Abstract
Introduction
Depression can suppress immune function, leading to lower resistance against infection and longer healing times in depressed individuals. Sexuality may also influence immune function, with evidence that sexual activity is associated with lowered immune function in women and mixed results in men. Immune mediators like immunoglobulin A (IgA) are immediately relevant to sexual health, since they are the first line of defense against pathogens at mucous membranes like the vagina.
Aim
This study aims to determine if and how depression, sexual activity, and their interaction impact salivary IgA (SIgA) in men and women.
Methods
In Study 1, a community-based sample of 84 women and 88 men provided saliva samples and completed questionnaires on their demographic background, level of depression, and frequency of partnered and solitary sexual activity. Study 2, conducted separately in an undergraduate student sample of 54 women and 52 men, had similar methods.
Main Outcome Measures
The main outcome measures were scores on the General Well-Being Schedule depression subscale, reported frequency of sexual activity, and SIgA levels as measured by enzyme immunoassay.
Results
Across studies, higher levels of partnered sexual activity were associated with lower SIgA for women with high depression scores, but not for women with low depression scores. In contrast, higher levels of partnered sexual activity were associated with higher SIgA for men with high depression scores, but not for men with low depression scores.
Conclusion
Our results show that partnered sexual activity is a risk factor for lowered immunity in women with depressive symptoms but a possible resilience factor for men with depressive symptoms. This suggests a role for sexual activity in determining the impact of depression on physical health parameters.
Keywords: Depression, IgA, Sex, Men, Women, Intercourse, Immune
Introduction
Depression negatively influences immune function [1], likely due to both biological and behavioral factors. Biological mechanisms include endocrine activity of the hypothalamic– pituitary–adrenal axis and upregulation of the sympathetic nervous system, both of which are impacted by stress and depressed mood and both of which directly modulate immune function [2]. Research on behavioral pathways has focused largely on health-related behaviors such as poor nutrition [3], alcohol and nicotine use [4], and sedentary lifestyles [5], all of which are hallmarks of depression and independently linked to poorer immune function [6]. One behavioral route that has received little empirical attention is sexual activity. It is relatively well established that depression has profound effects on sexuality [7], for example, lower interest in partnered sexual activity [8] but higher interest in masturbation [7,9], higher rates of clinically relevant sexual dysfunction [10,11] as well as subclinical sexual problems [12], and lower sexual pleasure and satisfaction [13]. It is less appreciated that sexuality may be linked to immunity, and the present study investigates the intersection of depression and sexual activity in immune function in women and men.
Sexual Activity and Immune Function: Gender/Sex Differences
There are gender/sex1 differences in both depression [14] and immune function [15–17]; women are more likely to become depressed, and also generally have higher immune system activity than men. A few studies suggest that there are gender differences in the link between sexual activity and immune mediators as well, with negative associations in women and positive associations in men.
Studies in women have generally shown an association between sexual activity and lower levels of immune markers. For example, Barousse et al. [18] found that adolescent women who had engaged in sexual activity in the last week had significantly lower concentrations of several vaginal immune parameters including interleukin (IL)-2, IL-4, and monocyte chemoattractant protein-1 (MCP-1) relative to those who had not engaged in sexual activity.
In men, findings are mixed, with studies in non-human animals suggesting sexual behavior is associated with lower immune parameters (see, e.g., McKean and Nunney [19]), possibly due to the energetic costs of sexual competition [20], and studies in humans generally suggesting sexual behavior is associated with higher immune parameters. One study [21] found that masturbation increased numbers of leukocytes in serum measures, especially natural killer (NK) cells. Another study showed that men undergoing a psychosocial intervention targeting number of sexual partners showed a decrease in immunoglobulin A (IgA) levels as they decreased their number of sexual partners [22], suggesting a positive association. A large study of men in the U.S. Army [23] included both sexual variables and immune parameters sampled from blood. It found that having 10 or more sexual partners in a year was significantly positively correlated with concentrations of T lymphocytes, helper/inducer CD4 cells, suppressor/cytotoxic CD8 cells, and B lymphocytes. Taken together, these findings suggest that sexual activity may increase immune parameters for men but decrease them in women.
Salivary Immunoglobulin A: A Noninvasive Immune Parameter
A limited number of immune parameters can be sampled noninvasively (i.e., without sampling blood or tissue), and one of these is salivary IgA (SIgA). SIgA, an antibody formed by B cells, attacks a broad array of pathogens both directly and by recruiting other components of the immune system such as macrophages. It is the first line of defense against infections at mucosal membranes [24], including both the mouth and the vagina, as it is the most abundant immunoglobulin in mucosal secretions [25,26]; it is thus especially relevant to prevention of sexually transmitted infections [27]. Higher levels of SIgA are associated with lower incidence of subsequent infection/illness [28,29], indicating SIgA levels are meaningful in assessing overall infection risk. While women tend to have higher levels of most immune parameters than do men [30], men tend to have higher baseline SIgA than do women [31–33].
Depression and stress can lead to alterations in SIgA levels specifically, as they can for immunity in general [34]. Depression has been associated with lower concentrations of SIgA in both men and women [35–38], with some sex/gender differences. In one study in children, the immunosuppressive effect of depression was more profound in girls than in boys [39]. Another study in adults found that women’s SIgA levels improved more than did men’s in response to depression treatment [40].
Sexual Activity and SIgA
Production of immune mediators is thought to be site-specific to some degree [41], but mediators sampled at one site are correlated with those sampled at others [26,42,43]. Critically, SIgA and vaginal IgA are correlated [44], suggesting IgA levels in saliva may tell us something about genital mucosal immunity and the subsequent risk of sexually transmitted infections.
However, although several studies have investigated the role of SIgA in the progression of STIs [45,46], only two studies have examined relationships between sexual activity and SIgA in healthy (preinfection) humans. One study found that adult premenopausal women who had engaged in sexual activity with women or men in the most recent menstrual cycle had significantly lower SIgA than abstinent women [47]. Charnetski and Brennan [48] showed a curvilinear relationship between sexual activity and SIgA, such that women and men engaging in a moderate frequency of sexual activity had higher salivary SIgA than individuals engaging in a low or high frequency of activity. However, Charnetski and Brennan’s analyses were mixed by gender/sex; as there are considerable gender/sex differences in baseline SIgA levels, and sexual frequency by gender/sex was not reported, it is possible these results were confounded by uneven numbers of men and women at different levels of sexual frequency. Nevertheless, these two studies confirm that sexual activity can influence SIgA levels.
Present Study
Given that depression impacts both immunity and sexuality, it is likely that there are considerable differences between depressed and nondepressed individuals in the impact of sexual behavior on immunity. We tested these differences using SIgA levels, a noninvasive measure of immune function.
Moreover, as there are sex and gender differences in the association between sexual behavior and immunity, we might expect men and women to differ in the association among depression, sexuality, and immune function. Partnered sexual activity introduces pathogens from the genitals and secretions of the partner (e.g., semen). The vagina is a mucosal surface that responds to these pathogens with an antibody response including production of IgA [49]. While the penis also has some mucosal immune responses to partnered sexual activity, they are much less marked than those of the vagina [50]. Thus, we expected different patterns of immune response in women and men in partnered sexual activity. Also, to test the specificity of the effect, we compared partnered and solitary sexual activity.
Aims
Our aim was to evaluate if sexual activity moderates the effect of depression on immune function in women and men. Because this research was exploratory, we tested associations between depression symptom scales and immunity in community-based samples. There is a dose– response curve between depression symptoms and immunosuppression [1]; thus, it is reasonable that clinically depressed individuals and individuals who score high on depression measures would differ in immunity only as a matter of degree [1]. Still, it should be noted that the term “high depression” throughout this paper refers to individuals scoring high on depression measures rather than individuals diagnosed clinically with depression.
Study 1
In this and Study 2, we used data collected as part of larger investigations of sexuality, health, and other parameters [51,52]. We thus conducted exploratory secondary analyses of data previously collected to address the link between sexuality and IgA.
Methods
Participants
Participants for the Partnering, Physiology, and Health study were recruited from the undergraduate psychology pool and the community through posters and online advertisements and were compensated with either course credit or US $10. Participants consisted of healthy men and women who were 18 or older, able to read and write in English, and not using any hormonal medications or supplements. Of the 121 men and 116 women who completed study procedures, 37 potential participants (16 men, 21 women) reported health conditions or medications that affect physiological parameters, and 30 (16 men, 14 women) reported current illness, with both categories applying to 7 participants. Of these, three participants had missing data on depression measures. Additionally, two outliers with SIgA levels over 3 SD from the mean were excluded from further analysis. Thus, the final sample included 84 women (Mage = 21.49, SD = 5.67) and 88 men (Mage = 23.80, SD = 9.22). Participants composed a diverse demographic sample in terms of race and ethnicity, occupation, sexual orientation, and relationship status (see Table 1).
Table 1.
Study 1 demographics
| Men (N = 88) |
Women (N = 84) |
Total (N = 172) |
||||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |||
| Age (years) | 23.55 | 9.17 | 21.49 | 5.68 | 22.54 | 7.71 | ||
| N | % | N | % | N | % | |||
| Race/ethnicity | ||||||||
| African American/Black | 9 | 11 | 8 | 11 | 17 | 10 | ||
| Asian | 20 | 23 | 28 | 33 | 48 | 28 | ||
| Bi/multiracial | 3 | 3 | 7 | 8 | 10 | 6 | ||
| Hispanic/Latino(a) | 3 | 3 | 2 | 2 | 5 | 3 | ||
| White/Caucasian | 50 | 57 | 38 | 45 | 88 | 50 | ||
| American Indian/Native Hawaiian | 1 | 1 | 0 | 0 | 1 | 1 | ||
| Unspecified/data missing | 2 | 2 | 1 | 1 | 3 | 2 | ||
| Sexual orientation | ||||||||
| Bisexual | 3 | 3 | 4 | 5 | 7 | 4 | ||
| Heterosexual | 60 | 68 | 68 | 81 | 128 | 74 | ||
| Gay/lesbian | 9 | 10 | 3 | 4 | 12 | 7 | ||
| Unspecified/data missing | 16 | 19 | 9 | 10 | 25 | 15 | ||
| Relationship status | ||||||||
| Single | 44 | 50 | 36 | 43 | 80 | 47 | ||
| Casual relationship(s) | 21 | 24 | 21 | 25 | 42 | 24 | ||
| Long-term relationship(s) | 23 | 26 | 27 | 32 | 50 | 29 | ||
| Highest education completed | ||||||||
| Some high school | 1 | 1 | 0 | 0 | 1 | 1 | ||
| High school diploma | 2 | 2 | 3 | 4 | 5 | 3 | ||
| Some college/undergraduate degree | 76 | 86 | 71 | 84 | 147 | 84 | ||
| Advanced degree | 5 | 7 | 8 | 10 | 13 | 8 | ||
| Other training | 2 | 2 | 1 | 1 | 3 | 2 | ||
| Unspecified/data missing | 2 | 2 | 1 | 1 | 3 | 2 | ||
| Student status | ||||||||
| Student | 73 | 83 | 74 | 88 | 147 | 86 | ||
| Nonstudent | 15 | 17 | 10 | 12 | 25 | 14 | ||
| Sexual frequency status | ||||||||
| Virgin | 8 | 9 | 4 | 5 | 12 | 7 | ||
| Non-virgin, not currently sexually active | 39 | 44 | 44 | 52 | 83 | 48 | ||
| Currently sexually active | 41 | 47 | 36 | 43 | 77 | 45 | ||
Materials
The study made use of a self-report questionnaire and of saliva samples from which SIgA levels were assessed.
Questionnaires
Participants completed a background, health, and demographics questionnaire so that we could characterize the sample and address possible confounds. The questionnaire also addressed relational and sexual variables. Participants reported frequency of partnered sexual activity, defined as sexual activity in which the participant’s genitals (penis, clitoris, vagina) were physically stimulated. Some cells contained low counts, and we were also interested in nonlinear effects; accordingly, we also coded participants categorically: no current partnered sexuality, low-frequency partnered sexuality (partnered sexual activity one to four times in the last month), or high-frequency partnered sexuality (partnered sexuality two or more times per week). Participants also reported frequency of masturbation in the last week.
Participants also completed the General Well-Being Schedule (GWBS [53]), which includes subscales on general and psychological health, including a depression-specific subscale (GWBSD). The GWBS has been widely used as an index of mental health, most notably depression [54]. The depression subscale has demonstrated good reliability and convergent validity with other measures of depression such as the Center for Epidemiological Studies Depression Scale [55,56]. For the depression subscale, a clinical cutoff score of 13 has been demonstrated to have good sensitivity and specificity [56]; participants scoring less than 13 are here termed to have “scores consistent with depression.” Reliability in the current sample was good (Cronbach’s alpha = 0.88).
Saliva Samples (Main Outcome Measure)
Participants rinsed their mouths with water and then provided saliva samples of at least 5 mL by spitting into polystyrene tubes. We froze samples until enzyme immunoassay, which was conducted with a commercially available kit from Salimetrics LLC (State College, PA, USA) at our Core Assay Facility, University of Michigan. The intra-assay coefficient of variance (CV) was 8.62, and the inter-assay CV was 9.2 over six assays. Samples were also assayed for other hormones to address other hypotheses [51,52].
Procedure
The university’s Institutional Review Board approved the study. Participants were tested in afternoon laboratory sessions from February to September, and asked to refrain from eating, drinking, smoking, brushing their teeth, or chewing gum for one hour prior to testing. Upon arrival, participants were taken to a private testing room to sign the consent form, which included a brief description of the study and statement of confidentiality. A research assistant provided participants instructions on providing a saliva sample and filling out the online questionnaire. We confirmed compliance to the pre-visit eating and drinking request by asking participants just before saliva collection. The participant was left alone and given as much time as needed to complete the study. Participants were asked to bring the saliva sample back to the lab when it reached the 5 mL mark on the polystyrene tube; they then returned to the private testing room to continue working on the questionnaire. Upon completion, the participant returned to the lab to receive the compensation and to be debriefed.
Analyses
Analyses were conducted with SPSS 18.0 (SPSS Inc., Chicago, IL, USA). We tested all associations in women and men separately. As frequency of partnered sexual activity was rated on an ordinal Likert scale (a rank-ordered noncontinuous scale), we tested associations with this variable using partial rank order correlations following the recommendations of Kendall and Gibbons [57]. Because this analysis required depression to be considered as a group-level variable, for this analysis only, we characterized participants as within the range consistent with depression (GWBS depression scores < 13) or not in the depressed range [56]; all other analyses used the full range of the GWBS scale. Masturbation frequency was rated on a continuous scale, so we tested associations with linear regression models. We also tested categorical-level differences in sexual activity with analyses of covariance (ancovas). We controlled for age and BMI in each analysis, as they can be expected to covary with SIgA levels, sexual measures, or both [33,58–61].
Results
Effects of Sexual Activity, Gender/Sex, and Depression on Immune Function
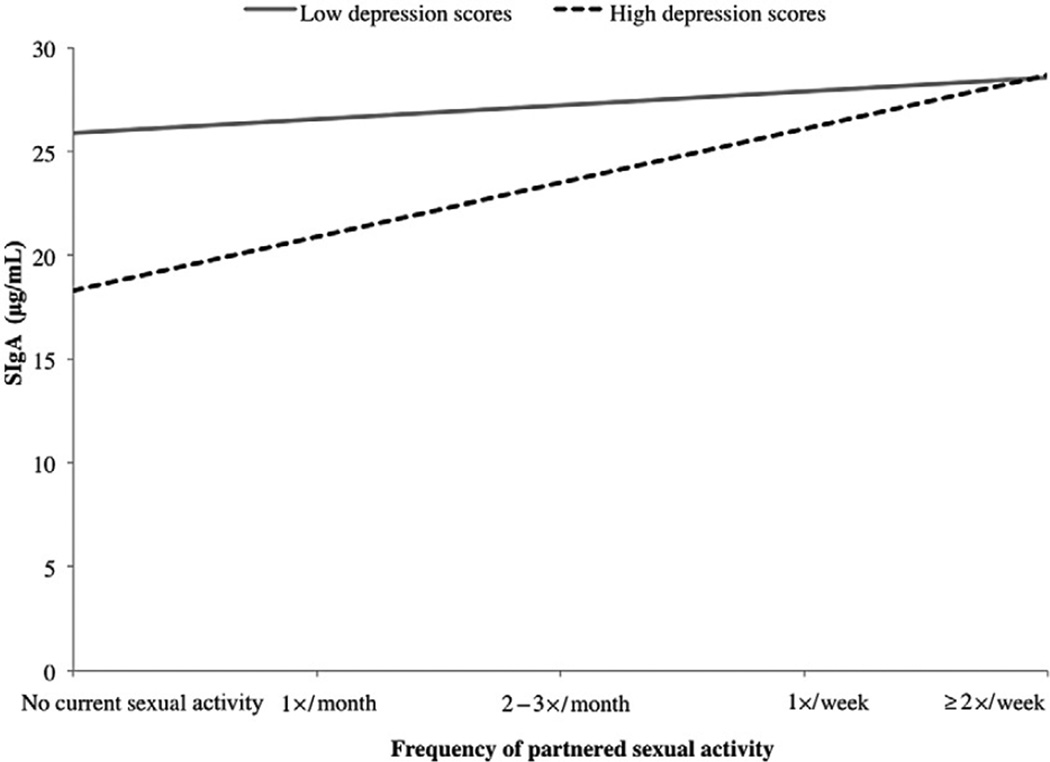
For women with GWBS scores consistent with depression, there was a significant negative correlation between frequency of sexual activity and SIgA levels (r[20] = −0.479, P = 0.024). For men with GWBS scores consistent with depression, however, there was a significant positive correlation between frequency of sexual activity and SIgA levels (r[19] = 0.462, P = 0.035). For women and men with GWBS scores not indicative of depression (i.e., low depression scores), the association between frequency of partnered sexual activity and SIgA levels was not significant (r[49] = −0.179, P = 0.208 and r[59] = 0.019, P = 0.885, respectively; see Figure 1).
Figure 1.
Study 1: Depression and sexual frequency interact to predict SIgA in men.
Follow-up analyses revealed that for men reporting no sexual activity, high depression was associated with significantly lower SIgA than low depression (F[1, 46] = 4.388, P = 0.042), suggesting that depression itself was associated with lower immune function. However, in men reporting partnered sexual activity at least once a month, men with high depression scores had levels of SIgA equivalent to those of men with low depression (F[1, 38] = 0.017, P = 0.896). Thus, frequency of partnered sexual activity ameliorated the potentially immunosuppressive effects of depression in men.
In women reporting no sexual activity, depression status was not significantly associated with SIgA levels (F[1, 46] = 0.003, P = 0.986). For women reporting partnered sexual activity at least once a month, however, women with high depression had significantly lower SIgA than those reporting low depression (F[1, 34] = 4.630, P = 0.039). This suggests that for women, sexual activity itself appeared to be associated with lower immune function, and depression amplified this.
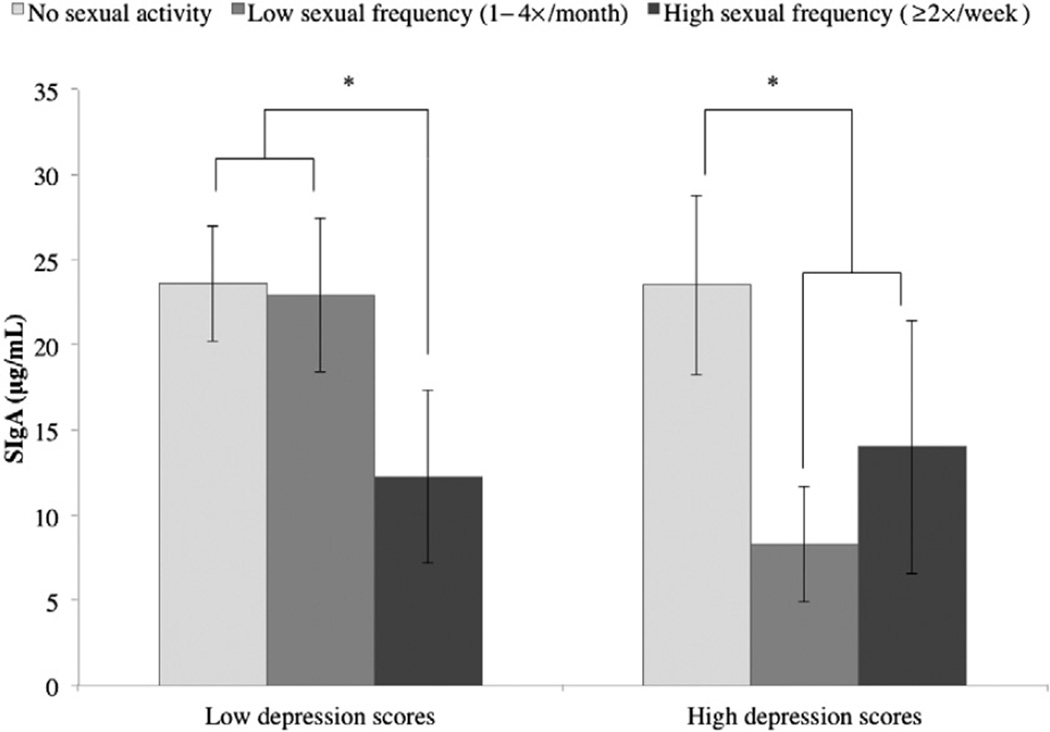
Group-Level Differences in Frequency of Sexual Activity
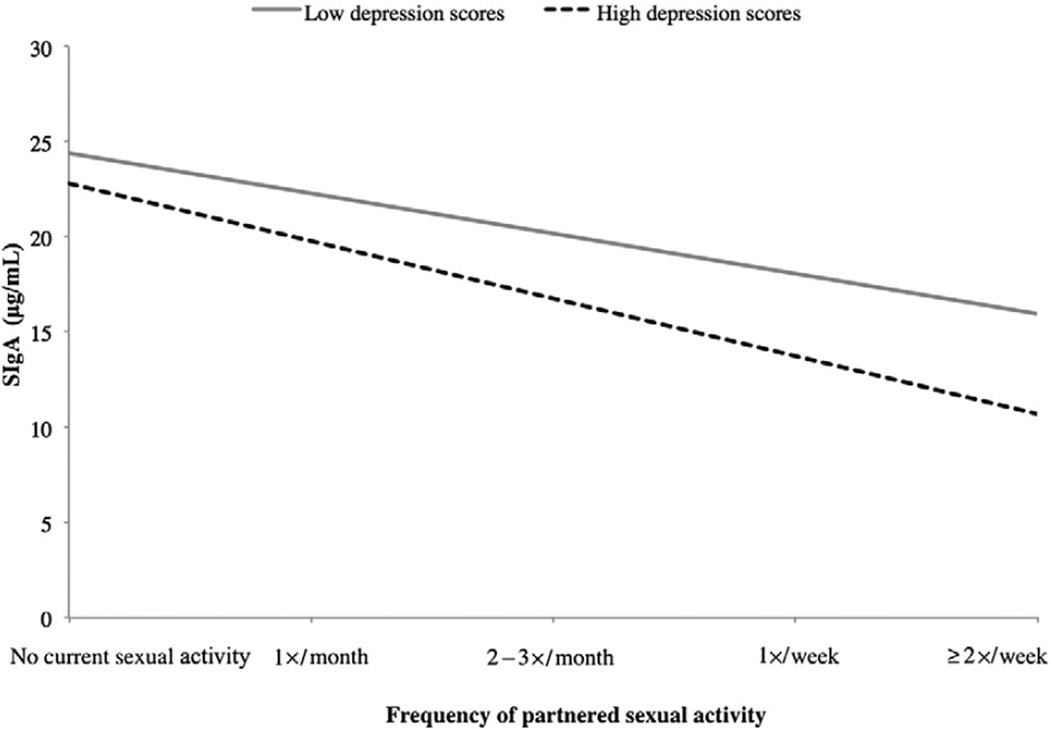
We then considered the same data, condensed into broader groups: no sexual activity, partnered sexual activity one to four times per month (low frequency), or partnered sexual activity two or more times per week (high frequency). In women, the interaction between depression and sexual frequency was significant (F[2, 79] = 3.159, P = 0.048; see Figure 2). For women with low depression, only high frequency of sexual activity was associated with lower SIgA, whereas for women with high depression, both low and high frequency were associated with lower SIgA. In men, the interaction between depression and sexual frequency was not significant (F[2, 84] = 0.187, P = 0.830).
Figure 2.
Study 1: Depression and sexual frequency interact to predict SIgA in women.
Effects of Masturbation, Gender/Sex, and Depression on Immune Function
As a control for aspects of sexual activity not related to pathogens from a partner (e.g., genital arousal), we considered the effects of masturbation on immune response. In contrast to the findings in partnered sexual activity, the interaction between masturbation frequency and SIgA levels was not significant in either men (R2 = 0.060, F[5, 83] = 1.007, P = 0.419) or women (R2 = 0.054, F[5, 73] = 0.789, P = 0.561). These results suggested that partnered, but not solitary, sexual activity affected immunity.
Study 2
For exploratory findings such as those of Study 1, it is especially important to confirm that patterns of results hold across multiple samples, in order to reduce the likelihood of findings being due to chance. Thus, we attempted to replicate the findings from Study 1 using data from an existing dataset from the ImPACT study. Surveys and saliva collection methods were identical to those reported above; the only differences were in recruitment and sample population.
Methods
Participants
First-year undergraduate students were recruited through e-mails to the entering class at the University of Michigan, fliers posted in student dormitories, and student events. Participants were compensated with up to US $130 and a chance to win two prizes, each worth US $100. As in Study 1, participants were required to be 18 years or older, able to read and write in English, not using any hormone-altering medications, with no physical conditions affecting hormones, and not experiencing any partner/spousal abuse with any present partners. We report here on data from 52 women (Mage = 18.08, SD = 0.33) and 54 men (Mage = 18.15, SD = 0.60) after excluding participants who were using medications or who had conditions affecting physiological parameters (N = 27), those who were not healthy at the time of the study (N = 26), outliers with SIgA levels over 3 SD from the mean (N = 4), and those with missing questionnaire data (N = 3), with eight participants affected by more than one category. As in Study 1, this sample was demographically diverse (see Table 2).
Table 2.
Study 2 demographics
| Men (N = 54) |
Women (N = 52) |
Total (N = 106) |
||||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |||
| Age (years) | 18.15 | 0.6 | 18.08 | 0.34 | 18.11 | 0.48 | ||
| N | % | N | % | N | % | |||
| Race/ethnicity | ||||||||
| African American/Black | 1 | 2 | 1 | 2 | 2 | 2 | ||
| Asian | 12 | 22 | 17 | 32 | 29 | 26 | ||
| Bi/multiracial | 3 | 6 | 5 | 9 | 8 | 8 | ||
| Hispanic/Latino(a) | 2 | 4 | 0 | 0 | 2 | 2 | ||
| White/Caucasian | 31 | 57 | 28 | 55 | 59 | 56 | ||
| Unspecified/data missing | 5 | 9 | 1 | 2 | 6 | 6 | ||
| Sexual orientation | ||||||||
| Bisexual | 3 | 6 | 4 | 8 | 7 | 7 | ||
| Heterosexual | 50 | 93 | 48 | 92 | 98 | 92 | ||
| Gay/lesbian | 1 | 2 | 0 | 0 | 1 | 1 | ||
| Relationship status | ||||||||
| Single | 25 | 46 | 22 | 43 | 47 | 44 | ||
| Casual relation hip(s) | 13 | 24 | 10 | 19 | 23 | 22 | ||
| Long-term relationship(s) | 16 | 30 | 20 | 38 | 36 | 24 | ||
| Sexual frequency status | ||||||||
| Virgin | 28 | 52 | 25 | 48 | 53 | 50 | ||
| Non-virgin, not currently sexually active | 14 | 26 | 16 | 31 | 30 | 28 | ||
| Currently sexually active | 12 | 22 | 11 | 21 | 23 | 22 | ||
All participants in Study 2 were first-year undergraduate students, and thus, student status and educational attainment are not listed.
Materials
Questionnaire
For the present analyses, we used data collected from the baseline session. All questionnaires were similar to those reported above.
Saliva Samples
As in Study 1, participants provided at least 5 mL of saliva by rinsing their mouths and then spitting into a polystyrene tube. These samples were frozen after collection and processing, and assayed using a Salimetrics kit at the Core Assay Facility. The intra-assay coefficients of variance (CVs) were 8.88% and 4.34%, and the inter-assay CVs were 9.25% and 9.65% over eight assays.
Procedure
Participants completed informed consent procedures and documented their consent, provided a saliva sample, and completed the questionnaires.
Analyses
In this study, frequency of partnered sexual activity and masturbation frequency were both rated on continuous scales, and therefore, we tested associations with linear regressions. As before, we tested group differences in partnered sexual activity with analyses of covariance (ancovas); however, as there were fewer participants reporting sexual activity than in Study 1, we characterized groups as no recent sexual activity vs. sexual activity in the last week. In these analyses, we controlled for BMI, but did not control for age, as all but eight participants reported their age as 18. Additionally, because there was a non-normal distribution of SIgA levels for this study (Shapiro–Wilk statistic = 0.934, P = 0.004), we transformed SIgA level values by taking the square root, leading to an adequately normal distribution (Shapiro–Wilk statistic = 0.974, P = 0.291).
Results
Effects of Partnered Sexual Activity, Gender/Sex, and Depression on Immune Function
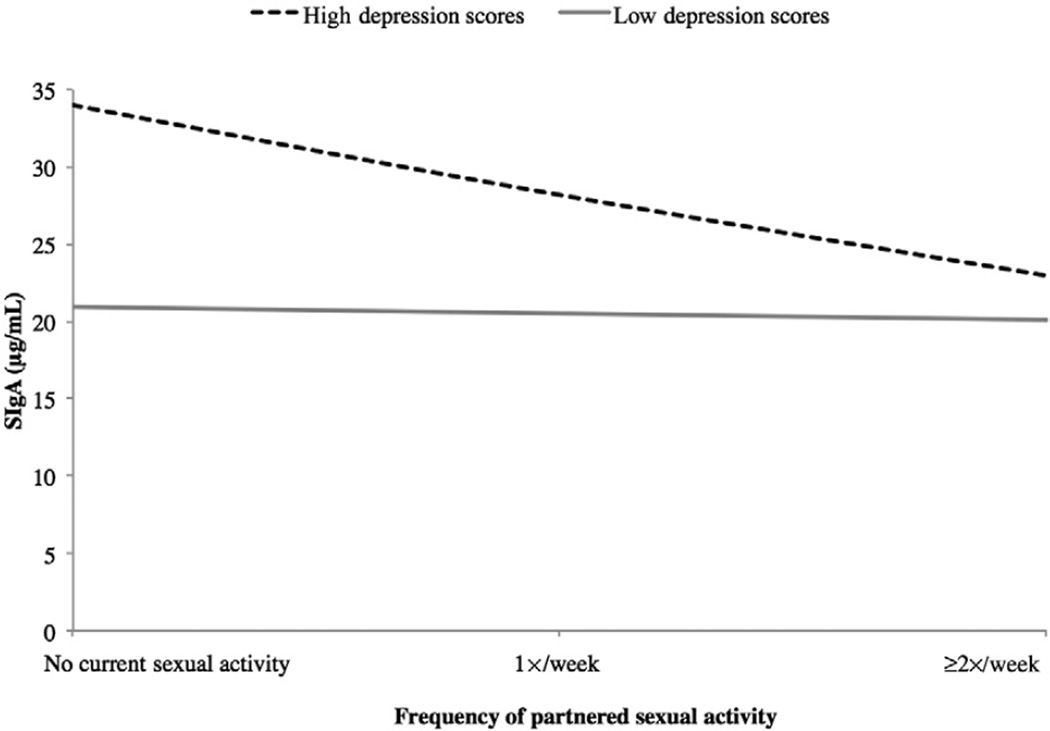
In women, the interaction of frequency of partnered sexual activity and depression was significant in predicting SIgA levels (model R2 = 0.600, F[4, 16] = 4.503, P = 0.019; addition of interaction term change in R2 = 0.200, F[1, 12] = 6.012, P = 0.030). From Figure 3, one can see that for women with low depression scores, frequency of partnered sexual activity had little effect on SIgA levels. For women with high depression scores, however, higher frequency of sexual activity was associated with lower SIgA. Follow-up tests showed that for women reporting no sexual activity, high-depression women had higher SIgA than low-depression women, but this difference was nonsignificant (F[1, 39] = 0.401, P = 0.530). Similarly, for women reporting at least some partnered sexual activity, high-depression women had non-significantly different SIgA from low-depression women (F[1, 10] = 4.554, P = 0.062).
Figure 3.
Study 1: Depression and frequency of partnered sexual activity interact to predict SIgA in women.
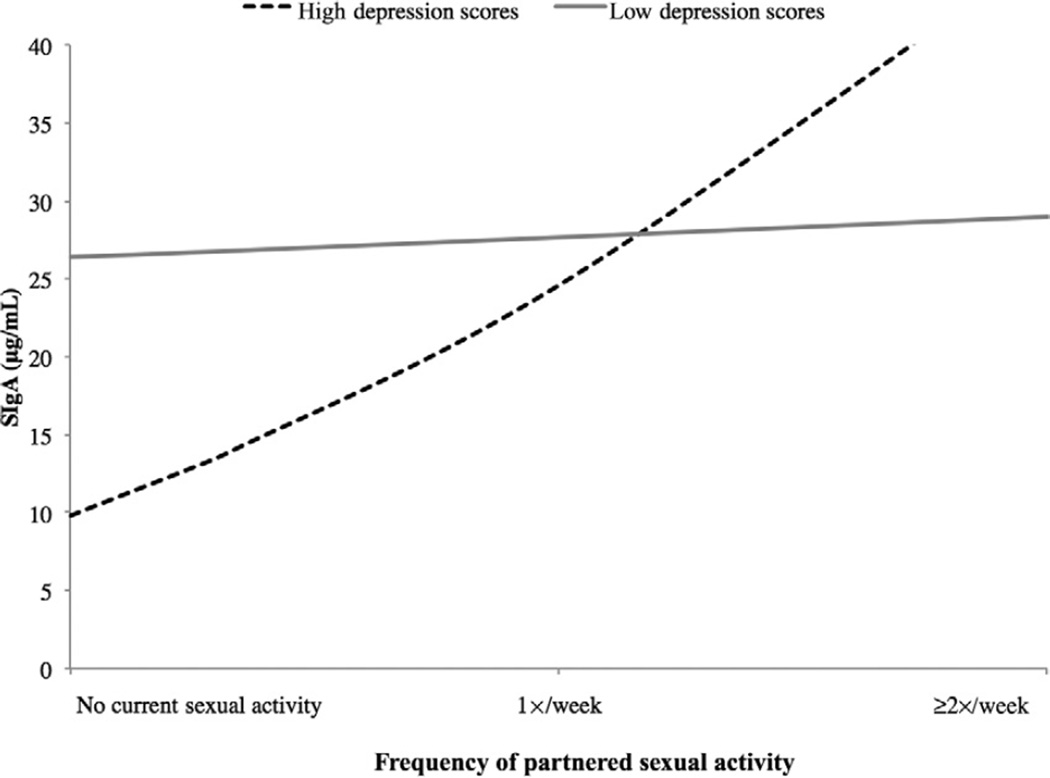
In men, the interaction of partnered sexual activity and depression was significant in predicting SIgA levels (model R2 = 0.468, F[4, 16] = 2.635, P = 0.087; addition of interaction term change in R2 = 0.268, F[1, 12] = 6.049, P = 0.030). As before, for men with low depression scores there was little effect of partnered sexual activity on SIgA levels, but for men with high depression scores, higher levels of sexual activity were associated with higher SIgA (Figures 4 and 5). Follow-up tests indicated that for men reporting no sexual activity, there was no significant difference in SIgA between high- and low-depression men (F[1, 40] = 0.619, P = 0.436). Likewise, for men reporting at least some partnered sexual activity, the difference in SIgA between high- and low-depressed men was nonsignificant (F[1, 11] = 0.119, P = 0.737).
Figure 4.
Study 2: Depression and sexual frequency interact to predict SIgA in men.
Figure 5.
Study 2: Depression and sexual frequency interact to predict SIgA in women.
Group-Level Differences in Frequency of Partnered Sexual Activity
When patients were broken down into groups according to sexual activity, the interaction of frequency of partnered sexual activity and SIgA levels was not significant for the men (F[1, 52] = 0.219, P = 0.642). In the women, the interaction was marginally significant (F[1, 49] = 3.932, P = 0.053), such that women reporting sexual activity in the last week had lower SIgA, particularly if they also had high depression scores.
Effects of Masturbation, Gender/Sex, and Depression on Immune Function
As in Study 1, the interaction of masturbation frequency and depression was not significant in predicting immune function in the men (R2 = 0.102, F[4, 49] = 1.282, P = 0.291; addition of interaction term change in R2 = 0.063, F[1, 45] = 3.178, P = 0.081). It was significant in the women (model R2 = 0.182, F[4, 46] = 2.341, P = 0.071; addition of interaction term change in R2 = 0.102, F[1, 42] = 5.244, P = 0.027); however, as only eight women reported any masturbation in the last week, this finding should be treated with caution.
Discussion
Across studies, we found similar interactions among frequency of sexual activity, depression, and SIgA levels, our index of mucosal immune function, and a similar moderation by gender/sex (see Table 3 for summary of results). For participants with high depression, there was a significant linear association between frequency of partnered sexuality and SIgA levels that was positive for men and negative for women. Findings in low-depression women and men differed slightly across studies, but generally showed frequency of partnered sexual activity being less strongly associated with SIgA levels than was seen in high-depression participants. Frequency of masturbation was not consistently associated with SIgA levels, suggesting that the effect is specific to partnered sexuality. This is the first empirical report investigating the interaction of gender/sex, depression, and sexual activity with immune function in humans, and these findings suggest that the effect of depression on immune function may depend on the level of sexual activity.
Table 3.
Summary of results across studies
| Men |
Women |
|||
|---|---|---|---|---|
| Low depression |
High depression |
Low depression |
High depression |
|
| Study 1 | . | ++ | − | − |
| Study 2 | . | ++ | . | − |
. = little to no effect;
− = decreasing SIgA with increasing sexual frequency;
4 = increasing SIgA with increasing sexual frequency
Similarities Across Studies
Our findings suggested that for men, high depression symptoms are an immunological challenge for which partnered sexual activity is a resilience factor. For women, partnered sexual activity itself appeared to be an immunological challenge, particularly for women with high depression. These differences could be due to differences in depression and sexual activity due to gender socialization, biological sex, or some combination of the two.
For example, it is possible testosterone may play a role. In men, depression is associated with low testosterone [62–64], and depressive symptoms may abate with testosterone treatments [65]. In women, the pattern is more complex, with moderate depression associated with low testosterone [66] and severe depression associated with high testosterone [67,68]. Frequency of sexual activity is also related to testosterone in a bidirectional fashion: higher levels of testosterone are associated with higher frequency of sexual activity, and sexual activity causes increases in testosterone [69–72]. Finally, testosterone is thought to be generally immunosuppressive in both women and men [73–75]. However, when data are controlled for cortisol levels, testosterone is associated with increased production of some immune parameters in men [20]. This may be because testosterone has dual effects in terms of increasing cortisol (immunosuppressing) and improving energy utilization through communication with fat-synthesizing cells (immunoenhancing). Accordingly, it may be that depression is associated with low testosterone (and high cortisol) in both men and women. Sexual activity may increase testosterone (or the ratio of testosterone to cortisol), which would have a large impact on depressed women and men (whose testosterone is lower) and a lesser impact on non-depressed men and women. This increased testosterone would be associated with improved immune function in men but decreased immune function in women.
Another possibility is that sexual activity influences relationship satisfaction and social support in gender-specific ways. At least one study has shown that while neuroticism (a tendency to experience negative emotion that is associated with depression [76]) has a negative impact on relationship satisfaction, high frequency of sexual activity can act as a buffer against these effects [77]. However, women are more likely to have multiple satisfying relationships outside their primary intimate relationship [78], and depressed men are more likely to rely on support from their intimate partner than nondepressed men or even depressed women [79]. Moreover, frequency of sexual activity does not appear to be associated with relationship satisfaction in depressed women [7,80]. Thus, it is possible that for men, frequent sex is a means by which the negative effects of depression on intimate relationships are buffered, and that it is this improved quality of social support which impacts immune health. For women, however, the increases in relationship quality may not play as much of a role, as they have multiple routes of social support, or the higher sexual activity may be unlinked to relationship quality.
That two different (and not mutually exclusive) mechanisms are equally plausible highlights the need for research investigating biopsychosocial mechanisms of sexuality and immune health in depressed patients.
Differences between Studies
Although the general pattern of results was similar across studies, there were some differences worth noting. In Study 1, it appeared that women with low depression followed a similar, though attenuated, trend to depressed women: though higher frequency of sexual activity was associated with lower SIgA in low-depression women, this was less pronounced than in high-depression women. In Study 2, however, there was little to no association between sexual frequency and SIgA levels in low-depression women. Interestingly, in Study 2, high-depression women had higher SIgA than low-depression women, though this difference was nonsignificant. Also, in Study 1, for men reporting no partnered sexual activity, depression was significantly associated with lower SIgA. At higher frequencies of sexual activity, however, the difference in SIgA levels between high- and low-depression men was smaller; in fact, if high-depression men reported at least some sexual activity, they did not have significantly lower SIgA than low-depression men. In Study 2, this trend was exaggerated such that high-depression men at the highest levels of sexual frequency had (nonsignificantly) higher SIgA than low-depression men at the same sexual frequency.
These differences between studies highlight the differences in the samples. Specifically, Study 1 was conducted in a large community sample encompassing a range of ages. Study 2 was conducted in a smaller sample with first-year undergraduate students only and thus restricted to young women and men. There is a negative association between age and SIgA levels, such that younger people tend to have higher SIgA than older people, even at comparable levels of health [33]. Moreover, by virtue of their stage of life, participants in Study 2 with high depression scores were less likely to have experienced chronic depression, and more likely to be experiencing depressive symptoms reactive to a major life transition. Immune responses to acute and chronic stressors differ significantly, with acute stress enhancing and chronic stress suppressing immune function [81–83]. Insofar as stress and depression are related, this may explain the higher SIgA levels in both high-depression women and men in Study 2. Similarly, participants in Study 2 were less likely to live with their partner, and more likely to be in new (or newly sexual) relationships. Women’s immune responses to sexual partners have been shown to be lower if they are in a long-term sexual relationship [84], particularly if they cohabit with their sexual partner [85]. This may explain why sexual frequency was associated with lower SIgA in both high- and low-depression women in Study 1, but only high-depression women in Study 2.
Limitations and Future Directions
Although our samples were drawn from a community rather than clinical population, we used a well-validated measure of depression, and, when relevant, classification into clinical and nonclinically relevant depression via proven cutoff points. Nevertheless, there may be unique features of immune function in severely or diagnosed depressed patients that we could not observe, and thus, generalizability of our findings to a clinical population is unknown. Also, as with all exploratory analyses, the findings reported here are preliminary and require further replication. Specifically, the studies reported here were secondary analyses conducted on previously collected data, and thus, mechanisms of these associations could not be directly tested. Examining the routes through which sexual activity modifies immunity in depressed men and women will be critical in developing clinical applications for these findings.
Our measure of depression (the GWBS-D), while well validated, is not as commonly used as other measures such as the Beck Depression Inventory [86], which makes comparison to other similar studies difficult. Also, SIgA is only one element of a complex system of responses from the immune system. It would be premature to conclude that sexual activity results in unilaterally improved immune response for depressed men, for instance, without examining other elements of the immune system such as inflammation and white blood cell production.
To confirm causality and examine the broad impact of sexual activity, we would need to experimentally manipulate level of sexual frequency in depressed and nondepressed individuals and observe the impact on a variety of immune markers. Our findings are strengthened by the consistent pattern across two samples collected separately; however it is possible that we were underpowered to detect true effects in the nondepressed sample. If this were the case, these effects would be quite small relative to the effects we observed in the depressed sample, and thus, the general conclusions of this report—that the effects of sexual activity on immunity are particularly relevant in depressed men and women—would be essentially preserved.
Conclusion
The need for an informed science of sexual health in depressed patients is critical. Rates of depression are high and rising [87] and yet little is known about sexuality in the context of depression. Considering the importance of sexuality for both mental and physical health outcomes, this is a serious obstacle to understanding and treating depression and its sequelae. Our findings underscore the importance of sexual activity for physical health of depressed women and men. Although many clinicians avoid asking depressed patients about their sexual practices [88], our findings suggest that this information may help predict immune health and thus be a critical element of taking a comprehensive health history. Moreover, our findings strongly support the need for models of health in depression that include sexuality as a key factor.
Footnotes
Given that it is plausible that either (or both) biological sex and gender role could influence depression, immune function, and sexuality, we use the term “gender/sex” to indicate a difference between men and women that cannot be attributed solely to either sex or gender.
Conflict of Interest: The authors report no conflicts of interest.
Statement of Authorship
-
Conception and DesignSari van Anders; Tierney Lorenz
-
Acquisition of DataSari van Anders
-
Analysis and Interpretation of DataTierney Lorenz; Sari van Anders
-
Drafting the ArticleTierney Lorenz; Sari van Anders
-
Revising It for Intellectual ContentTierney Lorenz; Sari van Anders
-
Final Approval of the Completed ArticleTierney Lorenz; Sari van Anders
References
- 1.Herbert TB, Cohen S. Depression and immunity: A meta-analytic review. Psychol Bull. 1993;113:472–486. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- 2.Blume J, Douglas SD, Evans DL. Immune suppression and immune activation in depression. Brain Behav Immun. 2011;25:221–229. doi: 10.1016/j.bbi.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodnar LM, Wisner KL. Nutrition and depression: Implications for improving mental health among childbearing-aged women. Biol Psychiatry. 2005;58:679–685. doi: 10.1016/j.biopsych.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Worthington J, Fava M, Agustin C, Alpert J. Consumption of alcohol, nicotine, and caffeine among depressed outpatients: Relationship with response to treatment. Psychosomatics. 1996;37:518–522. doi: 10.1016/S0033-3182(96)71515-3. [DOI] [PubMed] [Google Scholar]
- 5.Lobstein D, Mosbacher B, Ismail A. Depression as a powerful discriminator between physically active and sedentary middleaged men. J Psychosom Res. 1983;27:69–76. doi: 10.1016/0022-3999(83)90111-3. [DOI] [PubMed] [Google Scholar]
- 6.Weisse CS. Depression and immunocompetence: A review of the literature. Psychol Bull. 1992;111:475–489. doi: 10.1037/0033-2909.111.3.475. [DOI] [PubMed] [Google Scholar]
- 7.Cyranowski JM, Bromberger J, Youk A, Matthews K, Kravitz HM, Powell LH. Lifetime depression history and sexual function in women at midlife. Arch Sex Behav. 2004;33:539–548. doi: 10.1023/B:ASEB.0000044738.84813.3b. [DOI] [PubMed] [Google Scholar]
- 8.Phillips RL, Slaughter JR. Depression and sexual desire. Am Fam Physician. 2000;62:782–786. [PubMed] [Google Scholar]
- 9.Frohlich P, Meston C. Sexual functioning and self-reported depressive symptoms among college women. J Sex Res. 2002;39:321–325. doi: 10.1080/00224490209552156. [DOI] [PubMed] [Google Scholar]
- 10.Hayes RD, Dennerstein L, Bennett CM, Sidat M, Gurrin LC, Fairley CK. Risk factors for female sexual dysfunction in the general population: Exploring factors associated with low sexual function and sexual distress. J Sex Med. 2008;5:1681–1693. doi: 10.1111/j.1743-6109.2008.00838.x. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy SH, Dickens SE, Eisfeld BS, Bagby RM. Sexual dysfunction before antidepressant therapy in major depression. J Affect Disord. 1999;56:201–208. doi: 10.1016/s0165-0327(99)00050-6. [DOI] [PubMed] [Google Scholar]
- 12.Laumann EO, Nicolosi A, Glasser DB, Paik A, Gingell C, Moreira E, Wang T. Sexual problems among women and men aged 40–80 y: Prevalence and correlates identified in the Global Study of Sexual Attitudes and Behaviors. Int J Impot Res. 2004;17:39–57. doi: 10.1038/sj.ijir.3901250. [DOI] [PubMed] [Google Scholar]
- 13.Nicolosi A, Moreira ED, Villa M, Glasser DB. A population study of the association between sexual function, sexual satisfaction and depressive symptoms in men. J Affect Disord. 2004;82:235–243. doi: 10.1016/j.jad.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Culbertson FM. Depression and gender: An international review. Am Psychol. 1997;52:25–31. doi: 10.1037//0003-066x.52.1.25. [DOI] [PubMed] [Google Scholar]
- 15.Martin JT. Sexual dimorphism in immune function: The role of prenatal exposure to androgens and estrogens. Eur J Pharmacol. 2000;405:251–261. doi: 10.1016/s0014-2999(00)00557-4. [DOI] [PubMed] [Google Scholar]
- 16.Marriott I, Huet-Hudson YM. Sexual dimorphism in innate immune responses to infectious organisms. Immunol Res. 2006;34:177–192. doi: 10.1385/IR:34:3:177. [DOI] [PubMed] [Google Scholar]
- 17.Spinedi E, Gaillard RC, Chisari A. Sexual dimorphism of neuroendocrine-immune interactions. Front Horm Res. 2002;29:91–107. doi: 10.1159/000061059. [DOI] [PubMed] [Google Scholar]
- 18.Barousse MM, Theall KP, Van Der Pol B, Fortenberry JD, Orr DP, Fidel PL., Jr Susceptibility of middle adolescent females to sexually transmitted infections: Impact of hormone contraception and sexual behaviors on vaginal immunity. Am J Reprod Immunol. 2007;58:159–168. doi: 10.1111/j.1600-0897.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- 19.McKean KA, Nunney L. Increased sexual activity reduces male immune function in Drosophila melanogaster . Proc Natl Acad Sci USA. 2001;98:7904–7909. doi: 10.1073/pnas.131216398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muehlenbein MP, Bribiescas RG. Testosterone-mediated immune functions and male life histories. Am J Hum Biol. 2005;17:527–558. doi: 10.1002/ajhb.20419. [DOI] [PubMed] [Google Scholar]
- 21.Haake P, Krueger THC, Goebel MU, Heberling KM, Hartmann U, Schedlowski M. Effects of sexual arousal on lymphocyte subset circulation and cytokine production in man. Neuroimmunomodulation. 2004;11:293–298. doi: 10.1159/000079409. [DOI] [PubMed] [Google Scholar]
- 22.Coates TJ, McKusick L, Kuno R, Stites DP. Stress reduction training changed number of sexual partners but not immune function in men with HIV. Am J Public Health. 1989;79:885–887. doi: 10.2105/ajph.79.7.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Granger DA, Booth A, Johnson DR. Human aggression and enumerative measures of immunity. Psychosom Med. 2000;62:583–590. doi: 10.1097/00006842-200007000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Royle L, Roos A, Harvey DJ, Wormald MR, van Gijlswijk-Janssen D, Redwan el-RM, Wilson IA, Daha MR, Dwek RA, Rudd PM. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J Biol Chem. 2003;278:20140–20153. doi: 10.1074/jbc.M301436200. [DOI] [PubMed] [Google Scholar]
- 25.Fagarasan S, Honjo T. Regulation of IgA synthesis at mucosal surfaces. Curr Opin Immunol. 2004;16:277–283. doi: 10.1016/j.coi.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Brandtzaeg P. Do salivary antibodies reliably reflect both mucosal and systemic immunity? Ann NY Acad Sci. 2007;1098:288–311. doi: 10.1196/annals.1384.012. [DOI] [PubMed] [Google Scholar]
- 27.Woof JM, Mestecky J. Mucosal immunoglobulins. Immunol Rev. 2005;206:64–82. doi: 10.1111/j.0105-2896.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 28.Gleeson M, Bishop N, Oliveira M, McCauley T, Tauler P, Muhamad A. Respiratory infection risk in athletes: Association with antigen-stimulated IL-10 production and salivary IgA secretion. Scand J Med Sci Sports. 2011;22:410–417. doi: 10.1111/j.1600-0838.2010.01272.x. [DOI] [PubMed] [Google Scholar]
- 29.Volkmann ER, Weekes NY. Basal SIgA and cortisol levels predict stress-related health outcomes. Stress Health. 2006;22:11–23. [Google Scholar]
- 30.Klein S. The effects of hormones on sex differences in infection: From genes to behavior. Neurosci Biobehav Rev. 2000;24:627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 31.Gleeson M, Bishop N, Oliveira M, McCauley T, Tauler P. Sex differences in immune variables and respiratory infection incidence in an athletic population. Exerc Immunol Rev. 2011;17:122–135. [PubMed] [Google Scholar]
- 32.Weber-Mzell D, Kotanko P, Hauer A, Goriup U, Haas J, Lanner N, Erwa W, Ahmaida IA, Haitchi-Petnehazy S, Stenzel M, Lanzer G, Deutsch J. Gender, age and seasonal effects on IgA deficiency: A study of 7293 Caucasians. Eur J Clin Invest. 2004;34:224–228. doi: 10.1111/j.1365-2362.2004.01311.x. [DOI] [PubMed] [Google Scholar]
- 33.Evans P, Der G, Ford G, Hucklebridge F, Hunt K, Lambert S. Social class, sex, and age differences in mucosal immunity in a large community sample. Brain Behav Immun. 2000;14:41–48. doi: 10.1006/brbi.1999.0571. [DOI] [PubMed] [Google Scholar]
- 34.Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: A meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 35.Tsuboi H, Hamer M, Tanaka G, Takagi K, Kinae N, Steptoe A. Responses of ultra-weak chemiluminescence and secretory IgA in saliva to the induction of angry and depressive moods. Brain Behav Immun. 2008;22:209–214. doi: 10.1016/j.bbi.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Hucklebridge F, Lambert S, Clow A, Warburton D, Evans P, Sherwood N. Modulation of secretory immunoglobulin A in saliva: Response to manipulation of mood. Biol Psychol. 2000;53:25–35. doi: 10.1016/s0301-0511(00)00040-5. [DOI] [PubMed] [Google Scholar]
- 37.Graham N, Bartholomeusz R, Taboonpong N, La Brooy J. Does anxiety reduce the secretion rate of secretory IgA in saliva? Med J Aust. 1988;148:131–133. doi: 10.5694/j.1326-5377.1988.tb112773.x. [DOI] [PubMed] [Google Scholar]
- 38.Gold PW, Pavlatou MG, Carlson PJ, Luckenbaugh DA, Costello R, Bonne O, Csako G, Drevets WC, Remaley AT, Charney DS, Neumeister A, Kling MA. Unmedicated, remitted patients with major depression have decreased serum immunoglobulin A. Neurosci Lett. 2012;520:1–5. doi: 10.1016/j.neulet.2012.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keller PS, El-Sheikh M, Vaughn B, Granger DA. Relations between mucosal immunity and children’s mental health: The role of child sex. Physiol Behav. 2010;101:705–712. doi: 10.1016/j.physbeh.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura F, Shimizu K, Akama T, Akimoto T, Kuno S, Kono I. The effects of walking exercise training on immune response in elderly subjects. Int J Sport Health Sci. 2006;4(Special_Issue_2_2006):508–514. [Google Scholar]
- 41.Mestecky J, Fultz PN. Mucosal immune system of the human genital tract. J Infect Dis. 1999;179(suppl 3):S470–S474. doi: 10.1086/314806. [DOI] [PubMed] [Google Scholar]
- 42.Waldo FB, Van Den Wall Bake A, Mestecky J, Husby S. Suppression of the immune response by nasal immunization. Clin Immunol Immunopathol. 1994;72:30–34. doi: 10.1006/clin.1994.1103. [DOI] [PubMed] [Google Scholar]
- 43.Layward L, Finnemore A, Allen A, Harper S, Feehally J. Systemic and mucosal IgA responses to systemic antigen challenge in IgA nephropathy. Clin Immunol Immunopathol. 1993;69:306–313. doi: 10.1006/clin.1993.1185. [DOI] [PubMed] [Google Scholar]
- 44.Woof JM, Russell MW. Structure and function relationships in IgA. Mucosal Immunol. 2011;4:590–597. doi: 10.1038/mi.2011.39. [DOI] [PubMed] [Google Scholar]
- 45.Leigh JE, Shetty K, Fidel PL., Jr Oral opportunistic infections in HIV-positive individuals: Review and role of mucosal immunity. AIDS Patient Care STDS. 2004;18:443–456. doi: 10.1089/1087291041703665. [DOI] [PubMed] [Google Scholar]
- 46.Tjiong M, Out T, Schegget JT, Burger M, Van Der Vange N. Epidemiologic and mucosal immunologic aspects of HPV infection and HPV-related cervical neoplasia in the lower female genital tract: A review. Int J Gynecol Cancer. 2001;11:9–17. doi: 10.1046/j.1525-1438.2001.011001009.x. [DOI] [PubMed] [Google Scholar]
- 47.Brown SG, Morrison L, Calibuso MJ, Christiansen BA. The menstrual cycle and sexual behavior: Relationship to eating, exercise, sleep, and health patterns. Women Health. 2008;48:429–444. doi: 10.1080/03630240802575179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Charnetski CJ, Brennan FX. Sexual frequency and immunoglobulin A (IgA) Psychol Rep. 2004;94:839–844. doi: 10.2466/pr0.94.3.839-844. [DOI] [PubMed] [Google Scholar]
- 49.Laurence J. Repetitive and consistent cervicovaginal exposure to certain viral pathogens appears to protect against their sexual acquisition in some women: Potential mechanisms. J Reprod Immunol. 2003;58:79–91. doi: 10.1016/s0165-0378(02)00047-5. [DOI] [PubMed] [Google Scholar]
- 50.Pudney J, Anderson D. Innate and acquired immunity in the human penile urethra. J Reprod Immunol. 2011;88:219–227. doi: 10.1016/j.jri.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Anders SM, Goldey KL. Testosterone and partnering are linked via relationship status for women and “relationship orientation” for men. Horm Behav. 2010;58:820–826. doi: 10.1016/j.yhbeh.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 52.van Anders SM. Testosterone and sexual desire in healthy women and men. Arch Sex Behav. 2012;41:1471–1414. doi: 10.1007/s10508-012-9946-2. [DOI] [PubMed] [Google Scholar]
- 53.Fazio AF. Vital and Health Statistics Series 2, No 73. Hyattsville, MD: National Center for Health Statistics (US Department of Health, Education, and Welfare); 1977. A concurrent validational study of the NCHS General Well-Being Schedule. [PubMed] [Google Scholar]
- 54.McKenna SP, Hunt SM, Tennant A. Psychological well-being in depressed patients. Int J Methods Psychiatr Res. 1993;3:245–251. [Google Scholar]
- 55.Jonas BS, Franks P, Ingram DD. Are symptoms of anxiety and depression risk factors for hypertension? Longitudinal evidence from the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Fam Med. 1997;6:43–49. doi: 10.1001/archfami.6.1.43. [DOI] [PubMed] [Google Scholar]
- 56.Zonderman AB, Costa PT, McCrae RR. Depression as a risk for cancer morbidity and mortality in a nationally representative sample. JAMA. 1989;262:1191–1195. [PubMed] [Google Scholar]
- 57.Kendall MG, Gibbons JD. Rank correlation methods. 5th edition. London: Oxford University Press; 1990. [Google Scholar]
- 58.Vissink A, Spiikervet FK, Van Nieuw Amerongen A. Aging and saliva: A review of the literature. Spec Care Dentist. 1996;16:95–103. doi: 10.1111/j.1754-4505.1996.tb00842.x. [DOI] [PubMed] [Google Scholar]
- 59.Childers NK, Greenleaf C, Li F, Dasanavake AP, Powell WD, Michalek SM. Effect of age on immunoglobulin A subclass distribution in human parotid saliva. Oral Microbiol Immunol. 2003;18:298–301. doi: 10.1034/j.1399-302x.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- 60.Eliasson L, Birkhed D, Osterberg T, Carlen A. Minor salivary gland secretion rates and immunoglobulin A in adults and the elderly. Eur J Oral Sci. 2006;114:494–499. doi: 10.1111/j.1600-0722.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- 61.Bachour P, Yafawi R, Jaber F, Choueiri E, Abdel-Razzak Z. Effects of smoking, mother’s age, body mass index, and parity number on lipid, protein, and secretory immunoglobulin A concentrations of human milk. Breastfeed Med. 2012;7:179–188. doi: 10.1089/bfm.2011.0038. [DOI] [PubMed] [Google Scholar]
- 62.Barrett-Connor E, von Mühlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: The Rancho Bernardo Study. J Clin Endocrinol Metab. 1999;84:573–577. doi: 10.1210/jcem.84.2.5495. [DOI] [PubMed] [Google Scholar]
- 63.Seidman SN, Walsh BT. Testosterone and depression in aging men. Am J Geriatr Psychiatry. 1999;7:18–33. [PubMed] [Google Scholar]
- 64.Andersen ML, Alvarenga TF, Mazaro-Costa R, Hachul HC, Tufik S. The association of testosterone, sleep, and sexual function in men and women. Brain Res. 2011;1416:80–104. doi: 10.1016/j.brainres.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 65.Pope HG, Cohane GH, Kanayama G, Siegel AJ, Hudson JI. Testosterone gel supplementation for men with refractory depression: A randomized, placebo-controlled trial. Am J Psychiatry. 2003;160:105–111. doi: 10.1176/appi.ajp.160.1.105. [DOI] [PubMed] [Google Scholar]
- 66.Rohr U. The impact of testosterone imbalance on depression and women’s health. Maturitas. 2002;41:S25–S46. doi: 10.1016/s0378-5122(02)00013-0. [DOI] [PubMed] [Google Scholar]
- 67.Baischer W, Koinig G, Hartmann B, Huber J, Langer G. Hypothalamic-pituitary-gonadal axis in depressed premenopausal women: Elevated blood testosterone concentrations compared to normal controls. Psychoneuroendocrinology. 1995;20:553–559. doi: 10.1016/0306-4530(94)00081-k. [DOI] [PubMed] [Google Scholar]
- 68.Weber B, Lewicka S, Deuschle M, Colla M, Heuser I. Testosterone, androstenedione and dihydrotestosterone concentrations are elevated in female patients with major depression. Psychoneuroendocrinology. 2000;25:765–771. doi: 10.1016/s0306-4530(00)00023-8. [DOI] [PubMed] [Google Scholar]
- 69.Dabbs JM, Mohammed S. Male and female salivary testosterone concentrations before and after sexual activity. Physiol Behav. 1992;52:195–197. doi: 10.1016/0031-9384(92)90453-9. [DOI] [PubMed] [Google Scholar]
- 70.Morris NM, Udry JR, Khan-Dawood F, Dawood MY. Marital sex frequency and midcycle female testosterone. Arch Sex Behav. 1987;16:27–37. doi: 10.1007/BF01541839. [DOI] [PubMed] [Google Scholar]
- 71.Anderson R, Bancroft J, Wu F. The effects of exogenous testosterone on sexuality and mood of normal men. J Clin Endocrinol Metab. 1992;75:1503–1507. doi: 10.1210/jcem.75.6.1464655. [DOI] [PubMed] [Google Scholar]
- 72.Tsitouras PD, Martin CE, Harman SM. Relationship of serum testosterone to sexual activity in healthy elderly men. J Gerontol. 1982;37:288–293. doi: 10.1093/geronj/37.3.288. [DOI] [PubMed] [Google Scholar]
- 73.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 74.Besedovsky HO, Rey A. Physiology of psychoneuroimmunology: A personal view. Brain Behav Immun. 2007;21:34–44. doi: 10.1016/j.bbi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 75.Haddad JJ, Saad NE, Safieh-Garabedian B. Cytokines and neuro-immune-endocrine interactions: A role for the hypothalamic-pituitary-adrenal revolving axis. J Neuroimmunol. 2002;133:1–19. doi: 10.1016/s0165-5728(02)00357-0. [DOI] [PubMed] [Google Scholar]
- 76.Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am J Psychiatry. 2004;161:631–636. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- 77.Russell VM, McNulty JK. Frequent sex protects intimates from the negative implications of their neuroticism. Soc Psychol Pers Sci. 2011;2:220–227. [Google Scholar]
- 78.Antonucci TC, Akiyama H. An examination of sex differences in social support among older men and women. Sex Roles. 1987;17:737–749. [Google Scholar]
- 79.Vanfossen BE. Sex differences in depression: The role of spouse support. In: Hobfoll SE, editor. Stress, social support, and women. Washington, DC: Hemisphere Publishing Corporation; 1986. pp. 69–84. [Google Scholar]
- 80.De Judicibus MA, McCabe MP. Psychological factors and the sexuality of pregnant and postpartum women. J Sex Res. 2002;39:94–103. doi: 10.1080/00224490209552128. [DOI] [PubMed] [Google Scholar]
- 81.Evans P, Bristow M, Hucklebridge F, Clow A, Pang FY. Stress, arousal, cortisol and secretory immunoglobulin A in students undergoing assessment. Br J Clin Psychol. 1994;33:575–576. doi: 10.1111/j.2044-8260.1994.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 82.Dhabhar FS. Acute stress enhances while chronic stress suppresses skin immunity: The role of stress hormones and leukocyte trafficking. Ann NY Acad Sci. 2000;917:876–893. doi: 10.1111/j.1749-6632.2000.tb05454.x. [DOI] [PubMed] [Google Scholar]
- 83.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: A potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 84.Saracco A, Musicco M, Nicolosi A, Angarano G, Arici C, Gavazzeni G, Costigliola P, Gafa S, Gervasoni C, Luzzati R, et al. Man-to-woman sexual transmission of HIV: Longitudinal study of 343 steady partners of infected men. J Acquir Immune Defic Syndr. 1993;6:497–502. [PubMed] [Google Scholar]
- 85.Robillard PY, Périanin J, Janky E, Miri E, Hulsey T, Papiernik E. Association of pregnancy-induced hypertension with duration of sexual cohabitation before conception. Lancet. 1994;344:973–975. doi: 10.1016/s0140-6736(94)91638-1. [DOI] [PubMed] [Google Scholar]
- 86.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 87.Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, Wang P, Wells KB, Zaslavsky AM. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352:2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Temple-Smith M, Hammond J, Pyett P, Presswell N. Barriers to sexual history taking in general practice. Aust Fam Physician. 1996;25(9 suppl 2):S71–S74. [PubMed] [Google Scholar]