Abstract
The present study was conducted to evaluate whether supplementation of semen extender with glutathione (GSH) can maintain the quality of frozen-thawed canine spermatozoa. Eighteen ejaculates were obtained from 5 dogs and placed in extender (20% egg yolk, Tris, citric acid, lactose, raffinose, antibiotics and 6.5% glycerol) containing 0 (control), 2.5, 5, 7.5 or 10 mM GSH. The samples were cooled to 4 C and then frozen in liquid nitrogen vapor. Motility parameters of the sperm were evaluated at 0, 1, 2, 3, 4, 12 and 24 h after thawing. Sperm motility was higher in the 5 mM GSH group than in the control or 2.5 and 10 mM GSH groups; this effect was observed at 1 to 24 h after thawing (P < 0.05). The 5 mM GSH group had a higher sperm viability index at 12 and 24 h after thawing compared with the other groups (P < 0.05). Acrosome integrity, evaluated at 4 h after thawing, was greater in two of the GSH-treated groups (5 and 10 mM) compared with the control. Lipid peroxidation (LP) levels immediately after thawing were lower in the 5 and 10 mM GSH groups compared with the control, while those at 12 h after thawing did not differ significantly. Frozen-thawed semen in the 5 mM GSH group was used for transcervical insemination of 4 bitches, resulting in delivery of 5 puppies from 2 bitches. These results indicate that supplementation of semen extender with 5 mM GSH was effective in improving motility, longevity and acrosomal integrity and inhibiting LP levels in post-thaw canine spermatozoa, without any adverse impacts on full-term development after transcervical insemination.
Keywords: Canine sperm, Cryopreservation, Glutathione, Transcervical insemination
Artificial insemination (AI) with cryopreserved semen is widely applied in bovine reproduction, and it has resulted in a progressive improvement in the quality of cattle breeds. Development of an effective cryopreservation technique for canine semen might likewise offer the benefits of enabling maintenance of endangered canine breeds and species and of improving the utilization of specific genetic traits in male dogs of particular breeds. Recently, there has been considerable interest in the roles of various genes in determining the behavioral and personality characteristics of dogs, particularly assistance dogs such as guide dogs for the blind [1]. Our growing understanding of dog genetics will undoubtedly be of use in optimizing the breeding of assistance dogs with the desired characteristics. For such breeding, the ability to use frozen sperm from selected dogs will be of considerable value. Although the first report of successful AI using frozen-thawed dog semen was published in 1969 [2], the rates of successful pregnancy with cryopreserved sperm are still highly variable and generally lower than those with fresh semen [3]. This indicates that the quality of dog sperm after thawing must be relatively poor and likely determines the low pregnancy rate [3]. Thus, there is still considerable scope for improvement in dog sperm cryopreservation techniques, and such improvements will clearly have a positive effect on practical dog breeding.
It is known that cold-induced injury of tissue is associated with intracellular formation of reactive oxygen species (ROS) [4]. Several preservation solutions have been developed to minimize the production of ROS. In clinical applications, the most successful of these media is the University of Wisconsin cold storage solution, which contains reduced glutathione (GSH) as an antioxidant [5]. Supplementation with GSH has also been successfully applied to embryo culture [6] and tissue preservation [7]. In spermatozoa, ROS are generated metabolically and are necessary for sperm functions such as capacitation and the acrosome reaction [8]. However, excess levels of ROS cause detrimental effects, such as membrane lipid peroxidation [9] and DNA fragmentation [10]. These effects are associated with lethal oxidative damage that reduces sperm motility and fertility [10] or causes cell death [11]. Moreover, oxidative damage in sperm can also adversely affect early embryo development after fertilization [12] and subsequent fetal development [13]. In order to reduce oxidative stress in sperm, supplementation of the freezing medium with antioxidants has been used in several mammalian species [14, 15]. The potential benefits of using antioxidants such as vitamin C, N-acetyl-L-cysteine, taurine, catalase, vitamin E and vitamin B16 [16] have also been investigated in dogs.
Previous studies have reported different optimal concentrations of GSH in sperm cryopreservation medium depending on the species or experimental conditions [17, 18]. Here, we investigated the effects of oxidative stress on dog sperm during the freeze-thaw process and evaluated the effect of GSH supplementation on post-thaw sperm motility, longevity, acrosomal integrity and lipid peroxidation levels. In addition, transcervical insemination (TCI) with GSH-treated frozen-thawed semen was performed to test whether the post-thaw sperm could fertilize oocytes and allow full-term development.
Materials and Methods
Animals
Twelve male and 4 female sexually mature dogs (Labrador retrievers and crossbreds Labrador retriever × golden retriever, 1 to 5 years of age) were used in this study. The 12 male dogs used were as follows: 5 dogs for experimental evaluation of frozen-thawed GSH-treated semen, 4 dogs for TCI with frozen-thawed semen, 1 dog for natural mating and 2 dogs for AI with freshly ejaculated semen. The males, whose fertility had been confirmed in previous mating, had been used by the East Japan Guide Dog Association as sires in a breeding program to generate guide dogs for the blind. All experiments in this study were performed in accordance with the Utsunomiya University Guide for Experimental Animals.
Semen collection and processing
A total of 18 semen samples were obtained after digital manipulation in the presence of a bitch in heat as a dummy. The sperm-rich fraction was isolated, although inevitably it contained small amounts of the first (pre-sperm) and third (prostatic) fractions. Ejaculates were kept at room temperature for transport to the laboratory (a total period of about 1.5 h). The quality of the fresh semen samples was assessed within 3 h of ejaculation. Only samples with a fresh sperm motility > 70%, abnormality < 8.9% and sperm concentration > 430 × 106 spermatozoa/ml were used in subsequent experiments.
The original Tris–egg yolk–citrate extender was based on that described previously [19,20,21]: 130 mM Tris, 40 mM citric acid, 40 mM lactose, 40 mM raffinose, 1% (v/v) antibiotics and 20% (v/v) egg yolk in ultrapure water. For the primary dilution, the ejaculate was divided into 2 to 5 aliquots and placed into extender supplemented with GSH (2.5, 5, 7.5 or 10 mM) or without GSH (control). The samples were allowed to equilibrate for 3 h in a refrigerator at 4 C. An equal volume of extender (with or without GSH ) containing 13% glycerol was then added to obtain a final concentration of 6.5% glycerol and 100 × 106 spermatozoa/ml.
Freezing and thawing
The sperm samples were loaded into 0.25 ml straws (IMV, L’Aigle, France) and frozen by placing them horizontally in a rack 6 cm above the surface of liquid nitrogen (LN2) in a closed styrene foam box (16 cm × 24 cm × 16 cm) for 15 min; the straws were frozen in LN2 vapor and plunged into the LN2 [22]. The straws were stored in LN2 for at least one week before being thawed for evaluation of the sperm. Thawing was carried out in a water bath at 70 C for 5 sec [23], and the thawed sperm samples were placed into sealed 1.5 ml polypropylene tubes at room temperature (approximately 24 C).
Assessment of semen quality parameters
Sperm motility and longevity: The motility of spermatozoa was estimated by phase-contrast microscopy (Olympus Optical, Tokyo, Japan) at a magnification of 200 × on a warmed slide (38 C) at 0, 1, 2, 3, 4, 12 and 24 h after thawing. Motility patterns were classified using the WHO grades with some modifications [24]: +++, progressively motile at a high speed; ++, progressively motile at a moderate or low speed; +, motile without progression; –, immotile. The proportions (%) of sperm in each grade were assessed independently by two observers, and the average value per sample was recorded as the final motility. Sperm progressive motility (SPM) scores were obtained from the sperm samples classified as having a +++ motility pattern. The effects of the different treatments were also compared using the sperm viability index (SVI). This index is based on the four patterns of sperm motility and their relative proportions in each group. SVI was calculated using a previously described formula [25] with some modifications: (% +++ sperm) + (% ++ sperm × 0.75) + (% + sperm × 0.5).
Acrosomal integrity: Acrosome status of frozen-thawed spermatozoa was evaluated at 4 h after thawing by using combined Hoechst 33258/chlortetracycline staining (H258/CTC), which was performed with a modified version of the protocol described by Fraser et al. [26]. Briefly, the suspended samples from the 0, 5 and 10 mM GSH groups were stained with 0.1 µl/ml of Hoechst bis-benzimide 33258 in Bracket and Oliphant (BO) medium for 3 min at room temperature. Excess dye was removed by centrifugation at 600 × g for 10 min. The precipitated spermatozoa were resuspended in 45 μl of BO medium, mixed with the same volume of CTC solution (750 mM CTC, 5 mM cysteine, 130 mM NaCl and 20 mM Tris, pH 7.8) and then fixed with 8 μl of 12.5% (w/v) paraformaldehyde. The cells were analyzed using a fluorescence microscope (Olympus) at a magnification of 400 ×; a minimum of 100 spermatozoa per slide were screened. After H258/CTC staining, the spermatozoa were first categorized as live or dead according to their Hoechst staining using a 330 to 385 nm filter and a DM400 dichroic mirror (U-MWU2; Olympus). Only sperm judged as living (Hoechst negative) were examined for CTC staining using a 400 to 440 nm filter and a DM455 dichroic mirror (U-MWBV2; Olympus). Live spermatozoa were then further categorized according to the CTC fluorescence patterns as described by Hewitt et al. [27]. Three patterns of fluorescence were present in live spermatozoa: fluorescence over the whole head (F: non-capacitated), fluorescence-free band in the post-acrosomal region (B: capacitated); and almost no fluorescence over the whole head except for a thin band in the equatorial segment (AR: acrosome reacted). Viable spermatozoa with both F and B patterns were assumed to represent acrosomally intact spermatozoa.
Lipid peroxidation (LP): The levels of lipid peroxidation were assessed at 0 and 12 h after thawing by determining malondialdehyde (MDA) production using a thiobarbituric acid (TBA) assay kit (TBARS Assay Kit, Cayman Chemical, Ann Arbor, MI, USA) [28]. Semen samples from 0, 5 and 10 mM GSH groups were diluted with BO medium to yield 20 × 106 spermatozoa/ml. To detect MDA-TBA adduct formation, 0.1 ml of SDS solution and 4 ml of color reagent (10% (v/v) acetic acid, 5% (v/v) sodium hydroxide and 0.53% (w/v) TBA in ultrapure water) were added to 0.1 ml of the diluted sperm suspension. The mixture was boiled for 60 min, cooled in an ice bath for 10 min and then centrifuged at 1400 × g for 10 min. After stabilization at room temperature for 30 min, the supernatant from each vial was loaded, and the level of fluorescence at 485 excitation/535 emission was determined using a microplate reader (ARVO X4, Perkin-Elmer, Waltham, MA, USA). The results are presented as µM MDA/20 × 106 spermatozoa.
Transcervical insemination (TCI) of bitches
To demonstrate the capacity of spermatozoa in GSH-treated semen to fertilize oocytes, 4 bitches (Labrador retrievers) aged 2 to 5 years old were artificially inseminated with post-thaw spermatozoa supplemented with 5 mM GSH. The onset of proestrus in each bitch was taken as the day when a serosanguineous vaginal discharge could first be identified. The estrus cycles of the bitches were monitored using vaginal smears [29] and by plasma progesterone concentrations determined by enzyme-linked fluorescence assay (mini VIDAS, Sysmex bioMérieux, Tokyo, Japan). The day when the plasma progesterone concentration exceeded 2 ng/ml was presumed to be the day of the LH surge [30]. The bitches were inseminated or mated naturally at 3 to 6 days after identification of the LH surge [3, 31]. In order to confirm the fertility of each bitch and to avoid failure of conception, the bitches were inseminated or mated 2 to 4 times during estrus; the schedule for TCI with cryopreserved semen, vaginal AI with fresh semen and natural mating (NM) is detailed later.
TCI was performed nonsurgically without anesthesia using 5mM GSH-treated frozen-thawed semen. Immediately after thawing of the straws, 0.8 to 2.4 ml of semen was used for each TCI using a urethral catheter (8 Fr; Nippon Sherwood, Tokyo, Japan) equipped with a laparoscope (HOPKINS telescope, TELE PACK VET X; Karl Storz, Tuttlingen, Germany); the semen samples were deposited into the corpus uteri through the cervical canal. For AI with fresh sperm, the whole ejaculate was deposited deep into the vagina through a urethral catheter (8 Fr; Nippon Sherwood), and the hindquarters of the bitches were kept elevated for 20 min. Pregnancy was confirmed at approximately 30 days after insemination by ultrasonographic diagnosis.
Parentage tests were performed by PCR analyses of microsatellite polymorphisms using genomic DNAs extracted from the umbilical cord, oral mucosa or blood samples. Paternity was established using the microsatellite markers FH2309, FH3377, FH2321, FH2263, PEZ16, PEZ17, FH2293, CXX2001, CXX2010 and CXX2054 as previously described [32,33,34,35].
Statistical analysis
Results were expressed as means ± SEM and statistically analyzed using the StatView v. 5.0 software (Abacus Concepts, Berkeley, CA, USA). Factorial ANOVA with the Tukey-Kramer method was used to compare semen parameters. Differences with values of P < 0.05 were considered to be statistically significant.
Results
Motility-based data analysis
The effects of different GSH concentrations on post-thaw SPM over a 24 h period are shown in Fig. 1. The 5 mM GSH treatment group showed a significantly higher SPM than the 0 (control), 2.5 and 10 mM GSH groups at 1 h and up to 24 h after thawing (P < 0.05). The 7.5 mM GSH treatment group also showed increases at 2 and 3 h after thawing compared with the 0 (control), 2.5 and 10 mM GSH groups (P < 0.05). In addition, the 5 mM GSH treatment group showed a significantly higher SPM at 24 h than any of the other treatment groups (P < 0.05). The 0, 2.5 and 10 mM GSH supplementations showed no significant differences among the groups at any sampling point.
Fig. 1.
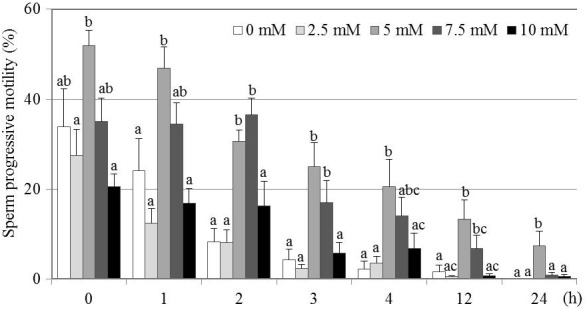
Effect of various GSH concentrations on SPM of frozen-thawed canine spermatozoa. Mean ± SEM. Four to seven replicate experiments were performed (control, n = 7; 2.5 mM, n = 4; 5 mM, n = 4; 7.5 mM, n = 5; and 10 mM, n = 4). Different letters (a–c) indicate significant differences at each time point (P < 0.05).
The effects of different GSH concentrations on post-thaw SVI over a 24 h period are shown in Fig. 2. There were no significant differences in the SVI between the 5 and 7.5 mM GSH treatment groups up to 4 h after thawing. However, at 12 and 24 h after thawing, the 5 mM treatment group showed a significantly higher SVI compared with the other groups (P < 0.05). Thus, the highest level of long-term survival at 24 h was observed in the 5 mM GSH treatment group. The 0, 2.5 and 10 mM GSH supplementations showed no significant differences among them at any sampling point.
Fig. 2.
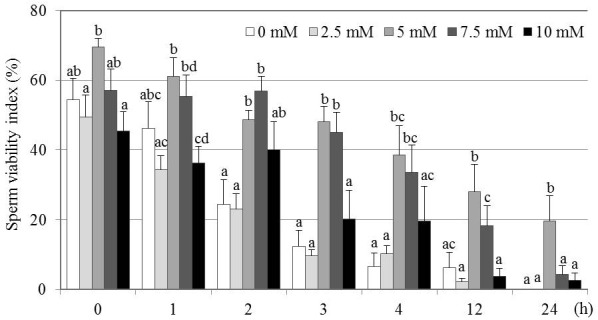
Effect of various GSH concentrations on SVI of frozen-thawed canine spermatozoa. Mean ± SEM. Four to seven replicate experiments were performed (control, n = 7; 2.5 mM, n = 4; 5 mM, n = 4; 7.5 mM, n = 5; and 10 mM, n = 4). Different letters (a–d) indicate significant differences among concentrations at each time point (P < 0.05).
Acrosomal integrity and lipid peroxidation
The proportions of spermatozoa with an intact acrosome were significantly higher in the 10 mM GSH treatment group compared with the control (P < 0.05; Fig. 3). The proportion in the 5 mM GSH treatment group did not differ significantly from those of either the control or the 10 mM GSH treatment group. In all groups, more than 80% of the acrosome-intact spermatozoa were categorized as capacitated (pattern B).
Fig. 3.
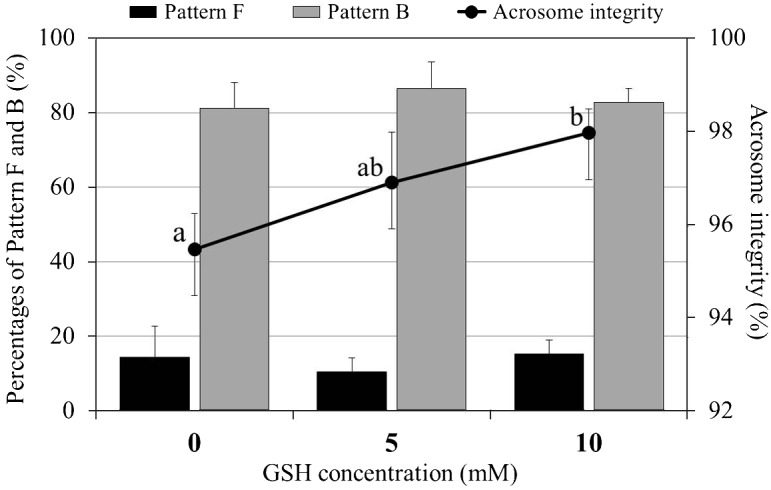
Effect of GSH supplementation on acrosomal status in frozen-thawed canine spermatozoa. Mean ± SEM. Acrosome integrity is expressed as the sum of pattern F (non-capacitated) and pattern B (capacitated). Five replicate experiments were performed. Different letters (a, b) indicate significant differences among the 3 concentrations at 4 h after thawing (P < 0.05).
The LP levels, determined by the MDA concentration, in the GSH treatment groups were significantly lower than that in the control group immediately after thawing (P < 0.05; Fig. 4). However, after 12 h of incubation, there were no significant differences in the LP level among the treatment groups.
Fig. 4.
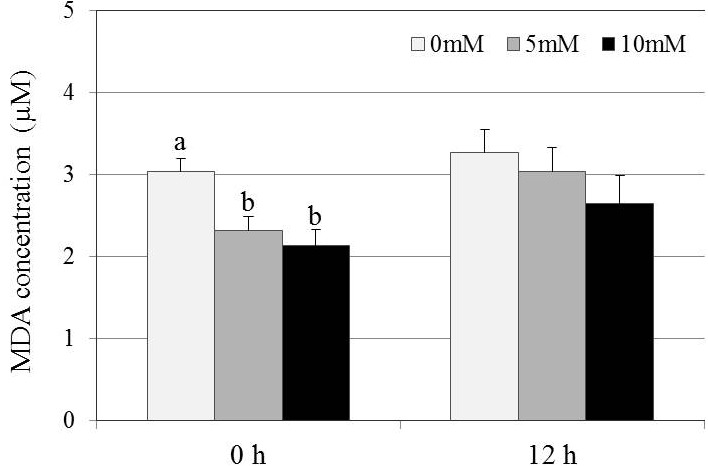
Effect of GSH supplementation on lipid peroxidation levels in frozen-thawed canine spermatozoa. Mean ± SEM. Five replicate experiments were performed. Different letters (a, b) indicate significant differences among the 3 concentrations of GSH at each of 0 and 12 h after thawing (P < 0.05).
Puppy production by transcervical insemination
Ten cycles of TCI in 4 bitches with frozen-thawed semen of the 5 mM GSH treatment group resulted in pregnancy in 2 bitches and subsequent delivery of 5 puppies (Table 1). There were no significant differences in the average birth weight of puppies among the 3 types of breeding (NM, 431.7 ± 15.4 g, n = 7; AI, 443.8 ± 20.1 g, n = 13; and TCI, 390.0 ± 30.8 g, n = 5). All of the TCI-derived puppies exhibited normal appearance and growth at least until 10 months after birth.
Table 1. Outcome of heterospermic insemination with freshly ejaculated sperm and GSH-treated frozen-thawed sperm in Labrador retrievers.
| Bitch no. | Days after LH surge |
Profile of FT-TCI |
Number of puppies |
|||||
| 3 | 4 | 5 | 6 | Total volume of semen nseminated (ml) * |
Post-thaw progressive motility (%) |
FT-TCI | Fr-AI·NM | |
| 1 | ― | ― | FT-TCI | NM | 1.5 | 30 | 0 | 7 |
| FT-TCI | ||||||||
| 2 | ― | ― | Fr-AI | Fr-AI | 3 | 45 | 0 | 7 |
| FT-TCI | FT-TCI | |||||||
| 3 | Fr-AI | Fr-AI | Fr-AI | Fr-AI | 4.8 | 22.5 | 4 | 6 |
| FT-TCI | FT-TCI | |||||||
| 4 | FT-TCI | FT-TCI | FT-TCI | FT-TCI | 6 | 50 | 1 | 0 |
FT-TCI: transcervical insemination with frozen-thawed semen. Fr-AI: artificial insemination with fresh ejaculate. NM: natural mating. * Concentrations of GSH-treated frozen-thawed sperm: 100 × 106 sperm/ml.
Discussion
The objective of this study was to determine whether adding GSH to semen extender had a positive effect on the post-thaw quality of cryopreserved canine sperm. A secondary objective was to identify the optimal GSH concentration for supplementation of the cryopreservation medium for canine sperm. Additionally, we checked whether supplementation of sperm freezing extender with GSH had no adverse influence on in vivo development of embryos produced with these sperm. Our analyses showed that SPM and SVI were highest in the 5 mM GSH treatment group at 24 h after thawing. The proportion of sperm showing normal acrosome integrity was also higher in the GSH-treated groups, and the LP levels were lower. The TCI performed with 5 mM GSH-treated semen resulted in successful production of 5 puppies, suggesting no adverse influence on fertilization and embryonic development in vivo. These results indicate that supplementation of the medium for freezing canine sperm with GSH improves post-thaw sperm quality; the optimal concentration of GSH appeared to be 5 mM. Monteiro et al. [36] reported that supplementation of canine semen extender with 5 mM GSH improved post-thaw sperm quality compared with supplementation with 1 mM GSH and 50 and 250 µM ascorbic acid. Strezezek et al. [37] reported that the physiologically normal GSH concentration in canine semen was closer to 5 mM, which is recommended here for supplementation of semen extender/freezing solution.
In our study, a wider range of GSH concentrations was investigated, and the highest concentration of GSH (10 mM) resulted in a lower SPM and SVI. One possible explanation for this may come from the report of Silva et al. [38], who found that addition of 7 mM GSH to frozen-thawed ram sperm induced membrane swelling, poorer mitochondrial integrity and a reduction in motility. Osmotic shock can induce many types of damage to sperm cells, including disruption of the plasma membrane lipid structure and changes in intracellular signaling [39]. These findings raise the possibility that osmotic stress might have contributed to the effects on SPM and SVI we observed in the highest GSH treatment group.
The time-course analyses of post-thaw SPM and SVI indicated that the 5 mM GSH treatment group showed good results up to 24 h after thawing at room temperature. In dogs, ovulation occurs over a period of 12–36 h [40, 41], and a maturation process occurs during the following 48–96 h [42]. Thus, there is a wide time range during which fertilization may occur. Following insemination, a selected sperm population is stored in the uterotubal junction, and small numbers of spermatozoa are released continuously into the caudal oviduct; this continues until the the oocytes have matured to metaphase II [42]. Therefore, for cryopreserved sperm to remain functionally competent until the time of fertilization, long-term maintenance of post-thaw motility and viability is necessary, which might contribute to some extent to the longevity of inseminated sperm in the female dog reproductive tract. SVI can be used to assess all motile spermatozoa including those with low motility, whereas SPM can be used to assess progressively motile sperm only. Therefore, the SVI parameter together with the SPM could be used to evaluate the long-term fertility of the whole post-thawed sperm population. Incubation at room temperature may enable long-term evaluation of motile spermatozoa [43]. However, it should be noted that the profile of post-thaw SPM and SVI during preservation at room temperature (24 C) may not fully reflect the true sperm viability under in vivo conditions (39 C).
Maintenance of the sperm’s capacity to fertilize an oocyte during transit through the genital tract is also important. It is believed that the oviductal epithelium acts to select sperm that are normal, viable and uncapacitated. Thus, a too rapid or high hyperactivation response during in vitro incubation might cause destabilization and result in reduced fertilization ability [44]. In the present study, sperm from the 5 mM GSH treatment group were preserved longer with normal acrosome integrity and high motility. A similar high rate of acrosome maintenance was found in a previous study, although there was a difference in the culture conditions used [27].
A significant reduction in the LP level was observed immediately after thawing when canine sperm were frozen-thawed in the presence of GSH. Improved motility parameters immediately after thawing may be reflected by the GSH effect on canine sperm before or during freezing. Moreover, the difference in motility between GSH-treated and non-treated sperm may have been amplified by the prolonged incubation period (12–24 h). The improved motility at 12 h after thawing under similar LP suppression levels can be explained by the time lag from the incidence of lipid peroxidation to the loss of motility. Spermatozoa are sensitive to lipid peroxidation due to their high polyunsaturated fatty acid content. Lipid peroxidation is well known to induce serious oxidative damage that can reduce sperm motility and mitochondrial membrane potential and to cause detrimental effects such as loss of fertility or cell death [45]. Consequently, the antioxidative effects of GSH might suppress lipid peroxidation, leading to a notable advantage for canine sperm cryopreservation. In bull sperm, peroxidative damage was located mainly in the midpiece and tail [46]. Lower levels of lipid peroxidation in the mitochondrial midpiece, which is necessary for sperm movement or metabolism, and the tail, which is associated with flagellar movement, might be responsible for the improvement of SPM and SVI in the GSH-treated semen samples in the present study.
Paternity analyses indicated that 5 normal pups were produced after heterospermic TCI that included 5 mM GSH-treated sperm. This outcome confirmed that GSH-treated sperm have normal fertilizing ability and that the embryos that they fertilize can develop normally. For successful AI using frozen semen, it is important to assess accurately the quality of the sperm after thawing. Selles et al. [47] showed that the IVF technique could be used on boar semen to assess gametic interactions, including sperm binding and penetration of the zona pellucida, sperm-oocyte membrane fusion and male pronuclear formation; these assessments provided a better prediction of fertility for frozen-thawed semen than routine laboratory evaluation. However, dog sperm fertility cannot be assessed directly by IVF, as a robust and reliable method for in vitro maturation of dog oocytes has not yet been established [48]. AI/TCI might be able to overcome the abovementioned problem. Moreover, it is well known that a higher conception rate following insemination with frozen-thawed semen is obtained when semen is deposited in the uterus through the cervix versus in the vagina. Therefore, the TCI technique, capable of noninvasive semen deposition into the uterus without anesthesia, would be a beneficial option for application of cryopreserved canine spermatozoa.
A tripeptide GSH (γ-glutamyl cysteinyl glycine) is one of the water-soluble, nonenzymatic antioxidants that are ubiquitously distributed in living cells. It can act both as a cofactor for glutathione peroxidase and singly as an antioxidant to preserve SH groups through disulfide interchange in proteins in the reduced state. Previous studies have demonstrated that the GSH content of bull [49] and boar [50] semen is decreased after cryopreservation. It is likely that a similar decrease occurs in dog semen, which also contains GSH [37]. In the present study, we presumed that supplementation with GSH would influence the creation of ROS during cryopreservation either directly using its own SH group or indirectly by acting as a cofactor of the enzymatic antioxidants systems that protect dog sperm. GSH might also help to prevent reduction in the antioxidant systems that might occur during cryopreservation of dog semen.
In conclusion, we demonstrated that addition of 5 mM GSH to the freezing medium used for dog sperm improves subsequent motility, longevity and acrosomal integrity and reduces lipid peroxidation after thawing. Moreover, semen treated in this way had a normal capacity to fertilize oocytes and did not adversely affect subsequent fetal development; this is the first report regarding successful birth of puppies using GSH-treated frozen-thawed canine sperm and TCI. Improvement in conception rates after AI/TCI with GSH-treated and cryopreserved canine semen could provide a means to effectively utilize specific genetic traits in male dogs and to improve the breeding of guide dogs for the blind.
Acknowledgments
This work was supported by the grant of Young Scholarship, VBL, Utsunomiya University (Tochigi, Japan). We thank Dr Hiroshi Takano (Takano Veterinary Hospital, Tochigi, Japan) for performance of TCI and helpful discussion and Kenji Nishihara, Naoko Sugane, Seigo Suzuki, Yuki Nishihara, Yoshinori Yanagisawa and Ryota Fukui (East Japan Guide Dog Association, Tochigi, Japan) for their technical assistance in semen collection and TCI.
References
- 1.Takeuchi Y, Hashizume C, Arata S, Inoue-Murayama M, Maki T, Hart BL, Mori Y. An approach to canine behavioural genetics employing guide dogs for the blind. Anim Genet 2009; 40: 217–224. [DOI] [PubMed] [Google Scholar]
- 2.Seager SWJ. Successful pregnancies utilizing dog semen. Artif Insem Dig 1969; 17: 6–16. [Google Scholar]
- 3.Thomassen R, Sanson G, Krogenaes A, Fougner JA, Berg KA, Farstad W. Artificial insemination with frozen semen in dogs: a retrospective study of 10 years using a non-surgical approach. Theriogenology 2006; 66: 1645–1650. [DOI] [PubMed] [Google Scholar]
- 4.Rauen U, de Groot H. New insights into the cellular and molecular mechanisms of cold storage injury. J Investig Med 2004; 52: 299–309. [DOI] [PubMed] [Google Scholar]
- 5.Southard JH, Belzer FO. Organ preservation. Annu Rev Med 1995; 46: 235–247. [DOI] [PubMed] [Google Scholar]
- 6.Ishizuka Y, Nishimura M, Matsumoto K, Miyashita M, Takeo T, Nakagata N, Hosoi Y, Anzai M. The influence of reduced glutathione in fertilization medium on the fertility of in vitro-matured C57BL/6 mouse oocytes. Theriogenology 2013; 80: 421–426. [DOI] [PubMed] [Google Scholar]
- 7.Nagao Y, Harada Y, Yamaguchi M, Igarashi A, Ooshima Y, Kato Y. Antioxidant treatment during preservation of bovine ovaries increased the development potential of embryos. Zygote 2010; 18: 315–321. [DOI] [PubMed] [Google Scholar]
- 8.Rivlin J, Mendel J, Rubinstein S, Etkovitz N, Breitbart H. Role of hydrogen peroxide in sperm capacitation and acrosome reaction. Biol Reprod 2004; 70: 518–522. [DOI] [PubMed] [Google Scholar]
- 9.Gomez E, Irvine DS, Aitken RJ. Evaluation of a spectrophotometric assay for the measurement of malondialdehyde and 4-hydroxyalkenals in human spermatozoa: relationships with semen quality and sperm function. Int J Androl 1998; 21: 81–94. [DOI] [PubMed] [Google Scholar]
- 10.Aitken RJ, Baker MA. Oxidative stress, sperm survival and fertility control. Mol Cell Endocrinol 2006; 250: 66–69. [DOI] [PubMed] [Google Scholar]
- 11.Gandini L, Lombardo F, Paoli D, Caponecchia L, Familiari G, Verlengia C, Dondero F, Lenzi A. Study of apoptotic DNA fragmentation in human spermatozoa. Hum Reprod 2000; 15: 830–839. [DOI] [PubMed] [Google Scholar]
- 12.Burruel V, Klooster KL, Chitwood J, Ross PJ, Meyers SA. Oxidative damage to rhesus macaque spermatozoa results in mitotic arrest and transcript abundance changes in early embryos. Biol Reprod 2013; 89: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lane M, McPherson NO, Fullston T, Spillane M, Sandeman L, Kang WX, Zander-Fox DL. Oxidative stress in mouse sperm impairs embryo development, fetal growth and alters adiposity and glucose regulation in female offspring. PLoS ONE 2014; 9: e100832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarthy MJ, Meyers SA. Antioxidant treatment in the absence of exogenous lipids and proteins protects rhesus macaque sperm from cryopreservation-induced cell membrane damage. Theriogenology 2011; 76: 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peña FJ, Johannisson A, Wallgren M, Rodriguez Martinez H. Antioxidant supplementation in vitro improves boar sperm motility and mitochondrial membrane potential after cryopreservation of different fractions of the ejaculate. Anim Reprod Sci 2003; 78: 85–98. [DOI] [PubMed] [Google Scholar]
- 16.Michael A, Alexopoulos C, Pontiki E, Hadjipavlou-Litina D, Saratsis P, Boscos C. Effect of antioxidant supplementation on semen quality and reactive oxygen species of frozen-thawed canine spermatozoa. Theriogenology 2007; 68: 204–212. [DOI] [PubMed] [Google Scholar]
- 17.Estrada E, Rodríguez-Gil JE, Rocha LG, Balasch S, Bonet S, Yeste M. Supplementing cryopreservation media with reduced glutathione increases fertility and prolificacy of sows inseminated with frozen-thawed boar semen. Andrology 2014; 2: 88–99. [DOI] [PubMed] [Google Scholar]
- 18.Gadea J, García-Vazquez F, Matás C, Gardón JC, Cánovas S, Gumbao D. Cooling and freezing of boar spermatozoa: supplementation of the freezing media with reduced glutathione preserves sperm function. J Androl 2005; 26: 396–404. [DOI] [PubMed] [Google Scholar]
- 19.Andersen K. Insemination with frozen dog semen based on a new insemination technique. Zuchthygiene 1975; 10: 1–4. [DOI] [PubMed] [Google Scholar]
- 20.Salamon S, Maxwell WM. Storage of ram semen. Anim Reprod Sci 2000; 62: 77–111. [DOI] [PubMed] [Google Scholar]
- 21.Silva AR, de Cássia Soares Cardoso R, Uchoa DC, MacHado da Silva LD. Effect of tris-buffer, egg yolk and glycerol on canine semen freezing. Vet J 2002; 164: 244–246. [DOI] [PubMed] [Google Scholar]
- 22.Okano T, Murase T, Asano M, Tsubota T. Effects of final dilution rate, sperm concentration and times for cooling and glycerol equilibration on post-thaw characteristics of canine spermatozoa. J Vet Med Sci 2004; 66: 1359–1364. [DOI] [PubMed] [Google Scholar]
- 23.Nöthling JO, Shuttleworth R. The effect of straw size, freezing rate and thawing rate upon post-thaw quality of dog semen. Theriogenology 2005; 63: 1469–1480. [DOI] [PubMed] [Google Scholar]
- 24. World Health Organization WHO laboratory manual for the examination of human semen and semen-cervical mucus interaction. Cambridge University Press1999; 4th edition. [Google Scholar]
- 25.Fukui Y, Togawa M, Abe N, Takano Y, Asada M, Okada A, Iida K, Ishikawa H, Ohsumi S. Validation of the sperm quality analyzer and the hypo-osmotic swelling test for frozen-thawed ram and minke whale (Balaenoptera bonarensis) spermatozoa. J Reprod Dev 2004; 50: 147–154. [DOI] [PubMed] [Google Scholar]
- 26.Fraser LR, Abeydeera LR, Niwa K. Ca(2+)-regulating mechanisms that modulate bull sperm capacitation and acrosomal exocytosis as determined by chlortetracycline analysis. Mol Reprod Dev 1995; 40: 233–241. [DOI] [PubMed] [Google Scholar]
- 27.Hewitt DA, England GC. An investigation of capacitation and the acrosome reaction in dog spermatozoa using a dual fluorescent staining technique. Anim Reprod Sci 1998; 51: 321–332. [DOI] [PubMed] [Google Scholar]
- 28.Zakošek Pipan M, Mrkun J, Kosec M, Nemec Svete A, Zrimšek P. Superoxide dismutase: a predicting factor for boar semen characteristics for short-term preservation. Biomed Res Int 2014; 2014: 105280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farstad W. The correlation between a cyclus coefficient based on cytological indices in the vaginal smear and circulating progesterone in oestrous bitches. Reprod Domest Anim 1984; 19: 211–217. [Google Scholar]
- 30.Hase M, Hori T, Kawakami E, Tsutsui T. Plasma LH and progesterone levels before and after ovulation and observation of ovarian follicles by ultrasonographic diagnosis system in dogs. J Vet Med Sci 2000; 62: 243–248. [DOI] [PubMed] [Google Scholar]
- 31.Suwa Y, Abe Y, Lee DS, Ueta YY, Suzuki H. Individual fertility differences in the frozen-thawed spermatozoa among semen donors in the Labrador Retriever. Reprod Med Biol 2009; 8: 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abe Y, Suwa Y, Asano T, Ueta YY, Kobayashi N, Ohshima N, Shirasuna S, Abdel-Ghani MA, Oi M, Kobayashi Y, Miyoshi M, Miyahara K, Suzuki H. Cryopreservation of canine embryos. Biol Reprod 2011; 84: 363–368. [DOI] [PubMed] [Google Scholar]
- 33.Ichikawa Y, Takagi K, Tsumagari S, Ishihama K, Morita M, Kanemaki M, Takeishi M, Takahashi H. Canine parentage testing based on microsatellite polymorphisms. J Vet Med Sci 2001; 63: 1209–1213. [DOI] [PubMed] [Google Scholar]
- 34.Kanthaswamy S, Tom BK, Mattila AM, Johnston E, Dayton M, Kinaga J, Erickson BJ, Halverson J, Fantin D, DeNise S, Kou A, Malladi V, Satkoski J, Budowle B, Smith DG, Koskinen MT. Canine population data generated from a multiplex STR kit for use in forensic casework. J Forensic Sci 2009; 54: 829–840. [DOI] [PubMed] [Google Scholar]
- 35.Mellersh CS, Langston AA, Acland GM, Fleming MA, Ray K, Wiegand NA, Francisco LV, Gibbs M, Aguirre GD, Ostrander EA. A linkage map of the canine genome. Genomics 1997; 46: 326–336. [DOI] [PubMed] [Google Scholar]
- 36.Monteiro JC, Gonçalves JS, Rodrigues JA, Lúcio CF, Silva LC, Assumpção ME, Vannucchi CI. Influence of ascorbic acid and glutathione antioxidants on frozen-thawed canine semen. Reprod Domest Anim 2009; 44(Suppl 2): 359–362. [DOI] [PubMed] [Google Scholar]
- 37.Strzezek R, Koziorowska-Gilun M, Kowalówka M, Strzezek J. Characteristics of antioxidant system in dog semen. Pol J Vet Sci 2009; 12: 55–60. [PubMed] [Google Scholar]
- 38.Silva SV, Soares AT, Batista AM, Almeida FC, Nunes JF, Peixoto CA, Guerra MM. In vitro and in vivo evaluation of ram sperm frozen in tris egg-yolk and supplemented with superoxide dismutase and reduced glutathione. Reprod Domest Anim 2011; 46: 874–881. [DOI] [PubMed] [Google Scholar]
- 39.Meyers SA. Spermatozoal response to osmotic stress. Anim Reprod Sci 2005; 89: 57–64. [DOI] [PubMed] [Google Scholar]
- 40.Boyd JS, Renton JP, Harvey MJ, Nickson DA, Eckersall PD, Ferguson JM. Problems associated with ultrasonography of the canine ovary around the time of ovulation. J Reprod Fertil Suppl 1993; 47: 101–105. [PubMed] [Google Scholar]
- 41.Tsutsui T. Gamete physiology and timing of ovulation and fertilization in dogs. J Reprod Fertil Suppl 1989; 39: 269–275. [PubMed] [Google Scholar]
- 42.Rijsselaere T, England G, Freeman S, Maes D, Van Soom A. Current knowledge on the transport and fate of spermatozoa in the reproductive tract of the bitch. Reprod Domest Anim 2014; 49(Suppl 2): 2–7. [DOI] [PubMed] [Google Scholar]
- 43.Batista M, Santana M, Alamo D, González F, Niño T, Cabrera F, Gracia A. Effects of incubation temperature and semen pooling on the viability of fresh, chilled and freeze-thawed canine semen samples. Reprod Domest Anim 2012; 47: 1049–1055. [DOI] [PubMed] [Google Scholar]
- 44.Petrunkina AM, Waberski D, Günzel-Apel AR, Töpfer-Petersen E. Determinants of sperm quality and fertility in domestic species. Reproduction 2007; 134: 3–17. [DOI] [PubMed] [Google Scholar]
- 45.Sanocka D, Kurpisz M. Reactive oxygen species and sperm cells. Reprod Biol Endocrinol 2004; 2: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brouwers JF, Gadella BM. In situ detection and localization of lipid peroxidation in individual bovine sperm cells. Free Radic Biol Med 2003; 35: 1382–1391. [DOI] [PubMed] [Google Scholar]
- 47.Sellés E, Gadea J, Romar R, Matás C, Ruiz S. Analysis of in vitro fertilizing capacity to evaluate the freezing procedures of boar semen and to predict the subsequent fertility. Reprod Domest Anim 2003; 38: 66–72. [DOI] [PubMed] [Google Scholar]
- 48.Luvoni GC, Chigioni S, Allievi E, Macis D. Factors involved in vivo and in vitro maturation of canine oocytes. Theriogenology 2005; 63: 41–59. [DOI] [PubMed] [Google Scholar]
- 49.Stradaioli G, Noro T, Sylla L, Monaci M. Decrease in glutathione (GSH) content in bovine sperm after cryopreservation: comparison between two extenders. Theriogenology 2007; 67: 1249–1255. [DOI] [PubMed] [Google Scholar]
- 50.Gadea J, Sellés E, Marco MA, Coy P, Matás C, Romar R, Ruiz S. Decrease in glutathione content in boar sperm after cryopreservation. Effect of the addition of reduced glutathione to the freezing and thawing extenders. Theriogenology 2004; 62: 690–701. [DOI] [PubMed] [Google Scholar]


