Abstract
Oak powdery mildew (Erysiphe alphitoides) is a major foliar pathogen of Quercus robur often infecting entire tree stands. In this study, foliage photosynthetic characteristics and constitutive and induced volatile emissions were studied in Q. robur leaves asking whether the changes in foliage physiological traits are quantitatively associated with the degree of leaf infection, and whether infection changes the light responses of physiological traits. Infection by E. alphitoides reduced net assimilation rate by 3.5-fold, and isoprene emission rate by 2.4-fold and increased stomatal conductance by 1.6-fold in leaves with the largest degree of infection of ca. 60%. These alterations in physiological activity were quantitatively associated with the fraction of leaf area infected. In addition, light-saturation of net assimilation and isoprene emission was reached at lower light intensity in infected leaves, and infection also reduced the initial quantum yield of isoprene emission. Infection-induced emissions of lipoxygenase pathway volatiles and monoterpenes were light-dependent and scaled positively with the degree of infection. Overall, this study indicates that the reduction of foliage photosynthetic activity and constitutive emissions and the onset of stress volatile emissions scales with the degree of infection, but also that infection modifies the light responses of foliage physiological activities.
Keywords: green leaf volatiles, induced emissions, isoprene emission, light dependency, monoterpene emission, photosynthesis, quantitative responses, volatile organic compounds
1. Introduction
Plants in field are exposed to a plethora of biotic stresses that strongly curb their photosynthesis, growth and survival. Among key biotic stresses, fungal infections constitute a major stress factor, especially under humid conditions that support the spread of fungal pathogens. There is a large difference in virulence of different fungal pathogens, and many of them are highly host-specific (Poland et al., 2009; Schulze-Lefert and Panstruga, 2011). Thus, infection by a compatible pathogen can result in major disease outbreaks in their host species (Gururani et al., 2012), thereby selectively reducing the competitive potential of the host (Alexander and Holt, 1998).
Pedunculate oak (Quercus robur) is the most susceptible species to infections of powdery mildew caused by Erysiphe alphitoides (formerly Microsphaera alphitoides). In fact, powdery mildew caused by E. alphitoides is one of the major foliar diseases of Quercus robur in Europe (Desprez-Loustau et al., 2011) that can significantly reduce tree growth and trigger tree decline (Mougou-Hamdane et al., 2010). Usually young plants are more susceptible to infections, but severe disease outbreaks can occur also in older plants in years with optimum weather conditions for E. alphitoides (Mougou-Hamdane et al., 2010). Although powdery mildew infection constitutes an important stress in Q. robur, physiological responses to powdery mildew infection have been studied only in a few cases. It has been demonstrated that Erysiphe alphitoides infection decreases net assimilation rates and stomatal conductance (Hajji et al., 2009), and reduces the rate of isoprene emission (Brüggemann and Schnitzler, 2001). However, quantitative relationships between these physiological modifications and the degree of infection have not been studied. As the shape of stress severity vs. plant response can differ for various stresses (Beauchamp et al., 2005; Niinemets, 2010a; Niinemets, 2010b), predicting stress responses requires understanding of quantitative relationships between stress severity and plant physiological response.
Apart from stress effects on photosynthesis and constitutive isoprenoid emissions, biotic and abiotic stresses result in a multitude of additional stress responses operating at molecular, cellular, leaf and whole plant level. Among the early stress responses activated upon the contact with pathogenic elicitors are Ca2+ signals, phosphorylation/dephosphorylation of proteins, and production of signaling molecules such as salicylic acid, jasmonic acid, ethylene, and reactive oxygen species ultimately leading to gene expression and defense responses (Berger et al., 2007; Koornneef and Pieterse, 2008; Pieterse and Dicke, 2007). Activation of lipoxygenases and resulting emission of volatile products of lipoxygenase pathway (LOX products) consisting of various C6 aldehydes and alcohols, also called green leaf volatiles, also belongs to the early stress responses that can be involved in subsequent alterations in gene expression patterns (Farag and Paré, 2002; Jansen et al., 2009; Matsui, 2006). Stress-elicited LOX product emission is a ubiquitous stress response and a sensitive indicator of the presence of abiotic and/or biotic stress (Harrison et al., 2013; Niinemets, 2010a; Possell and Loreto, 2013). Stress-induced emissions of LOX products are typically followed by emissions of volatile isoprenoids such as mono- and sesquiterpenes and benzenoids such as methyl salicylate, whereas the induced emission blend is stress-dependent, reflecting selective activation of different set of genes (Copolovici et al., 2012; Hartikainen et al., 2009; Jansen et al., 2009; Loreto and Schnitzler, 2010; Semiz et al., 2012; Staudt et al., 2010; Vuorinen et al., 2007).
There is evidence that stress-induced emissions of LOX products and volatile isoprenoids scale with the severity of different abiotic stresses such as heat, ozone (Beauchamp et al., 2005) and mechanical damage (Brilli et al., 2011), but also with biotic stresses such as the degree of herbivory infestation (Copolovici et al., 2014)(Copolovici et al. 2011). Presence of such quantitative relationships provides an important means to scale up the emissions of biogenic volatiles from leaves to vegetation and assess the role of stress-elicited volatiles in air quality and aerosol formation processes in biosphere-atmosphere system (Grote et al., 2013).
In powdery-mildew infected Beta vulgaris leaves, quantum yield of photosynthesis was moderately reduced (Gordon and Duniway, 1982). However, no effects on chlorophyll content and PSII quantum yield were observed in powdery mildew infected Vitis vinifera (Moriondo et al., 2005) leaves. As isoprene emissions are strongly associated with photosynthetic metabolism (Monson et al., 2012; Rasulov et al., 2009), any possible modification in the quantum efficiency of photosynthesis is also expected to result in alterations in the quantum efficiency of isoprene emission. Furthermore, induced monoterpene emissions are typically light-dependent (Niinemets et al., 2010b) and can compete for the same chloroplastic precursor pool, potentially modifying the light dependence of isoprene emissions. Such potential modifications in the shape of constitutive isoprenoid emission due to pathogen infection are not considered in contemporary isoprene emission models.
We investigated the influences of E. alphitoides infection on foliage photosynthetic characteristics, constitutive emissions of isoprene, induced emissions of LOX volatiles and monoterpenes in Quercus robur, hypothesizing that: 1) there are quantitative relationships between the degree of infection and reductions in foliage photosynthetic activity and isoprene emission, increases in stress volatile emission, and that 2) infection results in modifications in the shapes of the light responses of photosynthesis and isoprene emission.
2. Material and methods
2.1. Study site and plant material
The study was conducted in the vicinity of Tartu, Estonia (58°23′ N, 27°05′ E, elevation ca. 40 m above the sea level) in the middle of September 2011 and 2013. Both years were exceptionally warm and humid with average air temperatures of 18.3 °C for (year 2011) and 18.2 °C (2013) for the summer months June-August and 12.7 °C (2011) and 11.5 °C (2013) for September (data of Laboratory of Environmental Physics, Institute of Physics, University of Tartu, http://meteo.physic.ut.ee). These averages are 2.6-2.5 °C higher for June-August and 2.4 °C (for 2011) higher for September than the corresponding long-term averages for 1971-2000 (the Estonian Environment Agency, http://www.emhi.ee). Analogously, in 2011, the average values of relative air humidity of 81.8% for June-August and 93% for September were higher than corresponding long-term averages of 75% for June-August and 83% for September (the Estonian Environment Agency). Due to the favorable whether conditions for fungal development, almost all oak trees were infected with Erysiphe alphitoides and exhibited visible signs of damage.
South-exposed leaves of varying degree of visual infection symptoms were collected from three trees for the measurements. The twigs (around 25 cm long) with multiple leaves were excised under water, maintained with the cut ends immersed in water and immediately transported to the laboratory for the measurements. The cut ends were maintained in water and a representative leaf was clamped in the cuvette.
2.2. Foliage gas-exchange measurements
To measure gas-exchange and volatile organic compound (VOC) emission rates from Q. robur leaves with varying degree of powdery mildew infection, we used a custom-made gas-exchange system described in detail in Copolovici and Niinemets (2010). Briefly, the system used has an 1.2 L temperature-controlled double-walled glass chamber with glass and stainless steel bottom. The flow rate through the system was 1.4 L min−1, and synthetic air was used mixing purified N2 (80%), O2 (20%) and CO2 (385 μmol mol−1) by mass flow controllers (Bronkhorst, Ruurlo, Netherlands). The chamber air was vigorously mixed with a fan installed in the system, resulting in fully turbulent conditions such that the half-time of the chamber air was ca. 30 sec. assuming a first-order decay kinetics (Niinemets et al., 2011). The air was humidified with a custom-made humidifier to a relative humidity of 60%. Light intensity was set at 1000 μmol m−2 s−1, and leaf temperature at 25 °C. CO2 and H2O concentrations at the chamber in- and outlets were measured with an infra-red dual-channel gas analyzer operated in differential mode (CIRAS II, PP-systems, Amesbury, MA, USA). After enclosure of the leaf in the chamber, the leaf was stabilized under the measurement conditions until steady-state gas-exchange rates were observed (in ca. 30 min after enclosure), and the steady-state values of foliage gas-exchange and volatile emission rates (see the next section) were recorded. Altogether 25 leaves with different degrees of infection were measured (xx in 2011 and xx in 2013).
The light response curves of leaf gas-exchange and VOC emission were measured with a portable gas-exchange/fluorescence system (GFS-3000, Heinz Walz GmbH, Effeltrich, Germany). This system has a clip-on type leaf cuvette with 8 cm2 window area. The LED illumination (10 % blue, 90 % red) is provided by the leaf chamber fluorimeter, and the chamber temperature is regulated by Peltier elements. The measurements of the light response curve were carried out in the following sequence (light intensities in μmol m−2 s−1):
Light response curve measurements were carried out in triplicate for both control and seriously infected leaves (ca. 60% leaf area infected).
Equations of von Caemmerer and Farquhar (1981) were used to calculate the rates of net assimilation (A), transpiration (E), and stomatal conductance to water vapor (gs) per unit projected leaf area enclosed in the chamber.
2.3. Measurement of the emission rates of volatiles
Emission of volatile organic compounds was measured in parallel with physiological parameters by diverting a part of the outgoing air to a Proton-Transfer Reaction Mass Spectrometer (high sensitivity version of PTR-QMS, Ionicon, Innsbruck, Austria). Isoprene was detected as protonated parent ion at m/z of 69, while monoterpenes were calculated from measurements of masses 81 (the major monoterpene fragment) and 137 (for details see (Copolovici et al., 2005; Filella et al., 2007)). The sum of volatile octadecanoid pathway products (LOX products also called green leaf volatiles), consisting of a variety of C6 aldehydes, alcohols and derivatives produced by C13 hydroperoxide lyases was found as the sum of the individual mass signals at m/z 83, m/z 85, m/z 99 and m/z 101 (for more details see Copolovici and Niinemets, 2010).
To asses the composition of emitted terpenes, volatiles were also sampled for GC-MS analyses onto stainless steel tubes with three-bed filling consisting of different Carbotrap fractions optimized to quantitatively adsorb all volatiles in C5-C15 range. 4 L air was sampled at a constant-flow rate of 0.2 L min−1 using a air sample pump (1003-SKC, SKC Inc., Houston, TX, USA) (Niinemets et al., 2011). Adsorbent cartridges were analyzed for lipoxygenase (LOX) pathway products, mono-, homo- and sesquiterpene emissions with a combined Shimadzu TD20 automated cartridge desorber and Shimadzu 2010 plus GC-MS instrument (Shimadzu Corporation, Kyoto, Japan) using a method as detailed in Toome et al. (2010) and Kännaste et al. (2014). The emission rates of isoprene, monoterpenes and volatile LOX products per unit projected leaf area were calculated according to the equations of Niinemets et al. (2011).
2.4. Quantification of the degree of infection
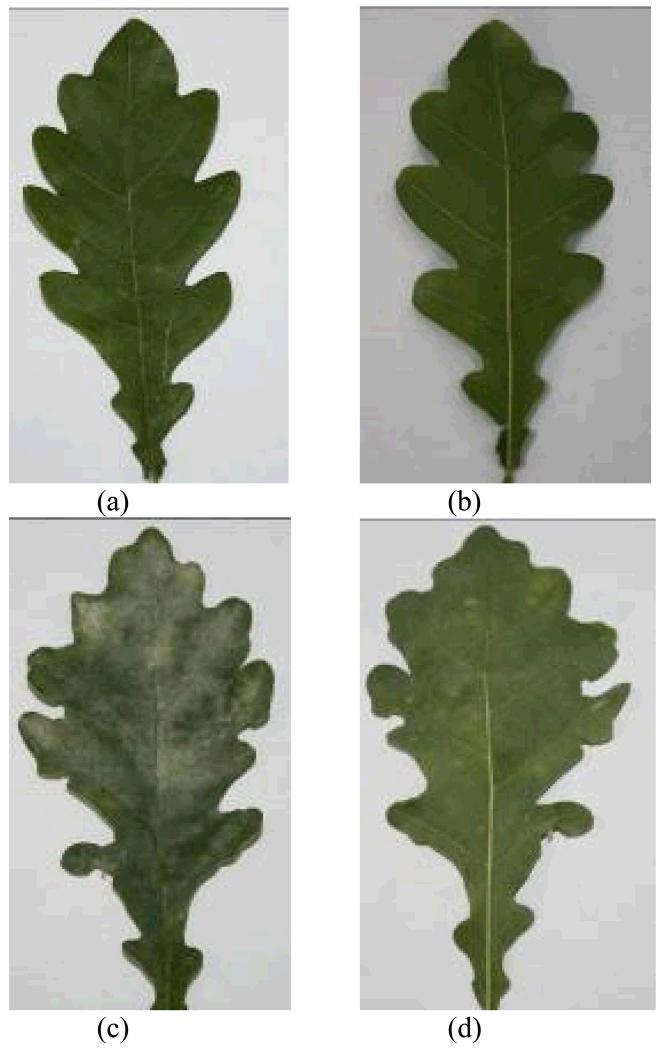
As E. alphitoides primarily grows on leaf surface (Mougou et al., 2008) the coverage of foliage by white fungal hyphae can be used to assess the level of infection. To estimate the fraction of leaf area infected (percentage of leaf area covered by the hyphae of E. alphitoides), both sides of the leaf were photographed (Fig. 1 for an example of a control leaf and a heavily infected leaf) at the end of each experiment and the infected areas were determined by UTHSCSA ImageTool 2.0 (Dental Diagnostic Science, The University of Texas Health Science Center, San Antonio, Texas, USA). The degree of infection was separately estimated for the lower and upper leaf surface, and also an average degree of infection was calculated for the entire leaf.
Figure 1.
Representative photographs of control (a, b) and oak powdery mildew (Erysiphe alphitoides) infected (c, d) leaves of Quercus robur. Both the images for the upper (a, c) and lower side (b, d) of the leaves are demonstrated.
2.5. Data analyses
The light (Q) response curves of emission rates (I) were fitted by an hyperbolic equation in the form of:
| (1) |
where α is the quantum yield, and Imax,Q is the emission capacity (Sun et al., 2012). We note that in VOC emission studies, light responses are often fitted to normalized data (so-called Guenther et al. model) (Grote et al., 2013; Guenther et al., 1993). However, in the original Guenther et al. model, the yield parameter is implicitly dependent on the emission capacity (Grote et al., 2013; Monson et al., 2012), and we therefore favor Eq. 1 that includes the true quantum yield.
The statistical dependencies between the degree of infection and emission of volatiles were explored by linear regressions. Separate regressions with the percentage of infection visible in the upper and lower leaf surface and the average for upper and lower surface damage were calculated to compare the descriptive power of different damage indicators. The parameters of Eq. 1 were compared among different compounds and for infected and non-infected plants using ANOVA. ANOVA was also used to analyze the differences in the content of various volatiles among control and heavily infected (ca. 60% infection) plants. A paired samples t-test was used to assess the statistical differences in the degree of infection of the upper and lower surface. All statistical tests were considered significant at P < 0.05.
3. Results
3.1. Variation in the degree of oak powdery mildew (Erysiphe alphitoides) infection
The infection by E. alphitoides was more prevalent on the upper leaf surface (average ± SE = 49.5 ± 4.1% across all leaves) than on the lower leaf surface (28.8 ± 4.1%, means are significantly different at P < 0.001). The degrees of infection of the lower and upper surface were positively, but relatively weakly correlated (r2 = 0.32, P < 0.03). The correlations of the degree of infection with foliage physiological characteristics and emission rates were stronger with the degree of upper leaf surface infection than with the degree of lower leaf surface infection or average degree of infection of both surfaces (data not shown). Thus, in the following, we only report the correlations with the degree of infection of the upper leaf surface.
3.2. Photosynthesis characteristics of leaves infected with E. alphitoides
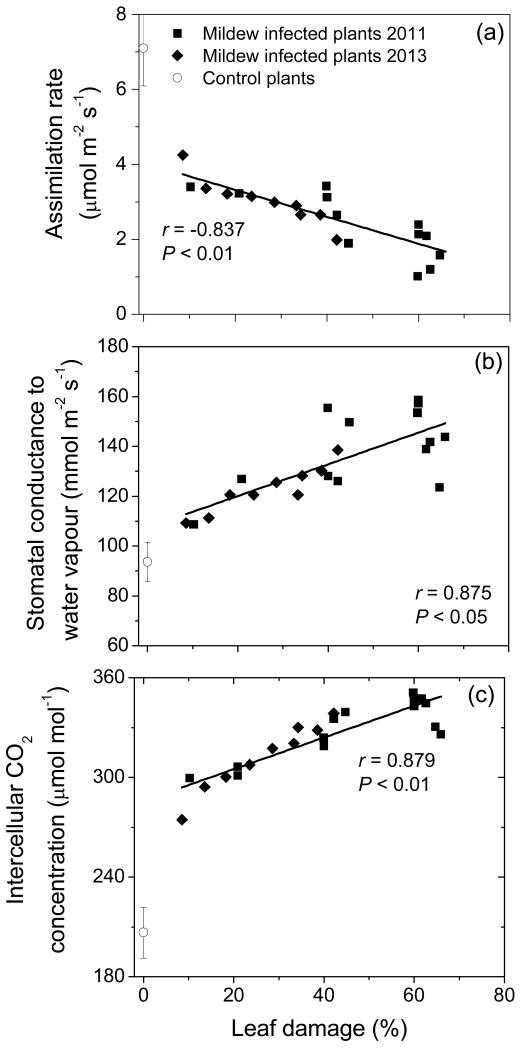
Infection by E. alphitoides reduced leaf net CO2 assimilation rates, and even a moderate infection of 10-20% leaf area infected resulted in significantly reduced net assimilation rates to values of about 4 μmol m−2 s−1 (Fig. 2a). With further increases in infection, net assimilation rate decreased, reaching values of 1-2 μmol m−2 s−1 in leaves with ca. 60% infection.
Figure 2.
Leaf net CO2 assimilation rate (a), stomatal conductance to water vapor (b) and intercellular CO2 concentration in relation to the percentage of leaf area (upper surface) infected with Erysiphe alphitoides in Quercus robur. The data correspond to different leaves and the relationships were fitted by linear regressions. Values for visually completely healthy non-infected leaves are shown by open symbols. Data are shown separately for 2011 and 2013 measurement campaigns.
Differently from net assimilation rate, stomatal conductance to water vapor increased with increasing the percentage of leaf infection by more than 60%, from 91 ± 11 mmol m−2 s−1 (average ± SE) in the control plants to about 150 mmol m−2 s−1 in leaves with more than 50% infection (Fig. 2b). As the result of the decreases in net assimilation rate and increases in stomatal conductance, the intercellular CO2 concentration increased with increasing the degree of leaf infection (Fig. 2c).
3.3. Modification of light response of photosynthesis by mildew infection
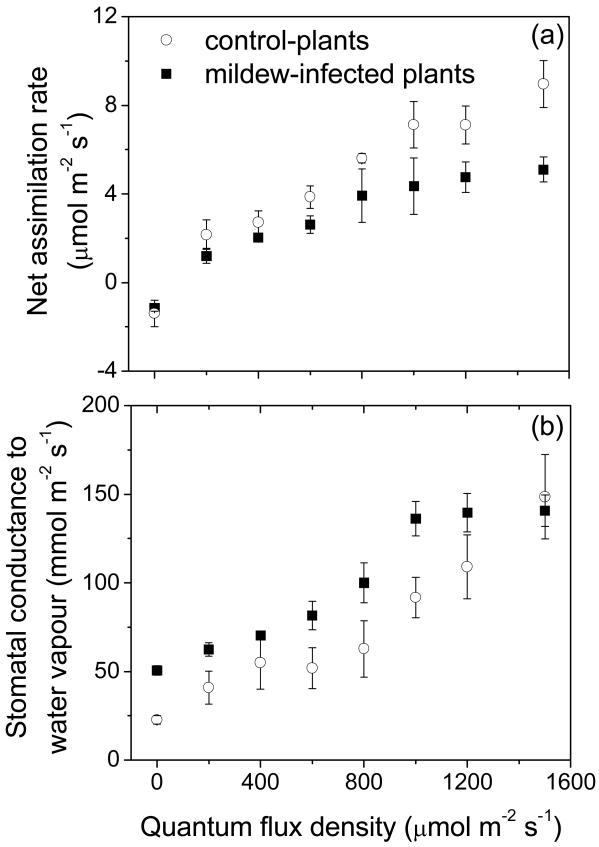
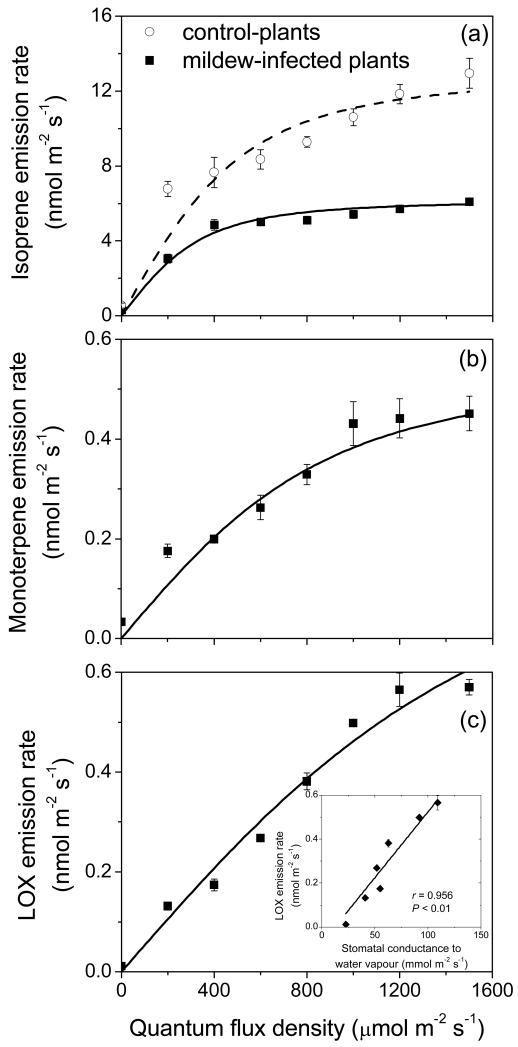
Dark respiration rate was similar, 1.6 μmol m−2 s−1 for both control and mildew-infected leaves, but mildew infection resulted in moderate reductions in the light-limited photosynthesis between light intensities 200-600 μmol m−2 s−1, and major changes in net assimilation rates at high light, ca. 2.3-fold from ca. 9 μmol m−2 s−1 in control to 3.9 ± 0.8 μmol m−2 s−1 (average ± SE) in infected leaves (Fig. 3a). Furthermore, net assimilation rates in infected leaves saturated at lower quantum flux density of ca. 800 μmol m−2 s−1, while the saturation light intensity was reached at more than 1500 μmol m−2 s−1 in control plants (Fig. 3a).
Figure 3.
Comparison of representative light responses of net CO2 assimilation rate (a) and stomatal conductance to water vapor (b) among Quercus robur control (non-infected) and E. alphitoides-infected leaves (percentage of leaf area infected ca. 60%, see Table 2). Data are averages ± SE (n = 3 for both control and infected leaves).
Stomatal conductance increased with increasing light intensity similarly to net assimilation rates (Fig. 3b). As with net assimilation rates, stomatal conductance reached a light saturated value at a lower light intensity in infected leaves (cf. Figs. 3a and 3b). Thus, while the values of stomatal conductance in infected leaves exceeded those in the control leaves over most of the light range, similar stomatal conductance of 140 ± 8 mmol m−2 s−1 (average ± SE) was reached at the highest light intensity of 1500 μmol m−2 s−1 (Fig. 3b).
3.4. Effects of E. alphitoides infection on emissions of isoprene and stress elicited volatile emissions
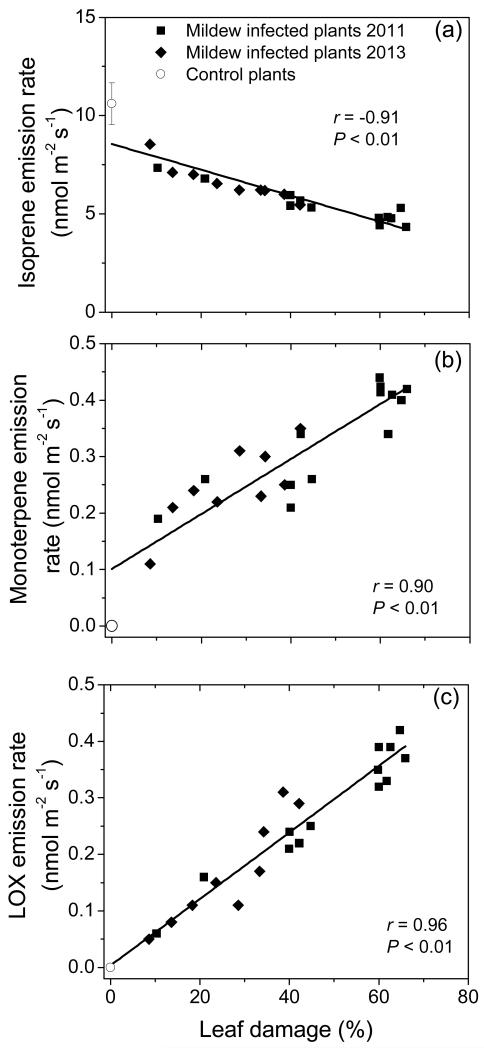
Infection by E. alphitoides led to reduction of isoprene emission from 10.6 ± 0.6 nmol m−2 s−1 (average ± SE) in healthy leaves of Q. robur to 4-5 nmol m−2 s−1 in heavily infected leaves (50-60% leaf area infected), and a strong negative correlation between isoprene emission rate and percentage of leaf damage was observed (Fig. 4a).
Figure 4.
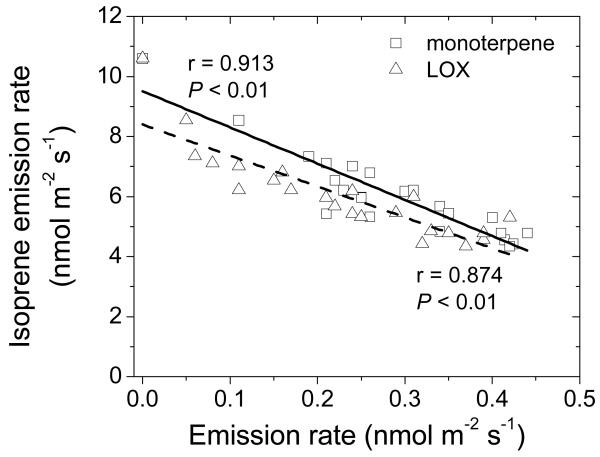
Correlations of isoprene (a, ΦIso), monoterpene (b, ΦMon) and lipoxygenase pathway volatile (LOX, ΦLox, various C6 alcohols, aldehydes and derivatives, also called green leaf volatiles) (c) emission rates with the percentage of E. alphitoides infection (upper surface) in Quercus robur leaves. The sum of all monoterpenes and LOX volatiles is reported in Table 1. Data presentation and fitting as in Fig. 2. The regressions with the degree of infection (DI) are: ΦIso = 8.91 −0.0743DI; ΦMon = 0.0732 + 0.00532DI; ΦLox = 0.00213 + 0.00624DI.
Emission of a variety of compounds was induced by infection, including volatiles of the lipoxygenase pathway [(Z)-3-hexenol, (E)-2-hexenal, (Z)-3-hexenyl acetate and 1-hexanol), ubiquitous monoterpenes (α-pinene, camphene, 3-carene, limonene and β-phellandrene), typical stress monoterpenes such as (E)-β-ocimene, linalool and monoterpene derivative geranyl acetone and benzenoid methyl salicylate (Table 1). The emissions of total monoterpenes (Fig. 4b), and the sum of lipoxygenase pathway products (LOX products, Fig. 4c) increased from close to zero level in control leaves to values as high as ca. 0.4 nmol m−2 s−1 in strongly infected leaves (Fig. 4b, c). Isoprene emission was negatively correlated with both monoterpene and LOX product emission rates for all leaves with different degrees of infection pooled (Fig. 5).
Table 1.
Volatile emission rates (nmol m−2 s−1) of Quercus robur control leaves and oak powdery mildew (Erysiphe alphitoides) infected leaves
| Compound | Control | Mildew-infected |
|---|---|---|
| Green leaf volatiles (lipoxygenase pathway volatiles, LOX) | ||
| (Z)-3-hexenol | 0.0139 ± 0.0022 | 0.0926 ± 0.0043*** |
| (E)-2-hexenal | 0.021 ± 0.006 | 0.149 ± 0.007*** |
| (Z)-3-hexenyl acetate | 0.0121 ± 0.0011 | 0.098 ± 0.007*** |
| 1-hexanol | 0.0119 ± 0.0018 | 0.082 ± 0.006*** |
| Isoprenoids and derivatives | ||
| Isoprene | 10.6 ± 0.6 | 5.36 ± 0.16** |
| α-Pinene | 0.0184 ± 0.0038 | 0.150 ± 0.007*** |
| Camphene | 0.0070 ± 0.0018 | 0.056 ± 0.005*** |
| β-Pinene | 0.0034 ± 0.0005 | 0.0177 0.0018*** |
| 3-Carene | 0.00363 ± 0.00041 | 0.0520 ± 0.0020*** |
| Limonene | 0.0080 ± 0.0009 | 0.0678 ± 0.0036*** |
| (E)-β-Ocimene | 2.5 10-5 ± 0.8 10-5 | 0.0004 ± 0.00005*** |
| β-Phellandrene | 0.0036 ± 0.0008 | 0.0206 ± 0.0009*** |
| Linalool | nd | 0.0164 ± 0.0027*** |
| Geranyl acetone | 0.0072 ± 0.0013 | 0.0112 ± 0.0006* |
| Aromatics | ||
| Benzaldehyde | 0.079 ± 0.012 | 0.122 ± 0.016 |
| Methyl salicylate | nd | 0.437 ± 0.011*** |
Control leaves had essentially no visible symptoms of infection (infection level <5%), while in the case of infected leaves, leaves with the infection level >60% (59.8-65.9%, on average ± SE of 61.6 ± 0.9%) were included in the analysis (see Fig. 4). The means were compared by ANOVA and the significance is denoted as P < 0.001 (***), P < 0.01 (**) or P < 0.05 (*)
Figure 5.
Correlations between isoprene emission rate and monoterpene and LOX volatile emission rates among the Quercus robur leaves with different degree of E. alphitoides infection. Data were fitted by linear regressions (solid line - relationship with monoterpene emission; dashed line - relationship with LOX volatile emission).
3.5. Light responses of volatile emissions in control and infected plants
Infection by E. alphitoides resulted in both reduced light-saturated isoprene emission rate and initial quantum yield (Fig. 6a, Table 2). In addition, isoprene emission in infected leaves also saturated at a lower quantum flux density of ca. 600-800 μmol m−2 s−1 than in the non-infected control leaves where the emission rate was still increasing at the highest light level of 1500 μmol m−2 s−1 used (Fig. 6a). Both monoterpene (Fig. 6b, Table 2) and LOX product (Fig. 6c, Table 2) emission rates also increased with increasing light intensity. The rate of emission of LOX products increased particularly strongly with light without apparent light saturation (Fig. 6c). The increase of LOX emission rate through the light response was close to linearly correlated with light-dependent changes in stomatal conductance (inset in Fig. 6c). The initial quantum yields for monoterpene and LOX product emissions in infected leaves were lower than those for isoprene emission in control and infected leaves (Table 2).
Figure 6.
Light response curves of isoprene (a), monoterpene (b) and LOX volatile (c) emissions in control and E. alphitoides-infected leaves of Quercus robur. Data were fitted by Eq. 1 (Table 2 for the parameters). The inset in (c) demonstrates the correlation between LOX volatile emission rate and stomatal conductance (gs) through the light response curve (Fig. 3b for the light response of stomatal conductance).
Table 2.
Comparison of light response curve parameters, the initial quantum yield (α) and the emission capacity (Imax,Q, Eq. 1) for different compound classes and among the control and E. alphitoides-infected leaves of Q. robur
| Compound | α (μmol mol−1) | Imax,Q (nmol m−2 s−1) |
|---|---|---|
| Isoprene (control) | 23 ± 6 A | 12.8 ± 0.6 A |
| Isoprene (infected) | 16.2 ± 3.5 B | 6.14 ±0.06 B |
| Monoterpenes (infected) | 0.586 ± 0.023 C | 0.55 ± 0.07 C |
| LOX volatiles (infected) | 0.532 ± 0.016 C | 0.73 ± 0.06 C |
Infected leaves had the infection level >60% (59.8-65.9%, on average ± SE of 61.6 ± 0.9%). Light response curve parameters for monoterpenes and LOX emissions are only provided for the infected leaves. The emission rates of these compound classes in control leaves were close to the detection limit (Table 1). Means of the parameter values were compared by ANOVA and different letters denote statistically significant differences at P < 0.05.
4. Discussion
4.1. Alteration of photosynthetic characteristics of Q. robur by E. alphitoides infection
Our results for Q. robur (Fig. 2) are in agreement with previous observations demonstrating strong reduction in net assimilation rate by various powdery mildew species (Gordon and Duniway, 1982; Hajji et al., 2009; Moriondo et al., 2005). Furthermore, reduction of photosynthesis is a general response to infection by different pathogens across a variety of species. For example, photosynthesis was reduced in Vaccinium ashei and V. corymbosum affected by leaf spot fungus Septoria albopunctata (Roloff et al., 2004), sugarcane infected with orange rust Puccinia kuehnii (Zhao et al., 2011) and Prunus cerasus affected by cherry leaf spot fungus Blumeriella jaapii (Gruber et al., 2012), in Quercus fusiformis infected by oak wilt Ceratocystis fagacearum (Anderson et al., 2000), and in Salix burjatica x S. dasyclados infected by rust fungus Melampsora epitea (Toome et al., 2010). In contrast, in some cases, compensatory photosynthesis has been demonstrated. For example, infection by the rust fungus Puccinia hordei increased photosynthesis in Hordeum distichum compared with control plants (Scholes and Farrar, 1986). Such compensatory responses have sometimes also been observed in herbivory-fed leaves where increases in photosynthetic rate of remaining leaf areas can compensate for consumed leaf area.
Our study further demonstrated that the degree of reduction in assimilation rate was quantitatively associated with the degree of leaf infection (Fig. 2). Such a response can result from uniform reduction in foliage assimilation rate across leaf surface in a coordinated manner analogously to senescing leaves undergoing programmed cell death (Munné-Bosch, 2008; Niinemets et al., 2012). Such a response can also be explained by reductions in net assimilation rate in infected leaf regions, especially in the case of necrotrophic pathogens, while the assimilation rate is maintained almost constant in non-infected leaf regions. In fact, reduction in net assimilation rate by Vaccinium infected by leaf spot fungus Septoria albopunctata was correlated with the fraction of necrotic area (Roloff et al., 2004). However, net assimilation rates reached close to zero level already at the percentage of necrotic area of ca. 50% (Roloff et al., 2004), suggesting that severe infection also resulted in overall reduction in net assimilation rate. In our study, net assimilation rate reached very low values of 1-2 μmol m−2 s−1 in severely infected leaves (50-60% leaf area infected), suggesting that both mechanisms were likely responsible for reduction in net assimilation rates in E. alphitoides infected leaves.
Multiple physiological mechanisms can be responsible for the reduction in photosynthesis in fungal-infected leaves. Among these mechanisms, changes characteristic to programmed cell death including inhibition of photosynthetic electron transport and reductions in the amount or activity of photosynthetic rate-limiting enzymes and light-harvesting pigments have been demonstrated for different fungal infections (Gordon and Duniway, 1982; Mandal et al., 2009; Tang et al., 1996; Zhao et al., 2011). Pathogenic infections can also induce reductions in stomatal conductance by rendering stomata in necrotic areas nonfunctional and by affecting stomatal functioning in surrounding leaf tissues (Lopes and Berger, 2001; Pinkard and Mohammed, 2006). In fact, decreases in stomatal conductance have often been observed in fungal-infected plants, especially when infection led to necrotic lesions (Mandal et al., 2009; Moriondo et al., 2005; Roloff et al., 2004; Toome et al., 2010). However, in our study, stomatal conductance actually increased with increasing the degree of infection (Fig. 2b) and this was associated with increased intercellular CO2 concentration (Fig. 2c). This suggests that the reduction in net assimilation rate in E. alphitoides infected Q. robur leaves was associated with reductions in the amount and/or activity of rate-limiting proteins such as ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco). Impairment of stomatal conductance and photosynthesis and resulting enhanced water loss in infected plants have also been observed in other studies (Major et al., 2009; Robert et al., 2005). In addition, despite the parallel reductions in net assimilation rate and stomatal conductance in downy mildew Peronospora plantaginis infected Plantago ovata leaves, intercellular CO2 concentration actually increased in infected leaves also indicating that the demand for CO2 decreased relatively more than the supply of CO2 (Mandal et al., 2009). On the other hand, in Vitis vinifera leaves infected by powdery mildew Uncinula necator, intercellular CO2 concentration decreased in infected leaves (Moriondo et al., 2005).
Overall, this evidence collectively indicates that the photosynthetic responses to infection are strongly host and pathogen specific. In the case of biotrophic pathogen E. alphitoides infecting Q. robur leaves, clearly a partial loss of stomatal control increased intercellular CO2 concentration and reduced the control of assimilation rates by CO2 concentration. However, the light-saturation of net assimilation rate (when cross-over from light-limitation to Rubisco limitation occurred) was observed at a lower light intensity in the infected leaves compared to control leaves (Fig. 3). This suggests that Rubisco activity decreased relative more than the activity of photosynthetic electron transport in the infected leaves. Analogously, a stronger effect of infection on high-light photosynthesis than on light-limited photosynthesis has been reported in several plant species including rice (Oryza sativa) (Bastiaans and Roumen, 1993), soybean (Glycine max) (Kumudini et al., 2008) and sugar beet (Beta vulgaris) (Gordon and Duniway, 1982).
4.2. Emission of isoprene and terpenoids from leaves infected with E. alphitoides
Reduction of isoprene emissions by powdery mildew infection (Fig. 4a) is in agreement with observations of Brüggemann and Schnitzler (2001). Analogously, isoprene emissions were reduced by oak wilt Ceratocystis fagacearum infected Quercus fusiformis (Anderson et al., 2000), and in rust fungus Melampsora epitea infected Salix (Toome et al., 2010). Our study further demonstrates that the reduction of isoprene emission in powdery mildew infected leaves is quantitatively associated with the degree of propagation of the infection (Fig. 4a).
However, compared with net assimilation rate, the reduction in isoprene emission was less under the most sever infection (cf. Figs. 2a and 4a), indicating that the fraction of carbon going into isoprene emission increased. On the other hand, isoprene emission is more tightly linked to the rate of photosynthetic electron transport than to light-saturated net assimilation (Harrison et al., 2013; Niinemets et al., 1999; Rasulov et al., 2011; Rasulov et al., 2009). Thus, lower sensitivity of light-limited photosynthesis to infection, can provide an explanation for smaller decreases in isoprene emissions than in photosynthetic capacity.
Nevertheless, isoprene emission rates also depend on intercellular CO2 concentration according to a curve with a maximum at ca. 100 μmol mol−1 (Loreto and Sharkey, 1993; Rasulov et al., 2009; Sun et al., 2012). This reduction has been associated with CO2 effects on the pool size of the substrate for isoprene synthesis, dimethylallyl diphosphate (DMADP) (Possell and Hewitt, 2011; Rasulov et al., 2009). Thus, increases in intercellular CO2 concentration in E. alphitoides leaves might have also suppressed isoprene emissions by reduction in DMADP pool size. In fact, reduction in the initial quantum yield of isoprene emission (Fig. 6a, Table 2) also suggests that the DMADP pool size was reduced in the infected leaves. We note that the relationships among isoprene emission, photosynthesis rate and CO2 concentration differ among infected and non-infected leaves. In particular, in non-infected leaves, the reduction of isoprene emissions at higher CO2 is characteristically associated with increased photosynthesis rate (Loreto and Sharkey 1990, Morfopoulos et al. 2014, Niinemets et al. 2010), but this was not the case in infected leaves where both photosynthesis and isoprene emission rates were reduced.
Apart from the CO2 effects, DMADP pool size depends on the competition among isoprene emission and other DMADP consuming chloroplastic pathways such as synthesis of larger isoprenoids including monoterpenes, diterpenes and carotenoids (Dudareva et al., 2013; Rasulov et al., 2013). Because geranyl diphosphate synthase, the initial enzyme in the synthesis of longer chain-length isoprenoids has much higher affinity towards DMADP than the isoprene synthase (Rajabi Memari et al., 2013; Rasulov et al., 2014), competition by alternative reactions can significantly curb isoprene emission. Thus, part of the inhibition of isoprene emission can be associated with elicitation of monoterpene emissions from the infected leaves. In fact, close to proportional negative correlation between isoprene emission and monoterpene emission was observed through all leaves of varying degree of infection (Fig. 5), supporting the hypothesis of the competition of different biochemical processes for DMADP.
4.3. Powdery mildew induced volatile emissions
Studies with different fungal pathogens have demonstrated that infection leads to induction of emissions of several classes of stress volatiles. For example, infection of willow (Salix) leaves by the rust fungus Melampsora epitea resulted in emissions of volatiles of the lipoxygenase (LOX) pathway, and mono- and sesquiterpenes (Toome et al., 2010). Analogously, silver birch (Betula pendula) infected with the leaf spot fungus Marssonina betulae emitted an increased amount of (Z)-ocimene and (E)-β-ocimene (Vuorinen et al., 2007). Tomato (Solanum lycopersicum) plants inoculated with necrotrophic fungus Botrytis cinerea emitted a different number and amount of volatiles after inoculation compared to control plants (Jansen et al., 2009).
Although E. alphitoides itself could emit different VOCs, as an obligate pathogen, its VOC emissions cannot be measured in the absence of plant in vivo, and thus, we were unable to determine the profile of possibly emitted fungal volatiles. According to Peñuelas et al. (2014), fungal emissions are dominated by alcohols, benzenoids, aldehydes and ketones and there are only marginal emission of terpenes. However, there is a large variability in the emission profiles among different fungal species (Müller et al. 2013).
In our study, the main compounds emitted were the LOX products, monoterpenes and methyl salicylate that are characteristic to fungal infections. As a result of an attack by pathogens, specific elicitor molecules are generated by chemical or physical damage to plant cell walls and cellular membranes. Usually the response of the plants to the stress includes formation of different reactive compounds which can be detected and recognized as a signal and initiate the activation of secondary elicitors and transcription of defense genes (Panka et al., 2013). Rapid emissions of LOX products are triggered by the release of free fatty acids from cell membranes, and their peroxidation by lipoxygenase enzymes (Liavonchanka and Feussner, 2006). As inoculation experiments with fungal pathogens demonstrate (Jansen et al., 2009; Toome et al., 2010), LOX products are emitted within hours after inoculation. This is somewhat slower compared with mechanical damage or herbivory damage (see Introduction), and likely reflects differences in time when the inoculation signal is perceived. In the case of herbivory damage, it has been demonstrated that LOX product emission rate scales quantitatively with the degree of leaf damage (Copolovici et al., 2014; Copolovici et al., 2011; Niinemets et al., 2013). In the case of herbivory, LOX emissions have been associated with continuous increase of damaged leaf parts that serve as sites of emission. In the case of mildew infection, sustained LOX emissions likely reflect propagation of cellular damage to leaf areas not yet infected (Toome et al., 2010) as well as continuous repair and damage of infected leaf regions. We suggest that detailed anatomical studies are needed to gain insight into the nature of powdery mildew caused LOX emissions at ultrastructural level.
Surprisingly, LOX product emission rates were light-dependent (Fig. 6c). Such light-dependence has not yet been demonstrated, and we suggest that several factors can be responsible for the light-dependence of these emissions. First, the increase of emissions of green leaf volatiles with light intensity can result from light-dependent formation of reactive oxygen species (ROS) due to photooxidative stress, and accordingly greater lipoxygenase activity and higher level of lipid peroxidation. In fact, an increase in lipoxygenase activity with increasing light level has been observed in Phalaenopsis (Ali et al., 2005). Second, as water-soluble compounds, several LOX products can be temporarily controlled by stomata (Harley, 2013; Niinemets and Reichstein, 2003), and thus, light-dependent changes in stomatal conductance can be partly responsible for the light-dependence of LOX product emission. This suggestion is corroborated by a strong positive correlation between stomatal conductance and LOX product emission (Fig. 6c).
Activation of defense pathways following infection is typically associated with the release of volatile isoprenoids, in particular, with light-dependent emissions of monoterpenes (Toome et al., 2010; Vuorinen et al., 2007) as was also demonstrated in powdery mildew infected Q. robur (Table 1, Fig. 4b, Fig. 6b). As discussed above, these emissions likely compete with constitutive isoprene synthesis for chloroplastic DMADP. However, while the DMADP pool size likely decreased with increasing the degree of infection as suggested by the reductions in the initial quantum yield for isoprene emission (Table 2), monoterpene emission rate increased with increasing the degree of infection (Fig. 4b). This indicates that the activity of monoterpene synthases scaled positively with the degree of leaf infection. On the other hand, the light dependence of monoterpene emission (Fig. 6b) suggests that the light-dependent changes in the monoterpene precursor pool size did affect monoterpene emission. This might reflect the competition by isoprene synthase that at any light level consumed more DMADP than monoterpene synthases.
5. Conclusions
This study provides important evidence of strong effects of pathogenic infections on constitutive and induced volatile emissions in Quercus robur. As the study demonstrates, powdery mildew infection in oak is associated with infection-dependent reductions in photosynthesis, and isoprene emission and increases in stomatal conductance and induced emissions. These results provide encouraging evidence that the severity of pathogen stress can be assessed by monitoring the emissions of stress-elicited volatiles. Such quantitative relationships form a valuable basis to incorporate pathogen stress in isoprene emission models and in models of induced volatile emissions.
Acknowledgements
Funding for this study has been provided by the Estonian Ministry of Science and Education (institutional grant IUT-8-3), the European Commission through the European Regional Fund (the Center of Excellence in Environmental Adaptation) and the European Research Council (advanced grant 322603, SIP-VOL+), and the Romanian National Authority for Scientific Research, CNCS – UEFISCDI (project number PN-II-RU-TE-2011-3-0022). We thank Kadri Põldmaa (University of Tartu) for the identification of the powdery mildew species.
References
- Alexander HM, Holt RD. The interaction between plant competition and disease. Perspect Plant Ecol Evol Syst. 1998;1-2:206–220. [Google Scholar]
- Ali MB, Hahn EJ, Paek KY. Effects of light intensities on antioxidant enzymes and malondialdehyde content during short-term acclimatization on micropropagated Phalaenopsis plantlet. Environ Exper Bot. 2005;54:109–120. [Google Scholar]
- Anderson LJ, Harley PC, Monson RK, Jackson RB. Reduction of isoprene emissions from live oak (Quercus fusiformis) with oak wilt. Tree Physiol. 2000;20:1199–1203. doi: 10.1093/treephys/20.17.1199. [DOI] [PubMed] [Google Scholar]
- Bastiaans L, Roumen EC. Effect on leaf photosynthetic rate by leaf blast for rice cultivars with different types and levels of resistance. Euphytica. 1993;66:81–87. [Google Scholar]
- Beauchamp J, Wisthaler A, Hansel A, Kleist E, Miebach M, Niinemets Ü , Schurr U, Wildt J. Ozone induced emissions of biogenic VOC from tobacco: relations between ozone uptake and emission of LOX products. Plant Cell Environ. 2005;28:1334–1343. [Google Scholar]
- Berger S, Sinha AK, Roitsch T. Plant physiology meets phytopathology: plant primary metabolism and plant-pathogen interactions. J Exp Bot. 2007;58:4019–4026. doi: 10.1093/jxb/erm298. [DOI] [PubMed] [Google Scholar]
- Brilli F, Ruuskanen TM, Schnitzhofer R, Müller M, Breitenlechner M, Bittner V, Wohlfahrt G, Loreto F, Hansel A. Detection of plant volatiles after leaf wounding and darkening by proton transfer reaction “time-of-flight” mass spectrometry (PTR-TOF) PloS ONE. 2011;6:e20419. doi: 10.1371/journal.pone.0020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüggemann N, Schnitzler JP. Influence of powdery mildew (Microsphaera alphitoides) on isoprene biosynthesis and emission of pedunculate oak (Quercus robur L.) leaves. J Appl Bot-Angew Bot. 2001;75:91–96. [Google Scholar]
- Copolovici L, Kännaste A, Remmel T, Niinemets Ü . Volatile organic compound emissions from Alnus glutinosa under interacting drought and herbivory stresses. Environ Exp Bot. 2014;100:55–63. doi: 10.1016/j.envexpbot.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolovici L, Niinemets Ü . Flooding induced emissions of volatile signalling compounds in three tree species with differing waterlogging tolerance. Plant Cell Environ. 2010;33:1582–1594. doi: 10.1111/j.1365-3040.2010.02166.x. [DOI] [PubMed] [Google Scholar]
- Copolovici LO, Filella I, Llusià J, Niinemets Ü , Peñuelas J. The capacity for thermal protection of photosynthetic electron transport varies for different monoterpenes in Quercus ilex. Plant Physiol. 2005;139:485–496. doi: 10.1104/pp.105.065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprez-Loustau ML, Feau N, Mougou-Hamdane A, Dutech C. Interspecific and intraspecific diversity in oak powdery mildews in Europe: coevolution history and adaptation to their hosts. Mycoscience. 2011;52:165–173. [Google Scholar]
- Dudareva N, Klempien A, Muhlemann JK, Kaplan I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013;198:16–32. doi: 10.1111/nph.12145. [DOI] [PubMed] [Google Scholar]
- Farag MA, Paré PW. C6-green leaf volatiles trigger local and systemic VOC emissions in tomato. Phytochemistry. 2002;61:545–554. doi: 10.1016/s0031-9422(02)00240-6. [DOI] [PubMed] [Google Scholar]
- Filella I, Wilkinson MJ, Llusià J, Hewitt CN, Peñuelas J. Volatile organic compounds emissions in Norway spruce (Picea abies) in response to temperature changes. Physiol Plantarum. 2007;130:58–66. [Google Scholar]
- Gordon TR, Duniway JM. Effects of powdery mildew infection on the efficiency of CO2 fixation and light utilization by sugar beet leaves. Plant Physiol. 1982;69:139–142. doi: 10.1104/pp.69.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote R, Monson RK, Niinemets Ü . Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 315–355. [Google Scholar]
- Gruber BR, Kruger EL, McManus PS. Effects of cherry leaf spot on photosynthesis in tart cherry ‘Montmorency’ foliage. Phytopathology. 2012;102:656–661. doi: 10.1094/PHYTO-12-11-0334. [DOI] [PubMed] [Google Scholar]
- Guenther AB, Zimmerman PR, Harley PC, Monson RK, Fall R. Isoprene and monoterpene emission rate variability: model evaluations and sensitivity analyses. J Geophys Res. 1993;98:12609–12617. [Google Scholar]
- Gururani MA, Venkatesh J, Upadhyaya CP, Nookaraju A, Pandey SK, Park SW. Plant disease resistance genes: current status and future directions. Physiol Mol Plant Pathol. 2012;78:51–65. [Google Scholar]
- Hajji M, Dreyer E, Marcais B. Impact of Erysiphe alphitoides on transpiration and photosynthesis in Quercus robur leaves. Eur J Plant Pathol. 2009;125:63–72. [Google Scholar]
- Harley PC. The roles of stomatal conductance and compound volatility in controlling the emission of volatile organic compounds from leaves. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 181–208. [Google Scholar]
- Harrison SP, Morfopoulos C, Dani KGS, Prentice IC, Arneth A, Atwell BJ, Barkley MP, Leishman MR, Loreto F, Medlyn BE, Niinemets Ü , Possell M, Peñuelas J, Wright IJ. Volatile isoprenoid emissions from plastid to planet. New Phytol. 2013;197:49–57. doi: 10.1111/nph.12021. [DOI] [PubMed] [Google Scholar]
- Hartikainen K, Nerg A-M, Kivimäenpää M, Kontunen-Sopplea S, Mäenpää M, Oksanen E, Rousi M, Holopainen T. Emissions of volatile organic compounds and leaf structural characteristics of European aspen (Populus tremula) grown under elevated ozone and temperature. Tree Physiol. 2009;29:1163–1173. doi: 10.1093/treephys/tpp033. [DOI] [PubMed] [Google Scholar]
- Jansen RMC, Miebach M, Kleist E, van Henten EJ, Wildt J. Release of lipoxygenase products and monoterpenes by tomato plants as an indicator of Botrytis cinerea-induced stress. Plant Biol. 2009;11:859–868. doi: 10.1111/j.1438-8677.2008.00183.x. [DOI] [PubMed] [Google Scholar]
- Koornneef A, Pieterse CMJ. Cross talk in defense signaling. Plant Physiol. 2008;146:839–844. doi: 10.1104/pp.107.112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumudini S, Prior E, Omielan J, Tollenaar M. Impact of Phakopsora pachyrhizi infection on soybean leaf photosynthesis and radiation absorption. Crop Sci. 2008;48:2343–2350. [Google Scholar]
- Liavonchanka A, Feussner N. Lipoxygenases: Occurrence, functions and catalysis. J Plant Physiol. 2006;163:348–357. doi: 10.1016/j.jplph.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Lopes DB, Berger RD. The effects of rust and anthracnose on the photosynthetic competence of diseased bean leaves. Phytopathology. 2001;91:212–220. doi: 10.1094/PHYTO.2001.91.2.212. [DOI] [PubMed] [Google Scholar]
- Loreto F, Schnitzler J-P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010;15:154–166. doi: 10.1016/j.tplants.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Loreto F, Sharkey TD. On the relationship between isoprene emission and photosynthetic metabolites under different environmental conditions. Planta. 1993;189:420–424. doi: 10.1007/BF00194440. [DOI] [PubMed] [Google Scholar]
- Major IT, Nicole MC, Duplessis S, Seguin A. Photosynthetic and respiratory changes in leaves of poplar elicited by rust infection. Photosynth Res. 2009;104:41–48. doi: 10.1007/s11120-009-9507-2. [DOI] [PubMed] [Google Scholar]
- Mandal K, Saravanan R, Maiti S, Kothari IL. Effect of downy mildew disease on photosynthesis and chlorophyll fluorescence in Plantago ovata Forsk. J Plant Dis Protect. 2009;116:164–168. [Google Scholar]
- Matsui K. Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr Opin Plant Biol. 2006;9:274–280. doi: 10.1016/j.pbi.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Monson RK, Grote R, Niinemets Ü , Schnitzler J-P. Tansley review. Modeling the isoprene emission rate from leaves. New Phytol. 2012;195:541–559. doi: 10.1111/j.1469-8137.2012.04204.x. [DOI] [PubMed] [Google Scholar]
- Moriondo M, Orlandini S, Giuntoli A, Bindi M. The effect of downy and powdery mildew on grapevine (Vitis vinifera L.) leaf gas exchange. J Phytopathol. 2005;153:350–357. [Google Scholar]
- Mougou-Hamdane A, Giresse X, Dutech C, Desprez-Loustau M-L. Spatial distribution of lineages of oak powdery mildew fungi in France, using quick molecular detection methods. Ann For Sci. 2010;67 [Google Scholar]
- Mougou A, Dutech C, Desprez-Loustau ML. New insights into the identity and origin of the causal agent of oak powdery mildew in Europe. Forest Pathol. 2008;38:275–287. [Google Scholar]
- Munné-Bosch S. Do perennials really senesce? Trends Plant Sci. 2008;13:216–220. doi: 10.1016/j.tplants.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü . Mild versus severe stress and BVOCs: thresholds, priming and consequences. Trends Plant Sci. 2010a;15:145–153. doi: 10.1016/j.tplants.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü . Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: past stress history, stress interactions, tolerance and acclimation. Forest Ecol Manag. 2010b;260:1623–1639. [Google Scholar]
- Niinemets Ü , García-Plazaola JI, Tosens T. Photosynthesis during leaf development and ageing. In: Flexas J, Loreto F, Medrano H, editors. Terrestrial photosynthesis in a changing environment. A molecular, physiological and ecological approach. Cambridge University Press; Cambridge: 2012. pp. 353–372. [Google Scholar]
- Niinemets Ü , Kuhn U, Harley PC, Staudt M, Arneth A, Cescatti A, Ciccioli P, Copolovici L, Geron C, Guenther A, Kesselmeier J, Lerdau MT, Monson RK, Peñuelas J. Estimations of isoprenoid emission capacity from enclosure studies: measurements, data processing, quality and standardized measurement protocols. Biogeosciences. 2011;8:2209–2246. [Google Scholar]
- Niinemets Ü , Reichstein M. Controls on the emission of plant volatiles through stomata: sensitivity or insensitivity of the emission rates to stomatal closure explained. J Geophys Res- Atm. 2003;108:4208. doi:4210.1029/2002JD002620. [Google Scholar]
- Niinemets Ü , Tenhunen JD, Harley PC, Steinbrecher R. A model of isoprene emission based on energetic requirements for isoprene synthesis and leaf photosynthetic properties for Liquidambar and Quercus. Plant Cell Environ. 1999;22:1319–1336. [Google Scholar]
- Panka D, Piesik D, Jeske M, Baturo-Ciesniewska A. Production of phenolics and the emission of volatile organic compounds by perennial ryegrass (Lolium perenne L.)/Neotyphodium lolii association as a response to infection by Fusarium poae. J Plant Physiol. 2013;170:1010–1019. doi: 10.1016/j.jplph.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Asensio D, Tholl D, Wenke K, Rosenkranz M, Piechulla B, Schnitzler JP. Biogenic volatile emissions from soil. Plant Cell Environ. 2014;37:1866–1891. doi: 10.1111/pce.12340. [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Dicke M. Plant interactions with microbes and insects: from molecular mechanisms to ecology. Trends Plant Sci. 2007;12:564–569. doi: 10.1016/j.tplants.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Pinkard EA, Mohammed CL. Photosynthesis of Eucalyptus globulus with Mycosphaerella leaf disease. New Phytol. 2006;170:119–127. doi: 10.1111/j.1469-8137.2006.01645.x. [DOI] [PubMed] [Google Scholar]
- Poland JA, Balint-Kurti PJ, Wisser RJ, Pratt RC, Nelson RJ. Shades of gray: the world of quantitative disease resistance. Trends Plant Sci. 2009;14:21–29. doi: 10.1016/j.tplants.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Possell M, Hewitt CN. Isoprene emissions from plants are mediated by atmospheric CO2 concentrations. Global Change Biol. 2011;17:1595–1610. [Google Scholar]
- Possell M, Loreto F. The role of volatile organic compounds in plant resistance to abiotic stresses: responses and mechanisms. In: Niinemets Ü , Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 209–235. [Google Scholar]
- Rajabi Memari H, Pazouki L, Niinemets Ü . The biochemistry and molecular biology of volatile messengers in trees. In: Niinemets Ü , Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 47–93. [Google Scholar]
- Rasulov B, Bichele I, Laisk A, Niinemets Ü . Competition between isoprene emission and pigment synthesis during leaf development in aspen. Plant Cell Environ. 2014;37:724–741. doi: 10.1111/pce.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Laisk A, Niinemets Ü . Induction of a longer-term component of isoprene release in darkened aspen leaves: origin and regulation under different environmental conditions. Plant Physiol. 2011;156:816–831. doi: 10.1104/pp.111.176222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Välbe M, Laisk A, Niinemets Ü . Evidence that light, carbon dioxide and oxygen dependencies of leaf isoprene emission are driven by energy status in hybrid aspen. Plant Physiol. 2009;151:448–460. doi: 10.1104/pp.109.141978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Bancal MO, Ney B, Lannou C. Wheat leaf photosynthesis loss due to leaf rust, with respect to lesion development and leaf nitrogen status. New Phytol. 2005;165:227–241. doi: 10.1111/j.1469-8137.2004.01237.x. [DOI] [PubMed] [Google Scholar]
- Roloff I, Scherm H, van Iersel MW. Photosynthesis of blueberry leaves as affected by Septoria leaf spot and abiotic leaf damage. Plant Dis. 2004;88:397–401. doi: 10.1094/PDIS.2004.88.4.397. [DOI] [PubMed] [Google Scholar]
- Scholes JD, Farrar JF. Increased rates of photosynthesis in localized regions of a barley leaf infected with Brown Rust. New Phytol. 1986;104:601–612. doi: 10.1111/j.1469-8137.1986.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Schulze-Lefert P, Panstruga R. A molecular evolutionary concept connecting nonhost resistance, pathogen host range, and pathogen speciation. Trends Plant Sci. 2011;16:117–125. doi: 10.1016/j.tplants.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Semiz G, Blande J, Heijari J, Işik K, Niinemets Ü , Holopainen JK. Manipulation of VOC emissions with methyl jasmonate and carrageenan in evergreen conifer Pinus sylvestris and evergreen broadleaf Quercus ilex. Plant Biol. 2012;14:57–65. doi: 10.1111/j.1438-8677.2011.00485.x. [DOI] [PubMed] [Google Scholar]
- Staudt M, Jackson B, El-Aouni H, Buatois B, Lacroze J-P, Poëssel J-L, Sauge M-H. Volatile organic compound emissions induced by the aphid Myzus persicae differ among resistant and susceptible peach cultivars and a wild relative. Tree Physiol. 2010;10:1320–1334. doi: 10.1093/treephys/tpq072. [DOI] [PubMed] [Google Scholar]
- Sun Z, Niinemets Ü , Hüve K, Noe SM, Rasulov B, Copolovici L, Vislap V. Enhanced isoprene emission capacity and altered light responsiveness in aspen grown under elevated atmospheric CO2 concentration. Glob Change Biol. 2012;18:3423–3440. [Google Scholar]
- Tang X, Rolfe SA, Scholes JD. The effect of Albugo candida (white blister rust) on the photosynthetic and carbohydrate metabolism of leaves of Arabidopsis thaliana. Plant Cell Environ. 1996;19:967–975. [Google Scholar]
- Toome M, Randjärv P, Copolovici L, Niinemets Ü , Heinsoo K, Luik A, Noe SM. Leaf rust induced volatile organic compounds signalling in willow during the infection. Planta. 2010;232:235–243. doi: 10.1007/s00425-010-1169-y. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Vuorinen T, Nerg A-M, Syrjala L, Peltonen P, Holopainen JK. Epirrita autumnata induced VOC emission of silver birch differ from emission induced by leaf fungal pathogen. Arthropod-Plant Interact. 2007;1:159–165. [Google Scholar]
- Zhao D, Glynn NC, Glaz B, Comstock JC, Sood S. Orange rust effects on leaf photosynthesis and related characters of sugarcane. Plant Dis. 2011;95:640–647. doi: 10.1094/PDIS-10-10-0762. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Remmel T, Vislap V, Niinemets Ü. Volatile emissions from Alnus glutinosa induced by herbivory are quantitatively related to the extent of damage. Journal of Chemical Ecology. 2011;37:18–28. doi: 10.1007/s10886-010-9897-9. [DOI] [PubMed] [Google Scholar]
- Kännaste A, Copolovici L, Niinemets Ü. Gas chromatography mass-spectrometry method for determination of biogenic volatile organic compounds emitted by plants. In: Rodríguez-Concepción M, editor. Plant isoprenoids: methods and protocols. Humana Press; New York: 2014. pp. 161–169. [DOI] [PubMed] [Google Scholar]
- Loreto F, Sharkey TD. A gas-exchange study of photosynthesis and isoprene emission in Quercus rubra L. Planta. 1990;182:523–531. doi: 10.1007/BF02341027. [DOI] [PubMed] [Google Scholar]
- Morfopoulos C, Sperlich D, Peñuelas J, Filella I, Llusià J, Medlyn BE, Niinemets Ü, Possell M, Sun Z, Prentice IC. A model of plant isoprene emission based on available reducing power captures responses to atmospheric CO2. The New Phytologist. 2014;203:125–139. doi: 10.1111/nph.12770. [DOI] [PubMed] [Google Scholar]
- Müller A, Faubert P, Hagen M, zu Castell W, Polle A, Schnitzler J-P, Rosenkranz M. Volatile profiles of fungi - chemotyping of species and ecological functions. Fungal Genetics and Biology. 2013;54:25–33. doi: 10.1016/j.fgb.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Monson RK, Arneth A, Ciccioli P, Kesselmeier J, Kuhn U, Noe SM, Peñuelas J, Staudt M. The leaf-level emission factor of volatile isoprenoids: caveats, model algorithms, response shapes and scaling. Biogeosciences. 2010;7:1809–1832. [Google Scholar]