Abstract
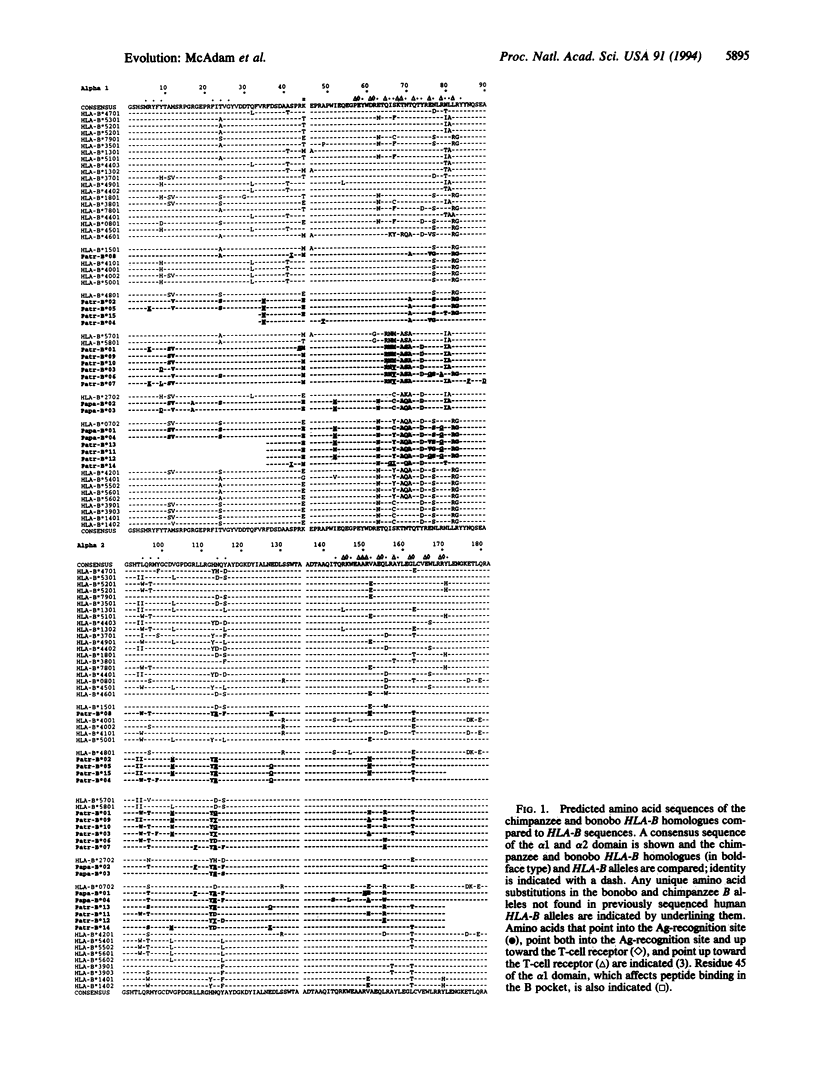
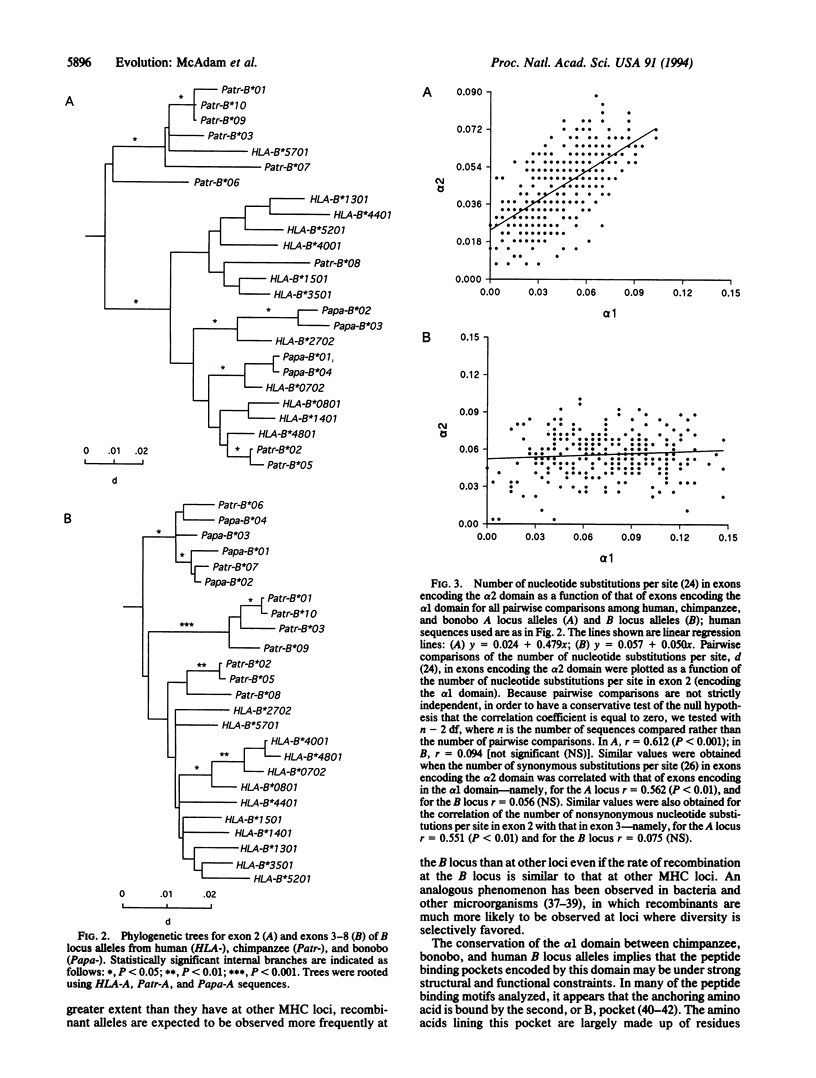
Major histocompatibility complex (MHC) loci are some of the most polymorphic genes in the animal kingdom. Recently, it has been suggested that although most of the human MHC loci are relatively stable, the HLA-B locus can undergo rapid changes, especially in isolated populations. To investigate the mechanisms of HLA-B evolution we have compared the sequences of 19 HLA-B homologues from chimpanzees and bonobos to 65 HLA-B sequences. Analysis of the chimpanzee and bonobo HLA-B homologues revealed that despite obvious similarities between chimpanzee and human alleles in exon 2, there was little conservation of exon 3 between humans and the two chimpanzee species. This finding suggests that, unlike all other HLA loci, recombination has characterized the HLA-B locus and its homologues for over 5 million years.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belich M. P., Madrigal J. A., Hildebrand W. H., Zemmour J., Williams R. C., Luz R., Petzl-Erler M. L., Parham P. Unusual HLA-B alleles in two tribes of Brazilian Indians. Nature. 1992 May 28;357(6376):326–329. doi: 10.1038/357326a0. [DOI] [PubMed] [Google Scholar]
- Bender B. S., Croghan T., Zhang L., Small P. A., Jr Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med. 1992 Apr 1;175(4):1143–1145. doi: 10.1084/jem.175.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Chen Z. W., Hughes A. L., Ghim S. H., Letvin N. L., Watkins D. I. Two more chimpanzee Patr-A locus alleles related to the HLA-A1/A3/A11 family. Immunogenetics. 1993;38(3):238–240. doi: 10.1007/BF00211524. [DOI] [PubMed] [Google Scholar]
- Ennis P. D., Zemmour J., Salter R. D., Parham P. Rapid cloning of HLA-A,B cDNA by using the polymerase chain reaction: frequency and nature of errors produced in amplification. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2833–2837. doi: 10.1073/pnas.87.7.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Fan W. M., Kasahara M., Gutknecht J., Klein D., Mayer W. E., Jonker M., Klein J. Shared class II MHC polymorphisms between humans and chimpanzees. Hum Immunol. 1989 Oct;26(2):107–121. doi: 10.1016/0198-8859(89)90096-7. [DOI] [PubMed] [Google Scholar]
- Flynn J. L., Goldstein M. M., Triebold K. J., Koller B., Bloom B. R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett T. P., Saper M. A., Bjorkman P. J., Strominger J. L., Wiley D. C. Specificity pockets for the side chains of peptide antigens in HLA-Aw68. Nature. 1989 Dec 7;342(6250):692–696. doi: 10.1038/342692a0. [DOI] [PubMed] [Google Scholar]
- Geluk A., Elferink D. G., Slierendregt B. L., van Meijgaarden K. E., de Vries R. R., Ottenhoff T. H., Bontrop R. E. Evolutionary conservation of major histocompatibility complex-DR/peptide/T cell interactions in primates. J Exp Med. 1993 Apr 1;177(4):979–987. doi: 10.1084/jem.177.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Ancient roots for polymorphism at the HLA-DQ alpha locus in primates. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9986–9990. doi: 10.1073/pnas.86.24.9986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U. B., Lashkari D., Erlich H. A. Allelic diversification at the class II DQB locus of the mammalian major histocompatibility complex. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1835–1839. doi: 10.1073/pnas.87.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U. B., Sundvall M., Erlich H. A. Allelic diversity is generated by intraexon sequence exchange at the DRB1 locus of primates. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3686–3690. doi: 10.1073/pnas.88.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. V., Allsopp C. E., Kwiatkowski D., Anstey N. M., Twumasi P., Rowe P. A., Bennett S., Brewster D., McMichael A. J., Greenwood B. M. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991 Aug 15;352(6336):595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- Hill A. V., Elvin J., Willis A. C., Aidoo M., Allsopp C. E., Gotch F. M., Gao X. M., Takiguchi M., Greenwood B. M., Townsend A. R. Molecular analysis of the association of HLA-B53 and resistance to severe malaria. Nature. 1992 Dec 3;360(6403):434–439. doi: 10.1038/360434a0. [DOI] [PubMed] [Google Scholar]
- Hou S., Doherty P. C., Zijlstra M., Jaenisch R., Katz J. M. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J Immunol. 1992 Aug 15;149(4):1319–1325. [PubMed] [Google Scholar]
- Hughes A. L., Nei M. Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature. 1988 Sep 8;335(6186):167–170. doi: 10.1038/335167a0. [DOI] [PubMed] [Google Scholar]
- Hughes A. L. Positive selection and interallelic recombination at the merozoite surface antigen-1 (MSA-1) locus of Plasmodium falciparum. Mol Biol Evol. 1992 May;9(3):381–393. doi: 10.1093/oxfordjournals.molbev.a040730. [DOI] [PubMed] [Google Scholar]
- Jardetzky T. S., Lane W. S., Robinson R. A., Madden D. R., Wiley D. C. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Kenter M., Otting N., Anholts J., Jonker M., Schipper R., Bontrop R. E. Mhc-DRB diversity of the chimpanzee (Pan troglodytes). Immunogenetics. 1992;37(1):1–11. doi: 10.1007/BF00223539. [DOI] [PubMed] [Google Scholar]
- Kenter M., Otting N., Anholts J., Leunissen J., Jonker M., Bontrop R. E. Evolutionary relationships among the primate Mhc-DQA1 and DQA2 alleles. Immunogenetics. 1992;36(2):71–78. doi: 10.1007/BF00215282. [DOI] [PubMed] [Google Scholar]
- Klein J. Origin of major histocompatibility complex polymorphism: the trans-species hypothesis. Hum Immunol. 1987 Jul;19(3):155–162. doi: 10.1016/0198-8859(87)90066-8. [DOI] [PubMed] [Google Scholar]
- Klein J., Satta Y., O'hUigin C., Takahata N. The molecular descent of the major histocompatibility complex. Annu Rev Immunol. 1993;11:269–295. doi: 10.1146/annurev.iy.11.040193.001413. [DOI] [PubMed] [Google Scholar]
- Lawlor D. A., Ward F. E., Ennis P. D., Jackson A. P., Parham P. HLA-A and B polymorphisms predate the divergence of humans and chimpanzees. Nature. 1988 Sep 15;335(6187):268–271. doi: 10.1038/335268a0. [DOI] [PubMed] [Google Scholar]
- Lawlor D. A., Warren E., Ward F. E., Parham P. Comparison of class I MHC alleles in humans and apes. Immunol Rev. 1990 Feb;113:147–185. doi: 10.1111/j.1600-065x.1990.tb00040.x. [DOI] [PubMed] [Google Scholar]
- Mayer W. E., Jonker M., Klein D., Ivanyi P., van Seventer G., Klein J. Nucleotide sequences of chimpanzee MHC class I alleles: evidence for trans-species mode of evolution. EMBO J. 1988 Sep;7(9):2765–2774. doi: 10.1002/j.1460-2075.1988.tb03131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer W. E., O'hUigin C., Zaleska-Rutczynska Z., Klein J. Trans-species origin of Mhc-DRB polymorphism in the chimpanzee. Immunogenetics. 1992;37(1):12–23. doi: 10.1007/BF00223540. [DOI] [PubMed] [Google Scholar]
- Muller D., Koller B. H., Whitton J. L., LaPan K. E., Brigman K. K., Frelinger J. A. LCMV-specific, class II-restricted cytotoxic T cells in beta 2-microglobulin-deficient mice. Science. 1992 Mar 20;255(5051):1576–1578. doi: 10.1126/science.1347959. [DOI] [PubMed] [Google Scholar]
- Nei M., Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986 Sep;3(5):418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- Nelson K., Selander R. K. Evolutionary genetics of the proline permease gene (putP) and the control region of the proline utilization operon in populations of Salmonella and Escherichia coli. J Bacteriol. 1992 Nov;174(21):6886–6895. doi: 10.1128/jb.174.21.6886-6895.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P. J., Gorski J., White G. C., 2nd, Gidwitz S., Cretney C. J., Aster R. H. Enzymatic amplification of platelet-specific messenger RNA using the polymerase chain reaction. J Clin Invest. 1988 Aug;82(2):739–743. doi: 10.1172/JCI113656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otting N., Kenter M., van Weeren P., Jonker M., Bontrop R. E. Mhc-DQB repertoire variation in hominoid and Old World primate species. J Immunol. 1992 Jul 15;149(2):461–470. [PubMed] [Google Scholar]
- Parham P., Lomen C. E., Lawlor D. A., Ways J. P., Holmes N., Coppin H. L., Salter R. D., Wan A. M., Ennis P. D. Nature of polymorphism in HLA-A, -B, and -C molecules. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4005–4009. doi: 10.1073/pnas.85.11.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzhetsky A., Nei M. Statistical properties of the ordinary least-squares, generalized least-squares, and minimum-evolution methods of phylogenetic inference. J Mol Evol. 1992 Oct;35(4):367–375. doi: 10.1007/BF00161174. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Slierendregt B. L., van Noort J. T., Bakas R. M., Otting N., Jonker M., Bontrop R. E. Evolutionary stability of transspecies major histocompatibility complex class II DRB lineages in humans and rhesus monkeys. Hum Immunol. 1992 Sep;35(1):29–39. doi: 10.1016/0198-8859(92)90092-2. [DOI] [PubMed] [Google Scholar]
- Smith N. H., Beltran P., Selander R. K. Recombination of Salmonella phase 1 flagellin genes generates new serovars. J Bacteriol. 1990 May;172(5):2209–2216. doi: 10.1128/jb.172.5.2209-2216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarleton R. L., Koller B. H., Latour A., Postan M. Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature. 1992 Mar 26;356(6367):338–340. doi: 10.1038/356338a0. [DOI] [PubMed] [Google Scholar]
- Watkins D. I., Garber T. L., Chen Z. W., Toukatly G., Hughes A. L., Letvin N. L. Unusually limited nucleotide sequence variation of the expressed major histocompatibility complex class I genes of a New World primate species (Saguinus oedipus). Immunogenetics. 1991;33(2):79–89. doi: 10.1007/BF00210819. [DOI] [PubMed] [Google Scholar]
- Watkins D. I., McAdam S. N., Liu X., Strang C. R., Milford E. L., Levine C. G., Garber T. L., Dogon A. L., Lord C. I., Ghim S. H. New recombinant HLA-B alleles in a tribe of South American Amerindians indicate rapid evolution of MHC class I loci. Nature. 1992 May 28;357(6376):329–333. doi: 10.1038/357329a0. [DOI] [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N. E., Loring J. M., Raulet D. H., Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990 Apr 19;344(6268):742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]



