google Scholar]
- 12.Gengrinovitch S, Greenberg SM, Cohen T, Gitay-Goren H, Rockwell P, Maione TE, Levi BZ, and Neufeld G: Platelet factor-4 inhibits the mitogenic activity of VEGF121 and VEGF165 using several concurrent mechanisms. J Biol Chem 1995;270:15059–15065 [DOI] [PubMed] [Google Scholar]
- 13.Han ZC, Sensebe L, Abgrall JF, and Briere J: Platelet factor 4 inhibits human megakaryocytopoiesis in vitro. Blood 1990;75:1234–1239 [PubMed] [Google Scholar]
- 14.Lecomte-Raclet L, Alemany M, Sequira-Le Grand A, Amiral J, Quentin G, Vissac AM, Caen JP, and Han ZC: New insights into the negative regulation of hematopoiesis by chemokine platelet factor 4 and related peptides. Blood 1998;91:2772–2780 [PubMed] [Google Scholar]
- 15.Dudek AZ, Nesmelova I, Mayo K, Verfaillie CM, Pitchford S, and Slungaard A: Platelet factor 4 promotes adhesion of hematopoietic progenitor cells and binds IL-8: novel mechanisms for modulation of hematopoiesis. Blood 2003;101:4687–4694 [DOI] [PubMed] [Google Scholar]
- 16.Vrij AA, Rijken J, Van Wersch JW, and Stockbrugger RW: Platelet factor 4 and beta-thromboglobulin in inflammatory bowel disease and giant cell arteritis. Eur J Clin Invest 2000;30:188–194 [DOI] [PubMed] [Google Scholar]
- 17.Schofield L, and Grau GE: Immunological processes in malaria pathogenesis. Nat Rev Immunol 2005;5:722–735 [DOI] [PubMed] [Google Scholar]
- 18.Trocme C, Marotte H, Baillet A, Pallot-Prades B, Garin J, Grange L, Miossec P, Tebib J, Berger F, Nissen MJ, Juvin R, Morel F, and Gaudin P: Apolipoprotein A-I and platelet factor 4 are biomarkers for infliximab response in rheumatoid arthritis. Ann Rheum Dis 2009;68:1328–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cervi D, Yip TT, Bhattacharya N, Podust VN, Peterson J, Abou-Slaybi A, Naumov GN, Bender E, Almog N, Italiano JE, Jr, Folkman J, and Klement GL: Platelet-associated PF-4 as a biomarker of early tumor growth. Blood 2008;111:1201–1207 [DOI] [PubMed] [Google Scholar]
- 20.Luo S, Liang G, Zhang P, Zhao M, and Lu Q: Aberrant histone modifications in peripheral blood mononuclear cells from patients with Henoch-Schonlein purpura. Clin Immunol 2013;146:165–175 [DOI] [PubMed] [Google Scholar]
- 21.Lapchak PH, Ioannou A, Rani P, Lieberman LA, Yoshiya K, Kannan L, Dalle Lucca JJ, Kowalska MA, and Tsokos GC: The role of platelet factor 4 in local and remote tissue damage in a mouse model of mesenteric ischemia/reperfusion injury. PLoS One 2012;7:e39934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg ID, Stemerman MB, and Handin RI: Vascular permeation of platelet factor 4 after endothelial injury. Science 1980;209:611–612 [DOI] [PubMed] [Google Scholar]
- 23.Sachais BS, Turrentine T, Dawicki McKenna JM, Rux AH, Rader D, and Kowalska MA: Elimination of platelet factor 4 (PF4) from platelets reduces atherosclerosis in C57Bl/6 and apoE-/- mice. Thromb Haemost 2007;98:1108–1113 [PubMed] [Google Scholar]
- 24.Sepulveda J, and Shoemaker CB: Design and testing of PCR primers for the construction of scFv libraries representing the immunoglobulin repertoire of rats. J Immunol Methods 2008;332:92–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, Marra F, Romagnani S, Serio M, and Romagnani P: An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med 2003;197:1537–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldwin WM, 3rd, Kuo HH, and Morrell CN: Platelets: versatile modifiers of innate and adaptive immune responses to transplants. Curr Opin Organ Transplant 2011;16:41–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davi G, and Patrono C: Platelet activation and atherothrombosis. N Engl J Med 2007;357:2482–2494 [DOI] [PubMed] [Google Scholar]
- 28.Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O'Donnell E, Farndale RW, Ware J, and Lee DM: Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 2010;327:580–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshida H, and Granger DN: Inflammatory bowel disease: a paradigm for the link between coagulation and inflammation. Inflamm Bowel Dis 2009;15:1245–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eslin DE, Zhang C, Samuels KJ, Rauova L, Zhai L, Niewiarowski S, Cines DB, Poncz M, and Kowalska MA: Transgenic mice studies demonstrate a role for platelet factor 4 in thrombosis: dissociation between anticoagulant and antithrombotic effect of heparin. Blood 2004;104:3173–3180 [DOI] [PubMed] [Google Scholar]
- 31.Woller G, Brandt E, Mittelstadt J, Rybakowski C, and Petersen F: Platelet factor 4/CXCL4-stimulated human monocytes induce apoptosis in endothelial cells by the release of oxygen radicals. J Leukoc Biol 2008;83:936–945 [DOI] [PubMed] [Google Scholar]
- 32.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, and Ley K: Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med 2003;9:61–67 [DOI] [PubMed] [Google Scholar]
- 33.Massberg S, Brand K, Gruner S, Page S, Muller E, Muller I, Bergmeier W, Richter T, Lorenz M, Konrad I, Nieswandt B, and Gawaz M: A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med 2002;196:887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao J, Gao J, Qian L, Wang X, Wu M, Zhang Y, Ye H, Zhu S, Yu Y, and Han W: Activation of p38-MAPK by CXCL4/CXCR3 axis contributes to p53-dependent intestinal apoptosis initiated by 5-fluorouracil. Cancer Biol Ther 2014;15(8) [DOI] [PMC free article] [PubMed] [Google Scholar]