Abstract
Mitochondria are highly dynamic organelles that undergo constant fusion/fission as well as activities orchestrated by large dynamin-related GTPases. These dynamic mitochondrial processes influence mitochondrial morphology, size and function. Therefore, this study was conducted to evaluate the effects of mitochondrial fission inhibitor, mdivi-1, on developmental competence and mitochondrial function of porcine embryos and primary cells. Presumptive porcine embryos were cultured in PZM-3 medium supplemented with mdivi-1 (0, 10 and 50 μM) for 6 days. Porcine fibroblast cells were cultured in growth medium with mdivi-1 (0 and 50 μM) for 2 days. Our results showed that the rate of blastocyst production and cell growth in the mdivi-1 (50 μM) treated group was lower than that of the control group (P < 0.05). Moreover, loss of mitochondrial membrane potential in the mdivi-1 (50 μM) treated group was increased relative to the control group (P < 0.05). Subsequent evaluation revealed that the intracellular levels of reactive oxygen species (ROS) and the apoptotic index were increased by mdivi-1 (50 μM) treatment (P < 0.05). Finally, the expression of mitochondrial fission-related protein (Drp 1) was lower in the embryos and cells in the mdivi-1-treated group than the control group. Taken together, these results indicate that mdivi-1 treatment may inhibit developmental competence and mitochondrial function in porcine embryos and primary cells.
Keywords: Embryos, Fibroblast cells, Mdivi-1, Mitochondria, Pig
Pigs have long been used as experimental models for investigation of organ xenotransplantation and human disease because of their physioanatomical similarities to humans. Moreover, porcine embryos are commonly used in investigations of the production of transgenic and somatic cell nuclear transfer embryos in animals [1, 2]. However, the production of in vitro porcine embryos has various problems, including incomplete culture conditions, chromosome abnormalities, polyspermy and mitochondrial dysfunction [3, 4].
Mitochondria are the major source of energy in mammalian cells and are responsible for production of adenosine triphosphate (ATP) through oxidative phosphorylation and the tricarboxylic acid cycle [3]. Mitochondria also regulate cell signaling, cell growth, reactive oxygen species (ROS) and apoptosis [5, 6]. On the other hand, mitochondria in healthy cells constantly undergo fusion and fission, which modulate mitochondrial morphology and structure via large dynamin-related GTPases [7, 8]. Therefore, dynamic dysfunction of mitochondrial fusion/fission causes mitochondria disruption and cell death [6].
In mammalian cells, mitochondrial fusion/fission dynamics are governed by a number of GTPase dynamin family proteins. Mitochondria fusion dynamics are modulated by the mitofusin 1 (Mfn 1), mitofusin 2 (Mfn 2) and optic atrophy 1 (Opa 1) proteins [6]. Mfn 1 and 2 proteins are located in the outer mitochondrial membrane (OMM), while Opa 1 is located in the inner mitochondrial membrane (IMM), where it acts as a key to fusion [9, 10]. In contrast, mitochondrial fission dynamics are mediated by a dynamin-related protein (Drp 1) and fission 1 (Fis 1) protein [6]. Drp 1 exists in the cytoplasm and is activated by posttranslational modifications, including phosphorylation, ubiquitylation, and sumoylation. Activated Drp 1 is recruited from the cytoplasm to the mitochondria OMM receptor and regulates fission through an interaction with Fis 1 [5]. The activity of Drp 1 plays an important role in regulation of cell survival, apoptosis and mitophagy [11]. It has also been suggested that Drp 1-mediated mitochondrial fission is essential to cell survival and maintenance of cellular homeostasis through mitophagy [12]. Inoue-Yamauchi and Oda [11] showed that Drp 1-depleted cells had increased apoptosis and reduced mitochondrial membrane potential . Despite the importance of Drp 1 in mitochondrial function, its role is not well understood in porcine embryos and fibroblast cells . The mitochondrial fission inhibitor, mdivi-1, has been reported to block mitochondrial fission [13]. Mdivi-1 inhibits GTPase activity by blocking the self-assembly of Drp 1 in vitro, which causes the rapid, reversible and dose-dependent formation of netlike mitochondria in wild-type cells [14, 15]. However, to the best of our knowledge, the effects of mdivi-1 on the developmental potential of porcine embryos and fibroblast cells have not yet been reported.
The mitochondrial membrane potential is a central indicator of cellular viability that reflects indicators of metabolic activity such as oxidative phosphorylation and the electron transport process [16]. Aberrant changes in mitochondrial membrane potential decrease the developmental competence in mouse embryos [3]. Under pathological conditions, uncoupling of oxidative phosphorylation and disruption of mitochondrial membrane potential lead to excessive reactive oxygen species (ROS) production from the respiratory chain [17]. In damaged cells exposed to oxidative stress, increased ROS production leds to a disturbance in mitochondrial dynamics that induces mitochondrial fragmentation and cell death [18]. Apoptosis is a programmed cell death mechanisms that plays important roles in a variety of biological events, such as cellular homeostasis and the removal of damaged cells [19]. Apoptosis also induces caspase activation, chromosome fragmentation and dysfunction of the mitochondria such as DNA damage and disruption [20, 21].
The present study was conducted to investigate the effects of the addition of mdivi-1 to culture medium on the developmental ability and quality of porcine embryos and fibroblast cells. We also examined the mitochondrial membrane potential and expression of mitochondrial dynamics-related proteins in embryos and fibroblast cells with and without mdivi-1 treatment.
Materials and Methods
Chemicals
Unless otherwise indicated, all chemicals were purchased from Sigma-Aldrich Korea (Yongin, Republic of Korea).
In vitro maturation (IVM)
Experiments were conducted according to the Animal Care and Use Committee of the Daegu University. Porcine ovaries were collected from a local slaughterhouse and transported to the laboratory at 30–35 C in 0.9% saline supplemented with 75 μg/ml potassium penicillin G. Cumulus oocyte complexes (COCs) were aspirated through an 18 gauge needle into a disposable 10 ml syringe from follicles 3 to 6 mm in diameter [22]. After washing three times with Tyrode’s lactate (TL)-N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES) medium, approximately 50 immature COCs were matured in 500 μl of IVM medium in a four-well multidish (Nunc, Roskilde, Denmark) at 38.5 C in 5% CO2 in air. Oocyte maturation was conducted in North Carolina State University (NCSU) 23 medium supplemented with 10% follicular fluid, 0.57 mM cysteine, 10 ng/ml β-mercaptoethanol, 10 ng/ml epidermal growth factor, 10 IU/ml pregnant mare's serum gonadotropin (PMSG) and 10 IU/ml human chorionic gonadotropin (hCG) [23]. After 22 h of culture, oocytes were washed three times and then further cultured in maturation medium without hormone supplementation (PMSG and hCG) for 22 h.
In vitro fertilization (IVF)
After IVM, the oocytes were subjected to IVF as described by Abeydeera and Day [24]. The IVF medium, modified Tris-buffered medium (mTBM), consisted of 113.1 mM NaCl, 3 mM KCl, 7.5 mM CaCl2, 5 mM sodium pyruvate, 11 mM glucose, 20 mM Tris, 2.5 mM caffeine sodium benzoate and 1 mg/ml BSA. Fresh semen was kindly supplied twice a week by an artificial insemination company (Darby Pig AI Center, Anseong, Republic of Korea) and kept at 17 C for 5 days. Semen was washed three times by centrifugation with Dulbecco’s phosphate buffered saline (DPBS; Gibco-BRL, Grand Island, NY, USA) supplemented with 1 mg/ml bovine serum albumin (BSA; Fraction V), 100 mg/ml penicillin G and 75 mg/ml streptomycin sulfate. After washing, the spermatozoa were suspended in mTBM at pH 7.8. Oocytes were then washed three times in mTBM with 2.5 mM caffeine sodium benzoate and 1 mg/ml BSA (fatty acid free), after which they were placed into 48 μl of mTBM under paraffin oil. Diluted spermatozoa (2 μl) were subsequently added to a 48 μl drop of medium containing 15–20 oocytes to give a final concentration of 1.5 × 105 sperms/ml. The oocytes were then co-incubated with the spermatozoa for 6 h at 38.5 C in 5% CO2 in air.
In vitro culture (IVC)
In all experiments, the embryos were cultured in 50 μl drops of porcine zygote medium (PZM) 3 supplemented with 3 mg/ml BSA at 38.5 C in 5% CO2 in air for 6 days. To modulate mitochondrial fission, fertilized embryos were treated with mdivi-1 (0, 10 and 50 μM) that was added to the culture medium. The rates of cleavage- and blastocyst-stage embryos were determined on days 2 and 6, respectively.
Mitochondrial membrane potential analysis
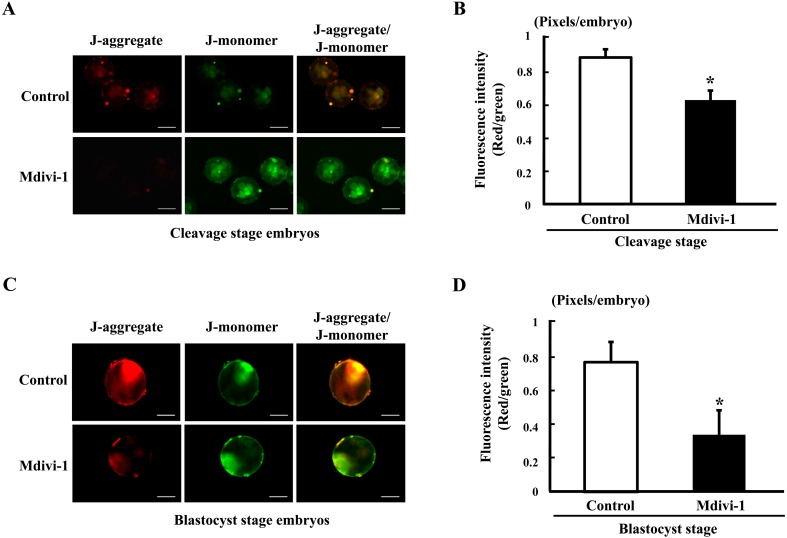
A mitochondrial membrane potential assay kit with JC-1 (Cayman Chemical, Ann Arbor, MI, USA) was used to measure mitochondrial membrane potential in embryos and fibroblast cells. Embryos derived from cleavage- (day 2) and blastocyst-stage (day 6) embryos were collected and then stained with JC-1 (1:100) in culture medium for 30 min at 38.5 C. After staining, the embryos were washed three times with 0.1% polyvinyl alcohol (PVA) in DPBS, after which they were placed in 3 μl drops of IVC medium on a slide. Fibroblast cells adhered to the coverslip were recovered from mdivi-1 treatment for 2 days and then stained with JC-1 (1:100) in growth medium for 30 min at 38.5 C. After staining, the cells were washed three times with DPBS and then placed on a slide. The embryos and fibroblast cells were subsequently examined under an epifluorescence microscope (IX 51, Olympus, Tokyo, Japan). High mitochondrial polarization was indicated by red fluorescence due to J-aggregate formation by the concentrated dye. Low mitochondrial polarization was indicated by green fluorescence. Mitochondrial membrane potential was assessed as the ratio of red fluorescence to green fluorescence using the ImageJ 1.38 software (National Institutes of Health, Bethesda, MD, USA) [25]. A total of 20 cleavage embryos and 20 blastocysts were examined in each treatment group and harvested cells.
Measurement of ROS levels
The level of ROS in each embryo was measured using the dichlorodihydrofluorescein diacetate method (H2DCFDA; Invitrogen, Molecular Probes, Eugene, OR, USA) as previously described [26]. At day 6, blastocysts produced in vitro were recovered and used for the experiment. After washing three times in IVC medium, blastocysts were transferred into IVC medium containing 5 μM H2DCFDA for 20 min at 38.5 C in 5% CO2 in air. A stock solution of H2DCFDA dissolved in dimethylsulfoxide (DMSO) was then diluted in IVC medium, after which the permeabilized blastocysts in H2DCFDA were washed three times with 0.1% PVA in DPBS, placed into a 50 μl drop and covered with mineral oil. The fluorescent emissions from the embryos were recorded with a fluorescent microscope (Olympus) equipped with a cooled charge-coupled device (CCD) camera in which filters at 488 nm and 520 nm were used for excitation and emission, respectively. The recorded fluorescent images were analyzed by subtracting the background and measuring the integrated density using the ImageJ software (version 1.38). A total of 20 blastocysts were examined in each treatment group.
TUNEL assay
Apoptotic cells in blastocysts were detected using an In Situ Cell Death Detection Kit (Roche Diagnostics, Mannheim, Germany). Briefly, blastocysts were recovered from IVC after 6 days, washed three times with 0.1% PVA in DPBS and then fixed in 4% (v/v) formaldehyde diluted in DPBS for 1 h at 4 C. For membrane permeabilization, the fixed embryos were incubated in DPBS containing 0.1% (v/v) Triton X-100 for 30 min at 4 C. The fixed embryos were subsequently incubated in TUNEL reaction medium for 1 h at 38.5 C in the dark, after which they were washed and transferred into 2 mg/ml of DAPI and mounted on slides. After TUNEL and DAPI staining, whole-mount embryos were examined under an epifluorescence microscope (Olympus) to determine the number of apoptotic nuclei and total number of nuclei. A total of 20 blastocysts were examined in each treatment group.
Cell culture
Porcine ear skin fibroblasts (pESFs) of passages 3 were used in this study. Cells were cultured in growth medium containing Dulbecco’s modified Eagle’s medium (DMEM, Welgene, Daegu, Republic of Korea) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL), 1% nonessential amino acids (Gibco-BRL) and 500 μg/ml penicillin/streptomycin (HyClone, Thermo Scientific, Tewksbury, MA, USA) under 5% CO2 at 38.5 C. The pESFs cells were seeded at 0.8 × 106 cells per 60 mm culture dish and treated with 50 μM mdivi-1 for 48 h.
Western blot analysis
Immunoblot analysis was carried out on 30 embryos per group and harvested cells. Briefly, samples were lysed in radioimmunoprecipitation assay (RIPA) buffer with protease inhibitor and phosphatase inhibitor. Sample aliquots containing equal amounts of protein were then boiled in sample loading buffer and separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), after which they were transferred onto polyvinylidene fluoride (PVDF)membranes (Immobilon-P, Millipore). Subsequently, membranes were blocked with 3% BSA (fatty-acid free) in TBS buffer containing 0.1% Tween 20 (TBST) at 4 C overnight, rinsed in TBST and probed with the following primary antibodies at 4 C overnight: rabbit anti-Drp 1 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-Mfn 1 (1:1000; Santa Cruz Biotechnology), rabbit anti-Mfn 2 (1:1000; Santa Cruz Biotechnology) and mouse anti-actin (1:5000; Santa Cruz Biotechnology). The blot then was washed with TBST and incubated with the following secondary antibodies at 4 C overnight: goat anti-mouse (1:4000; Thermo Scientific, Rockford, IL, USA) and goat anti-rabbit (1:4000; Thermo Scientific). Finally, the membranes were visualized using enhanced chemiluminescence detection reagent (Advansta, Menlo Park, CA, USA) according to the manufacturer's instructions.
Statistical analysis
Each experiment was repeated more than three times, and all percentage data and data sets obtained in the present study are presented as the mean ± standard deviation (SD). The Student’s t-test was used to compare the means of two samples, while ANOVA was used to compare the means of multiple samples. A P < 0.05 was considered to indicate statistical significance.
Results
Effects of mdivi-1 treatment on developmental competence of porcine embryos
No previous studies have investigated the effect of mdivi-1 on the preimplantation development of porcine embryos. Thus, we investigated the appropriate mdivi-1 concentration of culture medium for preimplantation development of porcine embryos. After fertilization, presumptive porcine zygotes were cultured in IVC medium supplemented with 0, 10 and 50 μM mdivi-1 for 6 days. As shown in Table 1, the rate of blastocyst formation in the presence of 50 μM mdivi-1 was significantly lower than that of the control (31.6 ± 3.4% vs. 21.3 ± 1.6%; P < 0.05). Otherwise, no difference was observed in cleavage rates between the treatment and nontreatment groups.
Table 1. Effect of different concentrations of mdivi-1 treatment on developmental competence in porcine embryos.
| Mdivi-1 (μM) | No. of embryos cultured |
% of embryos cleaved (n) | % of blastocysts (n) |
| 0 | 227 | 94.9 ± 2.9 (216) | 31.6 ± 3.4 (71)a |
| 10 | 219 | 92.7 ± 2.4 (203) | 28.9 ± 3.9 (63)a |
| 50 | 220 | 92.0 ± 3.6 (203) | 21.3 ± 1.6 (47)b |
Data are shown as the mean ± SD. Values were obtained from ten replicates. a,b Values with different superscripts are significantly different relative to the other groups (P < 0.05).
Effects of mdivi-1 treatment on mitochondrial membrane potential of the cleavage- and blastocyst-stage embryos
We next assessed the mitochondrial membrane potential of preimplantation porcine embryos by treatment with mdivi-1 during culture periods. The mitochondrial membrane potential was assessed by JC-1 staining of cleavage- (4–8 cells) and blastocyst-stage embryos in the mdivi-1 treated and nontreated groups (Fig. 1). The cleavage- and blastocyst-stage embryos in the mdivi-1-treated group showed significantly lower mitochondrial membrane potential than those in the nontreated group (P < 0.05). Thus, this result suggests that mdivi-1 treatment during embryo culture periods may induces mitochondrial dysfunction.
Fig. 1.
Epifluorescent images of mitochondrial membrane potential in the cleavage- and blastocyst-stage embryos. Fluorescence microscopy imaging of mitochondrial membrane potential and fluorescence intensity in the porcine cleavage-stage (A and B) and blastocyst-stage (C and D) embryos. Data are shown as the mean ± SD. Statistically significant differences are indicated by asterisks (P < 0.05). Scale bar = 100 μm.
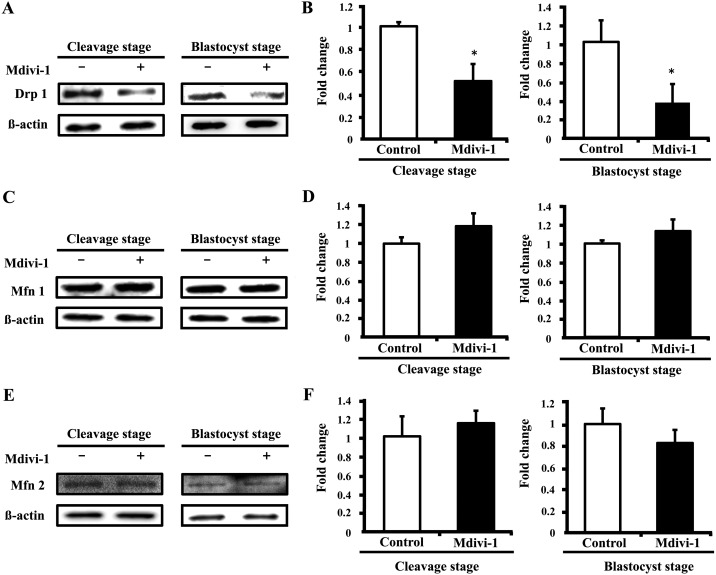
Effects of mdivi-1 treatment on expression of mitochondrial fission- and fusion-related proteins in porcine embryos
We first assessed the expression levels of mitochondrial fission- and fusion-related proteins, including Drp 1, in cleavage- (4–8 cells) and blastocyst-stage embryos. When compared with the nontreated group, the expression levels of Drp 1 in the cleavage- and blastocyst-stage embryos were significantly lower in the mdivi-1-treated group (Fig. 2A and B). However, the expression levels of mitochondrial fusion-related proteins (Mfn 1 and Mfn 2) did not differ significantly between the control and mdivi-1-treated groups (Fig. 2C, D, E and F). These results suggest that mdivi-1 treatment of porcine preimplantation embryos results in reduced fission protein expression of preimplantation stage embryos without changing the expression of mitochondrial fusion proteins.
Fig. 2.
Comparative analysis of the expression of mitochondrial fission proteins in cleavage- and blastocyst-stage embryos. Representative Western blot analysis of Drp 1 (A), Mfn 1 (C), Mfn 2 (E), and β-actin protein. Quantification of signals from Western blots showing the fold change in Drp 1/β-actin protein (B), Mfn 1/β-actin protein (D), and Mfn 2/β-actin protein (F) expression. Values are shown as the mean ± SD. Statistically significant differences are indicated by asterisks (P < 0.05).
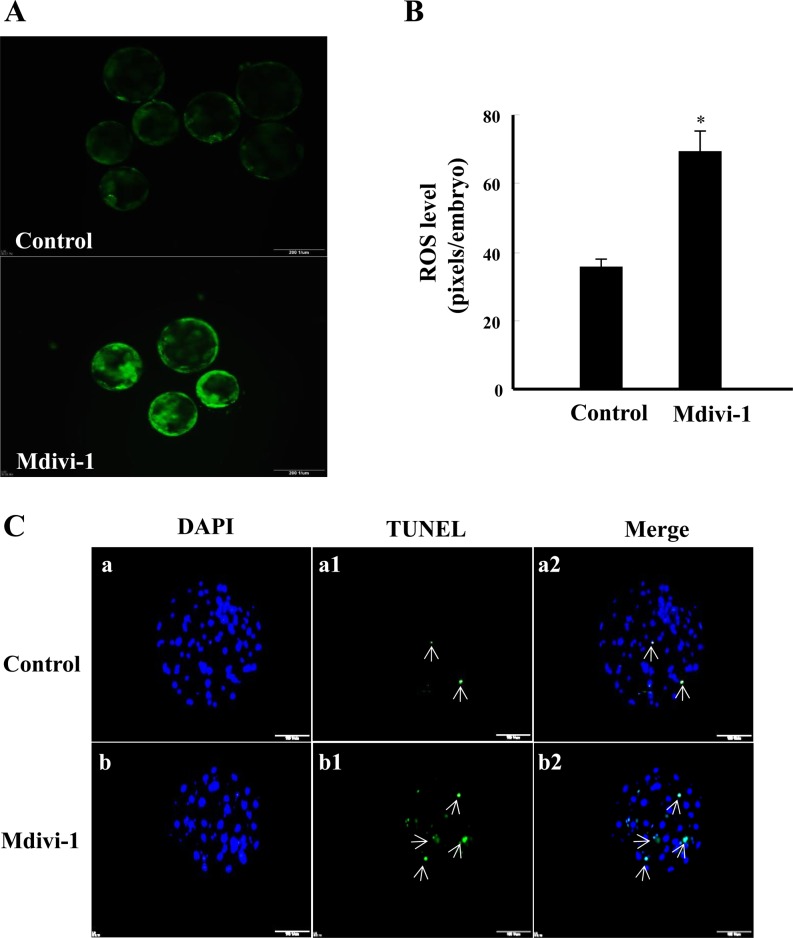
Effects of mdivi-1 treatment on expression levels of ROS and the apoptotic index in porcine embryos
We next investigated the intracellular levels of ROS and the apoptotic index in mdivi-1-treated blastocysts. As shown in Fig. 3A and B, the intracellular levels of ROS were significantly increased in blastocysts derived from 50 μM mdivi-1-treated embryos when compared with the nontreated group (P < 0.05). In addition, the number of TUNEL-positive nuclei and the apoptosis index was increased in blastocyst derived from the 50 μM mdivi-1-treated group when compared with the nontreated group (P < 0.05; Table 2, Fig. 3C). Moreover, the total cell number was decreased in blastocyst-stage embryos cultured in the presence of mdivi-1 when compared with those cultured in the absence of mdivi-1 (P < 0.05; Table 2, Fig. 3C).
Fig. 3.
Fluorescence microscopy imaging of intracellular ROS expression (A), fluorescence intensity (B) and apoptotic pattern (C) in blastocysts. The chromatin content is stained by DAPI (blue, a and b), fragmented DNA is labeled by the TUNEL reaction (green and white arrow, a1 and b1), and colocalization with DAPI appears sky blue (a2 and b2). Data are shown as the mean ± SD. Statistically significant differences are indicated by asterisks (P < 0.05). Scale bar = 200 μm.
Table 2. Effect of mdivi-1 treatment on the apoptotic index in porcine blastocysts.
| Mdivi-1 (μM) |
No. of blastocysts |
No. of cells |
Apoptotic index (%) |
|
| Total | TUNEL positive | |||
| 0 | 22 | 45.0 ± 7.2a | 1.2 ± 1.1a | 2.6 ± 2.6a |
| 50 | 21 | 35.0 ± 6.3b | 3.0 ± 1.8b | 8.3 ± 4.3b |
Data are shown as the mean ± SD. Values were obtained from ten replicates. a,b Values with different superscripts are significantly different relative to the other group (P < 0.05).
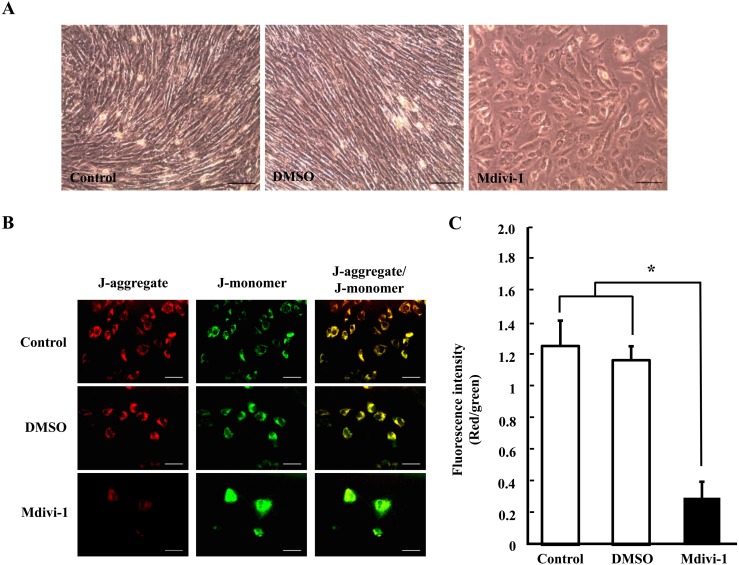
Effect of mdivi-1 treatment on morphology and mitochondrial membrane potential of pig fibroblast cells
We also investigated the cell morphology and mitochondrial membrane potential in fibroblast cells treated with mdivi-1. As shown in Fig. 4A, the cell proliferation pattern was inhibited in the mdivi-1-treated group when compared with nontreated groups. In addition, mitochondrial membrane potential was significantly decreased in the mdivi-1 treatment group (P < 0.05; Fig. 4B and C). These results demonstrated that mdivi-1 treatment during culture periods affects growth and mitochondrial membrane potential of fibroblast cells.
Fig. 4.
Representative photograph of the morphology and growth pattern in porcine fibroblast cells (A). Fluorescence microscopy imaging of mitochondrial membrane potential (B) and fluorescence intensity (C) in porcine fibroblast cells. Data are shown as the mean ± SD. Statistically significant differences are indicated by asterisks (P < 0.05). Scale bar = 100 μm.
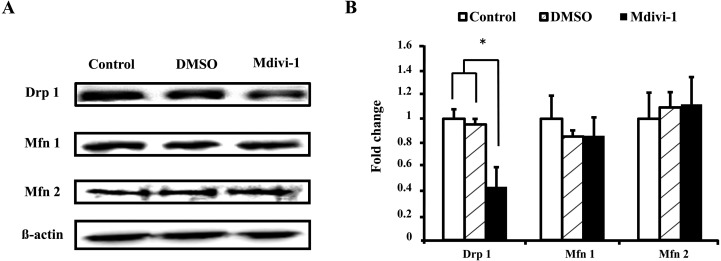
Effects of mdivi-1 treatment on expression of mitochondrial dynamics-related proteins in pig fibroblast cells
Western blot was also used to determine the relative abundance of Drp 1, Mfn 1 and Mfn 2 proteins in primary cells cultured in the presence or absence of mdivi-1. As shown in Fig. 5, the protein expression levels of Drp 1 were lower in the mdivi-1-treated group (P < 0.05). However, there was no difference in the expression levels of Mfn 1 and Mfn 2. These results suggest that mdivi-1 treatment during cell proliferation inhibits the expression of Drp 1.
Fig. 5.
Comparative analysis of the expression of mitochondrial fusion/fission proteins in porcine fibroblast cells. Representative Western blot analysis of Drp 1, Mfn 1, Mfn 2, and β-actin protein (A). Quantification of signals from Western blots showing the fold change in Drp 1/β-actin protein, Mfn 1/β-actin protein and Mfn 2/β-actin protein expression (B). Values are shown as the means ± SD. Statistically significant differences are indicated by asterisks (P < 0.05).
Discussion
In the present study, we demonstrated that mdivi-1 treatment significantly decreased embryo development and impaired embryo quality by reducing mitochondrial membrane potential and blastocyst cell number and increasing ROS and apoptosis, which was followed by a decrease in the expression levels of Drp 1. We also confirmed that mdivi-1 in pESFs significantly reduced cell growth, mitochondrial membrane potential and the expression levels of Drp 1. On the other hand, because there has been no research that has dealt with mdivi-1 treatment in porcine early embryos and fibroblast cells, a concentration experiment was conducted. Based on two previous studies [27, 28], a concentration of 50 μM was applied to in in vitro fertilized embryos for 6 days and fibroblast cells for 2 days.
Mitochondrial membrane potential is a key indicator of mitochondrial health, reflecting metabolic activity and function, including production of ATP, ion transport and oxidative phosphorylation [16]. Hua et al. [25] showed that downregulation of Mfn 1 levels significantly decreased the mitochondrial membrane potential and developmental competence of bovine somatic cell nuclear transfer (SCNT) embryos. The imbalance in mitochondrial dynamics in response to cadmium treatment also induced excessive mitochondrial fragmentation and ROS, loss of mitochondrial membrane potential and production of ATP in the liver tissue of rats [8]. Ferree and Shirihai [29] also showed that loss of phosphatase and tensin homolog-induced kinase 1 (PINK 1), a mitochondrial dynamics regulator, induced an increase in damaged mitochondria, and decreased mitochondrial membrane potential and ATP synthesis. Furthermore, a previous investigation revealed that mdivi-1 treatment of myotubes decreased mitochondrial DNA content and mitochondrial membrane potential and inhibited myotube formation during myogenic differentiation [30]. The results of the present study showed that mdivi-1 treatment led to a significant decrease in the mitochondrial membrane potential in porcine embryos and fibroblast cells (Fig. 1 and 4). Overall, these findings indicate that the balance of mitochondrial fusion/fission might play an important role in the development of porcine embryos and fibroblast cells.
Within embryos and cells, mitochondria may exist as complex interconnected networks, as discrete individual structures or as a dynamic, interchangeable combination of the two [31]. Indeed, the structure of the mitochondrial network has been found to differ significantly between embryos and cell types [32]. The extent of mitochondrial interconnectivity is regulated cytoskeletal elements [33] and by the action of specific proteins, including Mfn 1, Mfn 2 and Opa 1, which are involved in the process of fusion, and Drp 1 and Fis 1, which have a role in mitochondrial fission [34]. These variations in dynamics appear to be necessary to ensure proper function and distribution of mitochondria, and defects in these processes have been linked to disease [35]. Preimplantation embryos and fibroblast cells also contain two distinct genomes. One is located in the nucleus (nDNA), whereas the other is located in mitochondria (mtDNA) and is transmitted by maternal inheritance. Furthermore, mitochondria are essential to the life and death of preimplantation embryos and fibroblast cells [36]; however, mitochondrial morphology and function, which are related to the changes in mitochondrial fission/fusion, differ between embryos and fibroblast cells. In fibroblast cells, mitochondria are elongated organelles containing swollen cristae and possess high membrane potential, and they provide cell energy with sufficient ATP through aerobic metabolism. But in oocytes and early cleavage-stage embryos, mitochondria are oval and spherical, and anaerobic metabolism serves as the principal source of ATP to meet the energy demand of life activities [25]. Parone et al. [37] showed that the depletion of Drp 1 impaired cell proliferation, levels of cellular ATP, levels of cellular ROS and autophagy in mammalian cells. Vazquez-Martin et al. [38] showed that mdivi-1 treatment of mouse embryonic fibroblasts impaired colony growth and morphology of induced pluripotent stem cells. Furthermore, treatment in HeLa cells with the mitochondrial fission inducer tyrphostin A9 induced formation of fragmented mitochondrial filaments, reduction of cellular ATP levels and collapse of the mitochondrial membrane potential [39]. On the other hand, Wakefield et al. [40] showed that treatment of mouse embryos with the mitochondria metabolism inhibitor aminooxyacetate decreased blastocyst development and inner cell mass and trophectoderm cell numbers and reduced ATP production. The results of the present study showed that mdivi-1 treatment led to a significant decrease in the developmental competence of porcine preimplantation embryos and proliferation of fibroblast cells (Table 1, Fig. 4). Therefore, these results suggest that mdivi-1 treatment induces an imbalance of mitochondrial dynamics and impairs developmental competence in porcine embryos and fibroblast cells.
ROS are primarily generated in mitochondria as by-products of normal cell metabolism, and the principal source of ROS is hydrogen peroxide [41]. Damaged mitochondria can increase ROS production [42]. Lower levels of ROS in embryos are necessary for normal cellular functions [43], whereas higher levels of ROS damage embryos [42]. Previous studies showed that depletion of Drp 1 in HeLa cells significantly increased the levels of cellular ROS [29]. Furthermore, mitochondrial fragmentation mediated by fission has been shown to induce increased ROS production levels under high glucose conditions [44]. Thus, the balance of mitochondrial dynamics is important for regulation of intracellular ROS production. The results of the present study showed that ROS levels were significantly higher in the mdivi-1-treated group than the nontreated group (Fig. 3A and B). Similarly, a previous study showed that downregulation of Mfn 1 levels led to increased ROS levels in bovine SCNT embryos [25]. Taken together, these findings suggest that an imbalance in mitochondria fusion/fission damages mitochondria, leading to the production of excessive ROS.
Apoptosis play a vital role in embryonic development and is an important indicator of the quality of produced blastocysts [45]. Song et al. [46] showed that overexpression of fatty acid-binding protein 3 (FABP 3) in embryonic cancer cells led to an imbalance in mitochondrial dynamics and increased apoptosis. Moreover, treatment of mouse embryos with Ochratoxin A, an inhibitor of mitochondrial transport systems, induced apoptosis through activation of a mitochondrion-dependent pathway [47]. Additionally, sodium butyrate treatment of human colorectal cancer cells was found to modulate mitochondrial fission and fusion by inhibiting activation of Drp 1, leading to G2 and M phase cell cycle arrest and apoptosis [48]. In the present study, the apoptotic index was significantly higher for the mdivi-1-treated group than the nontreated group (Table 2, Fig. 3C). Similarly, mdivi-1 treatment during myogenic differentiation increased apoptosis [30]. Also, the total numbers of cells in blastocysts derived from embryos cultured with mdivi-1 was lower than in the nontreated group (Table 2). Taken together, these results suggest that an imbalance in mitochondria fusion/fission may induce lower qualities of porcine blastocysts.
The balance of the mitochondrial dynamics is regulated by fusion/fission-related proteins. Drp 1-mediated mitochondrial fission is necessary for cell survival because it removes impaired mitochondria and maintains cellular homeostasis through mitophagy [12]. Additionally, mdivi-1 binds outside the GTPase domain to a surface involved in oligomeric assembly and thereby inhibits Dnm 1/Drp 1 GTPase activation, and it displays less Drp 1 translocation from the cytosol onto mitochondria [49], indicating that mdivi-1 inhibits Drp 1 translocation from cytosol to mitochondria but does not inhibit the protein level of Drp 1. However, in the present study, though Mfn 1 and 2 did not show differences in the protein analysis, because the Drp 1 levels showed differences, which led to mdivi-1 especially suppressing Drp 1 among the mitochondrial fission proteins, resulting in the balance of the mitochondrial dynamics being upset, it was concluded that the developmental competence and quality of porcine embryos and fibroblast cells were affected. This is the first report regarding the action of mdivi-1 on the inhibition of Drp 1 expression in porcine embryos and fibroblast cells, and the definite mechanism of our findings should be confirmed by further experiments. In addition, the changes in the expression levels of both fusion and fission proteins might be an important factor in cellular metabolism and mitochondrial function.
In conclusion, the results of the present study suggest that treatment of porcine preimplantation embryos and fibroblast cells with mdivi-1 results in decreased blastocyst production and cell growth. Furthermore, our mdivi-1 treatment groups showed significantly decreased embryonic qualities, including mitochondrial membrane potential, ROS level and apoptosis, which affected the expression levels of Drp 1. Therefore, our results suggest that expression of Drp 1 protein regulates the developmental competence of porcine embryos and fibroblast cell proliferation by modulating mitochondrial dynamics.
Acknowledgments
This work was supported by grants from the Next-Generation BioGreen 21 Program (PJ01117604) and the Bio-industry Technology Development Program (112130031HD030) through the Rural Development Administration, the Ministry of Agriculture, Food and Rural Affairs, and the KRIBB Research Initiative Program (KGM4251314), Republic of Korea.
References
- 1.Muenthaisong S, Dinnyes A, Nedambale TL. Review of somatic cell nuclear transfer in pig. Afr J Biotechnol 2011; 10: 17384–17390. [Google Scholar]
- 2.Kang JT, Kwon DK, Park SJ, Kim SJ, Moon JH, Koo OJ, Jang G, Lee BC. Quercetin improves the in vitro development of porcine oocytes by decreasing reactive oxygen species levels. J Vet Sci 2013; 14: 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acton BM, Jurisicova A, Jurisica I, Casper RF. Alterations in mitochondrial membrane potential during preimplantation stages of mouse and human embryo development. Mol Hum Reprod 2004; 10: 23–32. [DOI] [PubMed] [Google Scholar]
- 4.Krisher RL. In vivo and in vitro environmental effects on mammalian oocyte quality. Annu Rev Anim Biosci 2013; 1: 393–417. [DOI] [PubMed] [Google Scholar]
- 5.Baixauli F, Martín-Cófreces NB, Morlino G, Carrasco YR, Calabia-Linares C, Veiga E, Serrador JM, Sánchez-Madrid F. The mitochondrial fission factor dynamin-related protein 1 modulates T-cell receptor signalling at the immune synapse. EMBO J 2011; 30: 1238–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi X, Qvit N, Su YC, Mochly-Rosen D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci 2013; 126: 789–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet 2005; 14 Spec No. 2: R283–R289. [DOI] [PubMed] [Google Scholar]
- 8.Xu S, Pi H, Chen Y, Zhang N, Guo P, Lu Y, He M, Xie J, Zhong M, Zhang Y, Yu Z, Zhou Z. Cadmium induced Drp1-dependent mitochondrial fragmentation by disturbing calcium homeostasis in its hepatotoxicity. Cell Death Dis 2013; 4: e540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, Bhattacharya SS, Wissinger B. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet 2000; 26: 211–215. [DOI] [PubMed] [Google Scholar]
- 10.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep 2007; 8: 939–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue-Yamauchi A, Oda H. Depletion of mitochondrial fission factor DRP1 causes increased apoptosis in human colon cancer cells. Biochem Biophys Res Commun 2012; 421: 81–85. [DOI] [PubMed] [Google Scholar]
- 12.Lee Y, Lee HY, Hanna RA, Gustafsson AB. Mitochondrial autophagy by Bnip 3 involves Drp 1-mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes. Am J Physiol Heart Circ Physiol 2011; 301: H1924–H1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park SW, Kim KY, Lindsey JD, Dai Y, Heo H, Nguyen DH, Ellisman MH, Weinreb RN, Ju WK. A selective inhibitor of drp1, mdivi-1, increases retinal ganglion cell survival in acute ischemic mouse retina. Invest Ophthalmol Vis Sci 2011; 52: 2837–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell 2008; 14: 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.So EC, Hsing CH, Liang CH, Wu SN. The actions of mdivi-1, an inhibitor of mitochondrial fission, on rapidly activating delayed-rectifier K+ current and membrane potential in HL-1 murine atrial cardiomyocytes. Eur J Pharmacol 2012; 683: 1–9. [DOI] [PubMed] [Google Scholar]
- 16.Kim JS, Cho YS, Song BS, Wee G, Park JS, Choo YK, Yu K, Lee KK, Han YM, Koo DB. Exogenous dibutyryl cAMP affects meiotic maturation via protein kinase A activation; it stimulates further embryonic development including blastocyst quality in pigs. Theriogenology 2008; 69: 290–301. [DOI] [PubMed] [Google Scholar]
- 17.Zhan M, Brooks C, Liu F, Sun L, Dong Z. Mitochondrial dynamics: regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int 2013; 83: 568–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makino A, Scott BT, Dillmann WH. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia 2010; 53: 1783–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong SY, Seol DW. The role of mitochondria in apoptosis. BMB reports 2008; 41: 11–22. [DOI] [PubMed] [Google Scholar]
- 20.Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev 2003; 17: 2481–2495. [DOI] [PubMed] [Google Scholar]
- 21.Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res 2007; 100: 460–473. [DOI] [PubMed] [Google Scholar]
- 22.Funahashi H, Cantley TC, Stumpf TT, Terlouw SL, Day BN. In vitro development of in vitro-matured porcine oocytes following chemical activation or in vitro fertilization. Biol Reprod 1994; 50: 1072–1077. [DOI] [PubMed] [Google Scholar]
- 23.Petters RM, Wells KD. Culture of pig embryos. J Reprod Fertil Suppl 1993; 48: 61–73. [PubMed] [Google Scholar]
- 24.Abeydeera LR, Day BN. Fertilization and subsequent development in vitro of pig oocytes inseminated in a modified tris-buffered medium with frozen-thawed ejaculated spermatozoa. Biol Reprod 1997; 57: 729–734. [DOI] [PubMed] [Google Scholar]
- 25.Hua S, Zhang H, Song Y, Li R, Liu J, Wang Y, Quan F, Zhang Y. High expression of Mfn1 promotes early development of bovine SCNT embryos: improvement of mitochondrial membrane potential and oxidative metabolism. Mitochondrion 2012; 12: 320–327. [DOI] [PubMed] [Google Scholar]
- 26.Choi J, Park SM, Lee E, Kim JH, Jeong YI, Lee JY, Park SW, Kim HS, Hossein MS, Jeong YW, Kim S, Hyun SH, Hwang WS. Anti-apoptotic effect of melatonin on preimplantation development of porcine parthenogenetic embryos. Mol Reprod Dev 2008; 75: 1127–1135. [DOI] [PubMed] [Google Scholar]
- 27.Qian W, Wang J, Roginskaya V, McDermott LA, Edwards RP, Stolz DB, Llambi F, Green DR, Van Houten B. Novel combination of mitochondrial division inhibitor 1 (mdivi-1) and platinum agents produces synergistic pro-apoptotic effect in drug resistant tumor cells. Oncotarget 2014; 5: 4180–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vazquez-Martin A1 Cufi S, Corominas-Faja B, Oliveras-Ferraros C, Vellon L, Menendez JA. Mitochondrial fusion by pharmacological manipulation impedes somatic cell reprogramming to pluripotency: new insight into the role of mitophagy in cell stemness. Aging (Albany, NY Online) 2012; 4: 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferree A, Shirihai O. Mitochondrial dynamics: the intersection of form and function. Adv Exp Med Biol 2012; 748: 13–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim B, Kim JS, Yoon Y, Santiago MC, Brown MD, Park JY. Inhibition of Drp 1-dependent mitochondrial division impairs myogenic differentiation. Am J Physiol Regul Integr Comp Physiol 2013; 305: R927–R938. [DOI] [PubMed] [Google Scholar]
- 31.Kuznetsov AV, Hermann M, Saks V, Hengster P, Margreiter R. The cell-type specificity of mitochondrial dynamics. Int J Biochem Cell Biol 2009; 41: 1928–1939. [DOI] [PubMed] [Google Scholar]
- 32.Bereiter-Hahn J, Vöth M, Mai S, Jendrach M. Structural implications of mitochondrial dynamics. Biotechnol J 2008; 3: 765–780. [DOI] [PubMed] [Google Scholar]
- 33.Anesti V, Scorrano L. The relationship between mitochondrial shape and function and the cytoskeleton. Biochim Biophys Acta 2006; 1757: 692–699. [DOI] [PubMed] [Google Scholar]
- 34.Scott I, Youle RJ. Mitochondrial fission and fusion. Essays Biochem 2010; 47: 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wassarman PM. Sperm receptors and fertilization in mammals. Mt Sinai J Med 2002; 69: 148–155. [PubMed] [Google Scholar]
- 36.Wang LY, Wang DH, Zou XY, Xu CM. Mitochondrial functions on oocytes and preimplantation embryos. J Zhejiang Univ Sci B 2009; 10: 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parone PA, Da Cruz S, Tondera D, Mattenberger Y, James DI, Maechler P, Barja F, Martinou JC. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS ONE 2008; 3: e3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vazquez-Martin A, Cufi S, Corominas-Faja B, Oliveras-Ferraros C, Vellon L, Menendez JA. Mitochondrial fusion by pharmacological manipulation impedes somatic cell reprogramming to pluripotency: new insight into the role of mitophagy in cell stemness. Aging (Albany, NY Online) 2012; 4: 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park SJ, Park YJ, Shin JH, Kim ES, Hwang JJ, Jin DH, Kim JC, Cho DH. A receptor tyrosine kinase inhibitor, Tyrphostin A9 induces cancer cell death through Drp1 dependent mitochondria fragmentation. Biochem Biophys Res Commun 2011; 408: 465–470. [DOI] [PubMed] [Google Scholar]
- 40.Wakefield SL, Lane M, Mitchell M. Impaired mitochondrial function in the preimplantation embryo perturbs fetal and placental development in the mouse. Biol Reprod 2011; 84: 572–580. [DOI] [PubMed] [Google Scholar]
- 41.Kirkinezos IG, Moraes CT. Reactive oxygen species and mitochondrial diseases. Semin Cell Dev Biol 2001; 12: 449–457. [DOI] [PubMed] [Google Scholar]
- 42.Favetta LA, St John EJ, King WA, Betts DH. High levels of p66shc and intracellular ROS in permanently arrested early embryos. Free Radic Biol Med 2007; 42: 1201–1210. [DOI] [PubMed] [Google Scholar]
- 43.Van Blerkom J. Mitochondria in early mammalian development. Semin Cell Dev Biol 2009; 20: 354–364. [DOI] [PubMed] [Google Scholar]
- 44.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA 2006; 103: 2653–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta MK, Uhm SJ, Han DW, Lee HT. Embryo quality and production efficiency of porcine parthenotes is improved by phytohemagglutinin. Mol Reprod Dev 2007; 74: 435–444. [DOI] [PubMed] [Google Scholar]
- 46.Song GX, Shen YH, Liu YQ, Sun W, Miao LP, Zhou LJ, Liu HL, Yang R, Kong XQ, Cao KJ, Qian LM, Sheng YH. Overexpression of FABP3 promotes apoptosis through inducing mitochondrial impairment in embryonic cancer cells. J Cell Biochem 2012; 113: 3701–3708. [DOI] [PubMed] [Google Scholar]
- 47.Hsuuw YD, Chan WH, Yu JS. Ochratoxin a inhibits mouse embryonic development by activating a mitochondrion-dependent apoptotic signaling pathway. Int J Mol Sci 2013; 14: 935–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tailor D, Hahm ER, Kale RK, Singh SV, Singh RP. Sodium butyrate induces DRP1-mediated mitochondrial fusion and apoptosis in human colorectal cancer cells. Mitochondrion 2014; 16: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka A, Youle RJ. A chemical inhibitor of DRP1 uncouples mitochondrial fission and apoptosis. Mol Cell 2008; 29: 409–410. [DOI] [PubMed] [Google Scholar]