Abstract
The prevalence of obesity has increased remarkably in the past four decades. Because obesity can promote the development of type 2 diabetes and cardiovascular disease, understanding the mechanisms that engender weight gain and discovering safe anti-obesity therapies are of critical importance. In particular, the gaseous signaling molecule, nitric oxide (NO), appears to be a central factor regulating adiposity and systemic metabolism. Obese and diabetic states are characterized by a deficit in bioavailable NO, with such decreases commonly attributed to downregulation of endothelial NO synthase (eNOS), loss of eNOS activity, or quenching of NO by its reaction with oxygen radicals. Gain-of-function studies, in which vascular-derived NO has been increased pharmacologically or genetically, reveal remarkable actions of NO on body composition and systemic metabolism. This review addresses the metabolic actions of eNOS and the potential therapeutic utility of harnessing its anti-obesogenic effects.
Keywords: nitric oxide, obesity, diabetes, adipose tissue, mitochondria, metabolism
Introduction
The rising prevalence of obesity is a principal health challenge in the United States and abroad. As of 2008, 10% of adults were obese, and approximately 1.5 billion were overweight (Ahima). In the United States, recent estimates indicate that greater than one-third of adults and 17% of children are obese (Ogden, Carroll, Kit, & Flegal). This is associated with an increase in pre-diabetic states, with >30% of the US population meeting the criteria for pre-diabetes (Ervin; Roger et al.). Furthermore, obesity is associated with multiple other co-morbidities including cardiovascular disease and cancer (Calle, Thun, Petrelli, Rodriguez, & Heath, 1999). The current high prevalence of obesity has also equated to a substantial economic burden of nearly $150 billion per year in health care costs (Zamosky).
While lifestyle changes and lack of exercise are undeniable risk factors for weight gain (Hu, Li, Colditz, Willett, & Manson; Robinson; Roger et al.; Smith et al.; Williamson et al.), an excess consumption of foods appears to be one of the key factors in the epidemic of obesity. In the US, the average human consumption of calories has increased by at least 200 kcal/d per person in the past three decades, and this is partly attributable to an increase in the intake of high-energy-density foods (Briefel & Johnson; Duffey & Popkin; Kant & Graubard; Nielsen & Popkin; Popkin et al.; Y. C. Wang, Bleich, & Gortmaker). Such dietary habits negatively affect a broad range of cardiovascular functions and promote the onset of T2D (Roger et al.).
Insulin resistance is a cardinal feature of T2D and has been identified in multiple prospective studies as the initial defect promoting development of disease (Reaven & Chen). It is typically defined as a decrease in sensitivity to the metabolic actions of insulin. Insulin maintains glucose homeostasis by promoting glucose uptake in skeletal muscle and by suppressing glucose production from the liver (Muniyappa, Montagnani, Koh, & Quon). Loss of insulin signaling therefore leads to hyperinsulinemia, hyperglycemia, and T2D. Hence, any treatment strategy to prevent diabetes must necessarily target insulin resistance.
Nitric oxide (NO) has emerged as a critical regulator of both adiposity and insulin sensitivity. In obese and diabetic states, the bioavailability of NO is decreased in both animal models (Bender, Herrick, Lott, & Klabunde, 2007; Kim et al., 2008) and adult and adolescent humans (Gruber et al., 2008; Higashi et al., 2001). Because the availability of NO is dependent upon its generation and degradation, lower levels observed in obese states may be due to downregulation of NOS, diminished NOS activity, or by reaction of NO with reactive oxygen species such as superoxide. In particular, eNOS abundance and activity is reported to decrease remarkably in obese and diabetic states and, as discussed below, is likely a central feature regulating body composition.
eNOS is important for regulating vascular and metabolic function
The nitric oxide synthase (NOS) family of enzymes catalyze NADPH- and O2-dependent oxidation of L-arginine to L-citrulline, producing NO in the process (Alderton, Cooper, & Knowles, 2001; Hill, Dranka, Bailey, Lancaster, & Darley-Usmar). NO synthesis depends also on the availability of several cofactors, including flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), tetrahydrobiopterin (BH4), as well as the prosthetic group, heme (H. Li & Poulos, 2005). Endothelial NOS (eNOS), the primary subject of this review, is expressed in the vascular endothelium, but has also been identified in neurons, epithelial cells, and cardiomyocytes (Dudzinski & Michel, 2007). Its activity is controlled by Ca2+ and calmodulin, post-translational modifications (Oess, Icking, Fulton, Govers, & Muller-Esterl, 2006; Sessa, 2004), and shear stress (Balligand, Feron, & Dessy, 2009; Kone, Kuncewicz, Zhang, & Yu, 2003). Other isoforms of NOS, i.e., neuronal NOS (nNOS) and inducible NOS (iNOS), are commonly expressed in different tissues and cells, and, in general produce higher quantities of NO (Hill et al.).
While NO is well known to have diverse biological actions (including regulation of learning and memory, platelet aggregation, leukocyte-endothelial interactions, immune function, and angiogenesis/arteriogenesis (Forstermann & Sessa)), it is most renowned for its vascular actions. The discovery that endothelial cells control acetylcholine-induced relaxation of smooth muscle (Furchgott & Zawadzki) was one of several impetuses responsible for the designation of NO as endothelial-derived relaxing factor (EDRF). Following Furchgott's findings, a series of studies showed that NO synthesized by eNOS in endothelial cells diffuses into the tunica media where it activates soluble guanylate cyclase (sGC), generating cyclic GMP (cGMP) and eliciting vessel relaxation (Forstermann, Mulsch, Bohme, & Busse; Gryglewski, Moncada, & Palmer; Moncada, Palmer, & Gryglewski; Palmer, Ferrige, & Moncada; Rajfer, Aronson, Bush, Dorey, & Ignarro; Rapoport, Draznin, & Murad; Rapoport & Murad).
Although NO's most-celebrated role is a result of its reaction with the metalloprotein sGC, NO and its oxidation species have the ability to react with other biomolecules as well. NO primarily reacts with ferrous iron and other radical species, with the highest affinity interactions being with the iron-containing proteins sGC, cytochrome c oxidase, and hemoglobin. The presence of other radical species such as superoxide (O2−) (Beckman, 2009; Pacher, Beckman, & Liaudet, 2007; Szabo, Ischiropoulos, & Radi, 2007; Trujillo, Ferrer-Sueta, & Radi, 2008) can result in the formation of products such as peroxynitrite (Beckman, 2009; Pacher et al., 2007; Szabo et al., 2007), which has different biomolecular targets and causes nitration of tyrosine residues (Hill et al.). NO can react also with O2 to form oxidized species such as N2O3, which can S-nitrosate or promote the S-oxidization of protein side chains (Hill & Bhatnagar; West, Hill, Xuan, & Bhatnagar). Also, NO reacts with thiyl radicals to form S-nitrosated proteins. Cysteinyl thiols of glutathione and proteins are commonly targets of NO and its oxidized species and become not only S-nitrosated or S-oxidized (SO2/3), but S-glutathiolated as well (Hill & Bhatnagar; West et al.). These modifications frequently modulate enzyme activity (Hill & Bhatnagar).
In addition to its vasodilatory actions, NO modulates oxygen delivery to cells and tissues by regulating oxygen binding and release from hemoglobin. It regulates oxygen consumption as well by binding and inhibiting cytochrome c oxidase, with such binding dependent on both mitochondrial activity and the O2 level (Cooper & Giulivi, 2007; Shiva et al., 2005). Hence, it can extend O2 gradients in tissues by regulating hemoglobin action and by inhibiting O2 consumption in mitochondria (Thomas, Liu, Kantrow, & Lancaster, 2001). Exposure of cells to relatively high concentrations of NO promotes mitochondrial biogenesis (Kelly & Scarpulla, 2004; Nisoli et al., 2003; Nisoli et al., 2004), thereby increasing overall respiratory capacity.
NO bioavailability is decreased in obese and diabetic states
Several studies link a decrease in eNOS-derived NO to diabetes. A T(-786)C variant of the eNOS gene is associated with insulin resistance (Ohtoshi et al.; Vecoli et al.; Yoshimura et al.), along with several other genetic variants in the eNOS locus, which are associated with T2D (Monti et al.). eNOS variants also appear to increase susceptibility for insulin resistance, hypertriglyceridemia, and low HDL (Gonzalez-Sanchez et al.), and worsen endothelial function in individuals prone to T2D (Rittig et al.).
Beyond eNOS polymorphisms, a primary mechanism by which NO is decreased in obesity is through diminished expression of eNOS. A decrease in eNOS abundance occurs in both adipose tissue and skeletal muscle of obese humans and rodents (Georgescu et al.; Kraus et al.; Perez-Matute, Neville, Tan, Frayn, & Karpe; Brian E. Sansbury et al.; Valerio et al.). In particular, it appears that the cytokine tumor necrosis factor-α (TNFα), which is implicated in the initiation of insulin resistance (Hotamisligil, Shargill, & Spiegelman), downregulates eNOS abundance (Anderson, Rahmutula, & Gardner; Lai, Mohamed, Monge, & Stewart; T. Michel & Lamas; Neumann, Gertzberg, & Johnson; Valerio et al.) by decreasing the stability of eNOS mRNA (Alonso, Sanchez de Miguel, Monton, Casado, & Lopez-Farre; Sanchez de Miguel et al.), thereby shortening its half-life (Yoshizumi, Perrella, Burnett, & Lee, 1993). Destabilization of Enos may be due, at least in part, to upregulation of elongation factor 1-α1 (Yan, You, Chen, Liao, & Sun).
The NO-producing activity of eNOS is also diminished in metabolic disease. Conditions related with nutrient excess were shown to upregulate caveolin-1, a negative regulator of eNOS (Ju, Zou, Venema, & Venema; J. B. Michel, Feron, Sacks, & Michel), in the aorta of obese rats (Yang et al.). Furthermore, ceramide,(which increases in abundance in obese states (Bikman & Summers)) decreases eNOS activity by disrupting the eNOS-Akt complex from HSP90 (Q. J. Zhang et al.).
Critical changes in eNOS phosphorylation occur in obesity as well. The eNOS enzyme can be phosphorylated at several sites, including: tyrosine (Y) resides—Y81 and Y567; serine (S) residues—S114, S615, S633, and S1177; and threonine (T) residues—T495 [for review, see (Kolluru, Siamwala, & Chatterjee; Rafikov et al.)]. In particular, the eNOS phosphorylation site—serine 1177 (Ser1176 in mice), the phosphorylation of which increases NO output from the enzyme (McCabe, Fulton, Roman, & Sessa)—is diminished by nutrient excess (Elrod et al.; Q. Li et al.; Taguchi, Kobayashi, Matsumoto, & Kamata; Zhong et al.) or high fat feeding (Kim et al.; Kim et al.; Brian E. Sansbury et al.; Symons et al.) in mice, and is similarly decreased in obese rats (Naruse et al.; Park et al.; Zecchin et al.) and pigs (Low Wang et al.). This eNOS phosphorylation site is regulated by Akt (Dimmeler et al.), which is stimulated by insulin (Hermann, Assmus, Urbich, Zeiher, & Dimmeler). Insulin receptor signaling stimulates the Akt-eNOS pathway, which is known to regulate post-prandial blood flow and nutrient disposition to peripheral tissues. Consequently, endothelial insulin resistance is sufficient to decrease NO bioavailability and promote endothelial dysfunction (Duncan et al.), and diminished eNOS phosphorylation due to insulin resistance appears to be responsible for diminished glucose uptake in skeletal muscle of high fat-fed mice (Kubota et al.).
Loss of eNOS phosphorylation under conditions of nutrient excess may be due to several factors, one of which is fatty acids, which can promote insulin resistance (Kim et al., 2008). Elevated free fatty acids (FFAs; e.g., palmitic acid) decrease NO production or availability in humans (Steinberg et al., 2000; Steinberg et al., 1997), animal models (X. Du et al., 2006), isolated arteries, and cultured cells (Kim et al., 2005) (Edirisinghe, McCormick Hallam, & Kappagoda, 2006). Insulin resistance due to FFAs is likely caused, at least in part, by activation of Toll-like receptor 2 (TLR2) (Jang, Kim, Hwang, Quon, & Kim) or Toll-like receptor 4 (TLR4) and NF-κB (Kim et al.; Kim et al.). In addition, hyperglycemia was suggested increase O-linked N-acetylglucosamine (OGlcNAc) modification of eNOS, which diminishes its activity (X. L. Du et al.). A PKCβII-mediated diminishment in Akt and eNOS responsiveness to insulin has also been reported (Naruse et al.; Park et al.), and an apparently Akt-independent impairment of eNOS phosphorylation may occur (Symons et al.). It is unclear whether or how each of these signaling pathways integrates to modulate NO production in obese and diabetic states.
eNOS activity and NO generation are dependent on proper enzyme coupling, which is regulated by cofactors, dimerization (Rodriguez-Crespo, Gerber, & Ortiz de Montellano; Rodriguez-Crespo & Ortiz de Montellano), and post-translational modifications (Alp & Channon; Forstermann & Sessa; H. Li & Forstermann; Zweier, Chen, & Druhan). The cofactor BH4 is critical for optimal eNOS activity, and it is depleted by excessive levels of reactive oxygen or nitrogen species (Channon). Obese and diabetic states are associated with decreased BH4 and increased levels of its oxidized form, BH2 (Cai, Khoo, & Channon; Chander et al.; Ding & Triggle; Pannirselvam, Verma, Anderson, & Triggle; Shinozaki et al.). This is important because deficiency in BH4 or elevations in BH2 uncouple NOS, resulting in superoxide production and peroxynitrite generation (Alp & Channon). Indeed, decreases in the BH4 to BH2 ratio are responsible for glucose-induced eNOS uncoupling (Crabtree, Smith, Lam, Goligorsky, & Gross) and replenishment of BH4 pools is an effective treatment in multiple pathologies (e.g., (Alp & Channon; Crabtree & Channon; Forstermann & Li; Kietadisorn, Juni, & Moens; H. Li & Forstermann)).
Commonly, 3-nitrotyrosine (3-NT) modifications are found at sites of eNOS uncoupling, and 3-NT-modified proteins are observed in abundance in tissues from obese and diabetic animals (Brodsky et al.; Chander et al.; Molnar et al.; Brian E. Sansbury et al.). In line with a potential role of peroxynitrite in regulating eNOS function, diabetic patients showed elevated levels 3-NT protein adducts, which localized with caveolae; these patients demonstrated diminished flow-mediated dilation of coronary arterioles (Cassuto et al.), which was rescued by the BH4 supplement, sepiapterin (Cassuto et al.). Numerous additional studies also demonstrate a role for reactive species such as peroxynitrite and superoxide to promote eNOS uncoupling (Bitar et al.; Dikalova et al.; Landmesser et al.; Satoh et al.; Xu, Xie, Reece, Pimental, & Zou). Nonetheless, the contribution of damaging reactive species to endothelial function remains unclear, as other studies suggest that, rather than uncoupling eNOS, superoxide activates the enzyme (Q. Zhang et al.), leaving open the possibility that loss of NO bioavailability could be due to quenching of NO and not to uncoupling of the enzyme. However, multiple other factors, such as asymmetric dimethyl arginine (ADMA), insufficient L-arginine levels or glutathio(ny)lation of the eNOS enzyme, can promote eNOS uncoupling and endothelial dysfunction also (Chen et al.; Forstermann & Sessa; Lei, Luo, Qin, & Xia; Risbano & Gladwin; Toutouzas, Riga, Stefanadi, & Stefanadis), suggesting the uncoupling of the enzyme is a contributor to decreases in NO production.
So, does obesity itself decrease NO availability? Obesity in humans is associated with decreased blood flow in response to shear stress (Arcaro et al.), bradykinin (Laine et al.; Van Guilder, Stauffer, Greiner, & Desouza), methacholine (Steinberg et al.), substance P and acetylcholine (Van Guilder et al.), and insulin (Tack, Ong, Lutterman, & Smits; Westerbacka et al.), which would appear to suggest that obesity is causally linked with decreased vascular NO bioavailability. Other studies support this hypothesis as well (Andersson et al.; Bhattacharjee, Alotaibi, Kheirandish-Gozal, Capdevila, & Gozal; Bhattacharjee et al.; Georgescu et al.; Grassi et al.; Gupta et al.; Han, Patel, Lteif, Chisholm, & Mather; Lambert et al.; Mahmud, Hill, Cuerden, & Clarson; Miadi-Messaoud et al.; Parikh et al.; Sturm et al.; Weil et al.). Nevertheless, the question remains: Does increased adiposity somehow decrease eNOS-derived NO and availability, or are losses in vascular NO due only to conditions associated with obesity? Several studies suggest that the state of being corpulent is not causative in decreasing vascular NO bioavailability. For example, morbidly obese humans appear to have endothelial dysfunction only when insulin resistance is present (El Assar et al.), and, severely obese humans, in the absence of insulin resistance, have better flow-mediated dilation compared with both normal and obese insulin-sensitive subjects (Biasucci et al.). Moreover, in overweight (insulin-sensitive) individuals, capillary recruitment may actually be higher compared with lean controls (Czernichow et al.). Hence, it appears that either insulin resistance or conditions directly linked with the insulin resistant phenotype are to blame for loss of NO bioavailability in obesity.
Regulation of obesity and insulin resistance by eNOS
Does eNOS-derived NO affect insulin resistance and obesity? This question has been addressed by multiple pharmacological studies and genetic studies, which, collectively, have helped clarify critical roles for eNOS-derived NO in regulating obesity and insulin resistance. Human studies show that L-arginine supplementation has favorable effects on adiposity and insulin sensitivity (Alizadeh et al.; Bogdanski et al.; Bogdanski et al.; Lucotti et al.; Monti et al.; Suliburska, Bogdanski, Szulinska, Pupek-Musialik, & Jablecka; Wascher et al.). Results from animal studies also show that L-arginine decreases fat mass, increases muscle mass, and improves insulin sensitivity (Clemmensen, Madsen, Smajilovic, Holst, & Brauner-Osborne). Dietary L-arginine supplementation in rats increases brown fat and skeletal muscle mass and reduces serum concentrations of triglycerides, glucose, homocysteine, free fatty acids, dimethylarginines, and leptin (Fu et al.; Jobgen et al.). L-arginine has a similar effect on pigs (Tan et al.).
Interestingly, sildenafil—which prevents the degradation of cGMP and is used to treat erectile dysfunction in humans—increases insulin sensitivity and prevents obesity in high fat-fed mice (Ayala et al.), potentially by promoting “browning” of white adipose tissue (Mitschke et al.). Sildenafil increases mitochondrial biogenesis in human adipose tissue ex vivo as well (De Toni et al.). Other compounds that activate the NO pathway also support a role for NO in improving insulin sensitivity. Beraprost (a stable prostaglandin analog), when given to endothelial-specific insulin receptor substrate 2 (Irs2) knockout mice, restored eNOS phosphorylation, capillary recruitment, and insulin and glucose delivery to skeletal muscle (Kubota et al.). Additionally, S-nitrosation in response to L-arginine, insulin, or sodium nitroprusside was shown to be important for regulating vascular endothelial insulin uptake and transendothelial transport (H. Wang, Wang, Aylor, & Barrett). Thus, it appears that NO may regulate obesity and insulin resistance by both cGMP-dependent and –independent pathways.
Although chronic treatment with NOS inhibitors causes weight loss and promotes insulin sensitivity in animals (Morley & Flood; Stricker-Krongrad, Beck, & Burlet; Tsuchiya et al.), their acute application causes systemic insulin resistance (Baron et al.), in part by promoting metabolic changes in the liver (Meshkani & Adeli). Furthermore, BH4, which is oxidized to BH2 in the diabetic state (Meininger et al.; Meininger et al.; Xu et al.), administered to STZ-treated mice lowered blood glucose levels in an eNOS-dependent manner. Increasing BH4 was shown to also improve glucose tolerance and insulin sensitivity in ob/ob mice (Abudukadier et al.). This was suggested to be due to eNOS-mediated activation of AMPK in the liver (Abudukadier et al.), which suppresses hepatic glucose production (Viollet et al.). Therefore, eNOS uncoupling in liver appears to negatively regulate systemic glucose metabolism in obese, diabetic states.
Genetic models in which eNOS has been deleted or overexpressed have helped to further elucidate the mechanisms by which NO regulates obesity and insulin resistance. Deletion of eNOS causes insulin resistance, hyperlipidemia, and hypertension (Duplain et al., 2001), and partial deletion of the gene can exaggerate insulin resistance, glucose intolerance, and hypertension under conditions of nutrient excess (Cook et al.; Cook et al.). Mice in which both eNOS and nNOS are absent show similar results, with deletion of eNOS appearing responsible for insulin resistance in both skeletal muscle and liver (Shankar, Wu, Shen, Zhu, & Baron). Similarly, mice lacking eNOS, nNOS, and iNOS (i.e., triple knockout mice), show increased visceral obesity, hypertension, hypertriglyceridemia, and impaired glucose tolerance (Nakata et al.).
The metabolic phenotype caused by eNOS deletion or otherwise low endothelial derived NO appears to relate directly to changes in substrate metabolism in liver, skeletal muscle and adipose tissue. In skeletal muscle, eNOS KO mice have lower mitochondrial content and fatty acid oxidation than WT mice, and they demonstrate markedly lower energy expenditure (Le Gouill et al., 2007). Supplementation of eNOS KO mice with nitrate, which can be reduced to nitrite and NO in the body, decreased not only blood pressure, but visceral adipose tissue and triglycerides as well (Carlstrom et al.).
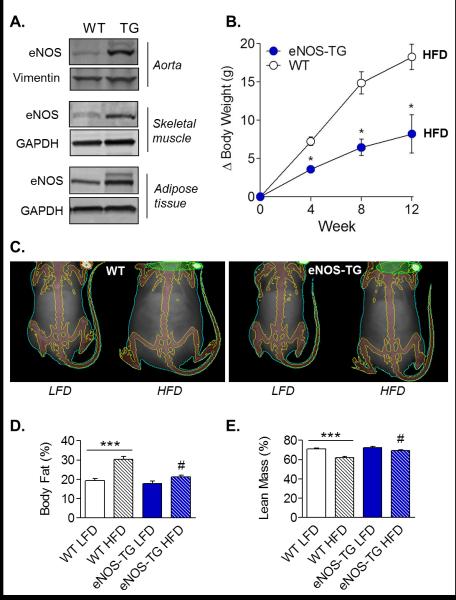
Our studies in mice overexpressing eNOS suggest a remarkable ability of eNOS to regulate metabolism and body composition. Mice overexpressing eNOS in the vasculature show an anti-obesogenic phenotype characterized by resistance to accumulation of white adipose tissue in response to a high fat diet, a higher metabolic rate, resistance to diet-induced hyperinsulinemia, and remarkably lower plasma levels of free fatty acids and triglycerides (B. E. Sansbury et al., 2012). As shown in Figure 1, overexpression of eNOS resulted in decreased weight gain on a high fat diet, which was due to diminished expansion of the adipose tissue.
Figure 1. Overexpression of eNOS prevents obesity.
(A) Immunoblot analysis: eNOS expression in aorta, skeletal muscle, and adipose tissue of wild-type (WT) and eNOS transgenic (eNOS-TG) mice. (B) Change in body weight during high fat feeding: mice were fed a high fat diet (HFD) for up to 12 weeks, and the change in body weight was measured. (C) Representative Dexascan images of WT and eNOS-TG mice fed a low fat diet (LFD) or HFD for 6 weeks. (D and E) Dexascan analysis of body fat and lean mass percentage. Figure adapted with permission from (Brian E. Sansbury et al.).
An eNOS phosphomimetic point mutant mouse model (Atochin & Huang; Kashiwagi et al.) showed a very similar phenotype: mutation of eNOS ser1176 to an aspartic acid increased endothelial NO production as well as promoted resistance to diet-induced weight gain and hyperinsulinemia, whereas mutation of this residue to an alanine promoted insulin resistance and permitted the development of an obese state (Huang; Kashiwagi et al.).
That eNOS KO mice have elevated plasma levels of triglycerides and free fatty acids compared with WT mice (Cook et al.; Duplain et al.), while eNOS transgenic mice show diminished abundance of the lipids (Brian E. Sansbury et al.) suggests that eNOS regulates lipid oxidation or synthesis. Indeed, eNOS KO mice show diminished fat oxidation capacity in skeletal muscle (Le Gouill et al.). Furthermore, administration of a NOS inhibitor to rats increases serum triglycerides and diminishes fatty acid oxidation in the liver (Khedara, Kawai, Kayashita, & Kato), potentially by decreasing carnitine palmitoyl transferase activity (Khedara, Goto, Morishima, Kayashita, & Kato). NOS inhibitor-dependent decreases in fatty acid oxidation occur in heart as well (Recchia et al.). In hepatocytes, NO donors increase β-oxidation in a cGMP-dependent manner by inhibiting acetyl CoA carboxylase, thereby stimulating the activity of carnitine palmitoyl transferase (Garcia-Villafranca, Guillen, & Castro). NO also diminishes fatty acid synthesis in hepatocytes (Garcia-Villafranca et al.), which is consistent with studies showing that inhibitors of NOS (Goto et al.) or deletion of eNOS increases lipid synthesis in liver (Schild et al.). Similarly, in skeletal muscle, loss of eNOS increases neolipogenic gene expression while decreasing those genes that promote fatty acid oxidation (Le Gouill et al.). These data suggest that eNOS may regulate peroxisome proliferator activated receptor (PPAR)-α, which is well known to regulate lipid metabolism (Lefebvre, Chinetti, Fruchart, & Staels). Indeed, our studies show that overexpression of eNOS increases PPARα expression in adipose tissue (Brian E. Sansbury et al.), suggesting that endothelial-derived NO increases the molecular machinery required to program cells to burn fat. However, it is possible that NO primes fat oxidation in other ways as well. For example, recent studies demonstrate S-nitrosation of multiple enzymes involved in metabolism. In particular, very long chain acyl-coA dehydrogenase (VLCAD), a liver enzyme important in β-oxidation, was shown to be nitrosated at Cys238, which increases the catalytic efficiency of the enzyme. This modification was dependent on eNOS activity, as nitrosation of the enzyme was absent in eNOS KO mice (Gould, Doulias, Tenopoulou, Raju, & Ischiropoulos). Lastly, it is possible that NO-induced increases in mitochondrial mass (Nisoli et al.; Nisoli et al.; Piantadosi & Suliman) could be sufficient to increase metabolic rate and prevent obesity. This would be consistent with studies showing that cGMP-dependent increases in mitochondrial biogenesis prevent obesity (Miyashita et al.) as well as several other studies demonstrating a link between augmented mitochondrial mass and resistance to diet-induced weight gain [e.g., (Fang et al.; Hwang et al.; Yadav et al.; Yamamoto et al.)].
Synopsis
Collectively, these studies suggest that eNOS-derived NO has powerful anti-obesity and insulin-sensitizing effects. It is likely that the enzyme increases fat oxidation and lipid synthesis in tissues such as liver, skeletal muscle, and fat. The relatively low levels of adiposity and plasma free fatty acids and triglycerides in models in which eNOS is overexpressed or permanently activated is consistent with this mechanism (Kashiwagi et al.; Brian E. Sansbury et al.). The favorable effects of eNOS on glucose metabolism and insulin sensitivity appear to be due to its ability to stimulate the transport of insulin and glucose to key peripheral tissues such as skeletal muscle and to regulate gluconeogenesis. In addition, eNOS overexpression or activation prevents diet-induced hyperinsulinemia (Kashiwagi et al.; Brian E. Sansbury et al.) suggesting that it could impact glucose metabolism by regulating insulin secretion. Exploiting the beneficial metabolic actions of eNOS is a promising prospect for anti-obesity therapies.
Acknowledgements
The authors acknowledge support from NIH grants GM103492 and HL078825.
References
- Abudukadier A, Fujita Y, Obara A, Ohashi A, Fukushima T, Sato Y, et al. Tetrahydrobiopterin has a glucose-lowering effect by suppressing hepatic gluconeogenesis in an endothelial nitric oxide synthase-dependent manner in diabetic mice. Diabetes. 2013;62(9):3033–3043. doi: 10.2337/db12-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS. Digging deeper into obesity. J Clin Invest. 2011;121(6):2076–2079. doi: 10.1172/JCI58719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357(Pt 3):593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh M, Safaeiyan A, Ostadrahimi A, Estakhri R, Daneghian S, Ghaffari A, et al. Effect of L-arginine and selenium added to a hypocaloric diet enriched with legumes on cardiovascular disease risk factors in women with central obesity: a randomized, double-blind, placebo-controlled trial. Ann Nutr Metab. 2012;60(2):157–168. doi: 10.1159/000335470. [DOI] [PubMed] [Google Scholar]
- Alonso J, Sanchez de Miguel L, Monton M, Casado S, Lopez-Farre A. Endothelial cytosolic proteins bind to the 3' untranslated region of endothelial nitric oxide synthase mRNA: regulation by tumor necrosis factor alpha. Mol Cell Biol. 1997;17(10):5719–5726. doi: 10.1128/mcb.17.10.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24(3):413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- Anderson HD, Rahmutula D, Gardner DG. Tumor necrosis factor-alpha inhibits endothelial nitric-oxide synthase gene promoter activity in bovine aortic endothelial cells. J Biol Chem. 2004;279(2):963–969. doi: 10.1074/jbc.M309552200. [DOI] [PubMed] [Google Scholar]
- Andersson J, Sjostrom LG, Karlsson M, Wiklund U, Hultin M, Karpe F, et al. Dysregulation of subcutaneous adipose tissue blood flow in overweight postmenopausal women. Menopause. 2010;17(2):365–371. doi: 10.1097/gme.0b013e3181c12b26. [DOI] [PubMed] [Google Scholar]
- Arcaro G, Zamboni M, Rossi L, Turcato E, Covi G, Armellini F, et al. Body fat distribution predicts the degree of endothelial dysfunction in uncomplicated obesity. Int J Obes Relat Metab Disord. 1999;23(9):936–942. doi: 10.1038/sj.ijo.0801022. [DOI] [PubMed] [Google Scholar]
- Atochin DN, Huang PL. Endothelial nitric oxide synthase transgenic models of endothelial dysfunction. Pflugers Arch. 2010;460(6):965–974. doi: 10.1007/s00424-010-0867-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, Julien BM, Rottman JN, Fueger PT, Wasserman DH. Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes. 2007;56(4):1025–1033. doi: 10.2337/db06-0883. [DOI] [PubMed] [Google Scholar]
- Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev. 2009;89(2):481–534. doi: 10.1152/physrev.00042.2007. [DOI] [PubMed] [Google Scholar]
- Baron AD, Zhu JS, Marshall S, Irsula O, Brechtel G, Keech C. Insulin resistance after hypertension induced by the nitric oxide synthesis inhibitor L-NMMA in rats. Am J Physiol. 1995;269(4 Pt 1):E709–715. doi: 10.1152/ajpendo.1995.269.4.E709. [DOI] [PubMed] [Google Scholar]
- Beckman JS. Understanding peroxynitrite biochemistry and its potential for treating human diseases. Arch Biochem Biophys. 2009;484(2):114–116. doi: 10.1016/j.abb.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender SB, Herrick EK, Lott ND, Klabunde RE. Diet-induced obesity and diabetes reduce coronary responses to nitric oxide due to reduced bioavailability in isolated mouse hearts. Diabetes Obes Metab. 2007;9(5):688–696. doi: 10.1111/j.1463-1326.2006.00650.x. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee R, Alotaibi WH, Kheirandish-Gozal L, Capdevila OS, Gozal D. Endothelial dysfunction in obese non-hypertensive children without evidence of sleep disordered breathing. BMC Pediatr. 2010;10:8. doi: 10.1186/1471-2431-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee R, Kim J, Alotaibi WH, Kheirandish-Gozal L, Capdevila OS, Gozal D. Endothelial dysfunction in children without hypertension: potential contributions of obesity and obstructive sleep apnea. Chest. 2012;141(3):682–691. doi: 10.1378/chest.11-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasucci LM, Graziani F, Rizzello V, Liuzzo G, Guidone C, De Caterina AR, et al. Paradoxical preservation of vascular function in severe obesity. Am J Med. 2010;123(8):727–734. doi: 10.1016/j.amjmed.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Bikman BT, Summers SA. Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest. 2011;121(11):4222–4230. doi: 10.1172/JCI57144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitar MS, Wahid S, Mustafa S, Al-Saleh E, Dhaunsi GS, Al-Mulla F. Nitric oxide dynamics and endothelial dysfunction in type II model of genetic diabetes. Eur J Pharmacol. 2005;511(1):53–64. doi: 10.1016/j.ejphar.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Bogdanski P, Suliburska J, Grabanska K, Musialik K, Cieslewicz A, Skoluda A, et al. Effect of 3-month L-arginine supplementation on insulin resistance and tumor necrosis factor activity in patients with visceral obesity. Eur Rev Med Pharmacol Sci. 2012;16(6):816–823. [PubMed] [Google Scholar]
- Bogdanski P, Szulinska M, Suliburska J, Pupek-Musialik D, Jablecka A, Witmanowski H. Supplementation with L-arginine favorably influences plasminogen activator inhibitor type 1 concentration in obese patients. A randomized, double blind trial. J Endocrinol Invest. 2013;36(4):221–226. doi: 10.3275/8467. [DOI] [PubMed] [Google Scholar]
- Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr. 2004;24:401–431. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
- Brodsky SV, Gealekman O, Chen J, Zhang F, Togashi N, Crabtree M, et al. Prevention and reversal of premature endothelial cell senescence and vasculopathy in obesity-induced diabetes by ebselen. Circ Res. 2004;94(3):377–384. doi: 10.1161/01.RES.0000111802.09964.EF. [DOI] [PubMed] [Google Scholar]
- Cai S, Khoo J, Channon KM. Augmented BH4 by gene transfer restores nitric oxide synthase function in hyperglycemic human endothelial cells. Cardiovasc Res. 2005;65(4):823–831. doi: 10.1016/j.cardiores.2004.10.040. [DOI] [PubMed] [Google Scholar]
- Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341(15):1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- Carlstrom M, Larsen FJ, Nystrom T, Hezel M, Borniquel S, Weitzberg E, et al. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc Natl Acad Sci U S A. 2010;107(41):17716–17720. doi: 10.1073/pnas.1008872107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassuto J, Dou H, Czikora I, Szabo A, Patel VS, Kamath V, et al. Peroxynitrite Disrupts Endothelial Caveolae Leading to eNOS Uncoupling and Diminished Flow-Mediated Dilation in Coronary Arterioles of Diabetic Patients. Diabetes. 2013 doi: 10.2337/db13-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander PN, Gealekman O, Brodsky SV, Elitok S, Tojo A, Crabtree M, et al. Nephropathy in Zucker diabetic fat rat is associated with oxidative and nitrosative stress: prevention by chronic therapy with a peroxynitrite scavenger ebselen. J Am Soc Nephrol. 2004;15(9):2391–2403. doi: 10.1097/01.ASN.0000135971.88164.2C. [DOI] [PubMed] [Google Scholar]
- Channon KM. Tetrahydrobiopterin: a vascular redox target to improve endothelial function. Curr Vasc Pharmacol. 2012;10(6):705–708. doi: 10.2174/157016112803520819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, et al. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468(7327):1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmensen C, Madsen AN, Smajilovic S, Holst B, Brauner-Osborne H. L-Arginine improves multiple physiological parameters in mice exposed to diet-induced metabolic disturbances. Amino Acids. 2012;43(3):1265–1275. doi: 10.1007/s00726-011-1199-1. [DOI] [PubMed] [Google Scholar]
- Cook S, Hugli O, Egli M, Menard B, Thalmann S, Sartori C, et al. Partial gene deletion of endothelial nitric oxide synthase predisposes to exaggerated high-fat diet-induced insulin resistance and arterial hypertension. [Research Support, Non-U.S. Gov't]. Diabetes. 2004;53(8):2067–2072. doi: 10.2337/diabetes.53.8.2067. [DOI] [PubMed] [Google Scholar]
- Cook S, Hugli O, Egli M, Vollenweider P, Burcelin R, Nicod P, et al. Clustering of cardiovascular risk factors mimicking the human metabolic syndrome X in eNOS null mice. Swiss Med Wkly. 2003;133(25-26):360–363. doi: 10.4414/smw.2003.10239. [DOI] [PubMed] [Google Scholar]
- Cooper CE, Giulivi C. Nitric oxide regulation of mitochondrial oxygen consumption II: Molecular mechanism and tissue physiology. Am J Physiol Cell Physiol. 2007;292(6):C1993–2003. doi: 10.1152/ajpcell.00310.2006. [DOI] [PubMed] [Google Scholar]
- Crabtree MJ, Channon KM. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide. 2011;25(2):81–88. doi: 10.1016/j.niox.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am J Physiol Heart Circ Physiol. 2008;294(4):H1530–1540. doi: 10.1152/ajpheart.00823.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernichow S, Greenfield JR, Galan P, Bastard JP, Charnaux N, Samaras K, et al. Microvascular dysfunction in healthy insulin-sensitive overweight individuals. J Hypertens. 2010;28(2):325–332. doi: 10.1097/HJH.0b013e328333d1fc. [DOI] [PubMed] [Google Scholar]
- De Toni L, Strapazzon G, Gianesello L, Caretta N, Pilon C, Bruttocao A, et al. Effects of type 5-phosphodiesterase inhibition on energy metabolism and mitochondrial biogenesis in human adipose tissue ex vivo. J Endocrinol Invest. 2011;34(10):738–741. doi: 10.1007/BF03346724. [DOI] [PubMed] [Google Scholar]
- Dikalova AE, Gongora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2010;299(3):H673–679. doi: 10.1152/ajpheart.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399(6736):601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Ding H, Triggle CR. Endothelial dysfunction in diabetes: multiple targets for treatment. Pflugers Arch. 2010;459(6):977–994. doi: 10.1007/s00424-010-0807-3. [DOI] [PubMed] [Google Scholar]
- Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest. 2006;116(4):1071–1080. doi: 10.1172/JCI23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108(9):1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75(2):247–260. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffey KJ, Popkin BM. Shifts in patterns and consumption of beverages between 1965 and 2002. Obesity (Silver Spring) 2007;15(11):2739–2747. doi: 10.1038/oby.2007.326. [DOI] [PubMed] [Google Scholar]
- Duncan ER, Crossey PA, Walker S, Anilkumar N, Poston L, Douglas G, et al. Effect of endothelium-specific insulin resistance on endothelial function in vivo. Diabetes. 2008;57(12):3307–3314. doi: 10.2337/db07-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, et al. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation. 2001;104(3):342–345. doi: 10.1161/01.cir.104.3.342. [DOI] [PubMed] [Google Scholar]
- Edirisinghe I, McCormick Hallam K, Kappagoda CT. Effect of fatty acids on endothelium-dependent relaxation in the rabbit aorta. Clin Sci (Lond) 2006;111(2):145–151. doi: 10.1042/CS20060001. [DOI] [PubMed] [Google Scholar]
- El Assar M, Ruiz de Adana JC, Angulo J, Pindado Martinez ML, Hernandez Matias A, Rodriguez-Manas L. Preserved endothelial function in human obesity in the absence of insulin resistance. J Transl Med. 2013;11:263. doi: 10.1186/1479-5876-11-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod JW, Duranski MR, Langston W, Greer JJ, Tao L, Dugas TR, et al. eNOS gene therapy exacerbates hepatic ischemia-reperfusion injury in diabetes: a role for eNOS uncoupling. Circ Res. 2006;99(1):78–85. doi: 10.1161/01.RES.0000231306.03510.77. [DOI] [PubMed] [Google Scholar]
- Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006. Natl Health Stat Report. 2009;(13):1–7. [PubMed] [Google Scholar]
- Fang S, Suh JM, Atkins AR, Hong SH, Leblanc M, Nofsinger RR, et al. Corepressor SMRT promotes oxidative phosphorylation in adipose tissue and protects against diet-induced obesity and insulin resistance. Proc Natl Acad Sci U S A. 2011;108(8):3412–3417. doi: 10.1073/pnas.1017707108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstermann U, Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br J Pharmacol. 2011;164(2):213–223. doi: 10.1111/j.1476-5381.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstermann U, Mulsch A, Bohme E, Busse R. Stimulation of soluble guanylate cyclase by an acetylcholine-induced endothelium-derived factor from rabbit and canine arteries. Circ Res. 1986;58(4):531–538. doi: 10.1161/01.res.58.4.531. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–837. 837a–837d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, et al. Dietary L-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J Nutr. 2005;135(4):714–721. doi: 10.1093/jn/135.4.714. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Villafranca J, Guillen A, Castro J. Involvement of nitric oxide/cyclic GMP signaling pathway in the regulation of fatty acid metabolism in rat hepatocytes. Biochem Pharmacol. 2003;65(5):807–812. doi: 10.1016/s0006-2952(02)01623-4. [DOI] [PubMed] [Google Scholar]
- Georgescu A, Popov D, Constantin A, Nemecz M, Alexandru N, Cochior D, et al. Dysfunction of human subcutaneous fat arterioles in obesity alone or obesity associated with Type 2 diabetes. Clin Sci (Lond) 2011;120(10):463–472. doi: 10.1042/CS20100355. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sanchez JL, Martinez-Larrad MT, Saez ME, Zabena C, Martinez-Calatrava MJ, Serrano-Rios M. Endothelial nitric oxide synthase haplotypes are associated with features of metabolic syndrome. Clin Chem. 2007;53(1):91–97. doi: 10.1373/clinchem.2006.075176. [DOI] [PubMed] [Google Scholar]
- Goto T, Ohnomi S, Khedara A, Kato N, Ogawa H, Yanagita T. Feeding the nitric oxide synthase inhibitor L-N(omega)nitroarginine elevates serum very low density lipoprotein and hepatic triglyceride synthesis in rats. J Nutr Biochem. 1999;10(5):274–278. doi: 10.1016/s0955-2863(99)00008-x. [DOI] [PubMed] [Google Scholar]
- Gould N, Doulias PT, Tenopoulou M, Raju K, Ischiropoulos H. Regulation of protein function and signaling by reversible cysteine S-nitrosylation. J Biol Chem. 2013;288(37):26473–26479. doi: 10.1074/jbc.R113.460261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Scopelliti F, Dell'Oro R, Fattori L, Quarti-Trevano F, et al. Structural and functional alterations of subcutaneous small resistance arteries in severe human obesity. Obesity (Silver Spring) 2010;18(1):92–98. doi: 10.1038/oby.2009.195. [DOI] [PubMed] [Google Scholar]
- Gruber HJ, Mayer C, Mangge H, Fauler G, Grandits N, Wilders-Truschnig M. Obesity reduces the bioavailability of nitric oxide in juveniles. [Research Support, Non-U.S. Gov't]. Int J Obes (Lond) 2008;32(5):826–831. doi: 10.1038/sj.ijo.0803795. [DOI] [PubMed] [Google Scholar]
- Gryglewski RJ, Moncada S, Palmer RM. Bioassay of prostacyclin and endothelium-derived relaxing factor (EDRF) from porcine aortic endothelial cells. Br J Pharmacol. 1986;87(4):685–694. doi: 10.1111/j.1476-5381.1986.tb14586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AK, Cornelissen G, Greenway FL, Dhoopati V, Halberg F, Johnson WD. Abnormalities in circadian blood pressure variability and endothelial function: pragmatic markers for adverse cardiometabolic profiles in asymptomatic obese adults. Cardiovasc Diabetol. 2010;9:58. doi: 10.1186/1475-2840-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KA, Patel Y, Lteif AA, Chisholm R, Mather KJ. Contributions of dysglycaemia, obesity, and insulin resistance to impaired endothelium-dependent vasodilation in humans. Diabetes Metab Res Rev. 2011;27(4):354–361. doi: 10.1002/dmrr.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann C, Assmus B, Urbich C, Zeiher AM, Dimmeler S. Insulin-mediated stimulation of protein kinase Akt: A potent survival signaling cascade for endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20(2):402–409. doi: 10.1161/01.atv.20.2.402. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Chayama K, Oshima T. Effect of obesity on endothelium-dependent, nitric oxide-mediated vasodilation in normotensive individuals and patients with essential hypertension. Am J Hypertens. 2001;14(10):1038–1045. doi: 10.1016/s0895-7061(01)02191-4. [DOI] [PubMed] [Google Scholar]
- Hill BG, Bhatnagar A. Role of glutathiolation in preservation, restoration and regulation of protein function. IUBMB Life. 2007;59(1):21–26. doi: 10.1080/15216540701196944. [DOI] [PubMed] [Google Scholar]
- Hill BG, Bhatnagar A. Protein S-glutathiolation: redox-sensitive regulation of protein function. J Mol Cell Cardiol. 2012;52(3):559–567. doi: 10.1016/j.yjmcc.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill BG, Dranka BP, Bailey SM, Lancaster JR, Jr., Darley-Usmar VM. What part of NO don't you understand? Some answers to the cardinal questions in nitric oxide biology. J Biol Chem. 2010;285(26):19699–19704. doi: 10.1074/jbc.R110.101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289(14):1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- Huang PL. eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol Metab. 2009;20(6):295–302. doi: 10.1016/j.tem.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JH, Kim DW, Jo EJ, Kim YK, Jo YS, Park JH, et al. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes. 2009;58(4):965–974. doi: 10.2337/db08-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HJ, Kim HS, Hwang DH, Quon MJ, Kim JA. Toll-like receptor 2 mediates high-fat diet-induced impairment of vasodilator actions of insulin. Am J Physiol Endocrinol Metab. 2013;304(10):E1077–1088. doi: 10.1152/ajpendo.00578.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobgen W, Meininger CJ, Jobgen SC, Li P, Lee MJ, Smith SB, et al. Dietary L-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J Nutr. 2009;139(2):230–237. doi: 10.3945/jn.108.096362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272(30):18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- Kant AK, Graubard BI. Eating out in America, 1987-2000: trends and nutritional correlates. Prev Med. 2004;38(2):243–249. doi: 10.1016/j.ypmed.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Kant AK, Graubard BI. Secular trends in patterns of self-reported food consumption of adult Americans: NHANES 1971-1975 to NHANES 1999-2002. Am J Clin Nutr. 2006;84(5):1215–1223. doi: 10.1093/ajcn/84.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi S, Atochin DN, Li Q, Schleicher M, Pong T, Sessa WC, et al. eNOS phosphorylation on serine 1176 affects insulin sensitivity and adiposity. Biochem Biophys Res Commun. 2013;431(2):284–290. doi: 10.1016/j.bbrc.2012.12.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18(4):357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- Khedara A, Goto T, Morishima M, Kayashita J, Kato N. Elevated body fat in rats by the dietary nitric oxide synthase inhibitor, L-N omega nitroarginine. Biosci Biotechnol Biochem. 1999;63(4):698–702. doi: 10.1271/bbb.63.698. [DOI] [PubMed] [Google Scholar]
- Khedara A, Kawai Y, Kayashita J, Kato N. Feeding rats the nitric oxide synthase inhibitor, L-N(omega)nitroarginine, elevates serum triglyceride and cholesterol and lowers hepatic fatty acid oxidation. J Nutr. 1996;126(10):2563–2567. doi: 10.1093/jn/126.10.2563. [DOI] [PubMed] [Google Scholar]
- Kietadisorn R, Juni RP, Moens AL. Tackling endothelial dysfunction by modulating NOS uncoupling: new insights into its pathogenesis and therapeutic possibilities. Am J Physiol Endocrinol Metab. 2012;302(5):E481–495. doi: 10.1152/ajpendo.00540.2011. [DOI] [PubMed] [Google Scholar]
- Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100(11):1589–1596. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, et al. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. [Research Support, N.I.H., Extramural]. Arterioscler Thromb Vasc Biol. 2008;28(11):1982–1988. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim F, Tysseling KA, Rice J, Pham M, Haji L, Gallis BM, et al. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol. 2005;25(5):989–994. doi: 10.1161/01.ATV.0000160549.60980.a8. [DOI] [PubMed] [Google Scholar]
- Kolluru GK, Siamwala JH, Chatterjee S. eNOS phosphorylation in health and disease. Biochimie. 2010;92(9):1186–1198. doi: 10.1016/j.biochi.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Kone BC, Kuncewicz T, Zhang W, Yu ZY. Protein interactions with nitric oxide synthases: controlling the right time, the right place, and the right amount of nitric oxide. Am J Physiol Renal Physiol. 2003;285(2):F178–190. doi: 10.1152/ajprenal.00048.2003. [DOI] [PubMed] [Google Scholar]
- Kraus RM, Houmard JA, Kraus WE, Tanner CJ, Pierce JR, Choi MD, et al. Obesity, insulin resistance, and skeletal muscle nitric oxide synthase. J Appl Physiol (1985) 2012;113(5):758–765. doi: 10.1152/japplphysiol.01018.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 2011;13(3):294–307. doi: 10.1016/j.cmet.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Lai PF, Mohamed F, Monge JC, Stewart DJ. Downregulation of eNOS mRNA expression by TNFalpha: identification and functional characterization of RNA-protein interactions in the 3'UTR. Cardiovasc Res. 2003;59(1):160–168. doi: 10.1016/s0008-6363(03)00296-7. [DOI] [PubMed] [Google Scholar]
- Laine H, Yki-Jarvinen H, Kirvela O, Tolvanen T, Raitakari M, Solin O, et al. Insulin resistance of glucose uptake in skeletal muscle cannot be ameliorated by enhancing endothelium-dependent blood flow in obesity. J Clin Invest. 1998;101(5):1156–1162. doi: 10.1172/JCI1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert E, Sari CI, Dawood T, Nguyen J, McGrane M, Eikelis N, et al. Sympathetic nervous system activity is associated with obesity-induced subclinical organ damage in young adults. Hypertension. 2010;56(3):351–358. doi: 10.1161/HYPERTENSIONAHA.110.155663. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111(8):1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gouill E, Jimenez M, Binnert C, Jayet PY, Thalmann S, Nicod P, et al. Endothelial nitric oxide synthase (eNOS) knockout mice have defective mitochondrial beta-oxidation. Diabetes. 2007;56(11):2690–2696. doi: 10.2337/db06-1228. [DOI] [PubMed] [Google Scholar]
- Lefebvre P, Chinetti G, Fruchart JC, Staels B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116(3):571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Luo S, Qin H, Xia Y. Molecular Mechanisms of Endothelial NO Synthase Uncoupling. Curr Pharm Des. 2013 doi: 10.2174/13816128113196660746. [DOI] [PubMed] [Google Scholar]
- Li H, Forstermann U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr Opin Pharmacol. 2013;13(2):161–167. doi: 10.1016/j.coph.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Li H, Poulos TL. Structure-function studies on nitric oxide synthases. J Inorg Biochem. 2005;99(1):293–305. doi: 10.1016/j.jinorgbio.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Li Q, Atochin D, Kashiwagi S, Earle J, Wang A, Mandeville E, et al. Deficient eNOS phosphorylation is a mechanism for diabetic vascular dysfunction contributing to increased stroke size. Stroke. 2013;44(11):3183–3188. doi: 10.1161/STROKEAHA.113.002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low Wang CC, Lu L, Leitner JW, Sarraf M, Gianani R, Draznin B, et al. Arterial insulin resistance in Yucatan micropigs with diet-induced obesity and metabolic syndrome. J Diabetes Complications. 2013;27(4):307–315. doi: 10.1016/j.jdiacomp.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucotti P, Setola E, Monti LD, Galluccio E, Costa S, Sandoli EP, et al. Beneficial effects of a long-term oral L-arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2006;291(5):E906–912. doi: 10.1152/ajpendo.00002.2006. [DOI] [PubMed] [Google Scholar]
- Mahmud FH, Hill DJ, Cuerden MS, Clarson CL. Impaired vascular function in obese adolescents with insulin resistance. J Pediatr. 2009;155(5):678–682. doi: 10.1016/j.jpeds.2009.04.060. [DOI] [PubMed] [Google Scholar]
- McCabe TJ, Fulton D, Roman LJ, Sessa WC. Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. J Biol Chem. 2000;275(9):6123–6128. doi: 10.1074/jbc.275.9.6123. [DOI] [PubMed] [Google Scholar]
- Meininger CJ, Cai S, Parker JL, Channon KM, Kelly KA, Becker EJ, et al. GTP cyclohydrolase I gene transfer reverses tetrahydrobiopterin deficiency and increases nitric oxide synthesis in endothelial cells and isolated vessels from diabetic rats. FASEB J. 2004;18(15):1900–1902. doi: 10.1096/fj.04-1702fje. [DOI] [PubMed] [Google Scholar]
- Meininger CJ, Marinos RS, Hatakeyama K, Martinez-Zaguilan R, Rojas JD, Kelly KA, et al. Impaired nitric oxide production in coronary endothelial cells of the spontaneously diabetic BB rat is due to tetrahydrobiopterin deficiency. Biochem J. 2000;349(Pt 1):353–356. doi: 10.1042/0264-6021:3490353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem. 2009;42(13-14):1331–1346. doi: 10.1016/j.clinbiochem.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Miadi-Messaoud H, Chouchane A, Abderrazek E, Debbabi H, Zaouali-Ajina M, Tabka Z, et al. Obesity-induced impairment of endothelium-dependent vasodilation in Tunisian women. Int J Obes (Lond) 2010;34(2):273–279. doi: 10.1038/ijo.2009.231. [DOI] [PubMed] [Google Scholar]
- Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem. 1997;272(25):15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- Michel T, Lamas S. Molecular cloning of constitutive endothelial nitric oxide synthase: evidence for a family of related genes. J Cardiovasc Pharmacol. 1992;20(Suppl 12):S45–49. doi: 10.1097/00005344-199204002-00014. [DOI] [PubMed] [Google Scholar]
- Mitschke MM, Hoffmann LS, Gnad T, Scholz D, Kruithoff K, Mayer P, et al. Increased cGMP promotes healthy expansion and browning of white adipose tissue. FASEB J. 2013;27(4):1621–1630. doi: 10.1096/fj.12-221580. [DOI] [PubMed] [Google Scholar]
- Miyashita K, Itoh H, Tsujimoto H, Tamura N, Fukunaga Y, Sone M, et al. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58(12):2880–2892. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar J, Yu S, Mzhavia N, Pau C, Chereshnev I, Dansky HM. Diabetes induces endothelial dysfunction but does not increase neointimal formation in high-fat diet fed C57BL/6J mice. Circ Res. 2005;96(11):1178–1184. doi: 10.1161/01.RES.0000168634.74330.ed. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Gryglewski RJ. Mechanism of action of some inhibitors of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1986;83(23):9164–9168. doi: 10.1073/pnas.83.23.9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti LD, Barlassina C, Citterio L, Galluccio E, Berzuini C, Setola E, et al. Endothelial nitric oxide synthase polymorphisms are associated with type 2 diabetes and the insulin resistance syndrome. Diabetes. 2003;52(5):1270–1275. doi: 10.2337/diabetes.52.5.1270. [DOI] [PubMed] [Google Scholar]
- Monti LD, Casiraghi MC, Setola E, Galluccio E, Pagani MA, Quaglia L, et al. L-arginine enriched biscuits improve endothelial function and glucose metabolism: a pilot study in healthy subjects and a cross-over study in subjects with impaired glucose tolerance and metabolic syndrome. Metabolism. 2013;62(2):255–264. doi: 10.1016/j.metabol.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Morley JE, Flood JF. Effect of competitive antagonism of NO synthetase on weight and food intake in obese and diabetic mice. Am J Physiol. 1994;266(1 Pt 2):R164–168. doi: 10.1152/ajpregu.1994.266.1.R164. [DOI] [PubMed] [Google Scholar]
- Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. 2007;28(5):463–491. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- Nakata S, Tsutsui M, Shimokawa H, Suda O, Morishita T, Shibata K, et al. Spontaneous myocardial infarction in mice lacking all nitric oxide synthase isoforms. Circulation. 2008;117(17):2211–2223. doi: 10.1161/CIRCULATIONAHA.107.742692. [DOI] [PubMed] [Google Scholar]
- Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, et al. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes. 2006;55(3):691–698. doi: 10.2337/diabetes.55.03.06.db05-0771. [DOI] [PubMed] [Google Scholar]
- Neumann P, Gertzberg N, Johnson A. TNF-alpha induces a decrease in eNOS promoter activity. Am J Physiol Lung Cell Mol Physiol. 2004;286(2):L452–459. doi: 10.1152/ajplung.00378.2002. [DOI] [PubMed] [Google Scholar]
- Nielsen SJ, Popkin BM. Patterns and trends in food portion sizes, 1977-1998. JAMA. 2003;289(4):450–453. doi: 10.1001/jama.289.4.450. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299(5608):896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, et al. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci U S A. 2004;101(47):16507–16512. doi: 10.1073/pnas.0405432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oess S, Icking A, Fulton D, Govers R, Muller-Esterl W. Subcellular targeting and trafficking of nitric oxide synthases. Biochem J. 2006;396(3):401–409. doi: 10.1042/BJ20060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief. 2012;(82):1–8. [PubMed] [Google Scholar]
- Ohtoshi K, Yamasaki Y, Gorogawa S, Hayaishi-Okano R, Node K, Matsuhisa M, et al. Association of (-)786T-C mutation of endothelial nitric oxide synthase gene with insulin resistance. Diabetologia. 2002;45(11):1594–1601. doi: 10.1007/s00125-002-0922-6. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pannirselvam M, Verma S, Anderson TJ, Triggle CR. Cellular basis of endothelial dysfunction in small mesenteric arteries from spontaneously diabetic (db/db −/−) mice: role of decreased tetrahydrobiopterin bioavailability. Br J Pharmacol. 2002;136(2):255–263. doi: 10.1038/sj.bjp.0704683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh NI, Keyes MJ, Larson MG, Pou KM, Hamburg NM, Vita JA, et al. Visceral and subcutaneous adiposity and brachial artery vasodilator function. Obesity (Silver Spring) 2009;17(11):2054–2059. doi: 10.1038/oby.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Li Q, Rask-Madsen C, Mima A, Mizutani K, Winnay J, et al. Serine phosphorylation sites on IRS2 activated by angiotensin II and protein kinase C to induce selective insulin resistance in endothelial cells. Mol Cell Biol. 2013;33(16):3227–3241. doi: 10.1128/MCB.00506-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Matute P, Neville MJ, Tan GD, Frayn KN, Karpe F. Transcriptional control of human adipose tissue blood flow. Obesity (Silver Spring) 2009;17(4):681–688. doi: 10.1038/oby.2008.606. [DOI] [PubMed] [Google Scholar]
- Piantadosi CA, Suliman HB. Redox regulation of mitochondrial biogenesis. Free Radic Biol Med. 2012;53(11):2043–2053. doi: 10.1016/j.freeradbiomed.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin BM, Armstrong LE, Bray GM, Caballero B, Frei B, Willett WC. A new proposed guidance system for beverage consumption in the United States. Am J Clin Nutr. 2006;83(3):529–542. doi: 10.1093/ajcn.83.3.529. [DOI] [PubMed] [Google Scholar]
- Rafikov R, Fonseca FV, Kumar S, Pardo D, Darragh C, Elms S, et al. eNOS activation and NO function: structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity. J Endocrinol. 2011;210(3):271–284. doi: 10.1530/JOE-11-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajfer J, Aronson WJ, Bush PA, Dorey FJ, Ignarro LJ. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med. 1992;326(2):90–94. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- Rapoport RM, Draznin MB, Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983;306(5939):174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- Rapoport RM, Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Reaven GM, Chen YD. Insulin resistance, its consequences, and coronary heart disease. Must we choose one culprit? Circulation. 1996;93(10):1780–1783. doi: 10.1161/01.cir.93.10.1780. [DOI] [PubMed] [Google Scholar]
- Recchia FA, Osorio JC, Chandler MP, Xu X, Panchal AR, Lopaschuk GD, et al. Reduced synthesis of NO causes marked alterations in myocardial substrate metabolism in conscious dogs. Am J Physiol Endocrinol Metab. 2002;282(1):E197–206. doi: 10.1152/ajpendo.2002.282.1.E197. [DOI] [PubMed] [Google Scholar]
- Risbano MG, Gladwin MT. Therapeutics targeting of dysregulated redox equilibrium and endothelial dysfunction. Handb Exp Pharmacol. 2013;218:315–349. doi: 10.1007/978-3-642-38664-0_13. [DOI] [PubMed] [Google Scholar]
- Rittig K, Holder K, Stock J, Tschritter O, Peter A, Stefan N, et al. Endothelial NO-synthase intron 4 polymorphism is associated with disturbed in vivo nitric oxide production in individuals prone to type 2 diabetes. Horm Metab Res. 2008;40(1):13–17. doi: 10.1055/s-2007-1004527. [DOI] [PubMed] [Google Scholar]
- Robinson TN. Reducing children's television viewing to prevent obesity: a randomized controlled trial. JAMA. 1999;282(16):1561–1567. doi: 10.1001/jama.282.16.1561. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Crespo I, Gerber NC, Ortiz de Montellano PR. Endothelial nitric-oxide synthase. Expression in Escherichia coli, spectroscopic characterization, and role of tetrahydrobiopterin in dimer formation. J Biol Chem. 1996;271(19):11462–11467. doi: 10.1074/jbc.271.19.11462. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Crespo I, Ortiz de Montellano PR. Human endothelial nitric oxide synthase: expression in Escherichia coli, coexpression with calmodulin, and characterization. Arch Biochem Biophys. 1996;336(1):151–156. doi: 10.1006/abbi.1996.0543. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart Disease and Stroke Statistics--2012 Update: A Report From the American Heart Association. Circulation. 2011 doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez de Miguel L, Alonso J, Gonzalez-Fernandez F, de la Osada J, Monton M, Rodriguez-Feo JA, et al. Evidence that an endothelial cytosolic protein binds to the 3'-untranslated region of endothelial nitric oxide synthase mRNA. J Vasc Res. 1999;36(3):201–208. doi: 10.1159/000025643. [DOI] [PubMed] [Google Scholar]
- Sansbury BE, Cummins TD, Tang Y, Hellmann J, Holden CR, Harbeson MA, et al. Overexpression of Endothelial Nitric Oxide Synthase Prevents Diet-Induced Obesity and Regulates Adipocyte Phenotype. Circulation Research. 2012;111(9):1176–1189. doi: 10.1161/CIRCRESAHA.112.266395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Fujimoto S, Haruna Y, Arakawa S, Horike H, Komai N, et al. NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am J Physiol Renal Physiol. 2005;288(6):F1144–1152. doi: 10.1152/ajprenal.00221.2004. [DOI] [PubMed] [Google Scholar]
- Schild L, Dombrowski F, Lendeckel U, Schulz C, Gardemann A, Keilhoff G. Impairment of endothelial nitric oxide synthase causes abnormal fat and glycogen deposition in liver. Biochim Biophys Acta. 2008;1782(3):180–187. doi: 10.1016/j.bbadis.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Sessa WC. eNOS at a glance. J Cell Sci. 2004;117(Pt 12):2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- Shankar RR, Wu Y, Shen HQ, Zhu JS, Baron AD. Mice with gene disruption of both endothelial and neuronal nitric oxide synthase exhibit insulin resistance. Diabetes. 2000;49(5):684–687. doi: 10.2337/diabetes.49.5.684. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Kashiwagi A, Nishio Y, Okamura T, Yoshida Y, Masada M, et al. Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2-imbalance in insulin-resistant rat aorta. Diabetes. 1999;48(12):2437–2445. doi: 10.2337/diabetes.48.12.2437. [DOI] [PubMed] [Google Scholar]
- Shiva S, Oh JY, Landar AL, Ulasova E, Venkatraman A, Bailey SM, et al. Nitroxia: the pathological consequence of dysfunction in the nitric oxide-cytochrome c oxidase signaling pathway. Free Radic Biol Med. 2005;38(3):297–306. doi: 10.1016/j.freeradbiomed.2004.10.037. [DOI] [PubMed] [Google Scholar]
- Smith DE, Lewis CE, Caveny JL, Perkins LL, Burke GL, Bild DE. Longitudinal changes in adiposity associated with pregnancy. The CARDIA Study. Coronary Artery Risk Development in Young Adults Study. JAMA. 1994;271(22):1747–1751. [PubMed] [Google Scholar]
- Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97(11):2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes. 2000;49(7):1231–1238. doi: 10.2337/diabetes.49.7.1231. [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, et al. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997;100(5):1230–1239. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker-Krongrad A, Beck B, Burlet C. Nitric oxide mediates hyperphagia of obese Zucker rats: relation to specific changes in the microstructure of feeding behavior. Life Sci. 1996;58(1):PL9–15. doi: 10.1016/0024-3205(95)02260-0. [DOI] [PubMed] [Google Scholar]
- Sturm W, Sandhofer A, Engl J, Laimer M, Molnar C, Kaser S, et al. Influence of visceral obesity and liver fat on vascular structure and function in obese subjects. Obesity (Silver Spring) 2009;17(9):1783–1788. doi: 10.1038/oby.2009.81. [DOI] [PubMed] [Google Scholar]
- Suliburska J, Bogdanski P, Szulinska M, Pupek-Musialik D, Jablecka A. Changes in mineral status are associated with improvements in insulin sensitivity in obese patients following L-arginine supplementation. Eur J Nutr. 2014;53(2):387–393. doi: 10.1007/s00394-013-0533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, et al. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res. 2009;104(9):1085–1094. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6(8):662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- Tack CJ, Ong MK, Lutterman JA, Smits P. Insulin-induced vasodilatation and endothelial function in obesity/insulin resistance. Effects of troglitazone. Diabetologia. 1998;41(5):569–576. doi: 10.1007/s001250050948. [DOI] [PubMed] [Google Scholar]
- Taguchi K, Kobayashi T, Matsumoto T, Kamata K. Dysfunction of endothelium-dependent relaxation to insulin via PKC-mediated GRK2/Akt activation in aortas of ob/ob mice. Am J Physiol Heart Circ Physiol. 2011;301(2):H571–583. doi: 10.1152/ajpheart.01189.2010. [DOI] [PubMed] [Google Scholar]
- Tan B, Yin Y, Liu Z, Li X, Xu H, Kong X, et al. Dietary L-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids. 2009;37(1):169–175. doi: 10.1007/s00726-008-0148-0. [DOI] [PubMed] [Google Scholar]
- Thomas DD, Liu X, Kantrow SP, Lancaster JR., Jr. The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci U S A. 2001;98(1):355–360. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toutouzas K, Riga M, Stefanadi E, Stefanadis C. Asymmetric dimethylarginine (ADMA) and other endogenous nitric oxide synthase (NOS) inhibitors as an important cause of vascular insulin resistance. Horm Metab Res. 2008;40(9):655–659. doi: 10.1055/s-0028-1083814. [DOI] [PubMed] [Google Scholar]
- Trujillo M, Ferrer-Sueta G, Radi R. Peroxynitrite detoxification and its biologic implications. Antioxid Redox Signal. 2008;10(9):1607–1620. doi: 10.1089/ars.2008.2060. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K, Sakai H, Suzuki N, Iwashima F, Yoshimoto T, Shichiri M, et al. Chronic blockade of nitric oxide synthesis reduces adiposity and improves insulin resistance in high fat-induced obese mice. Endocrinology. 2007;148(10):4548–4556. doi: 10.1210/en.2006-1371. [DOI] [PubMed] [Google Scholar]
- Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, et al. TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest. 2006;116(10):2791–2798. doi: 10.1172/JCI28570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Guilder GP, Stauffer BL, Greiner JJ, Desouza CA. Impaired endothelium-dependent vasodilation in overweight and obese adult humans is not limited to muscarinic receptor agonists. Am J Physiol Heart Circ Physiol. 2008;294(4):H1685–1692. doi: 10.1152/ajpheart.01281.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecoli C, Andreassi MG, Liga R, Colombo MG, Coceani M, Carpeggiani C, et al. T(-786)-->C polymorphism of the endothelial nitric oxide synthase gene is associated with insulin resistance in patients with ischemic or non ischemic cardiomyopathy. BMC Med Genet. 2012;13:92. doi: 10.1186/1471-2350-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet B, Guigas B, Leclerc J, Hebrard S, Lantier L, Mounier R, et al. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol (Oxf) 2009;196(1):81–98. doi: 10.1111/j.1748-1716.2009.01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang AX, Aylor K, Barrett EJ. Nitric oxide directly promotes vascular endothelial insulin transport. Diabetes. 2013;62(12):4030–4042. doi: 10.2337/db13-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Bleich SN, Gortmaker SL. Increasing caloric contribution from sugar-sweetened beverages and 100% fruit juices among US children and adolescents, 1988-2004. Pediatrics. 2008;121(6):e1604–1614. doi: 10.1542/peds.2007-2834. [DOI] [PubMed] [Google Scholar]
- Wascher TC, Graier WF, Dittrich P, Hussain MA, Bahadori B, Wallner S, et al. Effects of low-dose L-arginine on insulin-mediated vasodilatation and insulin sensitivity. Eur J Clin Invest. 1997;27(8):690–695. doi: 10.1046/j.1365-2362.1997.1730718.x. [DOI] [PubMed] [Google Scholar]
- Weil BR, Westby CM, Van Guilder GP, Greiner JJ, Stauffer BL, DeSouza CA. Enhanced endothelin-1 system activity with overweight and obesity. Am J Physiol Heart Circ Physiol. 2011;301(3):H689–695. doi: 10.1152/ajpheart.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MB, Hill BG, Xuan YT, Bhatnagar A. Protein glutathiolation by nitric oxide: an intracellular mechanism regulating redox protein modification. FASEB J. 2006;20(10):1715–1717. doi: 10.1096/fj.06-5843fje. [DOI] [PubMed] [Google Scholar]
- Westerbacka J, Vehkavaara S, Bergholm R, Wilkinson I, Cockcroft J, Yki-Jarvinen H. Marked resistance of the ability of insulin to decrease arterial stiffness characterizes human obesity. Diabetes. 1999;48(4):821–827. doi: 10.2337/diabetes.48.4.821. [DOI] [PubMed] [Google Scholar]
- Williamson DF, Madans J, Anda RF, Kleinman JC, Kahn HS, Byers T. Recreational physical activity and ten-year weight change in a US national cohort. Int J Obes Relat Metab Disord. 1993;17(5):279–286. [PubMed] [Google Scholar]
- Xu J, Wu Y, Song P, Zhang M, Wang S, Zou MH. Proteasome-dependent degradation of guanosine 5'-triphosphate cyclohydrolase I causes tetrahydrobiopterin deficiency in diabetes mellitus. Circulation. 2007;116(8):944–953. doi: 10.1161/CIRCULATIONAHA.106.684795. [DOI] [PubMed] [Google Scholar]
- Xu J, Xie Z, Reece R, Pimental D, Zou MH. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler Thromb Vasc Biol. 2006;26(12):2688–2695. doi: 10.1161/01.ATV.0000249394.94588.82. [DOI] [PubMed] [Google Scholar]
- Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab. 2011;14(1):67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Habata Y, Matsumoto Y, Yasuhara Y, Hashimoto T, Hamajyo H, et al. Apelin-transgenic mice exhibit a resistance against diet-induced obesity by increasing vascular mass and mitochondrial biogenesis in skeletal muscle. Biochim Biophys Acta. 2011;1810(9):853–862. doi: 10.1016/j.bbagen.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Yan G, You B, Chen SP, Liao JK, Sun J. Tumor necrosis factor-alpha downregulates endothelial nitric oxide synthase mRNA stability via translation elongation factor 1-alpha 1. Circ Res. 2008;103(6):591–597. doi: 10.1161/CIRCRESAHA.108.173963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Ying C, Xu M, Zuo X, Ye X, Liu L, et al. High-fat diet up-regulates caveolin-1 expression in aorta of diet-induced obese but not in diet-resistant rats. Cardiovasc Res. 2007;76(1):167–174. doi: 10.1016/j.cardiores.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Hisatomi A, Kajihara S, Yasutake T, Ogawa Y, Mizuta T, et al. The relationship between insulin resistance and polymorphisms of the endothelial nitric oxide synthase gene in patients with coronary artery disease. J Atheroscler Thromb. 2003;10(1):43–47. doi: 10.5551/jat.10.43. [DOI] [PubMed] [Google Scholar]
- Yoshizumi M, Perrella MA, Burnett JC, Jr., Lee ME. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. [Research Support, Non-U.S. Gov't]. Circ Res. 1993;73(1):205–209. doi: 10.1161/01.res.73.1.205. [DOI] [PubMed] [Google Scholar]
- Zamosky L. The obesity epidemic. While America swallows $147 billion in obesity-related healthcare costs, physicians called on to confront the crisis. Med Econ. 2013;90(4):14–17. [PubMed] [Google Scholar]
- Zecchin HG, Priviero FB, Souza CT, Zecchin KG, Prada PO, Carvalheira JB, et al. Defective insulin and acetylcholine induction of endothelial cell-nitric oxide synthase through insulin receptor substrate/Akt signaling pathway in aorta of obese rats. Diabetes. 2007;56(4):1014–1024. doi: 10.2337/db05-1147. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Malik P, Pandey D, Gupta S, Jagnandan D, Belin de Chantemele E, et al. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arterioscler Thromb Vasc Biol. 2008;28(9):1627–1633. doi: 10.1161/ATVBAHA.108.168278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QJ, Holland WL, Wilson L, Tanner JM, Kearns D, Cahoon JM, et al. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes. 2012;61(7):1848–1859. doi: 10.2337/db11-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong JC, Yu XY, Huang Y, Yung LM, Lau CW, Lin SG. Apelin modulates aortic vascular tone via endothelial nitric oxide synthase phosphorylation pathway in diabetic mice. Cardiovasc Res. 2007;74(3):388–395. doi: 10.1016/j.cardiores.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Zweier JL, Chen CA, Druhan LJ. S-glutathionylation reshapes our understanding of endothelial nitric oxide synthase uncoupling and nitric oxide/reactive oxygen species-mediated signaling. Antioxid Redox Signal. 2011;14(10):1769–1775. doi: 10.1089/ars.2011.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]