Abstract
Importance
Because early stage kidney disease is asymptomatic and is associated with both, morbidity and mortality, laboratory measurements are required for its detection.
Objective
To summarize evidence supporting the use of laboratory tests for glomerular filtration rate (GFR) and albuminuria to detect and stage acute kidney injury (AKI), acute kidney diseases and disorders (AKD), and chronic kidney disease (CKD) in adults.
Evidence Review
We reviewed recent guidelines from various professional groups and systematically searched for other sources of evidence for selected topics.
Findings
The Kidney Disease Improving Global Outcomes KDIGO guidelines define and stage acute and chronic kidney diseases by GFR and albuminuria. For initial assessment of GFR, measuring serum creatinine and reporting estimated GFR (eGFRcr) based on serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2009 equation is recommended. If confirmation of GFR is required because of conditions that affect serum creatinine independent of GFR, such as extremes of muscle mass or diet, or interference with the assay, cystatin C should be measured and estimated GFR should be calculated and reported using both serum creatinine and cystatin C (eGFRcys and eGFRcr-cys) or GFR should be measured directly using a clearance procedure. Initial assessment of albuminuria includes measuring urine albumin and creatinine in an untimed “spot” urine collection and reporting albumin-to-creatinine ratio (ACR). If confirmation of albuminuria is required because of diurnal variation or conditions that affect creatinine excretion, such as extremes of muscle mass or diet, the albumin excretion rate (AER) should be measured from a timed urine collection.
Conclusions and Relevance
Detection and staging of acute and chronic kidney diseases can be relatively simple. Because of the morbidity and mortality associated with kidney disease, early diagnosis is important and should be pursued in at-risk populations.
Introduction
Acute and chronic kidney diseases are common in adults, and are associated with increased risk for kidney failure, complications and mortality (Table 1) 1-3. Acute kidney injury (AKI) affects 10-20% of hospitalized adults and chronic kidney disease (CKD) is found in more than 10% of non-hospitalized adults. Kidney failure is the end-stage of acute and chronic kidney disease, and may require treatment by dialysis or transplantation. Earlier stages of kidney disease are 10-1000 times more common in the population than kidney failure, depending on age and the clinical setting, and are associated with electrolyte and acid-base disorders, fluid overload, metabolic and endocrine complications, toxicity of drugs excreted by the kidneys, and cardiovascular disease.
Table 1.
Definitions, Stages and Burden of Kidney Disease*
| Acute Kidney Injury (AKI) | Acute Kidney Diseases and Disorders (AKD) | Chronic Kidney Disease (CKD) | |
|---|---|---|---|
| Duration | Within 2-7 days | ≤ 3 months | > 3 months |
| Functional Criterion | Rise in serum creatinine by 50% (7days) OR Rise in serum ceatinine by 0.3 mg/dl (26.5 micromol/l) (2 days) OR Urine output <0.5 for 6 hours |
GFR <60 ml/min/1.73 m2 OR Decline in GFR by >35% times baseline OR Rise in serum creatinine by >50% times baseline |
GFR <60 ml/min/1.73 m2 |
| Structural Criterion | None required | Marker of kidney damage (hematuria or pyuria are most common) | Marker of kidney damage (albuminuria is most common) |
| Examples** | Decreased kidney perfusion (“pre-renal disorders”) Urinary tract obstruction (“post-renal” disorders) Intrinsic kidney diseases (acute tubular necrosis, acute interstitial nephritis) |
Acute and rapidly progressive glomerulonephritis Acute presentations of nephrotic syndrome Acute pyelonephritis Partial obstruction of the urinary tract |
Diabetic kidney disease Hypertensive nephrosclerosis Chronic glomerulonephritis Chronic interstitial nephritis Chronic pyelonephritis Polycystic kidney disease Chronic heart failure Chronic liver disease |
| Staging | Serum creatinine (mg/dl) and urine output (ml/kg/h) categories: Stage 1: serum creatinine rise by ≥0.3 from baseline OR serum creatinine rise by 1.5 to1.9 times baseline OR urine output <0.5 for 6-12 hours. Stage 2: serum creatinine rise by 2.0 to 2.9 times baseline OR urine output <0.5 for ≥12 hours. Stage 3: serum creatinine rise by ≥ 3.0 times baseline OR serum creatinine ≥ 4 OR renal replacement therapy |
Not defined | Albuminuria categories (ACR mg/g approximately equivalent to AER mg/d) and related terms: A1: <30, normal to mildly increased A2: 30-300, moderately increased (formerly “microalbuminuria”) A3: >300, severely increased (includes nephrotic syndrome, >~ 2000) GFR categories (ml/min/1.73 m2) and related terms: G1: >90, normal or high G2: 60-89, mildly decreased G3a: 45-59, mildly to moderately decreased G3b: 40-44, moderately to severely decreased G4: 15-29, severely decreased G5:<15 OR treated by dialysis, kidney failure |
| Burden | Incidence 10-20 % among adults requiring hospitalization (0.3% requiring dialysis) | Unknown | Prevalence ~ 10% in non-hospitalized adults (higher in the elderly) Life time risk ~ 50% Prevalence of kidney failure treated by dialysis or transplantation ~0.3% (higher in the elderly) Life time risk of kidney failure 2-8% (higher in blacks) |
GFR, glomerular filtration rate; ACR, albumin-to-creatinine ratio; AER, albumin excretion rate. Rise in serum creatinine of 0.3 mg/dl is equivalent to 26.5 micromol/l. ACR of 30, 300 and 2000 mg/g are approximately equivalent to 3, 30 and 200 mg/mol, respectively. Modified from Eckardt KU, Coresh J, Devuyst O, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. Jul 13 2013; 382(9887):158-169.3
Additional diagnostic testing is required to determine the cause of disease and treatment.
Early detection facilitates the appropriate diagnosis and treatment of acute and chronic kidney diseases, but early stage kidney disease is usually asymptomatic requiring laboratory tests for detection. Measurement of serum creatinine and urine protein are frequently performed in the general medical evaluation of adults with acute and chronic illness. Serum creatinine is routinely measured in the basic metabolic panel and proteinuria is ascertained along with routine urinalyses. However, until recently, uncertainty and controversy existed regarding the definitions for acute and chronic kidney diseases, which tests should be obtained to diagnose these conditions and how the tests should be reported and interpreted (Box 1).
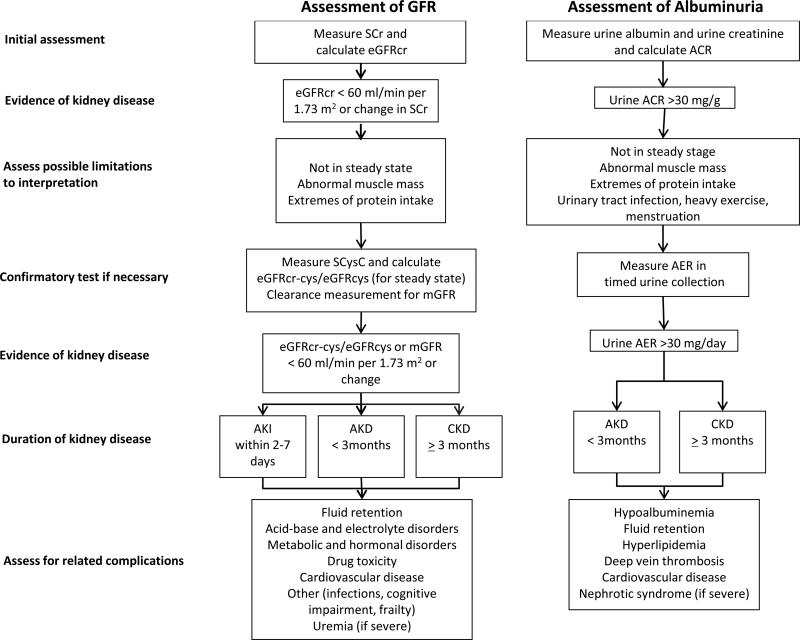
The international organization Kidney Disease Improving Global Outcomes (KDIGO) attempted to resolve these controversies by updating prior evidence based consensus definitions and staging systems for acute and chronic kidney diseases and for the proper laboratory evaluation of these diseases1,2. These guidelines use glomerular filtration rate (GFR), generally accepted as the best index of kidney function in health and disease, and albuminuria, a marker of kidney damage, as the principal kidney measures to define and stage acute and chronic kidney diseases. The guidelines also provide recommendations for the initial and confirmatory tests for these diseases (Figure 1). The evaluation of GFR and albuminuria is reviewed here in the context of the KDIGO guidelines, the guidelines are compared to other recent guidelines and more recently published literature, and areas of uncertainty are addressed.
Figure 1. Assessment of GFR and Albuminuria for Detection of Acute and Chronic Kidney Disease.
Figure illustrates stepwise use of initial and confirmatory tests for GFR and albuminuria for detection of acute and chronic kidney diseases and their association with complications.
Abbreviations: GFR, glomerular filtration rate: SCr, serum creatinine; SCysC, serum cystatin C; ACR albumin-to-creatinine ratio; AER, albumin excretion rate; CKD, chronic kidney disease; AKI, acute kidney injury; AKD, acute kidney diseases and disorders.
Sources of Evidence
The most recent KDIGO guidelines were reviewed1,2. These were developed by an independent and global group of volunteers with expertise in kidney disease supported by a professional evidence review team. Guidelines and evidence reviews are submitted for open review by experts, stakeholders and the public. The KDIGO guidelines on AKI and CKD were published in 2012 and 2013, respectively1,2. Additional searches of the literature focused on the evaluation of kidney disease through December 2014 were performed. The National Guideline Clearance House was searched for kidney disease testing guidelines. The creatinine-based GFR estimating equations were reviewed by Earley et al4 in 2011 and MEDLINE was searched for any newer literature on this topic. It was also searched for cystatin C-based equations that may have been reported after the 2012 KDIGO guideline. The review was restricted to equations developed and evaluated using standardized assays for creatinine and cystatin C. GFR measurement methods were reviewed by Stevens and Levey in 20095 and MEDLINE was searched for systematic reviews that may have appeared after that publication. Testing for CKD in high-risk populations was reviewed by Deo et al in 20106. The National Guideline Clearance House was searched for guidelines appearing after this publication regarding this topic. MEDLINE was searched for systematic reviews or meta-analyses on methods for albuminuria testing in high-risk populations that may have appeared subsequent to publication of the KDIGO CKD guideline2. For all MEDLINE searches, searches were limited to publications using human subjects and in the English language. (Additional information regarding the search strategies and number of articles reviewed is contained in the supplementary material.)
Definition, Staging and Burden of Acute and Chronic Kidney Disease
Acute and chronic kidney diseases encompass a spectrum of disorders that are defined by measures of kidney structure, function or disease duration, irrespective of the causes for kidney disease(Table 1)1-3. Acute kidney injury (AKI) is defined as a rise in serum creatinine within 2-7 days or oliguria1. Acute kidney diseases and disorders (AKD) refer to less than 3 months of having decreased kidney function or the presence of a marker of kidney damage and include AKI1. Chronic kidney disease (CKD) is defined by having more than 3 months of decreased GFR or evidence of kidney damage2. Markers of kidney damage include albuminuria, urine sediment abnormalities, electrolyte abnormalities related to tubular disorders, or structural abnormalities detected by histology or imaging.
Both CKD and AKI are classified (“staged”) by cause and severity of abnormal kidney measures, with more severe stages corresponding to poorer prognosis (Table 1). AKI is classified by the magnitude of the peak rise in serum creatinine concentration or nadir decline in urine output1. CKD is classified according to cause (C) and level of GFR (G) and albuminuria (A). referred to as the “CGA nomenclature” 2. For both conditions, additional diagnostic testing is required to determine the cause of kidney disease and guide its treatment7-9.
Recommendations for staging, evaluation and treatment have not yet been developed for AKD.
Origin and Rationale for the KDIGO Guidelines and Concurrence of Other Organizations
Defintions for acute and chronic kidney disease were proposed and generally accepted before the formation of KDIGO (Table 2) 1,2,10-19.
Table 2.
Origin of KDIGO Guideline Recommendations on the Definition and Staging of Acute and Chronic Kidney Diseases and Concurrence of Other Evidence-Based Guidelines and Commentaries
| Acute Kidney Injury (AKI) | Acute Kidney Diseases and Disorders (AKD) | Chronic Kidney Disease (CKD) | |
|---|---|---|---|
| KDIGO Guidelines | Harmonizes prior ADQI 10 and AKIN 11 definitions and staging based on rise in Scr and oliguria1 | New definition to include conditions that do not meet the criteria for AKI or CKD1 | Retains prior NKF-KDOQI16 definition and modifies staging to include cause and level of albuminuria in addition to GFR (“CGA” nomenclature)2 |
| NKF-KDOQI Commentary | Concurs with KDIGO definition criterion of 50% rise in SCr, but expresses reservations about criteria of oliguria and 0.3 mg/dl rise in SCr in patients with decreased baseline GFR. Concurs with KDIGO staging12. | Suggests evidence is not sufficient to implement the definition12 | Concurs with KDIGO definition. Expresses reservations that including cause of disease in staging will be difficult for non-kidney specialists 17. |
| CSN Commentary | Concurs with KDIGO definition based on 50% increase in serum creatinine. Concurs with KDIGO staging 13. | Suggests evidence is not sufficient to implement the definition13 | Concurs with KDIGO definition. Suggests including an additional albuminuria category for nephrotic range proteinuria18. |
| NICE Guideline | Concurs with KDIGO definition and staging14 | Not discussed14 | Concurs with KDIGO definition. Does not include cause of disease in staging19. |
| KHA-CARI Commentary | Concurs with KDIGO definition. Suggests staging based on duration rather than peak rise in SCr15. | Not discussed15 | NA |
Abbreviations: GFR glomerular filtration rate; SCr, serum creatinine; CGA, cause, GFR, albuminuria; NA, not applicable.
Worldwide Organizations:
KDIGO – Kidney Disease Improving Global Outcomes (http://kdigo.org)
ADQI – Acute Dialysis Quality Initiative (http://www.adqi.org)
AKIN – Acute Kidney Injury Network (http://www.akinet.org)
Regional and National Organizations:
NKF-KDOQI – National Kidney Foundation Kidney Disease Outcomes Quality Initiative, a US non-governmental organization (http://www.kidney.org/professionals/KDOQI).
NICE - National Institute for Clinical Excellence, a program within the Department of Health in England (http://www.nice.org.uk)
CSN - Canadian Society of Nephrology, a Canadian non-governmental organization (https://www.csnscn.ca/committees/clinical-practice-guidelines)
KHA-CARI – Kidney Health Australia Caring for Australasians with Renal Impairment, a non-governmental organization (http://www.cari.org.au)
AKI was first defined by increased serum creatinine of 1.5 times baseline within a 7 day time period or a urine volume of less than 0.5 ml/kg/h for 6 hours10. Subsequently, the definition was extended to include increased serum creatinine of ≥0.3 mg/dl (≥26.5 micromol/l) within 2 days.11 All criteria are retained by the KDIGO definition1 because they are all associated with increased risks for adverse short-term and long-term outcomes, including failure to recover kidney function, multi-organ failure, length of hospitalization and mortality.20-27.
AKD was first defined by KDIGO1 to include important presentations of kidney diseases and disorders that do not meet the criteria for either AKI or CKD. As with AKI and CKD, it was thought that acceptance of a nomenclature and definition for these conditions could potentially facilitate clinical communication, conduct of epidemiologic studies, and development of guidelines to improve care.
CKD was first defined by 3 months or longer of reduced GFR (less than 60 ml/min/1.73 m2) or having markers of kidney damage (e.g. albumin excretion rate (AER) more than 30 mg/d) and disease severity was staged only by GFR16. Because of uncertainty regarding threshold values for GFR and albuminuria in defining and staging CKD28, a series of meta-analysis were performed to better understand the relationship between these and outcomes of kidney failure and all-cause and CVD mortality29-38. Based on this analysis, KDIGO added staging by level of albuminuria in addition to level of GFR, and subdivided GFR stage 3 (30-59 ml/min/1.73 m2) into 3a and 3b (30-44 and 45-59 ml/min/1.73 m2, respectively) to better reflect prognosis (Table 1).2
Other groups subsequently expressed general agreement with the definitions of AKI and CKD, but expressed reservations about some components of the staging systems for AKI and CKD and felt there was not sufficient evidence to implement the definition of AKD (Table 2).12-15,17-19
Evaluation of GFR
Basic Principles
Glomerular filtration, the first step in urine formation, is the passive process of ultrafiltration of plasma from blood into Bowman's space as it traverses the glomerular capillaries. Because GFR varies by body size, it is indexed relative to an average body surface area (BSA) of 1.73 m2 and expressed as ml/min/per 1.73 m2. GFR also varies by time of day, protein intake, pregnancy, extracellular fluid status, blood pressure extremes, use of antihypertensive agents, and the presence and severity of kidney disease. The mean value is approximately 120-130 ml/min/1.73 m2 for adults younger than age 40 years and declines with age. A GFR of less than 60 ml/min/1.73 m2 is considered as moderately decreased for adults of any age.
GFR cannot be measured directly; it can be assessed from clearance measurements or estimated from serum levels of endogenous filtration markers, such as creatinine or cystatin C39,40. Clearance measurements require either multiple measurements of serum concentrations or timed urine collections and are not routinely performed. GFR is usually estimated from serum concentrations of a marker of filtration using GFR estimating equations. These equations account for non-GFR related factors that influence marker serum concentrations, including the rate of the generation, renal tubular reabsorption or secretion, and extra-renal elimination of the marker. The most accurate estimating equations were developed using standardized assays for creatinine and cystatin C measured in diverse populations. The estimating equations have minimal systematic bias (average deviation from the measured GFR), but are relatively imprecise, with approximately 10-20% of estimates deviating by more than 30% from the measured GFR.
Initial Tests
Serum creatinine by itself should not be relied on to assess kidney function. When serum creatinine is measured, estimated GFR (eGFRcr) should be calculated and reported by the clinical laboratory (Box 2)2. eGFRcr is best calculated using the 2009 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation41. This equation is preferable to the Modification of Diet in Renal Disease (MDRD) Study equation42 because it is more accurate in higher ranges of eGFR (>60 ml/min/1.73 m2) and allows reporting eGFRcr as a numeric value throughout the entire range. Other guidelines and commentaries concurred with the recommendation to use the CKD-EPI 2009 creatinine equation17,18,19. Our search revealed several new equations since the KDIGO guideline review, but none more accurate in North America, Europe and Australia than the CKD-EPI 2009 creatinine equation (see Supplement)
There are situations where eGFRcr may not be accurate (Table 3). These include clinical conditions that influence non-GFR factors that affect serum creatinine concentration, creatinine not being in a steady state (as occurs when GFR is rapidly changing, for example during AKI), and presence of substances in the blood that interfere with serum creatinine assays. Creatinine is a 113 dalton amino acid metabolite distributed throughout the total body water compartment and freely filtered by the glomeruli. Non-GFR factors include deviation in generation of creatinine generation due to extremes of muscle mass and ingestion in the diet, inhibition of secretion by the renal tubule (trimethoprim and fenofibrate), and interference with extra-renal elimination by gut bacteria (broad spectrum antibiotics). The 2009 CKD-EPI and MDRD Study equations both compute eGFR from serum creatinine plus age, sex and race (African American vs. non-African American) as surrogates for muscle mass and report values indexed to 1.73 m2 BSA. Deviation from expected values for creatinine generation by muscle mass and diet are major causes of error in eGFRcr. Even using standardized assays, interfering substances, such as ketones, can also lead to errors in eGFRcr.
Table 3.
Primary Use of Estimated GFR using Creatinine or Cystatin C and Urine Albumin-to-Creatinine Ratio and Sources of Error in Interpretation
| eGFRcr | eGFRcys | Urine ACR | |
|---|---|---|---|
| Primary use* | Initial test for assessment of GFR | Confirmatory test for assessment of GFR | Initial test for assessment of albuminuria |
| Non-steady state (AKI) | Change in eGFR lags behind the change in mGFR (eGFR overestimates mGFR when mGFR is declining and underestimates mGFR when mGFR is rising) | Change in eGFR lags behind the change in mGFR (eGFR overestimates mGFR when mGFR is declining and underestimates mGFR when mGFR is rising) | ACR overestimates AER when mGFR is declining (creatinine excretion is decreased) and underestimates AER when mGFR is rising (creatinine excretion is increased) |
| Non-GFR factors** | Directly measured in clinical studies | Hypothesized from clinical observations and epidemiologic studies | NA |
| Factors affecting generation | Decreased by large muscle mass, high protein diet, ingestion of cooked meat and creatine supplements Increased by small muscle mass, limb amputation, muscle wasting diseases | Decreased in hyperthyroidism, glucocorticoid excess, and possibly obesity, inflammation and smoking Increased in hypothyroidism |
Decreased by large muscle mass (higher urinary creatinine concentration) Increased by small muscle mass (lower urinary creatinine excretion). |
| Factors affecting tubular reabsorption of secretion | Decreased by drug-induced inhibition of secretion (trimethoprim, cimetidine, fenofibrate) | NA | NA |
| Factors affecting extra-renal elimination | Decreased by inhibition of gut creatininase by antibiotics Increased by dialysis, large losses of extracellular fluid (drainage of pleural fluid or ascites) |
Increased by large losses of extracellular fluid (drainage of pleural fluid or ascites) | NA |
| Range | Less precise at higher GFR, due to higher biological variability in non-GFR determinants relative to GFR, and larger measurement error in SCr and GFR | Less precise at higher GFR, due to higher biological variability in non-GFR determinants relative to GFR, and larger measurement error in SCysC and GFR | Less precise at lower ACR, due to higher biologic variability in AER, and larger measurement error in urine albumin concentration |
| Interference with assays | Spectral interferences (bilirubin, some drugs) Chemical interferences (glucose, ketones, bilirubin, some drugs) | NA | Very high urine albumin concentration (“prozone effect”) |
| Interfering conditions | NA | NA | Contamination with albumin in menstrual blood and lower urinary tract inflammation |
Reference test for GFR is measured GFR (mGFR) using clearance methods; reference test for albuminuria is albumin excretion rate (AER) in timed urine collection.
Effects of factors affecting non-GFR determinants refer to effects on eGFR
Abbreviations: GFR, glomerular filtration rate; ACR, albumin-to-creatinine ratio; AER, albumin excretion rate; SCr, serum creatinine; SCysC, serum cystatin C; AKI, acute kidney injury
Using the CKD-EPI 2009 creatinine equation, 50%, 100% and 200% increases in serum creatinine during steady state conditions reflect 39%, 57% and 74% decreases in eGFRcr. However, during AKI creatinine may be in the non-steady state condition, and the eGFRcr is a less accurate estimate of the measured GFR (Table 3). Nonetheless, reporting eGFRcr in AKI may be useful since changes in eGFR reflect both the direction and magnitude of kidney function changes in terms of GFR, simplifying the interpretation of changing kidney function in patients with decreased baseline GFR.
Confirmatory Tests
In clinical conditions when eGFRcr is anticipated not to be accurate enough for clinical decision making (as described above and in Table 3), confirmatory tests should be pursued to better estimate GFR. These include estimating GFR using serum cystatin C (with or without an accompanying serum creatinine) or a clearance measurement (Box 2).
Cystatin C is an alternative endogenous filtration marker that gained acceptance in recent years43. eGFRcys and eGFRcr-cys should reported using the CKD-EPI 2012 equations44 when serum cystatin C is measured (Box 2)1. There are several new equations for estimating eGFRcys and eGFRcr-cys, but none are more accurate than the CKD-EPI 2012 equations (see Supplement). Using a second endogenous filtration marker to estimate GFR improves the precision of GFR estimates over what can be achieved using only one marker. However, clinicians should understand the clinical settings in which eGFRcys and eGFRcr-cys are less accurate (Table 3). Cystatin C is a 13,300 dalton serum protein that is freely filtered, reabsorbed and extensively catabolized by the renal tubule. It is produced by all nucleated cells and distributed throughout the extracellular fluid. Serum concentrations of cystatin C are less influenced by muscle mass and diet than creatinine. Non-GFR factors that affect the serum concentrations of cystatin C are not well understood, but are thought to include thyroid and glucocorticoid hormones, obesity, inflammation and smoking. Differences between the non-GFR factors that affect serum cystatin C and creatinine probably account for the stronger association between diminished eGFRcys than eGFRcr and all-cause and CVD mortality45. Although a standardized reference material is now available, considerable variation remains among cystatin C assays 46,47 and it is more costly than serum creatinine measurements.
The KDIGO guideline focuses on a relatively common clinical situation when estimation of GFR using cystatin C in addition to creatinine may be helpful - confirmation of CKD2. In some patients, moderate-to-severe decrease in eGFRcr (45-59 ml/min/1.73 m2) may be the only indication for the diagnosis of CKD. In these patients, eGFRcr-cys <60 ml/min/1.73 m2 is associated with greater likelihood of mGFR <60 ml/min/1.73 m2 and a worse prognosis than patients with eGFRcr-cys ≥60 ml/min/1.73 m2 44,45. Confirmation of CKD may be particularly helpful for decisions whether or not to avoid agents and medications that are toxic to the kidneys (e.g. iodinated radiocontrast, non-steroid anti-inflammatory drugs, aminoglycoside antibiotics). Other situations in which it may be helpful to have a more accurate GFR estimate are to adjust the dose of a medication with systemic toxicity that is excreted by glomerular filtration (e.g., methotrexate or carboplatinum) or in the evaluation of kidney donors.
In patients in whom eGFRcr is likely to be inaccurate due to non-GFR factors affecting serum creatinine or interference with creatinine assays and in whom there are likely minimal non-GFR factors affecting cystatin C(as described above and in Table 3), it may be preferable to rely on eGFRcys rather than eGFRcr-cys. This has not been widely studied but a recent publication describes better performance of eGFRcys vs eGFRcr or eGFRcr-cys in amputees48. eGFRcys is less influenced by race and ethnicity than eGFRcr or eGFRcr-cys, potentially allowing GFR estimation without specification of race.
NICE concurred with the recommendation to use eGFRcr-cys for confirmation of CKD19. KDOQI and CSN agreed the cystatin C has promise as an alternative filtration marker, but recommended against widespread use because of concerns that incomplete understanding of non-GFR factors affecting its serum concentration and higher costs and incomplete standardization of assays17,18.
If even more accurate GFR assessment is required, the KDIGO guidelines recommend a clearance measurement using an exogenous filtration marker. A variety of exogenous filtration markers are available for use in either urinary or plasma clearance techniques. A recent systematic review evaluated the accuracy of alternative methods in comparison to the classic procedure of the urinary clearance of inulin49. Of note, urinary creatinine clearance did not meet the criteria for accuracy due to large systematic bias and imprecision.
In conclusion, serum creatinine and eGFRcr should be the intial test for the assessment of kidney function. If it is not sufficiently accurate for clinical decision making, cystatin C can be measured for estimation of eGFRcr-cys andr eGFRcys, or GFR can be measured using a clearance procedure.
Evaluation of Albuminuria
Principles
Normal urine contains a variety of proteins, including filtered serum proteins and proteins derived from the kidney and urinary tract. The glomerular capillary wall hinders the passage of albumin and other large serum proteins into Bowman's space. Larger body size, upright posture, pregnancy, exercise, fever and activation of the renin-angiotensin system are associated with higher albumin excretion, and there is significant diurnal and day-to-day variation. The mean value for albumin excretion rate (AER) is 5-10 mg per day in young adults and generally rises with age. AER more than 30 mg/d generally reflects an alteration in structure of the glomerular capillary wall.
Accurate assessment of the albumin excretion rate requires collection of a timed urine specimen, which is inconvenient and can be inaccurate due to errors in timing, incomplete bladder emptying, incomplete collections and spills. To overcome the difficulty in collecting a timed urine collection, albuminuria is generally assessed from measures of albuminuria in a “spot” urine sample (Table 3).
Initial assessment
A variety of methods are available. Measuring the albumin-tocreatinine ratio (ACR) in an untimed specimen is the preferred approach (Box 3)2. Measurement of albumin rather than total protein is preferred because the method for quantifying total urine protein cannot be standardized because of its variable composition. Recently, the international standard reference material for serum albumin measurement was adopted as the standard reference material for urine albumin measurement, enabling the standardization of urine albumin testing50. However, current methods for albumin measurement are based on immunoassays, which are more expensive than methods used for total urine protein measurement.
The rationale for preferring measurement of ACR and PCR (protein-to-creatinine ratio) to albumin and total protein concentration is to overcome variation in urine concentration and dilution. Many studies show high correlations between urine ACR and PCR in untimed “spot” samples with AER and PER (protein excretion rate) in timed urine specimens.16 Because average values of creatinine excretion exceed 1.0 g/day, urine ACR and PCR in mg/g generally exceed AER and PER in mg/day, but the relationship between them varies by body size and other factors affecting creatinine generation. Clinical laboratories should measure creatinine when urine albumin or total protein are requested, and express the results as ACR or PCR in addition to albumin or total protein concentration. To overcome variation by creatinine generation, some investigators have proposed estimating creatinine excretion rate (CER) and multiplying this quantity by ACR to estimate AER51.
Reagent strips allow inexpensive, point-of-care, semi-quantitative assessment of urine total protein concentration. Reagent strips (“dipsticks”) are more sensitive to albumin than other proteins, but lack specificity. Automated readers are more accurate than manual reading of reagent strips. For all methods, an early morning sample is preferred, as it minimizes variation due to diurnal variation in albumin excretion and in urine concentration. Other guidelines and commentaries concurred with these recommendations17,18.
Confirmatory Tests
Clinicians should understand the clinical settings in which urine ACR is less accurate (Table 3). They should know when confirmation of initial testing with additional untimed urine specimens or timed urine specimens is necessary and when a more accurate assessment of albuminuria is required. Examples include the detection of early diabetic kidney disease (previously termed “microalbuminuria”) or evaluation of potential kidney transplant donors.
Areas of Uncertainty
Indications for testing for acute and chronic kidney disease
Current guidelines do not recommend screening for kidney disease in the general population in the US52. However, most guidelines recommend testing for CKD in high-risk populations, including patients with hypertension, diabetes, cardiovascular disease, HIV infection, or a family history of kidney failure (see Supplement). Monitoring kidney function is also recommended during chronic therapy with many common medications, including diuretics and non-steroidal anti-inflammatory drugs, and many newer drugs used for HIV and cancer chemotherapy. Patients with acute medical conditions undergo frequent laboratory testing that can reveal the presence of acute and chronic kidney disease. The high prevalence of kidney diseases in the general population and the low cost of serum creatinine assays and urinalysis support these practices. However, like many accepted practices in clinical medicine, there have been not been large scale randomized trials to assess the benefits, harms and cost-effectiveness.
Assessment of duration of kidney disease
A frequent clinical problem is the initial evaluation of the patient in whom decreased eGFRcr or albuminuria is discovered for the first time. Current studies do not provide substantial information about the relative frequency of acute or chronic kidney disease in this setting. The importance of evaluation depends on the patient's clinical condition. AKI may require urgent attention to avoid life threatening complications, while CKD can generally be managed as an outpatient. The first step is to review prior medical records for past evidence of decreased GFR, albuminuria (or proteinuria) or other markers of kidney damage. In patients with acute illness, monitoring urine output and serum creatinine will allow early detection of AKI. In the absence of other data, repeat testing within 1-2 weeks is indicated. A renal ultrasound is helpful since detection of small kidneys provides unequivocal evidence of CKD.
Special populations (elderly, racial/ethnic minorities and obese)
Decreased GFR and albuminuria are common in the elderly, especially in association with CVD and CVD risk factors, but the cause of kidney disease is often not known with certainty. There is debate about whether these abnormalities reflect “aging” or “disease”53,54, and as with other common chronic conditions in the elderly, there is debate about “overdiagnosis”55. Nevertheless, it is prudent to assess decreased eGFR for purposes of drug dosing, and avoiding drugs that are toxic to the kidneys (as described before). Knowing the level of eGFR and urine ACR in combination with age and sex is also useful in predicting the risk of kidney failure56. Racial and ethnic minorities in the US are at increased risk for CKD. Racial/ethnic variation in creatinine generation leads to uncertainty in the diagnostic thresholds for disease definition or staging based on eGFRcr and ACR within these groups in the US and throughout the world. eGFRcys may be more accurate than eGFRcr in racial/ethnic groups other than whites and African Americans. Obesity is a risk factor for CKD. Body size influences GFR, and generation of both creatinine and cystatin C, making it difficult to interpret measured and estimated GFR, whether or not they are indexed by BSA57. At the present time, the GFR and albuminuria thresholds for the definition of kidney disease are based on risk associations and do not differ by age, race/ethnicity or body size, but this remains an important topic for research.
Drug dosing
There are conflicting recommendations about methods for assessment of kidney function for drug development and drug dosing58. The 2011 KDIGO clinical update on drug dosing in patients with acute and chronic kidney diseases recommended using GFR rather than creatinine clearance to evaluating kidney function for drug dosing. They also recommended use of the most accurate method for GFR evaluation for each patient rather than relying on estimated creatinine clearance from the Cockcroft-Gault equation59. The MDRD Study equation and CKD-EPI 2009 creatinine equation are both more accurate than the Cockcroft-Gault equation for estimating measured GFR40. Since drug dosing is based on body size, it is important to express GFR as ml/min, without indexing for BSA. To convert eGFR from ml/min/1.73 m2 to ml/min, multiply by BSA/1.73.
Methods for albuminuria testing in high risk populations
A recent meta-analysis showed that automated reagent strips had lower sensitivity than ACR for detecting albuminuria in patients with diabetes and hypertension60. Another meta-analysis showed that albumin concentration and ACR had similar performance characteristics in detecting albuminuria in patients with diabetes61. It is likely that ACR would be more accurate for distinguishing among categories of albuminuria as defined by KDIGO2 and monitoring changes overtime.
Evaluating CKD progression
There is no accepted definition for CKD progression. For clinical trials of drugs intended to slow the progression of of CKD, the US Food and Drug Administration uses a doubling of baseline serum creatinine, equivalent to a 57% decline in eGFR, as a surrogate endpoint for kidney failure. Unfortunately, doubling of serum creatinine is a late event in CKD. Data from recent meta-analyses suggest that smaller changes in eGFR (30% or 40%) or a doubling of urine albumin are associated with a higher risk of subsequent kidney failure and mortality62-64. It seems reasonable to use these measures in clinical practice.
Supplementary Material
Box 1. Summary of Key Messages.
Acute and chronic kidney diseases are common and can be detected by simple and inexpensive laboratory tests that are frequently performed in clinical practice (serum creatinine to and urinalysis).
Glomerular filtration rate (GFR) and albuminuria are the main kidney measures used for detection, staging and management of acute and chronic kidney disease. GFR is a measure of kidney function; albuminuria is a marker of kidney damage.
Current guidelines do not recommend screening for kidney disease in the general population in the US. However, most guidelines recommend testing for CKD in high-risk populations.
Initial tests for evaluation of GFR include serum creatinine to estimate GFR (eGFRcr) and urine albumin-to-creatinine ratio (ACR).
Confirmatory tests should be performed when there is uncertainty if the accuracy of initial tests is sufficient for clinical decision-making.
Confirmatory tests for GFR include serum cystatin C with or without creatinine to estimate GFR or clearance measurements. Confirmatory tests for albuminuria include albumin excretion rate in a timed urine collection.
Box 2.
KDIGO Recommendations for Evaluation of GFR and Comments*
| General recommendations | |
| Use serum creatinine and a GFR estimating equation for initial assessment (1A). | |
| Use additional tests (such as cystatin C or a clearance measurement) for confirmatory testing in specific circumstances when eGFR based on serum creatinine is less accurate (2B). | |
| Initial Testing Using Creatinine | |
| Recommendations to clinicians | Use a GFR estimating equation to derive GFR from serum creatinine (eGFRcr) rather than relying on the serum creatinine concentration alone (1B). Understand clinical settings in which eGFRcr is less accurate (1B). See Table 3. |
| Recommendations to clinical laboratories | Measure serum creatinine using a specific assay with calibration traceable to the international standard reference materials and minimal bias compared to isotope-dilution mass spectrometry (IDMS) reference methodology (1B). Report eGFRcr in addition to the serum creatinine concentration in adults and specify the equation used whenever reporting eGFRcr (1B). Report eGFRcreat in adults using the 2009 CKD-EPI creatinine equation. An alternative creatinine-based GFR estimating equation is acceptable if it has been shown to improve accuracy of GFR estimates compared to the 2009 CKD-EPI creatinine equation (1B). |
| Confirmatory Testing Using Cystatin C | |
| Recommendations to clinicians | Use a GFR estimating equation to derive GFR from serum cystatin C rather than relying on the serum cystatin C concentration alone (2C). Understand clinical settings in which eGFRcys and eGFRcr-cys are less accurate (2C). See Table 3. |
| Recommendations to clinical laboratories | Measure serum cystatin C using an assay with calibration traceable to the international standard reference material (1B). Report eGFR from serum cystatin C in addition to the serum cystatin C concentration in adults and specify the equation used whenever reporting eGFRcys and eGFRcr-cys (1B). Report eGFRcys and eGFRcr-cys in adults using the 2012 CKD-EPI cystatin C and 2012 CKD-EPI creatinine-cystatin C equations, respectively, or an alternative cystatin C-based GFR estimating equations if they have been shown to improve accuracy of GFR estimates compared to the 2012 CKD-EPI equations (1B). |
| Confirmation of CKD | Measure cystatin C in adults with eGFRcr 45-59 ml/min/1.73m2 who do not have markers of kidney damage (2C): If eGFRcys/eGFRcr-cys is also <60 ml/min/1.73 m2, the diagnosis of CKD is confirmed. If eGFRcys/eGFRcr-cys is ≥60 ml/min/1.73 m2, the diagnosis of CKD is not confirmed. |
| Confirmatory Testing Using Measured GFR | |
| Recommendations to clinicians | Measure GFR using an exogenous filtration marker under circumstances where more accurate ascertainment of GFR will impact on treatment decisions (2B). |
GFR, glomerular filtration rate; eGFR, estimated GFR; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration. KDIGO recommendations 1.4.3.1-8. From KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013 Jan; 3(1):1-150. Within each recommendation, the strength of recommendation is indicated as Level 1 (“We recommend ...”), Level 2 (“We suggest ...”), or Not Graded, and the quality of the supporting evidence is shown as A (high), B (moderate), C (low), or D (very low).
Box 3.
KDIGO Recommendations for Evaluation of Albuminuria*
| General recommendations | |
| Use untimed urine specimens for initial assessment | |
| Use repeat untimed urine specimens or timed urine specimens for confirmatory testing in specific circumstances when single measurements of untimed urine specimens are less accurate | |
| Initial Testing Using Untimed Urine Specimens | |
| Recommendations to clinicians | Use the following measurements for initial testing of proteinuria (in descending order of preference, in all cases an early morning urine sample is preferred) (2B): (1)urine albumin-to-creatinine ratio (ACR); (2)urine protein-to-creatinine ratio (PCR); (3)reagent strip urinalysis for total protein with automated reading; (4)reagent strip urinalysis for total protein with manual reading. |
| Recommendations to clinical laboratories | Report ACR and PCR in untimed urine samples in addition to albumin concentration or proteinuria concentrations rather than the concentrations alone (1B). The term microalbuminuria should no longer be used by laboratories (Not Graded). |
| Confirmatory Testing, Using Repeat Measurements of Untimed Urine Specimens or Timed Urine Specimens | |
| Recommendations to clinicians | Understand clinical settings that may affect interpretation of measurements of albuminuria and order confirmatory tests as indicated (Not Graded). See Table 3. Confirm reagent strip positive albuminuria and proteinuria by quantitative laboratory measurement and express as a ratio to creatinine wherever possible. Confirm ACR ≥30 mg/g (≥3mg/mmol) on a random untimed urine with a subsequent early morning urine sample. If a more accurate estimate of albuminuria or total proteinuria is required, measure albumin excretion rate or total protein excretion rate in a timed urine sample. |
| Confirmatory Testing for Non-Albumin Proteinuria | |
| Recommendations to clinicians | If significant non-albumin proteinuria is suspected, use assays for specific urine proteins (e.g., al-microglobulin, monoclonal heavy or light chains, [known in some countries as “Bence Jones”proteins]) (Not Graded). |
KDIGO recommendations 1.4.4.1-4. From KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013 Jan; 3(1):1-150. Within each recommendation, the strength of recommendation is indicated as Level 1 (“We recommend...”), Level 2 (“We suggest...”), or Not Graded, and the quality of the supporting evidence is shown as A (high), B (moderate), C (low), or D (very low).
Acknowledgement
Dr. Levey was chair of the workgroup for the 2002 KDOQI CKD guideline and member of the workgroup for the 2012 KDIGO CKD guidelines. Dr. Inker was co-chair of the workgroup for 2013 KDOQI commentary on the 2012 KDIGO CKD guideline. Dr. Levey and Inker are principal investigator and clinical director for the CKD-EPI research group, which developed the CKD-EPI equations for GFR estimation. The CKD-EPI research group has received grant support from the NIDDK U01 DK 053869. Drs. Levey and Inker have applied for a patent for precise estimation of GFR using a panel of filtration markers.
Contributor Information
Andrew S. Levey, Tufts Medical Center Boston, MA.
Cassandra Becker, Tufts Medical Center Boston, MA.
Lesley A. Inker, Tufts Medical Center Boston, MA.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney international. 2013;3(Supplement)(1):136–150. [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) KDIGO Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney inter. 2013;3(Suppl.)(1):1–150. [Google Scholar]
- 3.Eckardt KU, Coresh J, Devuyst O, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013 Jul 13;382(9887):158–169. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 4.Earley A, Miskulin D, Lamb EJ, Levey AS, Uhlig K. Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Annals of internal medicine. 2012 Jun 5;156(11):785–795. doi: 10.7326/0003-4819-156-11-201203200-00391. [DOI] [PubMed] [Google Scholar]
- 5.Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. Journal of the American Society of Nephrology : JASN. 2009 Nov;20(11):2305–2313. doi: 10.1681/ASN.2009020171. [DOI] [PubMed] [Google Scholar]
- 6.Deo A, Sarnak M, Uhlig K. US Guidelines on the Management of Chronic Kidney Disease. In: Daugirdas J, editor. Handbook of Chronic Kidney Disease Management 1ed. Lippincott Williams & Wilkins; 2011. pp. 566–580. [Google Scholar]
- 7.Levey AS, Inker LA. In: Definition and staging of chronic kidney disease in adults. Curhan GC, Forman JP, editors. UpToDate; Waltham, MA: 2014. [Google Scholar]
- 8.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012 Jan 14;379(9811):165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 9.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012 Aug 25;380(9843):756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 10.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004 Aug;8(4):R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013 May;61(5):649–672. doi: 10.1053/j.ajkd.2013.02.349. [DOI] [PubMed] [Google Scholar]
- 13.James M, Bouchard J, Ho J, et al. Canadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013 May;61(5):673–685. doi: 10.1053/j.ajkd.2013.02.350. [DOI] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence [January 3, 2015];Prevention, detection and management of acute kidney injury up to the point of renal replacement therapy. 2013 guidance.nice.org.uk/cg169.
- 15.Kidney Health Australia [January 3 2015];KHA-CARI adaptation of the KDIGO Clinical Practice Guideline for Acute Kidney Injury. 2014 doi: 10.1111/nep.12220. http://cari.org.au/CKD/CKD%20aki/Section_1_Definition_of%20AKI_Final.pdf. [DOI] [PubMed]
- 16.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2002 Feb;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 17.Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014 May;63(5):713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 18.Akbari A, Clase CM, Acott P, et al. Canadian Society of Nephrology Commentary on the KDIGO Clinical Practice Guideline for CKD Evaluation and Management. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014 Nov 4; doi: 10.1053/j.ajkd.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 19.National Institute for Health and Care Excellence [January 3 2015];Chronic kidney disease (partial update) Early identification and management of chronic kidney disease in adults in primary and secondary care. 2014 https://www.nice.org.uk/guidance/cg182. [PubMed]
- 20.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006 Jul;34(7):1913–1917. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- 21.Bagshaw SM, George C, Dinu I, Bellomo R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008 Apr;23(4):1203–1210. doi: 10.1093/ndt/gfm744. [DOI] [PubMed] [Google Scholar]
- 22.Kellum JA, Bellomo R, Ronco C. Classification of acute kidney injury using RIFLE: What's the purpose? Crit Care Med. 2007 Aug;35(8):1983–1984. doi: 10.1097/01.CCM.0000277518.67114.F8. [DOI] [PubMed] [Google Scholar]
- 23.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int. 2008 Mar;73(5):538–546. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 24.Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med. 2009 Sep;37(9):2552–2558. doi: 10.1097/CCM.0b013e3181a5906f. [DOI] [PubMed] [Google Scholar]
- 25.Joannidis M, Metnitz B, Bauer P, et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009 Oct;35(10):1692–1702. doi: 10.1007/s00134-009-1530-4. [DOI] [PubMed] [Google Scholar]
- 26.Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007 Aug;35(8):1837–1843. doi: 10.1097/01.CCM.0000277041.13090.0A. quiz 1852. [DOI] [PubMed] [Google Scholar]
- 27.Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. Journal of the American Society of Nephrology : JASN. 2007 Apr;18(4):1292–1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 28.Eckardt KU, Berns JS, Rocco MV, Kasiske BL. Definition and classification of CKD: the debate should be about patient prognosis--a position statement from KDOQI and KDIGO. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009 Jun;53(6):915–920. doi: 10.1053/j.ajkd.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney international. 2011 Jul;80(1):17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 30.Chronic Kidney Disease Prognosis Consortium. Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010 Jun 12;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney international. 2011 Jun;79(12):1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 32.Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney international. 2011 Jul;80(1):93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney international. 2011 Jun;79(12):1331–1340. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hallan SI, Matsushita K, Sang Y, et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA : the journal of the American Medical Association. 2012 Dec 12;308(22):2349–2360. doi: 10.1001/jama.2012.16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nitsch D, Grams M, Sang Y, et al. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ. 2013;346:f324. doi: 10.1136/bmj.f324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen CP, Matsushita K, Coresh J, et al. Relative risks of chronic kidney disease for mortality and end-stage renal disease across races are similar. Kidney international. 2014 Feb 12; doi: 10.1038/ki.2013.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahmoodi BK, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet. 2012 Nov 10;380(9854):1649–1661. doi: 10.1016/S0140-6736(12)61272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012 Nov 10;380(9854):1662–1673. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. The New England journal of medicine. 2006 Jun 8;354(23):2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 40.Levey AS, Inker LA, Coresh J. GFR Estimation: From Physiology to Public Health. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014 May;63(5):820–834. doi: 10.1053/j.ajkd.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Annals of internal medicine. 2006 Aug 15;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 43.Levey AS, Fan L, Eckfeldt JH, Inker LA. Cystatin C for glomerular filtration rate estimation: coming of age. Clinical chemistry. 2014 Jul;60(7):916–919. doi: 10.1373/clinchem.2014.225383. [DOI] [PubMed] [Google Scholar]
- 44.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England journal of medicine. 2012 Jul 5;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shlipak MG, Matsushita K, Arnlov J, et al. Cystatin C versus creatinine in determining risk based on kidney function. The New England journal of medicine. 2013 Sep 5;369(10):932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grubb A, Blirup-Jensen S, Lindstrom V, Schmidt C, Althaus H, Zegers I. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. 2010 Nov;48(11):1619–1621. doi: 10.1515/CCLM.2010.318. [DOI] [PubMed] [Google Scholar]
- 47.Eckfeldt JH, Karger AB, W.G. M, Rynders GP, Inker LA. Performance in Measurement of Serum Cystatin C by Laboratories Participating in the College of American Pathologists' 2014 CYS Survey. Archives of Pathology and Laboratory Medicine. 2014. doi: 10.5858/arpa.2014-0427-CP. In press. [DOI] [PubMed] [Google Scholar]
- 48.Thurlow JS, Abbott KC, Linberg A, Little D, Fenderson J, Olson SW. SCr and SCysC concentrations before and after traumatic amputation in male soldiers: a case-control study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014 Jan;63(1):167–170. doi: 10.1053/j.ajkd.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 49.Soveri I, Berg UB, Bjork J, et al. Measuring GFR: a systematic review. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014 Sep;64(3):411–424. doi: 10.1053/j.ajkd.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 50.Miller WG, Bruns DE, Hortin GL, et al. Current issues in measurement and reporting of urinary albumin excretion. Clinical chemistry. 2009 Jan;55(1):24–38. doi: 10.1373/clinchem.2008.106567. [DOI] [PubMed] [Google Scholar]
- 51.Inker LA. Albuminuria: time to focus on accuracy. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014 Mar;63(3):378–381. doi: 10.1053/j.ajkd.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Moyer VA. Screening for chronic kidney disease: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2012 Oct 16;157(8):567–570. doi: 10.7326/0003-4819-157-8-201210160-00533. [DOI] [PubMed] [Google Scholar]
- 53.de Boer IH. Chronic kidney disease-a challenge for all ages. JAMA. 2012 Dec 12;308(22):2401–2402. doi: 10.1001/jama.2012.30761. [DOI] [PubMed] [Google Scholar]
- 54.Stevens LA, Coresh J, Levey AS. CKD in the elderly--old questions and new challenges: World Kidney Day 2008. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2008 Mar;51(3):353–357. doi: 10.1053/j.ajkd.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Moynihan R, Glassock R, Doust J. Chronic kidney disease controversy: how expanding definitions are unnecessarily labelling many people as diseased. Bmj. 2013;347:f4298. doi: 10.1136/bmj.f4298. [DOI] [PubMed] [Google Scholar]
- 56.Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA : the journal of the American Medical Association. 2011 Apr 20;305(15):1553–1559. doi: 10.1001/jama.2011.451. [DOI] [PubMed] [Google Scholar]
- 57.Levey AS, Kramer H. Obesity, glomerular hyperfiltration, and the surface area correction. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010 Aug;56(2):255–258. doi: 10.1053/j.ajkd.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Stevens LA, Levey AS. Use of the MDRD study equation to estimate kidney function for drug dosing. Clinical pharmacology and therapeutics. 2009 Nov;86(5):465–467. doi: 10.1038/clpt.2009.124. [DOI] [PubMed] [Google Scholar]
- 59.Matzke GR, Aronoff GR, Atkinson AJ, Jr., et al. Drug dosing consideration in patients with acute and chronic kidney disease-a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney international. 2011 Sep 14;80(11):1122–1137. doi: 10.1038/ki.2011.322. [DOI] [PubMed] [Google Scholar]
- 60.McTaggart MP, Newall RG, Hirst JA, et al. Diagnostic accuracy of point-of-care tests for detecting albuminuria: a systematic review and meta-analysis. Annals of internal medicine. 2014 Apr 15;160(8):550–557. doi: 10.7326/M13-2331. [DOI] [PubMed] [Google Scholar]
- 61.Wu HY, Peng YS, Chiang CK, et al. Diagnostic performance of random urine samples using albumin concentration vs ratio of albumin to creatinine for microalbuminuria screening in patients with diabetes mellitus: a systematic review and meta-analysis. JAMA internal medicine. 2014 Jul;174(7):1108–1115. doi: 10.1001/jamainternmed.2014.1363. [DOI] [PubMed] [Google Scholar]
- 62.Lambers Heerspink HJ, Kropelin TF, Hoekman J, de Zeeuw D. Drug-Induced Reduction in Albuminuria Is Associated with Subsequent Renoprotection: A Meta-Analysis. Journal of the American Society of Nephrology : JASN. 2014 Nov 24; doi: 10.1681/ASN.2014070688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inker LA, Levey AS, Pandya K, Stoycheff N, Okparavero A, Greene T. Early Change in Proteinuria as a Surrogate End Point for Kidney Disease Progression: An Individual Patient Meta-analysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64(1):74–85. doi: 10.1053/j.ajkd.2014.02.020. 2014/05/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levey AS, Inker LA, Matsushita K, et al. GFR Decline as an End Point for Clinical Trials in CKD: A Scientific Workshop Sponsored by the National Kidney Foundation and the US Food and Drug Administration. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014 Dec;64(6):821–835. doi: 10.1053/j.ajkd.2014.07.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.