Abstract
To investigate the effect of endocrine disruption of 4-nitro-3-phenylphenol (PNMPP) on immature male Wistar-Imamichi rats, the rat pituitary was exposed to PNMPP (10–5–10–9 M) for 24 h with or without gonadotropin-releasing hormone (GnRH) in experiment I. In addition, the Leydig cells (10–5–10–9 M) were exposed to PNMPP for 24 h with or without human chronic gonadotropin (hCG) in experiment II. Our results showed that the PNMPP at 10–5–10–7 M suppressed follicle-stimulating hormone (FSH) and luteinizing hormone (LH) productions from GnRH-stimulated pituitary cells. At the same time, PNMPP 10–5–10–7 M induced an increase in testosterone production from the Leydig cells treated with or without hCG. Based on our results, it can be concluded that that PNMPP might have both androgen agonist action by decreasing FSH and LH production in the pituitary and anti-androgenic action by increasing testosterone production in the Leydig cell.
Keywords: 4-Nitro-3-phenylphenol, Gonadotropins, Leydig cell, Pituitary, Testosterone
There is currently increased interest in the endocrine disruption of diesel exhaust (DE) and diesel exhaust particles (DEPs) [1, 2]. Many studies have demonstrated that DE and DEPs induce problems of organs and hormones related reproduction in both humans [3] and animals [4,5,6,7,8,9,10].
It has previously been reported that 4-nitro-3-phenylphenol (PNMPP) isolated from DEPs had estrogenic action and affected reproductive function. Injection of PNMPP into ovariectomized immature female rats induced an increase in uterine weight, oxytocin-induced myometrium contractility [11], and a significant increase in the uterine luminal epithelium [12]. In in vitro studies, PNMPP provoked a proliferation of breast cancer cell line MCF-7 [13] and decreased the estradiol concentration but did not affect testosterone and cortisol secretion in human adrenal H295R cells [14]. Furthermore, PNMPP had an anti-androgenic effect by inhibiting 5α-dihydrotestosterone (DHT) binding to the androgen receptor (AR) [1].
We hypothesized that PNMPP might affect hormone synthesis and secretion in reproduction-related organs. Accordingly, this study investigated the effect of PNMPP on gonadotropin synthesis in the pituitary and testosterone synthesis on Leydig cells in cell cultures.
Materials and Methods
Chemicals
4-Nitro-3-phenylphenol (PNMPP), as shown in Fig. 1, was synthesized by the method described previously [2].
Fig. 1.
Chemical structure of 4-nitro-3-phenylphenol (PNMPP), a component of diesel exhaust particles.
Animals
Immature male Wistar-Imamichi rats at 28 days of age were purchased from the Imamichi Institute for Animal Reproduction, Ibaraki, Japan. They were maintained under conditions of controlled lighting (14 h: light 10 h dark, lights on 0500 h), temperature (22 ± 2 C), and humidity (50 ± 5%). Food (CE-2 commercial diet; Clea Japan, Tokyo, Japan) and water were available ad libitum. All procedures were carried out in accordance with guidelines established by the Tokyo University of Agriculture and Technology, for use of laboratory animals.
Experimental procedure
The rats were decapitated, and the anterior pituitary gland and Leydig cells were removed immediately.
Experiment I: Effect of PNMPP on hormone secretion from the anterior pituitary
The anterior pituitaries were placed in cold DMEM medium containing 10 g/l M5M, 6 g/l HEPES, 10% NaHCO3, and 10 ml/l MEM nonessential amino acid without enzymes. The pituitaries were minced and incubated with Dulbecco’s Modified Eagle’s Medium (DMEM, Invitrogen, Burlington, ON, Canada) with 2.8 mg/ml collagenase, 0.8 mg/ml hyaluronidase, 8 mg/ml bovine serum albumin (BSA), and 200 U/ml DNAse in a shaking incubator (150 cycles/min) at 34 C for approximately 20 min. Minced pituitary was washed by centrifugation at 1,500 rpm for 5 min at room temperature and then resuspended with DMEM with 10% Daigo’s GF21 solution (inhibin-free serum; Wako Pure Chemical Industries, Osaka, Japan), 100 U/ml penicillin and 100 µg/ml streptomycin (Invitrogen, Burlington, ON, Canada). The pituitary suspension was cultured and incubated for 78 h in 96-well culture plates at 37 C under an atmosphere of 95% air and 5% CO2. Then the culture media were changed, and the cells were exposed to PNMPP (10–9–10–5 M) dissolved in media. At 24 h after exposure to PNMPP, the cells were stimulated with and without 10 nm gonadotropin-releasing hormone (GnRH); National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, Torrance, CA, USA) for 4 h and then collected. The culture media were subsequently stored at –20 C until assayed for FSH and LH.
Experiment II: Effect of PNMPP on hormone secretion from the Leydig cells
The testes were trimmed free of fat and decapsulated. Then the testicular artery was removed from the decapsulated testes to eliminate red blood cells. After that, the testes were dissociated by incubation in M199 medium (Gibco®) containing 0.71 g/l sodium bicarbonate, 2.21 g/l HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 0.1% BSA, and 25 mg/l soybean trypsin inhibitor (STI), pH 7.4, with 0.25 mg/ml of collagenase (Worthington Biochemical, Freehold, NJ, USA) and then horizontal shaking (90 cycles/min) at 34 C for approximately 10–20 min. After dissociation, the seminiferous tubules were removed by filtration through 100 µm nylon mesh. The filtrated was centrifuged at 1,500 rpm for 5 min at room temperature to wash the dissociated cells. To separate the Leydig cells, the cells were mixed with a Percoll suspension and centrifuged at 3,000 rpm for 30 min at room temperature. The Leydig cells were resuspended in M199 medium with 1% fetal bovine serum (FBS) and without STI and collagenase enzymes. The Leydig cells in the medium (105 cells/well) were cultured and incubated for 48 h in 96-well culture plates at 37 C under an atmosphere of 95% air and 5% CO2 . Then the cells were exposed to PNMPP (10–9–10–5 M) for 24 h with or without 0.1 IU/ml human chronic gonadotropin (hCG) dissolved in media. After 4 h of hCG stimulation, the medium was collected for testosterone assay.
Hormonal assays
FSH and LH concentrations were measured using an NIDDK radioimmunoassay (RIA) kits (Torrance, CA, USA) for rat FSH and LH. The iodinated preparations were rat FSH-I-5 and LH-I-5. The antisera used were anti-rat FSH-S-11 and anti-rat LH-S-11. Results were expressed as rat FSH RP-2 and rat LH RP-3. The intra- and interassay coefficients of variations were 4.8 and 11.4% for FSH and 5.4 and 6.9% for LH, respectively.
Testosterone concentration was measured using a double-antibody RIA system with 125I-labeled radioligands as described previously [15]. Antisera against testosterone (GDN 250), provided by Dr GD Niswender (Colorado State University, Fort Collins, CO, USA), were used. The intra- and interassay coefficients of variations were 5.9 and 5.8%.
Statistical analysis
The data were expressed as means ± SE. One-way analysis of variance (ANOVA) was used to compare means among groups. Post hoc multiple comparison analyses were performed with the Least Significant Difference (LSD) test when the F ratio for the ANOVA was significant at P < 0.05.
Results
Effect of PNMPP on hormone production from the pituitary
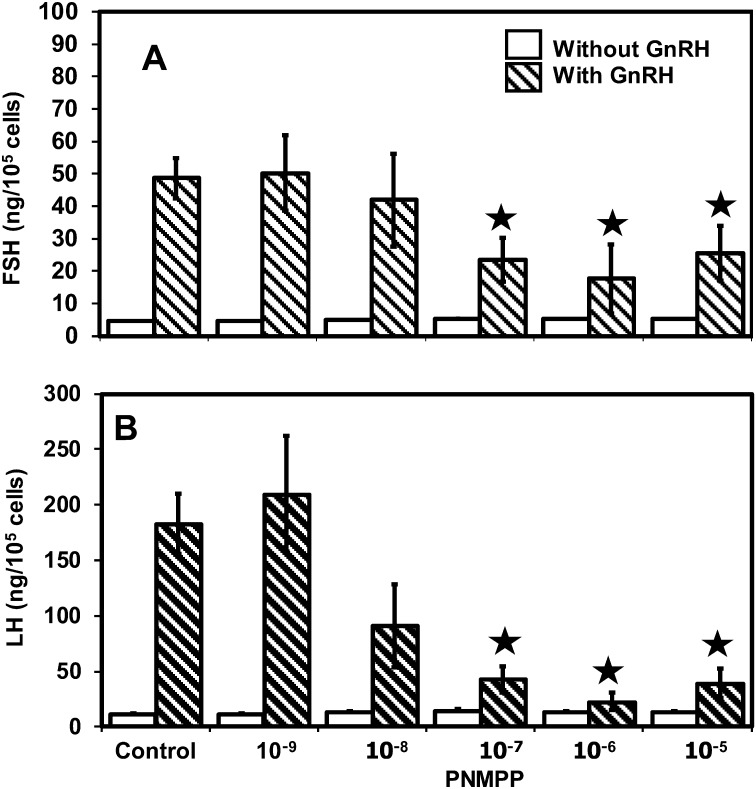
As shown in Fig. 2, PNMPP treatment could not increase the concentrations of FSH and LH secreted from the pituitary cells without GnRH stimulation. Conversely, 10–5–10–7 M of PNMPP could increase the FSH and LH concentration when the cells were stimulated with GnRH. On the other hand, 10–8–10–9 M of PNMPP could not increase FSH and LH concentrations, although the cells were stimulated with GnRH.
Fig. 2.
FSH and LH productions by pituitary cells incubated with PNMPP (10–5–10–9 M) in the presence or absence of GnRH. Each graph represents the means ± SE. The F ratio for the ANOVA compared with control was significant at  P < 0.05.
P < 0.05.
Effect of PNMPP on hormone production from Leydig cell culture
Testosterone concentrations were significantly increased, showing an inverted U shape, in cultures of Leydig cells stimulated with and without hCG when the cells were treated with PNMPP (Fig. 3).
Fig. 3.
Testosterone production by Leydig cells incubated with PNMPP (10–5–10–9 M) in the presence or absence of hCG. Graph represents the means ± SE. The F ratio for the ANOVA compared with control was significant at  P < 0.05 and
P < 0.05 and  P <0.001.
P <0.001.
Discussion
In the present study, PNMPP (10–5–10–7 M) reduced GnRH-stimulated FSH and LH secretions from anterior pituitary cells, but did not have any effect on the pituitary cells without GnRH stimulation. This result indicated that PNMPP played a role in decreasing FSH and LH secretion via GnRH stimulation. As we known, AR is found in the pituitary and affected by androgen administration and castration [16,17,18,19,20]. Androgen also has a role in control of GnRH released from the hypothalamus, as shown by the effect of testosterone treatment on reducing GnRH mRNA [17] and GnRH release [18]. In in vitro studies, androgen was found to suppress pituitary responsiveness to a hypothalamic extract and changed FSH and LH release from the anterior pituitary [20, 21]. Androgen acts by binding at receptor sites and then has a negative feedback action that decreases FSH and LH secretions by slowing the GnRH pulse generator and suppressing FSH and LH syntheses in the pituitary [22].
A single treatment of 3-methyl-4-nitrophenol (4-nitro-m-cresol; PNMC), which was extracted from DEPs, suppressed the plasma LH concentration in Japanese quails [6]. PNMC increased the plasma testosterone concentration and decreased the plasma FSH and LH concentrations, indicating that it acts on the hypothalamus-pituitary axis in adult male rats [23] and immature male rats [24].
Our previous study found that the chemical structure of PNMPP comprises a benzene ring, which is similar to steroid hormones including estrogen and androgen [1]. Hence, in the study of a pituitary cell culture containing GnRH, we might assume that PNMPP acts as androgen and reduce the effect of GnRH action on the secretions of FSH and LH, which would be the same as the effect of PNMC found in previous papers [1, 23, 24].
In the present study, PNMPP induced high secretion of testosterone in the Leydig cells cultured with and without hCG when compared with the control. From previous in vitro studies, DEPs have been reported to slightly increase the gene expression of the steroidogenic acute regulatory (StAR) protein in mouse Leydig cells [25]. Exposure to nanoparticle-rich diesel exhaust (NR-DE) enhanced cholesterol synthesis and increased the expression of gene that regulate steroid synthesis along with the testosterone concentration in testicular culture [26]. Consistent with the study in vitro, exposures to NR-DE for 1 or 2 months significantly increased StAR and cytochrome P450 side-chain cleavage (P450scc) mRNA and their protein expressions and increased the testosterone concentration in male rats and mice [26, 27]. Either NR-DE or DEPs have a direct effect on testosterone production by increasing mRNA expression and genes associated with testosterone cholesterol synthesis in Leydig cells [23, 27, 28]. Accordingly, we assume that PNMPP may have a direct effect on increasing the testosterone concentration in Leydig cells.
Furthermore, addition of procymidone, an anti-androgenic substance, to a Leydig cell culture stimulated with hCG increased testosterone production by elevating several steroidogenic enzymes including StAR, P450scc and cytochrome P450c17α (P450c17) [29]. Flutamide, an androgen receptor antagonist, also enhanced StAR mRNA expression from Leydig cells of adult rats treated with hCG [30]. Furthermore, it was shown previous that anti-androgen caused hypergonadotropic activation of testicular steroidogenesis [2]. PNMPP has been reported to inhibit DHT action by binding to the androgen receptor in a recombinant yeast screen assay [2]. It can be assumed that PNMPP had an anti-androgenic effect on testosterone production in the Leydig cells.
In summary, the present study clearly demonstrated that PNMPP had androgen agonist action by suppressing the effect of GnRH and then decreasing the FSH and LH concentrations in the pituitary cell culture. In addition, PNMPP had a direct effect on the increase in testosterone concentration in Leydig cell culture without hCG stimulation; moreover, it had androgen antagonist action by increasing the testosterone concentration in Leydig cell culture.
Acknowledgments
We are grateful to the Rat Pituitary Hormone Distribution Program, NIDDK, NIH, Bethesda, MD, USA, for providing RIA materials and Dr GD Niswender, Animal Reproduction and Biotechnology Laboratory, Colorado State University, Fort Collins, CO, USA, for providing antisera to testosterone (GDN 250). This study was supported by a grant in Aid for Scientific Research (C-26340037) from the Japan Society for the Promotion of Sciences.
This work was supported by a Grants-in-Aid for Scientific Research (the 21st Century Center of Excellence Program, E-I) from the Ministry of Education Culture, Sports, Science and Technology of Japan (B18310044) and the Japan Society for the Promotion of Science (P05480).
References
- 1.Taneda S, Kamata K, Hayashi H, Toda N, Seki K, Sakushima A, Yoshino S, Yamaki S, Yamaki K, Sakata M, Mori Y, Suzuki AK. Investigation of vasodilatory substances in diesel exhaust particles (DEP): isolation and identification of nitrophenol derivatives. J Health Sci 2004; 50: 133–141. [Google Scholar]
- 2.Taneda S, Mori Y, Kamata K, Hayashi H, Furuta C, Li C, Seki K, Sakushima A, Yoshino S, Yamaki K, Watanabe G, Taya K, Suzuki AK. Estrogenic and anti-androgenic activity of nitrophenols in diesel exhaust particles (DEP). Biol Pharm Bull 2004; 27: 835–837. [DOI] [PubMed] [Google Scholar]
- 3.Lewtas J. Air pollution combustion emissions: characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mutat Res 2007; 636: 95–133. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe N, Oonuki Y. Inhalation of diesel engine exhaust affects spermatogenesis in growing male rats. Environ Health Perspect 1999; 107: 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsukue N, Toda N, Tsubone H, Sagai M, Jin WZ, Watanabe G, Taya K, Birumachi J, Suzuki AK. Diesel exhaust (DE) affects the regulation of testicular function in male Fischer 344 rats. J Toxicol Environ Health A 2001; 63: 115–126. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Takahashi S, Taneda S, Furuta C, Watanabe G, Suzuki AK, Taya K. Impairment of testicular function in adult male Japanese quail (Coturnix japonica) after a single administration of 3-methyl-4-nitrophenol in diesel exhaust particles. J Endocrinol 2006; 189: 555–564. [DOI] [PubMed] [Google Scholar]
- 7.Li C, Taneda S, Suzuki AK, Furuta C, Watanabe G, Taya K. Effects of 3-methyl-4-nitrophenol in diesel exhaust particles on the regulation of testicular function in immature male rats. J Androl 2007; 28: 252–258. [DOI] [PubMed] [Google Scholar]
- 8.Izawa H, Kohara M, Watanabe G, Taya K, Sagai M. Diesel exhaust particle toxicity on spermatogenesis in the mouse is aryl hydrocarbon receptor dependent. J Reprod Dev 2007; 53: 1069–1078. [DOI] [PubMed] [Google Scholar]
- 9.Izawa H, Kohara M, Watanabe G, Taya K, Sagai M. Effects of diesel exhaust particles on the male reproductive system in strains of mice with different aryl hydrocarbon receptor responsiveness. J Reprod Dev 2007; 53: 1191–1197. [DOI] [PubMed] [Google Scholar]
- 10.Izawa H, Kohara M, Aizawa K, Suganuma H, Inakuma T, Watanabe G, Taya K, Sagai M. Alleviative effects of quercetin and onion on male reproductive toxicity induced by diesel exhaust particles. Biosci Biotechnol Biochem 2008; 72: 1235–1241. [DOI] [PubMed] [Google Scholar]
- 11.Furuta C, Suzuki AK, Taneda S, Kamata K, Hayashi H, Mori Y, Li C, Watanabe G, Taya K. Estrogenic activities of nitrophenols in diesel exhaust particles. Biol Reprod 2004; 70: 1527–1533. [DOI] [PubMed] [Google Scholar]
- 12.Furuta C, Li C, Taneda S, Suzuki AK, Kamata K, Watanabe G, Taya K. Immunohistological study for estrogenic activities of nitrophenols in diesel exhaust particles. Endocrine 2005; 27: 33–36. [DOI] [PubMed] [Google Scholar]
- 13.Furuta C, Suzuki AK, Watanabe G, Li C, Taneda S, Taya K. Nitrophenols isolated from diesel exhaust particles promote the growth of MCF-7 breast adenocarcinoma cells. Toxicol Appl Pharmacol 2008; 230: 320–326. [DOI] [PubMed] [Google Scholar]
- 14.Furuta C, Noda S, Li C, Suzuki AK, Taneda S, Watanabe G, Taya K. Nitrophenols isolated from diesel exhaust particles regulate steroidogenic gene expression and steroid synthesis in the human H295R adrenocortical cell line. Toxicol Appl Pharmacol 2008; 229: 109–120. [DOI] [PubMed] [Google Scholar]
- 15.Taya K, Watanabe G, Sasamoto S. Radioimmunoassay for progesterone, testosterone and estradiol-17β using 125I-iodohistamine radioligands. Jpn J Anim Reprod 1985; 31: 186–197. [Google Scholar]
- 16.Sar M, Lubahn DB, French FS, Wilson EM. Immunohistochemical localization of the androgen receptor in rat and human tissues. Endocrinology 1990; 127: 3180–3186. [DOI] [PubMed] [Google Scholar]
- 17.Richardson HN, Parfitt DB, Thompson RC, Sisk CL. Redefining gonadotropin-releasing hormone (GnRH) cell groups in the male Syrian hamster: testosterone regulates GnRH mRNA in the tenia tecta. J Neuroendocrinol 2002; 14: 375–383. [DOI] [PubMed] [Google Scholar]
- 18.Hileman SM, Jackson GL. Regulation of gonadotrophin-releasing hormone secretion by testosterone in male sheep. J Reprod Fertil Suppl 1999; 54: 231–242. [PubMed] [Google Scholar]
- 19.McGinnis MY, Davis PG, Meaney MJ, Singer M, McEwen BS. In vitro measurement of cytosol and cell nuclear androgen receptors in male rat brain and pituitary. Brain Res 1983; 275: 75–82 S. [DOI] [PubMed] [Google Scholar]
- 20.Sharma MK, Balasinor NL, Juneja HS. Modulation of pituitary gonadotropins and prolactin secretion by testosterone in vitro. J Endocrinol Invest 1992; 15: 549–558. [DOI] [PubMed] [Google Scholar]
- 21.Kao LW, Weisz J. Direct effect of testosterone and its 5alpha-reduced metabolites on pituitary LH and FSH release in vitro: change in pituitary responsiveness to hypothalamic extract. Endocrinology 1975; 96: 253–260. [DOI] [PubMed] [Google Scholar]
- 22.Okada Y, Fujii Y, Moore JP, Jr, Winters SJ. Androgen receptors in gonadotrophs in pituitary cultures from adult male monkeys and rats. Endocrinology 2003; 144: 267–273. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Taneda S, Taya K, Watanabe G, Li X, Fujitani Y, Ito Y, Nakajima T, Suzuki AK. Effects of inhaled nanoparticle-rich diesel exhaust on regulation of testicular function in adult male rats. Inhal Toxicol 2009; 21: 803–811. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Li C, Suzuki AK, Watanabe G, Taneda S, Taya K. Endocrine disruptive effect of 3-methyl-4-nitrophenol isolated from diesel exhaust particles in Hershberger assay using castrated immature rats. Biosci Biotechnol Biochem 2009; 73: 2018–2021. [DOI] [PubMed] [Google Scholar]
- 25.Komatsu T, Tabata M, Kubo-Irie M, Shimizu T, Suzuki K, Nihei Y, Takeda K. The effects of nanoparticles on mouse testis Leydig cells in vitro. Toxicol In Vitro 2008; 22: 1825–1831. [DOI] [PubMed] [Google Scholar]
- 26.Li C, Li X, Jigami J, Hasegawa C, Suzuki AK, Zhang Y, Fujitani Y, Nagaoka K, Watanabe G, Taya K. Effect of nanoparticle-rich diesel exhaust on testosterone biosynthesis in adult male mice. Inhal Toxicol 2012; 24: 599–608. [DOI] [PubMed] [Google Scholar]
- 27.Ramdhan DH, Ito Y, Yanagiba Y, Yamagishi N, Hayashi Y, Li C, Taneda S, Suzuki AK, Watanabe G, Taya K, Kamijima M, Nakajima T. Nanoparticle-rich diesel exhaust may disrupt testosterone biosynthesis and metabolism via growth hormone. Toxicol Lett 2009; 191: 103–108. [DOI] [PubMed] [Google Scholar]
- 28.Yamagishi N, Ito Y, Ramdhan DH, Yanagiba Y, Hayashi Y, Wang D, Li CM, Taneda S, Suzuki AK, Taya K, Watanabe G, Kamijima M, Nakajima T. Effect of nanoparticle-rich diesel exhaust on testicular and hippocampus steroidogenesis in male rats. Inhal Toxicol 2012; 24: 459–467. [DOI] [PubMed] [Google Scholar]
- 29.Svechnikov K, Supornsilchai V, Strand ML, Wahlgren A, Seidlova-Wuttke D, Wuttke W, Söder O. Influence of long-term dietary administration of procymidone, a fungicide with anti-androgenic effects, or the phytoestrogen genistein to rats on the pituitary-gonadal axis and Leydig cell steroidogenesis. J Endocrinol 2005; 187: 117–124. [DOI] [PubMed] [Google Scholar]
- 30.Houk CP, Pearson EJ, Martinelle N, Donahoe PK, Teixeira J. Feedback inhibition of steroidogenic acute regulatory protein expression in vitro and in vivo by androgens. Endocrinology 2004; 145: 1269–1275. [DOI] [PubMed] [Google Scholar]