Abstract
Influence of environmental stress factors on both crop and wild plants of nutritional value is an important research topic. The past research has focused on rising temperatures, drought, soil salinity and toxicity, but the potential effects of increased environmental contamination by human-generated electromagnetic radiation on plants have little been studied. Here we studied the influence of microwave irradiation at bands corresponding to wireless router (WLAN) and mobile devices (GSM) on leaf anatomy, essential oil content and volatile emissions in Petroselinum crispum, Apium graveolens and Anethum graveolens. Microwave irradiation resulted in thinner cell walls, smaller chloroplasts and mitochondria, and enhanced emissions of volatile compounds, in particular, monoterpenes and green leaf volatiles. These effects were stronger for WLAN-frequency microwaves. Essential oil content was enhanced by GSM-frequency microwaves, but the effect of WLAN-frequency microwaves was inhibitory. There was a direct relationship between microwave-induced structural and chemical modifications of the three plant species studied. These data collectively demonstrate that human-generated microwave pollution can potentially constitute a stress to the plants.
Keywords: Microwave, abiotic stress, essential oils, volatile organic compounds, aromatic plants
INTRODUCTION
Aromatic plants represent an important resource for human nutrition, due to their valuable properties, including medicinal benefits (Bonjar, 2004; Wong and Kitts, 2006; Bakkali et al., 2008; Ortan et al., 2009; Cornara et al., 2009). Therefore, understanding their chemical composition and how the properties of aromatic plants are affected by key climate change factors as well as human-generated pollution are research topics of major interest.
The key property of aromatic plants is the presence of essential oils that play important roles in plants acting as direct defenses against pathogen and herbivore attacks (Rhoades, 1977; Lewinsohn, 1991; Fugmann et al., 1997; Reddy et al., 2001). The essential oils are very complex natural mixtures that consist of molecules produced through different secondary metabolic pathways, characteristically containing terpenoids, benzenoids and sometimes aliphatic compounds (Bauer et al., 1998; Eggersdorfer, 1998; Cheng et al., 2007; Bakkali et al., 2008).
Both the composition and content of essential oils has been shown to strongly depend on plant species and environmental conditions (Langlille and MacLean, 1976; Letchamo and Gosselin, 1996; Zabaras et al., 2002; Manzan et al., 2003). These aspects are relevant because plants in natural conditions as well as in agricultural fields are exposed to a plethora of abiotic and biotic stresses and the importance of several biological and environmental stresses is expected to increase in the future (Peñuelas and Estiarte, 1998; Lobell, 2008; Craufurd and Wheeler, 2009; Jacob and Winner, 2009; Niinemets, 2010b).
The key abiotic stresses(Lobell, 2008; Craufurd and Wheeler, 2009; Jacob and Winner, 2009) of contemporary economical importance for plant growth worldwide are drought, heat, cold (chilling and freezing), high salinity, soil mineral deficiency and toxicity. Furthermore, diffuse environmental pollution, including air and soil pollution constitutes a major problem for agriculture and human health (Gauderman et al., 2004; WHO, 2004; Copaciu et al., 2013; Opriş et al., 2013). It was demonstrated that the blend of volatile organic compounds emitted by aromatic plants under stress factors is complex (Rodrigues-Navas et al., 2012). The complexity of volatile emissions in species having specialized storage structures for volatiles results from the circumstance that there may be emissions directly coming from storage and de novo emissions independent of storage (Staudt et al., 1997; Niinemets et al., 2010b; Monson et al., 2012; Grote et al., 2013; Li and Sharkey, 2013).
Among the novel potential pollution sources is the enhanced use of mobile phones and wireless devices generating an exponentially increased level of electromagnetic radiation in the microwave range of radiation frequencies (1-100 GHz). There have been some studies on microwave effects on plants showing no significant effects, while others have demonstrated important modifications in plant functioning. Laboratory growth experiments in plants subject to magnetic fields demonstrated that plants were taller and heavier (Martínez et al., 2003). Likewise, germination of Cicer arietinum L. seeds and early development were enhanced upon exposure to a moderate magnetic field (Vashisth and Nagarajan, 2008). It has been shown that electromagnetic radiation at broadcast-frequency (0.2-30 MHz) altered the cellular contents of calcium and sulfur, effect associated with the power of radiation (Balmori Martínez, 2003), while in animal cells has been observed that microwaves (frequency of 147 MHz, amplitude-modulated at 16 Hz) can influence the intercellular communication through altering the functioning of the calcium channels (Balmori Martínez, 2003). Exposure to microwaves (frequency of 9.75 GHz and low intensity) of wheat (Triticum aestivum) plants has resulted in cytogenetic changes (Pavel et al., 1998; Balmori Martínez, 2003). Studies have also shown alterations in condensed chromatin distribution of meristem cells exposed to low magnetic fields (Belyavskaya, 2001; Belyavskaya, 2004). In general, these studies collectively suggest that the effects of electromagnetic fields on plants can be variable.
It is, however, unclear what the mechanism of low-energy microwave irradiation effects on plant is. While high energy microwave-radiation can break the chemical bonds (Caldwell et al., 1995; Barnes and Cardoso-Vilhena, 1996), the quantum energy of microwave radiation is low and mainly can have thermal effects, heating up selectively plant structures and possibly also alter the conformation of biomolecules, such as proteins, nucleic acids and membrane lipids. Furthermore, modifications in biomolecular tertiary structure can importantly alter the rate of physiological processes, again implying that microwaves can lead to stress conditions in plants (Takeuchi and Thornber, 1994; Ha et al., 1997; Havaux, 1998). Thus, it is important to gain more conclusive insight into the effects of microwaves on plant performance.
The aim of the present study was to investigate the influence of microwave irradiation on the ultrastructure of leaves, the essential oil content and volatile organic compounds emission of three aromatic plant species of the Apiaceae family, parsley (Petroselinum crispum L.), dill (Anethum graveolens L. subsp. hortorum Alef.) and celery (Apium graveolens L.). The stress application consisted in three weeks microwave irradiation of plants at bands corresponding to wireless router (WLAN) and mobile devices (GSM). As the emissions of stress volatiles such as green leaf volatiles (GLV) and specific terpenes are enhanced upon exposure to different stresses (Heiden et al., 2003; Beauchamp et al., 2005; Copolovici et al., 2011; Niinemets et al., 2013), we hypothesized that microwave irradiation leads to enhanced emission of stress volatiles. In addition, we intend to investigate how microwave irradiation affects the leaf structure, content and composition of essential oils in these aromatic plants of nutritional and medicinal importance.
MATERIALS AND METHODS
Plant material and growth conditions
Plant material including parsley (Petroselinum crispum cv. Plain leaved 2) (P), dill (Anethum graveolens subsp. hortorum cv. Common) (D) and celery (Apium graveolens cv. Pascal Giant) (C) were grown in laboratory from seeds obtained from Agrosel (Câmpia Turzii, Romania). Fifteen seeds were sown in 150 mL plastic pots (height x diameter of 8.5 × 6.5 cm) filled with commercial garden soil.
Three weeks after seeding, the vessels with plants were placed in three identical anechoic chambers (Surducan et al., 2012) characterized by a degree of isolation of 60 dB at radio-frequency range between the exterior and interior. The fully-closed chambers were maintained under the same conditions of light intensity at 300 μmol m−2 s−1 (provided from four 4W MR16 LED lamps (every lamp consisting of 26 warm white SMD 5050 LED at 3300K), temperature (25°C), CO2 concentration (385 ± 20 ppmv) and humidity (65%). One chamber was for non-treated control plants, while plants in the other two chambers were subjected to microwave irradiation. The microwave irradiation was performed at bands corresponding to mobile devices (GSM) using a modified AP5200 generator (D-LINK, China), operating in four bands (860 – 910 MHz frequency range, Pout 29 dBm), and to wireless router (WLAN) using a D-LINK wireless router 802.11g/2.4 GHz (2.412 – 2.48 GHz frequency range, Pout 19 dBm). In the irradiation chamber there is one stick antenna placed in the center of the ceiling. The exposure levels where chosen in agreement with the microwave irradiation levels measured in open space for heavily used GSM networks (100mW/m2) and for indoor WLAN (70mW/m2) communication protocols. The power density to the base of chambers was measured with a spectrum analyzer SPECTRAN HF 4060, AARONIA AG (Germany). Both control and microwave-irradiated plants were watered every 2 days with 10 mL of bidistilled water (bidistillator AcquaMatic model AWC/4D, Hamilton Laboratory Glass Ltd., Kent, UK).
Irradiation was performed during three weeks, after which plants were removed from the chambers for measurements of volatile organic compound (VOC) emission and analyses of leaf structure and essential oil content. All measurements of volatile organic compound (VOC) emission and analyses of leaf structure and essential oil content have been replicated with eight different plants.
Transmission electron microscopy measurements
Samples for transmission electron microscopy (TEM) were contrasted with 2% uranyl acetate in 50% ethanol solution for 2 min and in 0.2% lead citrate in 0.1 M sodium hydroxide solution for 2 min. The samples were dehydrated in ethanol series and embedded in epoxy resin, Epon 812. The samples were cut in an Ultramicrotome, Leica UC6 with a diamond knife and the ultrathin samples (100 nm) were analyzed with a 120kV TEM Model JEM 1010 (Jeol USA Inc., Peabody, MA, USA). The number of chloroplasts, mitochondria, starch grains in the chloroplasts and nuclei were determined in the palisade mesophyll cells for replicate plants under each treatment.
Essential oil extraction
Samples of fresh plant material of 1 g were frozen in liquid nitrogen, pulverized and essential oils were extracted with 2 mL of 1:1 (v/v) mixture of HPLC-grade diethyl ether and n-hexane (Merck, Germany). For extraction, plant material was initially soaked for 10 minutes with the solvent mixture, and then extracted in an ultrasonic bath (Elmasonic S 15H, 37 kHz) for 30 minutes at 30°C. Each extraction was performed using five parallel samples. In all cases, extracts were decanted and filtered through nylon syringe filters (0.45 μm) before use.
Volatile organic compounds (VOC’s) sampling and photosynthetic parameters determinations
VOC sampling was performed using a portable gas exchange system GFS-3000 (Waltz GmbH, Effeltrich, Germany). The system has an environment-controlled cuvette with 8 cm2 window area and multiple leaves were enclosed in the cuvette to fill the whole cuvette window. A volume of 4 L of air exiting the cuvette was sampled in a multibed stainless steel cartridge (8.88 × 0.65 cm, Supelco, Bellefonte, PA, USA) filled with Carbopack adsorbents (C 20/40 mesh, C 40/60 mesh, and X 20/40 mesh). The chamber air was sampled at a flow rate of 200 mL min−1 for 20 min using a 1003-SKC constant flow sampling pump (SKC Inc., Houston, TX, USA) at room temperature. Background air samples were taken before and after the measurements using the same system without the leaves enclosed in the cuvette. Using the same system, CO2 and H2O concentrations have been measured. The rates of net assimilation (A) and stomatal conductance to water vapour (gs) were calculated per unit enclosed plant leaf area according to von Caemmerer and Farquhar (1981).
Essential oils and volatile organic compounds analysis
For both essential oils and VOC analysis, a Shimadzu QP2010 Plus gas chromatograph coupled with quadrupole mass spectrometer (GC–MS) (Kyoto, Japan) was used. The conditions for essential oils analysis were as follows: injector temperature was 215°C, initial oven temperature at 40°C was held for 1 min; ramped at 5°C min−1 up to 200°C, held at this temperature for 1 min; ramped at 10°C min−1 up to 220°C and held for further 5 min. Helium (purity 99.9999 %, Elmer Messer Gaas AS, Tallinn, Estonia) was employed as carrier gas with a constant flow rate of 1 mL min−1. The mass spectrometer was operated in electron-impact mode (EI) at 70 eV, in the scan range m/z 30 – 400, the transfer line temperature was set at 240°C and ion-source temperature at 150°C.
For VOC analysis, an automated cartridge desorber Shimadzu TD20 (Kyoto, Japan) was used. The volatiles were analyzed according to the method described in detail in Copolovici et al., 2009.
The essential oils and volatile organic compounds were identified by comparing the mass spectra of individual compounds with the spectra of GC purity external standards (Sigma Aldrich, St. Louis, MO, USA) and with the spectra of NIST Library.
Statistical analysis and data handling
For transmission electron microscopy (TEM) analyses, five replicate measurements, and for essential oils and volatile organic compound three replicates with independent samples of plants were available and we report means of the replications ± SE at each treatment. The means were statistically compared with Student ANOVAs followed by post hoc Tukey’s tests using ORIGIN 8 (OriginLab Corporation, Northampton, MA, USA). All statistical differences were considered significant at P < 0.05.
RESULTS AND DISCUSSION
Ultrastructural analyses and photosynthetic parameters
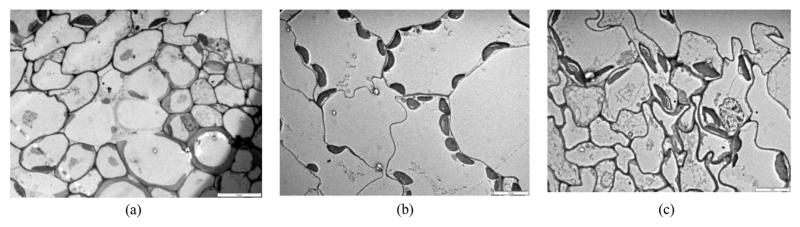
Irradiation resulted in both qualitative and quantitative modifications in leaf anatomy. Palisade and spongy-parenchyma cells exhibit slightly wavy walls in leaves of irradiated plants, while in leaves of control plants were straight-walled (Fig. 1).
Fig. 1.
TEM images of cell walls in leaves of microwave-irradiated and control parsley: a) Control; b) GSM irradiated; c) WLAN iradiated.
This indicates alterations in spatial arrangement of cells in leaf lamina cross-section and a moderate decrease of lamina turgidity. In fact, cell wall thickness was reduced by microwave-irradiation, and generally more strongly by the treatment with WLAN-frequency microwaves (Table 1).
Table 1.
Ultrastructural analysis of the leaves of studied plants. The average values (± SE) are replicates of six independent measurements with different plants.
| Treatment | Cell wall thickness (μm) | Chloroplast length (μm) | Chloroplast area (μm2) | Mitochondrion length (μm) | Ratio of starch grain area to chloroplast area (%) |
|---|---|---|---|---|---|
|
Petroselinum crispum
| |||||
| Control | 0.300 ± 0.07 | 6.78 ± 0.12 | 13.31 ± 0.22 | 1.00 ± 0.27 | 8.93 ± 0.13 |
| 860 – 910 MHz | 0.250 ± 0.06 | 6.76 ± 0.28 | 8.491 ± 0.06 | 0.90 ± 0.13 | 9.99 ± 0.12 |
| 2.4 - 2.5 GHz | 0.200 ± 0.05 | 6.50 ± 0.16 | 7.807 ± 0.11 | 0.70 ± 0.05 | 6.01 ± 0.08 |
|
| |||||
|
Anethum graveolens
| |||||
| Control | 0.187 ± 0.01 | 5.90 ± 0.13 | 8.43 ± 0.23 | 1.68 ± 0.13 | 5.21 ± 0.13 |
| 860 – 910 MHz | 0.175 ± 0.01 | 5.20 ± 0.17 | 8.08 ± 0.29 | 1.00 ± 0.25 | 8.13 ± 0.08 |
| 2.4 - 2.5 GHz | 0.175 ± 0.01 | 4.85 ± 0.31 | 7.04 ± 0.22 | 0.80 ± 0.10 | 0 |
|
| |||||
|
Apium graveolens
| |||||
| Control | 0.160 ± 0.01 | 5.80 ± 0.20 | 7.68 ± 0.14 | 1.57 ± 0.08 | 5.34 ± 0.28 |
| 860 – 910 MHz | 0.156 ± 0.01 | 4.33 ± 0.26 | 7.06 ± 0.35a | 0.55 ± 0.13 | 0 |
| 2.4 - 2.5 GHz | 0.136 ± 0.01 | 3.33 ± 0.26 | 6.68 ± 0.14 | 0.25 ± 0.13 | 0 |
Chloroplasts retained their ultrastructure and normal arrangement within cells, but chloroplasts tended to be smaller in irradiated leaves, especially in the case of WLAN frequency microwaves (Table 1). The ratio of starch grain area to chloroplast area was somewhat increased in treatment with GSM-frequency microwaves in P. crispum and A. graveolens, while it was reduced in WLAN-frequency microwave treatment in P. crispum (Table 1). Nevertheless, the differences among the treatments were relatively small, indicating moderate effects of microwave treatments on starch accumulation.
The mitochondrion length generally decreased in microwave-treated plants, especially in the case of plants irradiated with WLAN-frequency microwaves (Table 1). In addition, the number of mitochondrial cristae was also somewhat less, suggesting a certain decrease in their metabolic activity. Nuclei of most cells showed normal structure, however abundance of heterochromatin and presence of wavy contours tended to be greater in microwave-treated plants.
The anatomical modifications were qualitatively similar among species (Table 1). In all species, the treatment with WLAN-frequency microwaves resulted in greater anatomical changes than the treatment with GSM-frequency microwaves (Table 1). However, the microwave-induced changes were the strongest in Anethum graveolens, where all treatments differed from each other (Table 1) followed by P. crispum and Apium graveolens. In this species, chloroplast length did not differ among the treatments and mitochondrion length in GSM-frequency microwaves was not different from that in control plants (Table 1).
These data collectively demonstrate important alterations in foliage ultrastructure by microwave irradiation, and are in agreement with observations in wheat (Triticum aestivum) exhibiting pronounced cytogenetic changes in response to microwaves (Pavel et al., 1998; Balmori Martínez, 2003). It was shown that under the influence of low-intensity microwaves in the species of Triticum aestivum, as compared to the control plants different types of chromosomal aberrations appeared: delayed chromosomes, micronuclei, interchromosomal bridges, chromosomal fragments (Pavel et al., 1998). In meristem cells of Pisum sativum L. roots exposed to low-magnetic field were observed ultrastructural changes such as a noticeable accumulation of lipid bodies, development of a lytic compartment (vacuoles, cytosegresomes and paramural bodies), and reduction of phytoferritin in plastids. The most sensitive organelle to low-magnetic field application was mitochondria, whose size and relative volume in cells increased, matrix was electron-transparent, and cristae reduced (Belyavskaya, 2001).
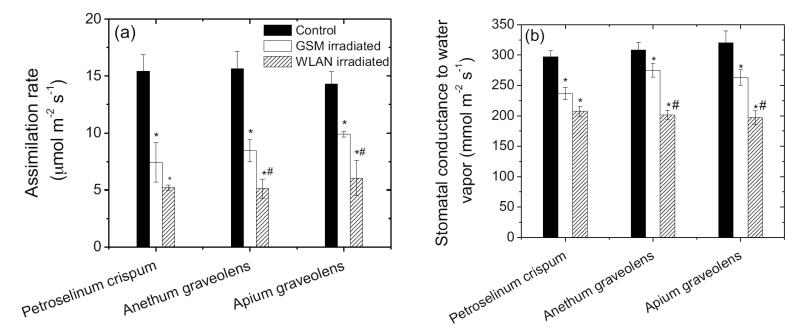
Photosynthesis parameters (assimilation rates and stomata conductance to water vapor) have been affected by microwaves exposure (Figure 2). Even more, both parameters are influenced by the strength of the stress.
Fig. 2.
Changes in net assimilation rate (a) and stomatal conductance to water vapour (b) in 3 aromatic plants in response to microwave stress. The data are expressed per unit projected leaf area. Each data point is the mean (± SE) of 8 independent replicate experiments with a different plant.
* and # demonstrates statistically significant differences between the microwave irradiated plants and control plants and between WLAN and GSM irradiated plants respectively (P < 0.05).
Overall, reduction in the size of organelles may indicate that photosynthesis and respiratory metabolism was somewhat impaired by microwave treatment (Louwerse and van der Zweerde, 1977; Lichtenthaler, 1981; Griffin et al., 2001; Terashima et al., 2011). On the other hand, reduction in cell wall thickness reduces mesophyll diffusion conductance to chloroplasts thereby potentially compensating for reduced physiological potentials and altered stomatal conductance to water vapor (Terashima et al., 2006; Tosens et al., 2012a; Tosens et al., 2012b; Tomás et al., 2013). However, reduced cell wall thickness reduces cellular resistance to low leaf water potentials (Niinemets, 2001). Thus, microwave irradiance may importantly decrease plant drought resistance.
Changes in essential oils content in response to microwave irradiation: general patterns
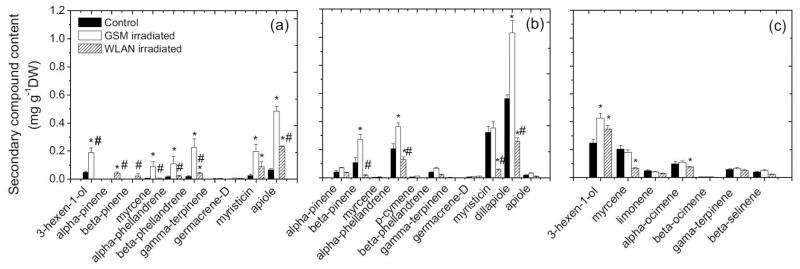
Many aromatic plants have specialized structures for the storage of volatiles and the composition of essential oils in these storage structures is often complex (Letchamo et al., 1995; Manzan et al. 2003; Rajabi et al., 2013). Our study also observed complex composition of essential oils in the studied species, 10 compounds were detected in P. crispum, 11 compounds in Anethum graveolens and 7 compounds in Apium graveolens. In all species, monoterpenes constituted a significant component of the essential oil (Fig. 3). In addition, several specific benzenoids were also dominating components of the oil: apiol in P. crispum, and myristicin and dillapiole in Anethum graveolens (Fig. 3). Lipoxygenase pathway compounds were important constituents in essential oil in P. crispum and Apium graveolens (Fig. 3). In addition, the main volatile compounds in Apium graveolens are 3-hexen-1-ol, myrcene, α-ocimene, γ-terpinene (Fig. 3). These results broadly agree with past observations of essential oils produced by these species (Deng et al., 2003; Orav et al., 2003).
Fig. 3.
Changes in terpene content (mg g−1 FW) in Petroselinum crispum (a), Anethum graveolens subsp. hortorum (b) and Apium graveolens (c) foliage in response to microwave irradiations at bands corresponding to wireless router (WLAN) and mobile devices (GSM). Each data point is the mean (± SE) of three independent replicate experiments with a different plant. * and # demonstrates statistically significant differences between the microwave irradiated plants and control plants and between WLAN and GSM irradiated plants respectively (P < 0.05).
Multiple environmental factors have been shown to modify the content of essential oil (Langlille and MacLean, 1976; Gershenzon, 1984; Letchamo et al., 1994; Wannaz et al., 2003; Peñuelas et al., 2011). Such modifications in essential oil content have been often explained on the basis of hypotheses linking growth, and primary and secondary metabolism (Bryant et al., 1983; Herms and Mattson, 1992; Peñuelas and Estiarte, 1998). According to these hypotheses, when sink activity (growth) rate decreases and carbon dioxide availability is in excess of that naturally occurring in the atmosphere, plants increase the rate of synthesis of secondary metabolites such as essential oils (Bryant et al., 1983; Herms and Mattson, 1992; Peñuelas and Estiarte, 1998). In agreement with this hypothesis, water deficit has been shown to increase the yield of essential oil and affected its relative composition in P. crispum (Petropoulos, 2008). Analogously, enhanced salinity increased the essential oil yield for Anethum graveolens plants under stress (Ghassemi-Golezani et al., 2011).
In our study, microwave irradiation by GSM-frequency microwaves generally increased the essential oil contents (Fig. 3), while the effect of WLAN-frequency microwaves was less clear, varying from positive or negative for different compounds and species (Fig. 3). In a like manner, ozone stress induced two distinct pathways in P. crispum (Eckey-Kaltenbach et al., 1994) suggesting that different types and severity of stress can lead to qualitatively different responses. Taken together, these results are in partial agreement with several past observations indicating enhanced production of essential oils under stress and also are in agreement with the evidence of impaired photosynthetic and respiratory metabolism under microwave irradiation (Table 1). However, differently from essential oils, the anatomical modifications were significant under WLAN microwave irradiation (cf. Table 1 and Fig. 1c).
Species and microwave-frequency effects on essential oils
Although the effects were broadly similar among species, important species differences were observed in individual compound responses to GSM- and WLAN-frequency microwaves. Among the three plant species tested in these experiments, the strongest effects of microwave irradiation on essential oils were observed on Anethum graveolens plants (Fig. 3).
For individual compounds in P. crispum, the microwave irradiation produced by GSM generator statistically increased 3-hexen-1-ol, myrcene, α-phellandrene, β-phellandrene, myristicin and apiole contents. Compared to the reference, the strongest increase in response to GSM-frequency irradiation was observed for apiole (more than seven times greater content, Fig. 3a). The WLAN-frequency microwaves statistically increased the content of α-pinene, β-phellandrene, myristicin and apiol in this species (Fig. 3a).
In Anethum graveolens irradiated with GSM microwaves, increased content was observed for β-pinene, α-phellandrene and dillapiole (Fig. 3b). However, WLAN-frequency microwaves reduced α-phellandrene, myristicin and dillapiole content, whereas the greatest reduction was observed for myristicin (approximately to the level 18% of that in reference plants, Fig. 3b).
In Apium graveolens, both types of microwaves used in this study increased 3-hexen-1-ol content (Fig. 3c). Irradiation by WLAN-frequency microwaves reduced myrcene (19%) and α-ocimene (21%) contents (Fig. 3c).
Species-differences in environmental responses to stress factors have been demonstrated (El-Keltawi and Croteau, 1986; Mangas et al., 2006) although interspecific studies have been rare. Species ranking according to anatomical modifications was similar to the ranking based on essential oil changes (cf. Table 1 and Fig. 3). The structure of Apium graveolens leaves was the least affected by microwave irradiation and the effect on leaf chemistry was also the least in this species.
General patterns in volatile organic compounds emissions
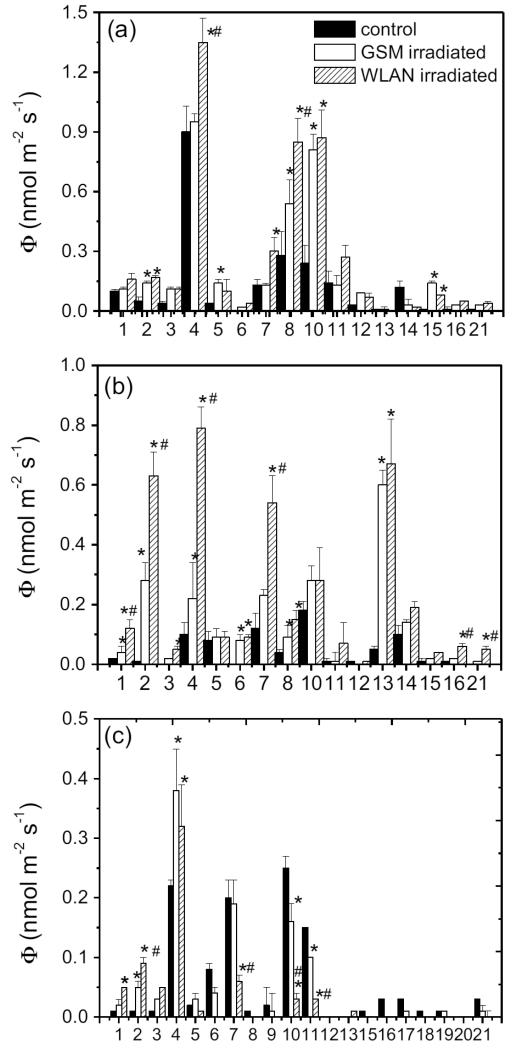
Our data demonstrate that the emissions observed did reflect a mixture of both storage emission consisting of compounds present in essential oils and de novo emissions. The blend of volatiles was very complex and, in all plant species, the non-stressed plants also emitted monoterpenes and benzenoids present in essential oils, in some cases even compounds not-present in essential oils (Fig. 4). The number of compounds detected in the emissions was greater than in the essential oils, and characteristic de novo released stress volatiles were observed (Fig. 4). 16 compounds were detected in the emissions of P. crispum, 16 compounds in Anethum graveolens and 20 compounds in Apium graveolens.
Fig. 4.
Alteration of the emission of volatile organic compounds (nmol m−2 s−1) from foliage of Petroselinum crispum (a), Anethum graveolens subsp. hortorum (b) and Apium graveolens (c) in response to microwave irradiations at bands corresponding to wireless router (WLAN) and mobile devices (GSM) (presentation of statistical differences as shown in Fig. 2). Each number corresponds to a particular volatile compound as follows: 1. 1-hexanol; 2. (Z)-3-hexen-1-ol; 3. (E)-2-hexenal; 4. α-pinene; 5. camphene; 6. β-myrcene; 7. β-pinene; 8. α-phellandrene; 9. Δ-3-carene; 10. D-limonene; 11. para-cymene; 12. β-phellandrene; 13. (E)-β-ocimene; 14. 1,8-cineol; 15. iso-bornyl acetate; 16. longicyclene; 17. caryophyllene oxide; 18. α-selinene; 19. (Z)-β-farnesene; 20. α-caryophyllene; 21. geranylacetone.
* and # demonstrates statistically significant differences between the microwave irradiated plants and control plants and between WLAN and GSM irradiated plants respectively (P < 0.05).
There was evidence of similar enhancement of essential oils and emissions for several monoterpenes, especially for GSM microwave treatments in P. crispum and Anethum graveolens (cf. Figs. 3 and 4). However, in these species, emissions were more strongly enhanced under WLAN microwave treatment, which appeared to have an inhibitory effect on the content of the same terpenoids, e.g. α-pinene and β-phellandrene (cf. Figs. 3 and 4). Although there was evidence of parallel changes in contents and emissions for some volatiles in species, and for some treatments, this evidence suggests that the storage and de novo emissions cannot be fully teased apart in the current study. Nevertheless, the data suggest that the total emissions and especially treatment differences reflect to a large degree the microwave-induced de novo synthesized plant volatiles.
Compound-class, species- and treatment-specific differences in volatile emissions
Among the de novo emissions, green leaf volatiles (GLV), also called volatiles of lipoxygenase pathway (LOX volatiles) are released in plants in response to different stresses (Copolovici and Niinemets, 2010; Copolovici et al., 2011; Copolovici et al., 2012). GLVs are formed in the hydroperoxide lyase pathway of oxylipin metabolism from free octadecanoic fatty acids and consist usually of a mixture of C6 aldehydes and ketones (Matsui, 2006). In our study, all microwave-irradiated plants emitted the following GLVs: (E)-2-hexenal, (Z)-3-hexenol, 1-hexanol, while the emissions of GLVs were very low at the level of detection limit of our device in control plants (Fig. 4).
In general, in all plant species studied, the emissions of GLV were greater for WLAN-frequency microwaves compared to GSM-frequency microwaves (Fig. 4, P < 0.001 for all). These results suggest greater stress in the case of WLAN microwave irradiation, and are in agreement with the more significant changes in anatomy of leaves induced by WLAN microwaves (Table 1). Stronger GLV emissions under more severe stress have been shown for water (Capitani et al., 2009), ozone (Beauchamp et al., 2005), herbivory attack (Allmann and Baldwin, 2010) and temperature (Copolovici et al., 2012) stresses.
The GLV emissions of the P. crispum and Anethum graveolens were dominated by the 1-hexanol (Fig. 4), while in Apium graveolens the main component was (Z)-3-hexenol that was also important constituent in the essential oil in this species (Figs. 3 and 4). The total GLV emission from P. crispum and Anethum graveolens was five times higher than from Apium graveolens. As with the essential oil content (Fig. 3), Apium graveolens was clearly less sensitive to the microwave fields than P. crispum and Anethum graveolens.
The monoterpenes detected in the emissions were α-pinene, β-pinene, camphene, limonene, 3-carene, para-cymene, β-phellandrene, (E)-β-ocimene, eucalyptol and bornyl acetate. In P. crispum, emission of α-pinene, β-pinene and β-phellandrene were dominant and enhanced by microwave irradiation, especially in the case of WLAN-frequency microwave treatment (Fig. 4a). Treatment effects on monoterpene emissions were similar for Apium graveolens and Anethum graveolens, but the main components are at some extent different (Figure 4b and 4c). Monoterpene emissions from Anethum graveolens were dominated by α-pinene, α-phellandrene and limonene, and these emissions were enhanced by microwave irradiation (Fig. 4b). In Apium graveolens, the emissions were almost four times lower than in the other species and were dominated by α-pinene, β-pinene and limonene (Fig. 4c). The emission of terpenes was inhibited by microwave irradiation similarly to the content of essential oils (Figs. 3 and 4).
Overall, these emitted monoterpenes are characteristic plant-released compounds and are not specific to stress-induced emissions (Staudt et al., 1997; Kesselmeier and Staudt, 1999; Staudt et al., 2000; Niinemets et al., 2010b). However, the emission rates of these typical monoterpenes is also often enhanced in stress conditions (Vuorinen et al., 2004; Blande et al., 2007; Heijari et al., 2008; Copolovici et al., 2011; Copolovici et al., 2012), implying that induced and constitutive emission are often difficult to separate. Among the characteristic induced monoterpenes (Staudt and Bertin, 1998; Hakola et al., 2001; Noe et al., 2006; Niinemets et al., 2010b), emissions of (E)-β-ocimene and 1,8-cineole were strongly enhanced by microwave-irradiation in Anethum graveolens (Fig. 4). In addition, both P. crispum and Anethum graveolens, emitted in low amounts longicyclene, a stress induced sesquiterpene, under WLAN-frequency irradiation.
CONCLUSIONS
The presented data collectively suggest that microwave irradiation constitute a stress to the plants, resulting in enhanced emissions of green leaf volatiles, up-regulation of terpenoid emissions and modification in essential oil content and foliage anatomy. Anatomical and emission traits suggested that WLAN-frequency irradiation resulted in more severe stress than GSM-frequency irradiation, but the effect of WLAN-frequency irradiation on essential oil was inhibitory. There was an agreement between anatomical and chemical traits with anatomically most resistant species Apium graveolens being chemically least responsive.
ACKNOWLEDGMENTS
This work was supported by grants of the Romanian National Authority for Scientific Research, CNCS-UEFISCDI, projects numbers PN-II-RU-TE-2011-3-0283 and PN-II-RUTE-2011-3-0022, Estonian Ministry of Science and Education (institutional grant IUT-8-3) and the European Commission through the European Regional Fund (the Center of Excellence in Environmental Adaptation) and the European Research Council (advanced grant 322603, SIP-VOL+). The authors thank to Dr. E. Surducan team (INCDTIM Cluj-Napoca) for all their experimental support with anechoic chambers.
Abbreviations
- VOC
volatile organic compounds
- GLV
green leaf volatiles
- WLAN
wireless router
- GSM
mobile devices
- TEM
transmission electron microscopy
REFERENCES
- Allmann S, Baldwin IT. Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science. 2010;329:1075–8. doi: 10.1126/science.1191634. [DOI] [PubMed] [Google Scholar]
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils – A review. Food Chem Toxicol. 2008;46:446–75. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Balmori Martínez A. The effects of microwaves on the trees and other plants. Valladolid; Spain: 2003. Available online at buergerwelle. de. [Google Scholar]
- Barnes JD, Cardoso-Vilhena J. Interactions between electromagnetic radiation and the plant cuticle. In: Kerstiens G, editor. Plant cuticles. An integrated functional approach. Vol. 1996. Bios Scientific Publishers; Oxford: 1996. pp. 157–74. [Google Scholar]
- Bauer K, Garbe D, Surburh H. Ullmann’s encyclopedia of industrial chemistry. The CD-ROM edition Wiley-VCH; Berlin: 1998. Flavors and fragrances. [Google Scholar]
- Beauchamp J, Wisthaler A, Hansel A, Kleist E, Miebach M, Niinemets Ü , Schurr U, Wildt J. Ozone induced emissions of biogenic VOC from tobacco: relations between ozone uptake and emission of LOX products. Plant Cell Environ. 2005;28:1334–43. [Google Scholar]
- Belyavskaya NA. Ultrastructure and calcium balance in meristem cells of pea roots exposed to extremely low magnetic fields. Adv Space Res. 2001;28:645–50. doi: 10.1016/s0273-1177(01)00373-8. [DOI] [PubMed] [Google Scholar]
- Belyavskaya NA. Biological effects due to weak magnetic field on plants. Adv Space Res. 2004;34:1566–74. doi: 10.1016/j.asr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Blande JD, Tiiva P, Oksanen E, Holopainen JK. Emission of herbivore-induced volatile terpenoids from two hybrid aspen (Populus tremula x tremuloides) clones under ambient and elevated ozone concentrations in the field. Glob Change Biol. 2007;13:2538–50. [Google Scholar]
- Bonjar S. Evaluation of antibacterial properties of some medicinal plants used in Iran. J Ethnopharmacol. 2004;94:301–5. doi: 10.1016/j.jep.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Bryant JP, Chapin FS, III, Klein DR. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos. 1983;40:357–68. [Google Scholar]
- Caldwell MM, Teramura AH, Tevini M, Bornman JF, Björn LO, Kulandaivelu G. Effects of increased solar ultraviolet radiation on terrestrial plants. AMBIO: A Journal of the Human Environment. 1995;24:166–73. [Google Scholar]
- Capitani D, Brilli F, Mannina L, Proietti N, Loreto F. In situ investigation of leaf water status by portable unilateral Nuclear Magnetic Resonance. Plant Physiol. 2009;149:1638–47. doi: 10.1104/pp.108.128884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AX, Lou YG, Mao YB, Lu S, Wang LJ, Chen XY. Plant terpenoids: biosynthesis and ecological functions. J Integr Plant Biol. 2007;49:179–86. [Google Scholar]
- Copaciu F, Opriş O, Coman V, Ristoiu D, Niinemets Ü , Copolovici L. Diffuse water pollution by anthraquinone and azo dyes in environment importantly alters foliage volatiles, carotenoids and physiology in wheat (Triticum aestivum) Water Air Soil Pollut. 2013;224:1478. [Google Scholar]
- Copolovici L, Kännaste A, Niinemets Ü . Gas chromatography-mass spectrometry method for determination of monoterpene and sesquiterpene emissions from stressed plants. Studia Univ Babes-Bolyai, Chem. 2009;54:329–39. [Google Scholar]
- Copolovici L, Niinemets U. Flooding induced emissions of volatile signalling compounds in three tree species with differing waterlogging tolerance. Plant Cell Environ. 2010;33:1582–94. doi: 10.1111/j.1365-3040.2010.02166.x. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Remmel T, Vislap V, Niinemets Ü . Volatile emissions from Alnus glutinosa induced by herbivory are quantitatively related to the extent of damage. J Chem Ecol. 2011;37:18–28. doi: 10.1007/s10886-010-9897-9. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Pazouki L, Niinemets Ü . Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. J Plant Physiol. 2012;169:664–72. doi: 10.1016/j.jplph.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Cornara L, La Rocca A, Marsili S, Mariotti MG. Traditional uses of plants in the Eastern Riviera (Liguria, Italy) J Ethnopharmacol. 2009;125:16–30. doi: 10.1016/j.jep.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Craufurd PQ, Wheeler TR. Climate change and the flowering time of annual crops. J Exp Bot. 2009;60:2529–39. doi: 10.1093/jxb/erp196. [DOI] [PubMed] [Google Scholar]
- Deng C, Song G, Zheng X, Hu Y, Zhang X. Analysis of the volatile constituents of Apium graveolens L. and Oenanthe crocata L. by gas chromatography-mass spectrometry, using headspace solid-phase microextraction. Chromatographia. 2003;57:805–9. [Google Scholar]
- Eckey-Kaltenbach H, Ernst D, Heller W, Sandermann H. Biochemical-plant responses to ozone (IV. Cross-induction of defensive pathways in parsley (Petroselinum crispum L.) plants. Plant Physiol. 1994;104:67–74. doi: 10.1104/pp.104.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggersdorfer M. Ullmann’s encyclopedia of industrial chemistry. The CD-ROM edition Wiley-VCH Verlag; Berlin: 1998. Terpenes. [Google Scholar]
- El-Keltawi NE, Croteau R. Influence of ethephon and daminozide on growth and essential oil content of peppermint and sage. Phytochemistry. 1986;25:1285–8. [Google Scholar]
- Fugmann B, Lang-Fugmann S, Steglich W. Naturstoffe. Georg Thieme Verlag; Stuttgart - New York: 1997. [Google Scholar]
- Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, McConnell R, Kuenzli N, Lurmann F, Rappaport E, Margolis H, Bates D, Peters J. The effect of air pollution on lung development from 10 to 18 years of age. New Eng J Med. 2004;351:1057–67. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- Gershenzon J. Changes in the levels of plant secondary metabolites under water and nutrient stress. In: Timmermann BN, Steelink C, Loewus FA, editors. Phytochemical adaptations to stress. Plenum Press; New York - London: 1984. pp. 273–320. [Google Scholar]
- Ghassemi-Golezani K, Zehtab-Salmasi S, Dastborhan S. Changes in essential oil content of dill (Anethum graveolens) organs under salinity stress. J Med Plants Res. 2011;5:3142–5. [Google Scholar]
- Gog L, Berenbaum MR, DeLucia EH, Zangerl AR. Autotoxic effects of essential oils on photosynthesis in parsley, parsnip, and rough lemon. Chemoecology. 2005;15:115–9. [Google Scholar]
- Griffin KL, Anderson RO, Gastrich MD, Lewis JD, Lin G, Schuster W, Seemann JR, Tissue DT, Turnbull MH, Whitehead D. Plant growth in elevated CO2 alters mitochondrial number and chloroplast fine structure. P Natl Acad Sci USA. 2001;98:2473–8. doi: 10.1073/pnas.041620898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote R, Monson RK, Niinemets Ü . Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In: Niinemets Ü , Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 315–55. [Google Scholar]
- Ha MA, Apperley DC, Jarvis MC. Molecular rigidity in dry and hydrated onion cell walls. Plant Physiol. 1997;115:593–8. doi: 10.1104/pp.115.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakola H, Laurila T, Lindfors V, Hellen H, Gaman A, Rinne J. Variation of the VOC emission rates of birch species during the growing season. Boreal Environ Res. 2001;6:237–49. [Google Scholar]
- Havaux M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998;3:147–51. [Google Scholar]
- Heiden AC, Kobel K, Langebartels C, Schuh-Thomas G, Wildt J. Emissions of oxygenated volatile organic compounds from plants. Part I: Emissions from lipoxygenase activity. J Atmos Chem. 2003;45:143–72. [Google Scholar]
- Heijari J, Nerg AM, Kainulainen P, Vuorinen M, Holopainen JK. Long-term effects of exogenous methyl jasmonate application on Scots pine (Pinus sylvestris) needle chemical defence and diprionid sawfly performance. Entomol Exp Appl. 2008;128:162–71. [Google Scholar]
- Herms DA, Mattson WJ. The dilemma of plants: to grow or defend. Q Rev Biol. 1992;67:283–335. 1992. [Google Scholar]
- Jacob DJ, Winner DA. Effect of climate change on air quality. Atmos. Environ. 2009;43:51–63. [Google Scholar]
- Kesselmeier J, Staudt M. Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. J Atmos Chem. 1999;33:23–88. [Google Scholar]
- Langlille WM, MacLean KS. Some essential nutrient elements in forest plants as related to species, plant part, season and location. Plant Soil. 1976;45:17–26. [Google Scholar]
- Letchamo W, Gosselin A. Transpiration, essential oil glands, epicuticular wax and morphology of Thymus vulgaris are influenced by light intensity and water supply. J Hortic Sci. 1996;71:123–34. [Google Scholar]
- Letchamo W, Lu HL, Gosselin A. Variations in photosynthesis and essential oil in thyme. J Plant Physiol. 1995;147:29–37. [Google Scholar]
- Letchamo W, Marquard R, Hölzl J, Gosselin A. Effects of water supply and light intensity on growth and essential oil of two Thymus vulgaris selections. Angew Bot. 1994;68:83–8. [Google Scholar]
- Lewinsohn E, Gijzen M, Croteau R. Defense mechanisms of conifers. Differences in constitutive and wound-induced monoterpene biosynthesis among species. Plant Physiol. 1991;96:44–9. doi: 10.1104/pp.96.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Sharkey TD. Molecular and pathway controls on biogenic volatile organic compound emissions. In: Niinemets Ü , Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 119–51. [Google Scholar]
- Lichtenthaler HK. Adaptation of leaves and chloroplasts to high quanta fluence rates. In: Akoyunoglou G, editor. Photosynthesis and productivity, photosynthesis and environment. Balaban International Science Services; Philadelphia: 1981. pp. 273–87. [Google Scholar]
- Lobell DB, Burke MB, Tebaldi C, Mastrandrea MD, Falcon WP, Naylor RL. Prioritizing climate change adaptation needs for food security in 2030. Science. 2008;319:607–10. doi: 10.1126/science.1152339. [DOI] [PubMed] [Google Scholar]
- Louwerse W, van der Zweerde W. Photosynthesis, transpiration and leaf morphology of Phaseolus vulgaris and Zea mays grown at different irradiances in artificial and sunlight. Photosynthetica. 1977;11:11–21. [Google Scholar]
- Mangas S, Bonfill M, Osuna L, Moyano E, Tortoriello J, Cusido RM, Pinol MT, Palazon J. The effect of methyl jasmonate on triterpene and sterol metabolisms of Centella asiatica, Ruscus aculeatus and Galphimia glauca cultured plants. Phytochemistry. 2006;67:2041–49. doi: 10.1016/j.phytochem.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Manzan ACCM, Toniolo FS, Bredow E, Povh NP. Extraction of essential oil and pigments from Curcuma longa [L.] by steam distillation and extraction with volatile solvents. J Agric Food Chem. 2003;51:6802–7. doi: 10.1021/jf030161x. [DOI] [PubMed] [Google Scholar]
- Martínez E, Carbonell MV, Flórez M. Estimulación de la germinación y el crecimiento por exposición a campos magnéticos. Investigacion y Ciencia. 2003;324:24–8. [Google Scholar]
- Matsui K. Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr Opin Plant Biol. 2006;9:274–80. doi: 10.1016/j.pbi.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Monson RK, Grote R, Niinemets Ü , Schnitzler JP. Modeling the isoprene emission rate from leaves. New Phytologist. 2012;195:541–59. doi: 10.1111/j.1469-8137.2012.04204.x. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü . Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology. 2001;82:453–69. [Google Scholar]
- Niinemets Ü . Mild versus severe stress and BVOCs: thresholds, priming and consequences. Trends Plant Sci. 2010a;15:145–53. doi: 10.1016/j.tplants.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü . Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: past stress history, stress interactions, tolerance and acclimation. Forest Ecol Manag. 2010b;260:1623–39. [Google Scholar]
- Niinemets Ü , Arneth A, Kuhn U, Monson RK, Peñuelas J, Staudt M. The emission factor of volatile isoprenoids: stress, acclimation, and developmental responses. Biogeosciences. 2010a;7:2203–23. [Google Scholar]
- Niinemets Ü , Monson RK, Arneth A, Ciccioli P, Kesselmeier J, Kuhn U, Noe SM, Peñuelas J, Staudt M. The leaf-level emission factor of volatile isoprenoids: caveats, model algorithms, response shapes and scaling. Biogeosciences. 2010b;7:1809–32. [Google Scholar]
- Niinemets Ü , Kännaste A, Copolovici L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Frontiers in Plant Science. Frontiers in Plant-Microbe Interaction. 2013;4 doi: 10.3389/fpls.2013.00262. article 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe SM, Ciccioli P, Brancaleoni E, Loreto F, Niinemets Ü . Emissions of monoterpenes linalool and ocimene respond differently to environmental changes due to differences in physico-chemical characteristics. Atmos Environ. 2006;40:4649–62. [Google Scholar]
- Opriş O, Copaciu F, Soran ML, Ristoiu D, Niinemets Ü , Copolovici L. Influence of nine antibiotics on key secondary metabolites and physiological characteristics in Triticum aestivum: leaf volatiles as a promising new tool to assess toxicity. Ecotox Environ Safe. 2013;87:70–9. doi: 10.1016/j.ecoenv.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Orav A, Kailas T, Jegorova A. Composition of the essential oil of dill, celery, and parsley from Estonia. Proc Est Acad Sci Chem. 2003;52:147–54. [Google Scholar]
- Ortan A, Popescu ML, Gaita AL, Dinu-Pirvu C, Campeanu Gh. Contributions to the pharmacognostical study on Anethum graveolens, dill (Apiaceae) Rom Biotechnol Lett. 2009;14:4342–8. [Google Scholar]
- Pavel A, Ungureanu C, Bara I, Gassner P, Creanga D. Cytogenetic changes induced by low-intensity microwaves in the species Triticum aestivum. Rev Med Chir Soc Med Nat Iasi. 1998;102:89–92. [PubMed] [Google Scholar]
- Peñuela J, Estiarte M. Can elevated CO2 affect secondary metabolism and ecosystem functioning? Trends Ecol Evol. 1998;13:20–4. doi: 10.1016/s0169-5347(97)01235-4. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Llusià J. BVOCs: plant defense against climatic warming? Trends Plant Sci. 2003;8:105–9. doi: 10.1016/S1360-1385(03)00008-6. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Sardans J, Llusià J, Owen SM, Niinemets Ü . Lower P contents and more widespread terpene presence in old Bornean than in young Hawaiian tropical plant species guilds. Ecosphere. 2011;2 article 45. [Google Scholar]
- Petropoulos SA, Daferera D, Polissiou MG, Passm HC. The effect of water deficit stress on the growth, yield and composition of essential oils of parsley. Sci Hortic. 2008;115:393–7. [Google Scholar]
- Rajabi Memari H, Pazouki L, Niinemets Ü . The biochemistry and molecular biology of volatile messengers in trees. In: Niinemets Ü , Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 47–93. [Google Scholar]
- Reddy PS, Jamil K, Madhusudhan P, Anjani G, Das B. Antibacterial activity of isolates from Piper longum and Taxus baccata. Pharm Biol. 2001;39:236–8. [Google Scholar]
- Rhoades DF. The antiherbivore chemistry of Larrea. In: Mabry TJ, et al., editors. Creosote bush. Dowden, Hutchinson & Ross; Stroudsburg, Pennsylvania: 1977. pp. 135–75. [Google Scholar]
- Rodrigues-Navas C, Forteza R, Cerda V. Use of thermal desorption-gas chromatography-mass spectrometry (TD-GC-MS) on identification of odorant emission focus by volatile organic compounds characterization. Chemosphere. 2012;89:1426–36. doi: 10.1016/j.chemosphere.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Staudt M, Bertin N. Light and temperature dependence of the emission of cyclic and acyclic monoterpenes from holm oak (Quercus ilex L.) leaves. Plant Cell Environ. 1998;21:385–95. [Google Scholar]
- Staudt M, Bertin N, Frenzel B, Seufert G. Seasonal variation in amount and composition of monoterpenes emitted by young Pinus pinea trees - implications for emission modeling. J Atmos Chem. 2000;35:77–99. [Google Scholar]
- Staudt M, Bertin N, Hansen U, Seufert G, Ciccioli P, Foster P, Frenzel B, Fugit JL. Seasonal and diurnal patterns of monoterpene emissions from Pinus pinea (L.) under field conditions. Atmos Environ. 1997;31:145–56. [Google Scholar]
- Surducan E, Surducan V, Halmagyi A. Romanian Patent RO-125068B1. 2012.
- Takeuchi TS, Thornber JP. Heat-induced alterations in thylakoid membrane protein composition in barley. Aust J Plant Physiol. 1994;21:759–70. [Google Scholar]
- Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S. Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J Exp Bot. 2006;57:343–54. doi: 10.1093/jxb/erj014. [DOI] [PubMed] [Google Scholar]
- Terashima I, Hanba YT, Tholen D, Niinemets Ü . Leaf functional anatomy in relation to photosynthesis. Plant Physiol. 2011;155:108–16. doi: 10.1104/pp.110.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás M, Flexas J, Copolovici L, Galmés J, Hallik L, Medrano H, Tosens T, Vislap V, Niinemets Ü . Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. J Exp Bot. 2013;64:2269–81. doi: 10.1093/jxb/ert086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosens T, Niinemets Ü , Vislap V, Eichelmann H, Castro-Díez P. Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: how structure constrains function. Plant Cell Environ. 2012a;35:839–56. doi: 10.1111/j.1365-3040.2011.02457.x. [DOI] [PubMed] [Google Scholar]
- Tosens T, Niinemets Ü , Westoby M, Wright IJ. Anatomical basis of variation in mesophyll resistance in eastern Australian sclerophylls: news of a long and winding path. J Exp Bot. 2012b;63:5105–19. doi: 10.1093/jxb/ers171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashisth A, Nagarajan S. Exposure of seeds to static magnetic field enhances germination and early growth characteristics in chickpea (Cicer arietinum L.) Bioelectromagnetics. 2008;29:571–78. doi: 10.1002/bem.20426. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–87. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Vuorinen T, Reddy GVP, Nerg AM, Holopainen JK. Monoterpene and herbivore-induced emissions from cabbage plants grown at elevated atmospheric CO2 concentration. Atmos Environ. 2004;38:675–82. [Google Scholar]
- Wannaz ED, Zygaldo JA, Pignata ML. Air pollutants effects on monoterpenes composition and foliar chemical parameters in Schinus areira L. Sci Total Environ. 2003;305:177–93. doi: 10.1016/S0048-9697(02)00466-7. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Health aspects of air pollution - results from the WHO project “Systematic review of health aspects of air pollution in Europe”. WHO/Euro; Bonn: 2004. [Google Scholar]
- Wong PYY, Kitts DD. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006;97:505–15. [Google Scholar]
- Zabaras D, Spooner-Hart RN, Wyllie SG. Effects of mechanical wounding on concentration and composition of essential oil from Melaleuca alternifolia leaves. Biochem Syst Ecol. 2002;30:399–412. [Google Scholar]