Abstract
Background
Diffusion tensor imaging (DTI) studies in schizophrenia report widespread aberrations in brain white matter (WM). These appear related to poorer neurocognitive performance and higher levels of negative and positive symptomatology. However, identification of the most salient WM aberrations to neurocognition and clinical symptoms is limited by relatively small samples with divergent results.
Methods
We examined 53 well-characterized patients with schizophrenia and 62 healthy controls. All participants were administered a computerized neurocognitive battery, which evaluated performance in several domains. Patients were assessed for negative and positive symptoms. Fractional anisotropy (FA) of WM cortical regions and WM fiber tracts were compared across the groups. FA values were also used to predict neurocognitive performance and symptoms.
Results
We confirm widespread aberrant WM microstructure in a relatively large sample of well-characterized patients with schizophrenia in comparison to healthy participants. Moreover, we illustrate the utility of FA measures in predicting global neurocognitive performance in healthy participants and schizophrenia patients, especially for reaction time. FA was less predictive of clinical symptomatology.
Conclusions
Using a standardized computerized neurocognitive battery and diffusion tensor imaging we show that behavioral performance is moderated by a particular constellation of WM microstructure in healthy individuals that differs in schizophrenia.
Keywords: schizophrenia, diffusion-tensor imaging, neurocognition, clinical symptoms
1. Introduction
Disruptions in brain white matter (WM) organization in schizophrenia may alter neural communication critical for sustaining neurocognitive performance and may relate to the manifestation of clinical symptoms. Diffusion tensor imaging (DTI) has facilitated in vivo study of WM integrity, as measured by fractional anisotropy (FA). Reduced FA has been documented in multiple brain regions in schizophrenia (Kyriakopoulos et al., 2008). Studies of patients with chronic schizophrenia reported significant, widespread WM microstructural aberrations (Asami et al., 2014; Kyriakopoulos et al., 2008; Thomason and Thompson, 2011), while more recent investigations of early-onset psychosis (Epstein et al., 2013; Lee et al., 2013) and psychosis in adolescence (Davenport et al., 2010; White et al., 2007) have identified focal WM abnormalities. Typically, these findings are limited to major WM fiber tracts and recent evidence indicates that reductions in cortical WM microstructure are associated with cognition (Nazeri et al., 2013). However, the specific constellation of affected brain regions varies across studies and there is likely regional specificity that relates to neurocognition or clinical symptomatology.
Impaired cognition, a core feature of schizophrenia (Gur et al., 2001a), is associated with WM abnormalities (Gur et al., 2001a; Kubicki et al., 2007; Phillips et al., 2009; Szeszko et al., 2008). Global deficits in cognition are reflective of domain-specific impairments, which are associated with aberrant WM microstructure including working memory (Sugranyes et al., 2012), executive and motor function (Perez-Iglesias et al., 2010), and verbal and visual learning abilities (Liu et al., 2013). We recently reported smaller correlations between a global measure of neurocognition, across task within-individual variability, and WM microstructure in schizophrenia as compared to healthy participants (Roalf et al., 2013). Higher within individual variability in performance speed on a computerized neurocognitive battery was associated with lower FA in the left cingulum bundle and left inferior frontal-occipital fasciculus in healthy people, but not in patients with schizophrenia. Since WM connectivity is essential for maintaining effective communication among regions, deficits in neurocognitive performance may be related, in part, to complex patterns of disrupted WM microstructure.
The relation of WM findings and clinical variables has also been evaluated, including symptoms (Paillere-Martinot et al., 2001), medications (Lieberman et al., 2005) and treatment response (Marques et al., 2014). Clinical variables contribute to heterogeneity beyond demographic variables such as age and sex, which are associated with brain maturation tractography patterns (Asato et al., 2010; Ingalhalikar et al., 2014). Clinical findings evaluating major symptom dimensions suggest that positive symptoms, such as hallucinations, are related to increases in FA (Hubl et al., 2004; Seok et al., 2007), but see (Asami et al., 2014), while negative symptoms (Bai et al., 2009) and poor outcome (Mitelman et al., 2007) are associated with lower FA. A recent study correlated clinical symptoms with global and regional measures of FA and reported significant associations between lower FA in the left hemisphere and negative, but not positive, symptoms (Asami et al., 2014). Most of these investigations have focused on localized WM regions or tracts. Given the inconsistency in previous findings and limited sample sizes, better estimates of the relation between WM microstructure and clinical symptoms are needed.
While DTI is a powerful neuroimaging technique for measuring white matter structure, methodological concerns often make group inference challenging. For example, spatial normalization, or inter-subject registration, is affected by high data dimensionality and the orientation component of the tensors (Ingalhalikar, 2010). Several options exist for spatial normalization (Alexander et al., 2001; Cao et al., 2006; Yang et al., 2008; Yeo et al., 2008; Zhang et al., 2006), including a deformable registration using orientation and intensity descriptors (DROID; Ingalhalikar et al., 2010). DROID capitalizes on the structural geometry of the diffusion tensor (Westin, 2002) and incorporates orientation information to improve the matching of white matter fiber tracts by accounting for the underlying fiber orientation (Ingalhalikar et al., 2010). Here, DROID is used to register all data to a common template; this method is efficient and produces robust results.
The goal of this study was to 1) evaluate WM microstructural abnormalities in a large sample of patients with schizophrenia and healthy controls, and 2) relate these measures to neurocognitive performance and clinical symptoms. We hypothesized that: A) patients with schizophrenia will have lower FA values in diffuse cortical WM and along WM fiber tracts compared to healthy controls; B) prediction of performance using brain WM microstructure will result in non-overlapping networks in controls and patients; C) abnormalities in WM regions and tracts will be associated with greater symptom severity.
2. Materials and methods
2.1. Participants
The sample included 53 patients with schizophrenia and 62 healthy controls recruited through the Penn Schizophrenia Research Center. Table 1 presents the sample characteristics. Participants underwent standard medical, neurological and psychiatric screening and received the Structured Clinical Interview (SCID) for DSM-IV-TR Axis I Disorders, Patient or Non-patient Edition (First et al., 2002). Patients met DSM-IV diagnosis of schizophrenia and healthy controls did not meet any axis I diagnosis or axis II cluster A personality disorder, and did not have a family history of axis I psychotic disorder in a first-degree relative. All patients had a primary diagnosis of schizophrenia; 18 patients had a history of one comorbid condition, 12 patients had two comorbid conditions, and one had three comorbid conditions. Comorbid conditions included Mood (16 patients), Substance Dependence (17 patients), or Substance Use disorders (9 patients). These counts reflect total comorbidities and are not mutually exclusive.
Table 1.
Demographic Characteristics and Neurocognitive Performance by Diagnosis
| Controls (n=62) | Patients (n=53) | ||||
|---|---|---|---|---|---|
|
|
|||||
| Percentage | Proportion | Percentage | Proportion | p-value | |
|
|
|||||
| Gender (% Female) | 51.6 | 32F, 30M | 35.8 | 19F, 34M | 0.09 |
|
|
|||||
| Mean (SD) | Range | Mean (SD) | Range | p-value | |
|
|
|||||
| Age (years) | 36.1 (8.6) | 25–55 | 38.7 (9.8) | 25–55 | 0.14 |
| Education (years) | 14.9 (2.4) | 10–20 | 13.3 (2.6) | 6–20 | <0.001 |
| Parental Education (years) | 13.9 (3.1) | 6.5–20 | 13.4 (3.0) | 6–19.5 | 0.46 |
| Handedness (% Right) | 88.7% | 81.1% | - | 0.38 | |
| SANS Global Average | - | - | 1.4 (0.9) | 0–3.3 | - |
| SAPS Global Average | - | - | 1.4 (1.0) | 0–3.5 | - |
| Comorbid Diagnoses‡ | |||||
| SZ only | - | - | 22 | - | - |
| SZ + 1 | - | - | 18 | - | - |
| SZ + 2 | - | - | 12 | - | - |
| SZ + 3 | - | - | 1 | - | - |
| Age of Onset | - | - | 22.0 (6.4) | 9–37 | |
| Medication (Atypical/Typical) | - | - | 27/7 | - | - |
| Medication (CPZE)* | - | - | 428.4 (439.4) | - | - |
| GNP Accuracy (z-score) | 0.0 (0.6) | −2.0–1.3 | −0.6 (0.9) | −2.9–0.6 | - |
| GNP Speed (z-score) | 0.0 (0.6) | −2.0–0.9 | −0.9 (1.1) | −3.2–0.9 | - |
Comorbid diagnoses included: Mood disorders, Substance Dependence and Substance Use.
Sixteen patients were receiving mood stabilizers, but not antipsychotics ; six patients reported no antipsychotic medication; antipsychotic dosage was unknown for two patients.
Potential participants in either group were excluded for any medical condition that might affect brain function, any history of neurological disorder, head trauma with loss of consciousness, lifetime history of substance dependence, substance abuse within the preceding 6 months, or any contraindication for MRI. Symptoms were rated with the Scales for Assessment of Negative Symptoms (SANS; (Andreasen, 1984a) and Positive Symptoms (SAPS; (Andreasen, 1984b). The average of patients’ global items was used as a dependent measure. Trained clinical research assessors completed all evaluations and scales. The sample was predominately right-handed and the proportion was similar in each diagnostic group (χ2(1)=0.77, p=0.38; 88% of controls and 81% of patients). Written informed consent was obtained after all procedures were fully explained, in compliance with guidelines of the University of Pennsylvania Institutional Review Board and the Declaration of Helsinki.
2.2. Computerized Neurocognitive Battery (CNB)
The CNB examines performance accuracy and speed (response time) in five neurocognitive domains including executive function (abstraction and mental flexibility, attention, working memory), episodic memory (verbal, face, spatial), complex cognition (language reasoning, spatial processing), social cognition (emotion identification) and sensorimotor processing speed (Table 1). The CNB development, validation and application were described in healthy people (Gur et al., 2014; Gur et al., 2001b; Gur et al., 2012; Gur et al., 2010) and individuals with schizophrenia (Gur et al., 2001a; Gur et al., 2007). A global index of neurocognitive performance (GNP; (Roalf et al., in press) for accuracy and speed was calculated by averaging the standardized scores across all available CNB tests and this metric was the primary dependent variable in prediction analyses.
2.3. Diffusion tensor weighted imaging acquisition
Diffusion weighted imaging (DWI) and T1-weighted MRI scan volumes were acquired for all participants on the same Siemens 3T Verio scanner (Siemens Tim Medical Solutions, Erlangen, Germany) using a 32-channel head coil. The DTI acquisition was with TR/TE=8100/82 ms, voxel size = 1.9x1.9 mm, slice thickness = 2mm, 64 diffusion directions with b=1000 s/mm2 and 7 b=0 images. Seventy 2mm contiguous axial slices of 128×128 matrix yielded 1.875*1.875*2 mm data. T1 structural imaging was performed with TR/TE=1810/3.51 ms. Manual qualitative analysis of all images was performed.
2.4. DTI processing
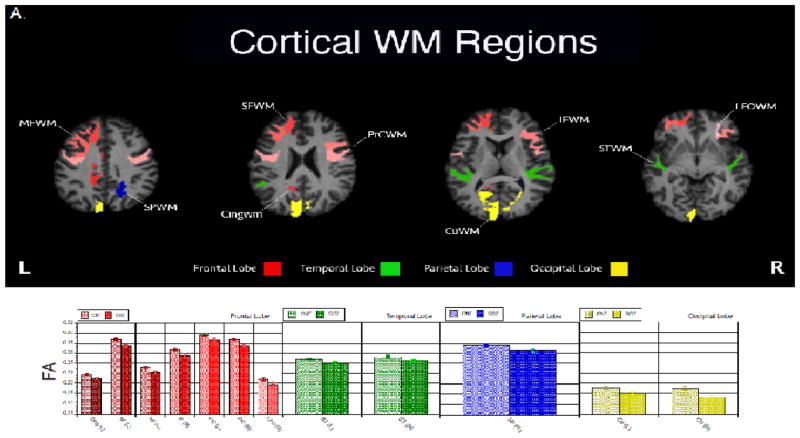
We followed an established pipeline to measure FA of each individual. The diffusion tensor images were reconstructed using multivariate linear fitting (Pierpaoli and Basser, 1996). All the tensor images were spatially normalized via a high dimensional elastic registration known as DROID (Ingalhalikar et al., 2010) to a standard atlas known as “EVE” (Mori et al., 2008; Mori et al., 2005) consisting of 176 anatomical ROIs (see http://cmrm.med.jhmi.edu/cmrm/atlas/human_data/). Following the spatial normalization, the mean FA was computed for each of the 176 ROIs. Subsequent analyses were restricted to only bilateral cortical WM regions (n=44 bilateral regions; Table 2A). In addition, FA of the major bilateral WM fiber tracts (n=34 bilateral tracts; Table 2B) was extracted in the same atlas space.
Table 2.
Cortical White Matter (A) and White Matter Fiber Tracts (B) ROI labels
| 2A. | ||
|---|---|---|
| Region | Label | Brain Lobe |
| Superior Frontal | SF | Frontal |
| Middle Frontal | MF | Frontal |
| Inferior Frontal | IF | Frontal |
| Precentral Gyral | PrC | Frontal |
| Lateral Fronto-Orbital | LFO | Frontal |
| Middle Fronto-Orbital | MFO | Frontal |
| Rectus Gyral | RG | Frontal |
| Cingulum Bundle | Cing | Frontal/Parietal |
| Superior Temporal | ST | Temporal |
| Inferior Temporal | IT | Temporal |
| Middle Temporal | MT | Temporal |
| Superior Parietal | SP | Parietal |
| Postcentral Gyrus | PoC | Parietal |
| Angular Gyral | AG | Parietal |
| Pre-Cuneus | PrCu | Parietal |
| Supramarginal | SM | Parietal |
| Cuneus | Cu | Occipital |
| Lingual Gyral | LG | Occipital |
| Fusiform Gyral | Fu | Occipital |
| Superior Occipital | SO | Occipital |
| Inferior Occipital | IO | Occipital |
| Middle Occipital | MO | Occipital |
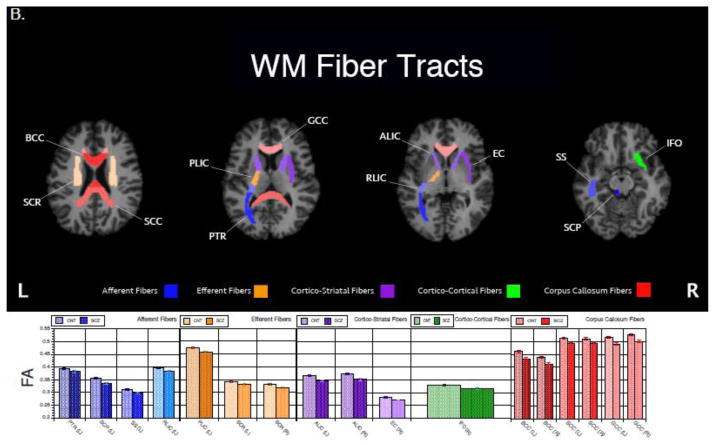
| 2B. | |||
|---|---|---|---|
| Fiber Tract | Label | Fiber Connections | Fiber Type |
| Superior Cerebellar Peduncle | SCP | Afferent | Projection Fiber |
| Posterior Thalamic Radiation | PTR | Afferent | Projection Fiber |
| Retrolenticular Part of Internal Capsule | RLIC | Afferent | Projection Fiber |
| Sagittal Stratum# | SS | Afferent | Association Fiber |
| Posterior Limb of Internal Capsule | PLIC | Efferent | Projection Fiber |
| Superior Corona Radiata | SCR | Efferent | Projection Fiber |
| Posterior Corona Radiata | PCR | Efferent | Projection Fiber |
| Anterior Limb of Internal Capsule | ALIC | Cortico-Striatal | Projection Fiber |
| Fornix (Cres) / Stria Terminalis | Fx/ST | Cortico-Striatal | Association Fiber |
| External Capsule | EC | Cortico-Striatal | Association Fiber |
| Cingulum (Cingulate Gyrus) | CGC | Cortico-Cortical | Association Fiber |
| Cingulum (Hippocampus) | CGH | Cortico-Cortical | Association Fiber |
| Superior Longitudinal Fasciculus | SLF | Cortico-Cortical | Association Fiber |
| Inferior Fronto-Occipital Fasciculus | IFO | Cortico-Cortical | Association Fiber |
| Genu of Corpus Callosum | GCC | Corpus Callosum | Commissural Fiber |
| Body of Corpus Callosum | BCC | Corpus Callosum | Commissural Fiber |
| Splenium of Corpus Callosum | SCC | Corpus Callosum | Commissural Fiber |
2.5. Statistical analyses
A comparison of FA between patients and controls was performed using MANCOVAs in SAS. Mean FA values of cortical WM regions were entered as dependent measures with Group (patients, controls) as a between factor, Region and Hemisphere as within-group factors, and age, sex and education as covariates. FDR correction (q<.05) was used to adjust for multiple comparisons. Post-hoc exploratory ANOVAs were used to compare regional differences between groups. The same analysis was performed using FA in fiber tracts. SAS 9.3 was used for all statistical analyses.
3. Results
3.1. Neurocognitive Performance
Global neurocognitive performance was lower in patients compared to healthy subjects (Table 1). Patients performed worse across most, but not all CNB tasks (Supplemental Table 1).
3.2. Cortical white matter
FA was significantly reduced in patients with schizophrenia compared to controls in nearly all cortical WM regions (Figure 1). The Group x Region x Hemisphere MANCOVA adjusted for age, sex and education showed significant main effects of Group: F (1,106)= 6.84, p = 0.01, and Region: F (21,86)= 8.50, p <.0001. There were also significant two-way interactions of Region x Hemisphere: F (21,86) = 3.75, p <.0001, and Group x Region: F (21,86)= 1.86, p = 0.02. Cortical WM regions that differ between patients and controls are shown in Figure 1A.
Figure 1.
White matter regions (A) and tracts (B) that differed significantly (p<.05) in FA by diagnostic group. Patients with schizophrenia had lower FA in all regions and tracts reported. Raw mean(sem) FA values are reported in the bar graphs. ROI abbreviations are detailed in Table 2.
3.3. Fiber tracts
FA was significantly reduced in patients compared to controls in nearly all tracts (Figure 1). The Group x Region x Hemisphere MANCOVA adjusted for age, sex and education showed significant main effects of Group: F (1,107)= 10.80, p = 0.01, and Region: F (16,92)= 33.35, p <.0001. There were also significant two-way interactions of Region x Hemisphere: F (16,92) = 2.44, p=0.004, Group x Region: F (16,133)= 2.19, p = 0.01. WM fiber tracts that differ between patients and controls are shown in Figure 1B.
3.4. Prediction of Cognitive Performance
To examine the relationship between WM microstructure and cognitive performance, we used a previously validated predictive modeling approach (Roalf et al., 2014). First, FA values and GNP were normalized for age, sex and education across the entire sample using linear regressions. These factors explained between 1–21% of variance in FA ROIs in healthy controls and between 1– 29% of the variance in FA ROIs in patients. The residual values were then used to predict neurocognitive performance from regional and tract FA values. This approach uses the least absolute shrinkage and selection operator (LASSO) method of variable selection (Efron et al., 2004) with tenfold cross-validation. LASSO is a shrinkage and selection method that minimizes the residual sum of squares, with a bound on the sum of absolute values of the coefficients. It can estimate a sparse model that has strong theoretical properties (Tibshirani, 1996). Model selection was achieved by minimizing the cross-validated predicted residual error sum of squares (CVPRESS) after the addition of each predictor (e.g., ROI). This combined approach was used to reduce the number of independent variables into a smaller set reliably associated with outcome. The LASSO model was run separately on controls and patients.
White matter FA values explained a substantial proportion of the variance in GNP accuracy (R2 = 0.40, adjusted R2 = 0.24) and speed (R2 = 0.23, adjusted R2 = 0.11) in controls after accounting for age, sex and education. Less variance was explained in patients for GNP accuracy (R2 = 0.30, adjusted R2 = 0.10), but more for GNP speed (R2 = 0.87, adjusted R2 = 0.61). The regions contributing towards the prediction of average performance in patients and controls are summarized in Table 3 (values for the specific cognitive domains are in Supplementary Table 2).
Table 3.
Location and parameter estimates of white matter region and tracts used to predict GNP accuracy and speed in healthy controls (HC) and patients with schizophrenia (SZ).
| Fiber Tracts | CIQ Accuracy | CIQ Speed | ||||||||||
|
| ||||||||||||
| HC | Hemi | PE | SZ | L/R | PE | HC | Hemi | PE | SZ | L/R | PE | |
|
| ||||||||||||
| SCP | L | 0.65 | SCP | R | 0.57 | |||||||
| SCP | R | 1.61 | SCP | R | 0.85 | PLIC | R | 0.24 | PLIC | R | 3.16 | |
| RLIC | L | -1.17 | CGC | L | -3.25 | |||||||
| PCR | L | -1.25 | SS | R | 6.44 | SS | R | -5.93 | ||||
| CGC | R | 7.65 | CGC | R | 9.69 | SLF | R | -1.91 | ||||
| CGH | R | 2.83 | CGH | R | 2.48 | PLIC | L | -0.78 | ||||
| SLF | R | 0.72 | PTR | L | 3.84 | |||||||
| EC | R | 1.42 | SCR | R | -2.42 | |||||||
| PTR | L | -1.79 | CGC | L | 2.70 | |||||||
| CGH | L | 1.84 | ||||||||||
| Fx/ST | L | 6.26 | ||||||||||
| Fx/ST | R | -6.45 | ||||||||||
| SLF | L | 4.02 | ||||||||||
| SS | L | 19.05 | ||||||||||
| SCC | L | 0.14 | ||||||||||
| RLIC | L | 1.10 | ||||||||||
| GCC | R | 0.95 | ||||||||||
| RLIC | R | 5.61 | ||||||||||
|
| ||||||||||||
| Cortical White Matter | IO | L | 0.75 | Cing | L | 3.36 | Cing | L | -14.25 | |||
| IO | R | -2.21 | PoC | L | -2.80 | |||||||
| LFO | L | 4.62 | LFO | L | 2.33 | RG | R | -4.85 | ||||
| IT | R | -0.02 | IF | L | -3.67 | |||||||
| LFO | R | 2.12 | PrC | L | -5.36 | |||||||
| LG | L | 0.61 | AG | L | -0.07 | |||||||
| MF | L | -9.54 | PrCu | L | 7.46 | |||||||
| MFO | R | -1.20 | LG | L | -2.61 | |||||||
| SM | R | -0.50 | Fu | L | 5.14 | |||||||
| SO | L | 2.21 | ||||||||||
| SM | L | -4.41 | ||||||||||
| SF | R | -6.77 | ||||||||||
| MF | R | -14.85 | ||||||||||
| PrC | R | -11.28 | ||||||||||
| PrCu | R | 5.20 | ||||||||||
| Cu | R | -5.60 | ||||||||||
| Fu | R | -7.41 | ||||||||||
| SO | R | 1.38 | ||||||||||
| IT | R | -7.21 | ||||||||||
| MT | R | 9.21 | ||||||||||
| Intercept: | 0.15 | -0.19 | -0.27 | 0.28 | ||||||||
Regions that overlap between patient and controls are in bold; Table ordered by tract (afferent, efferent, cortico-striatal, cortico-cortical, then corpus callosum) then region (frontal, temporal, parietal, occipital). ROI abbreviations are detailed in Table 2.
HC= Healthy control; SZ= patient with schizophrenia; Hemi = Hemisphere; L= left; R=right; PE= parameter estimate;
3.5. Prediction of symptom severity
A similar LASSO approach was used to examine the relation between FA and symptom severity, with FA values predicting the sum of the global negative and positive symptom ratings from the SANS and SAPS. The linear effects of age, sex and education were estimated from the subsample of patients only, since controls did not have clinical symptoms. FA values were predictive of both negative (R2 = 0.08, adjusted R2 = 0.03, intercept=1.48) and positive (R2 = 0.05 adjusted R2 = 0.03, intercept=1.51) symptom severity. Lower FA in the superior parietal white matter (parameter estimate (PE) =−4.07) was associated with higher severity of negative symptoms. Lower FA in the left superior cerebellar peduncle (PE=−1.52), right inferior frontal occipital fasciculus (PE=−3.40) and the rectal gyral white matter (PE=−1.17) was associated with higher positive symptom severity.
4. Discussion
The present study obtained robust brain-behavior associations in schizophrenia combining rigorous assessment of neurocognitive ability with measures of brain microstructure. We confirm widespread aberrant WM microstructure in a relatively large sample of stable, well-characterized schizophrenia patients. Cross-validated WM region-of-interest analysis indicated the utility of WM microstructure patterns in the prediction of neurocognitive performance, controlling for age, sex and education. More importantly, this analysis indicates that a disruption in a complex constellation of WM regions likely underlies deficits in neurocognitive performance in schizophrenia. We also find WM microstructural changes to be associated with clinical symptoms.
We report lower FA in 12 cerebral WM regions and 17 WM fiber tracts in patients with schizophrenia. Lower FA values in patients are consistent with the literature (Asami et al., 2014; Lim et al., 1999; Minami et al., 2003), particularly in studies of chronic schizophrenia, including a recent large study of 109 patients (Skudlarski et al., 2013). Skudlarski et al., (2013) report differences between patients with schizophrenia (and psychotic bipolar patients) and healthy comparison subjects in 29 WM fiber tracts, using a higher resolution parcellation method. Still, we find significant overlap between studies, including lower FA in: the body and splenium of the corpus callosum, anterior limb of internal capsule, sagittal stratum, and posterior thalamic radiation. Accumulating evidence, including data from the current study, suggests that WM disruption in schizophrenia is widespread, not focal. It is unlikely that singular disruptions in WM underlie the global neurocognitive deficits or clinical symptoms well documented in schizophrenia. Thus, it is necessary to consider FA measures from across the brain, and if possible their interrelation, when attempting to understand how FA abnormalities affect behavior in schizophrenia.
Numerous studies associate alterations in neurocognitive function with WM changes in schizophrenia. However, variability in these findings regarding the nature of WM impairment has emerged with some results indicating focal changes and others noting diffuse abnormalities (Melonakos et al., 2011). Such inconsistencies are likely influenced by the analytic approach that included examination of specific regions of interest (ROI), yielding focal abnormalities (Foong et al., 2002), VBM method examining whole brain (Melonakos et al., 2011), and source-based morphometry methods that examine brain features (SBM; Caprihan, 2011). However, the heterogeneity of these reports is probably attributable not only to post-acquisition processing, but other factors as well (e.g. clinical sample, limited clinical information, number of diffusion directions). We attempted to overcome some of these methodological issues by using robust DTI processing and a penalized regression approach. We demonstrated the possibility of predicting performance from FA values, after accounting for age, sex and education. Given that automated variable selection methods are often considered unreliable, because a large proportion of selected variables can be independent of the outcome (Derksen and Keselman, 1992; Flack and Chang, 1987), our combination of a k-folds cross-validation approach with sparse regression to select variables increases the confidence that can be placed on the predictors. In general, our results, in concert with the literature, suggest diffuse WM abnormalities in schizophrenia are associated with deficits in neurocognitive performance and, to a lesser degree, with clinical symptoms.
Patients with schizophrenia have deficits in neurocognitive ability (Gur et al., 2001a; Gur et al., 2007). However, elucidating brain-behavior relationships that underlie these deficits is challenging. Our predictive models were able to account for 24% of variance in global neurocognitive performance accuracy and 11% in speed in healthy adults using FA from both WM cortical regions and fiber tracts. This model is comprised of both cortical WM regions and WM fiber bundles suggesting that both intra- and inter-lobar connections facilitate neurocognitive performance. While the percent of variance explained may appear small, these predictions were achieved after accounting for age and sex, two factors known to significantly influence WM microstructure and neurocognitive performance. Prediction in patients was smaller for GNP accuracy (5%). This is reflected in overall reduction of model fit and a different constellation of WM regions associated with performance in patients. More heterogeneity of both accuracy performance and WM microstructure in the patient sample likely play a role in our reduced prediction. However, for GNP speed 61% of the variance was explained by FA measures from multiple regions, suggesting that inter-subject variability in overall speed of performance in schizophrenia is associated with alterations in the physiological properties of WM throughout the brain. Specifically, higher anisotropy of the cingulum bundle (CGG, CGH) and several frontal and occipital WM regions were associated with faster performance. The cingulum bundle is a major fiber tract that connects limbic and cortical brain regions, including the thalamus, amygdala, hippocampus, and dorsolateral and dorsomedial prefrontal cortex (Croxson et al., 2005; Di Rosa et al., 2008; Goldman-Rakic et al., 1984). Reduced FA of the cingulum in schizophrenia is a common finding (Wheeler and Voineskos, 2014) and it is linked to poorer ability to orient attention (Nestor et al., 2007), including longer reaction-times during the Stroop task (Takei et al., 2009) and with higher reaction time variability (Roalf et al., 2013).
In that latter study, we found that WM fiber tracts that were associated with the consistency in speed of performance in healthy controls were not related to performance in patients. However, we did not investigate these particular WM tracts or regions for associations in schizophrenia patients only. In the predictive models we report a combination of positively and negatively weighted predictors for neurocognitive performance indicating that both higher and lower FA in specific regions may portend better performance. Better performance was associated with more diffuse properties (negative associations) and greater directionality (positive associations) of regional WM. This balance of connection properties reflects the complex nature of information processing in the brain, as a network of different regions. For example, lower FA in certain regions may signify areas of crossing-fibers (Oouchi et al., 2007) or areas of diffuse local connections with surrounding cortex (Dong et al., 2004), either may be necessary for optimal performance. Notably, many regions with negative associations with performance are in frontal cortex, particularly in patients. A larger constellation of regions for patients for speed may be the result of disease-specific alterations culminating in a more distributed network of regions that underlie neurocognitive performance. Alternatively, this larger constellation of regions may reflect the heterogeneity of WM deficits found within schizophrenia.
There were only seven overlapping regions in controls and patients that aided in predicting accuracy or speed performance in healthy participants and patients. Importantly, for accuracy prediction each region was positively associated with performance in both groups. These data suggest common WM structures necessary for successful completion of neurocognitive tasks. However, for speed, the parameter estimates for overlapping regions were incongruent in direction, including the sagittal stratum and the cingulum white matter, indicating regions of potential importance for elucidating neurocognitive speed deficits in schizophrenia. Last, there are many regions that independently predict performance in either controls or patients, potentially signifying disease specific alterations in WM organization.
Prediction of clinical symptomatology using FA measures was small, but significant for negative (~3% of variance explained) and positive (~5% of variance explained) symptoms. In general, negative symptoms, in addition to age, are considered to be the best predictors of white matter change in schizophrenia (Mitelman et al., 2007; Wolkin et al., 2003). However, a recent finding suggests that age may be the driving force of these effects (Bijanki et al., 2014). Negative symptoms in schizophrenia are related to structural brain abnormalities (Sigmundsson et al., 2001), including deficits in WM regions (Nestor et al., 2008; Skelly et al., 2008) and lower FA values (Mitelman et al., 2009; Mitelman et al., 2006; Sigmundsson et al., 2001; Wible et al., 2001). Asami (2014) reported relationships between FA and negative symptoms at several anatomical levels, including whole brain, left hemispheric, and left frontal regions (Bijanki et al., 2014). In the current study we only find a negative association of FA in right superior parietal white matter and negative symptoms. The association between positive symptoms and brain FA is less consistent (Kelly et al., 2008; Lee et al., 2013; Whitford et al., 2010). We show that FA can increase the reliability of predicting positive symptoms from demographic characteristic in patients with schizophrenia to a small degree. All three significant regions (SCP, IFO, GR) associated with positive symptoms had negative parameter estimates, indicating lower FA is associated with higher positive symptom score. Moreover, of these regions the IFO (inferior frontal occipital fasciculus) is widely implicated in schizophrenia (Wheeler and Voineskos, 2014), and is associated with positive symptoms (Cheung et al., 2011). There was no overlap in brain regions in the prediction of both negative and positive symptoms suggesting distinct disruptions in WM microstructure. However, more systematic studies are necessary to further clarify these findings. In general, FA in WM regions is aids in predicting clinical symptoms, but to a lesser degree than neurocognitive ability.
Several limitations should be noted when considering the current results. Our sample, while larger than many DTI studies, is limited for prediction analyses. Statistical prediction suffers when multiple variables are used (Murtaugh, 1998) as is the case here with a number of WM brain regions and tracts. The number of predictors in the combined model is relatively large, which could affect the stability and predictive ability of the models. However, our use of a penalized regression method (LASSO) attempts to mitigate this issue. While we can explain a significant amount of variance in performance and clinical symptoms, more remains to be explained. In accordance with the literature (Rotarska-Jagiela et al., 2009; Skudlarski et al., 2013), we find greater age-related changes in FA in schizophrenia. This may alter WM associations with neurocognition, however we attempted to remove these effects prior to prediction. We only include the linear age effects in our analysis however, non-linear effects were tested and did not alter the MANCOVA or LASSO results. Ideally, prediction models would be directly compared across groups; yet, larger samples are required for meaningful statistical inferences. Comorbid diagnoses could influence our results, but more through assessment of these conditions is necessary to elucidate their impact. Assessing the role of antipsychotic medication was outside the scope of the current study, but previous studies (Asami et al., 2014; Kanaan et al., 2009; Kubicki et al., 2005; Lee et al., 2013) show no relationship between antipsychotic medication and FA measures. We report the average chlorpromazine equivalent and provide detailed information about medication. Exploratory correlation analysis indicated no relationship between medication and FA in the current sample. Finally, we used global scores for performance, which may be biased by the test instruments used and prediction may change by neurocognitive domain or specific task (See Supplemental Data).
The present study addresses the need for reliable, standardized neurocognitive assessments combined with neuroimaging methods establishing WM connectivity correlates of cognition. Using a standardized computerized battery and diffusion tensor imaging we show that behavioral performance is moderated by a particular constellation of WM microstructure in healthy individuals that differs in schizophrenia. In addition, we confirm that lower anisotropy in a few brain regions may contribute to negative and positive symptoms. We hope that such data and the approach employed may aid in the identification of aberrations in neural connectivity associated with neurocognitive deficiency and debilitating clinical symptoms in schizophrenia.
Supplementary Material
Acknowledgments
We thank the participants of this study, and all the members of the Recruitment, Assessment, and Data Teams, including Larry Macy and Chad T. Jackson for systems support, in the Schizophrenia Research Center and the Brain Behavioral Laboratory whose individual contributions collectively made this work possible.
Role of funding source
This work was supported by the National Institute of Mental Health (R01MH084856 to [RCG]; T32 MH019112 to [REG]).
Footnotes
Conflicts of interest
The authors report no conflict of interest.
Contributors
R.C.G. and R.E.G. developed the study concept and study design. Research coordinators performed testing and data collection and staff under the guidance of R.E.G., R.C.G. K.R. managed the database and helped with the analysis. R.V. and W.A.P. performed the ROI based analysis of the DTI data and corresponding sections. D.R.R. performed the statistical data analysis under the guidance of R.C.G. and K.R. and interpretation under the supervision of R.V., R.C.G. and R.E.G. R.C.G and D.R.R drafted the paper with K.R., M.Q., R.V., and W.A.P. All authors approved the final version of the paper for submission.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander DC, Pierpaoli C, Basser PJ, Gee JC. Spatial transformations of diffusion tensor magnetic resonance images. Medical Imaging, IEEE Transactions on. 2001;20(11):1131–1139. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Unversity of Iowa; Iowa City, Iowa: 1984a. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Unversity of Iowa; Iowa City, Iowa: 1984b. [Google Scholar]
- Asami T, Hyuk Lee S, Bouix S, Rathi Y, Whitford TJ, Niznikiewicz M, Nestor P, McCarley RW, Shenton ME, Kubicki M. Cerebral white matter abnormalities and their associations with negative but not positive symptoms of schizophrenia. Psychiatry Res: Neuroimaging. 2014;222(1):52–59. doi: 10.1016/j.pscychresns.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: a DTI study. Cereb Cortex. 2010;20(9):2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai YM, Chou KH, Lin CP, Chen IY, Li CT, Yang KC, Chou YH, Su TP. White matter abnormalities in schizophrenia patients with tardive dyskinesia: a diffusion tensor image study. Schizophr Res. 2009;109(1):167–181. doi: 10.1016/j.schres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Bijanki KR, Hodis B, Magnotta VA, Zeien E, Andreasen NC. Effects of age on white matter integrity and negative symptoms in schizophrenia. Schizophr Res. 2014 doi: 10.1016/j.schres.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Miller MI, Mori S, Winslow RL, Younes L. Diffeomorphic matching of diffusion tensor images. Computer Vision and Pattern Recognition Workshop, 2006. CVPRW'06. Conference on. IEEE; 2006. pp. 67–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung V, Chiu C, Law C, Cheung C, Hui C, Chan K, Sham P, Deng M, Tai K, Khong P. Positive symptoms and white matter microstructure in never-medicated first episode schizophrenia. Psychol Med. 2011;41(08):1709–1719. doi: 10.1017/S003329171000156X. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TE, Robson MD, Pinsk MA, Gross CG, Richter W, Richter MC, Kastner S, Rushworth MF. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J Neurosci. 2005;25(39):8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport ND, Karatekin C, White T, Lim KO. Differential fractional anisotropy abnormalities in adolescents with ADHD or schizophrenia. Psychiatry Res: Neuroimaging. 2010;181(3):193–198. doi: 10.1016/j.pscychresns.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen S, Keselman HJ. Bacward, forward and step-wise automated subset selection algorithms: frequency of obtaining authentic and noise variables. Br J Math Stat Psychol. 1992;45:265–282. [Google Scholar]
- Di Rosa E, Crow TJ, Chance SA. Axon bundle spacing in the anterior cingulate cortex of the human brain. J Clin Neurosci. 2008;15(12):1389–1392. doi: 10.1016/j.jocn.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Dong Q, Welsh RC, Chenevert TL, Carlos RC, Maly–Sundgren P, Gomez–Hassan DM, Mukherji SK. Clinical applications of diffusion tensor imaging. J Magn Reson Imaging. 2004;19(1):6–18. doi: 10.1002/jmri.10424. [DOI] [PubMed] [Google Scholar]
- Efron B, Hastie T, Johnstone I, Tibshirani R. Least angle regression. Ann Statist. 2004;32(2):407–499. [Google Scholar]
- Epstein KA, Cullen K, Mueller B, Lee S, Kumra S. White Matter Abnormalities and Cognitive Impairment in Early-Onset Schizophrenia-Spectrum Disorders. J Am Acad Child Adolesc Psychiatry. 2013 doi: 10.1016/j.jaac.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research. New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) [Google Scholar]
- Flack VF, Chang PC. Frequency of selecting noise variabile in subset regression analysis: a simulation study. Am Stat. 1987;41(1):84–86. [Google Scholar]
- Foong J, Symms MR, Barker GJ, Maier M, Miller DH, Ron MA. Investigating regional white matter in schizophrenia using diffusion tensor imaging. Neuroreport. 2002;13(3):333–336. doi: 10.1097/00001756-200203040-00017. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12(3):719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Gur RC, Calkins ME, Satterthwaite TD, Ruparel K, Bilker WB, Moore TM, Savitt AP, Hakonarson H, Gur RE. Neurocognitive growth charting in psychosis spectrum youths. JAMA psychiatry. 2014 doi: 10.1001/jamapsychiatry.2013.4190. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: II. The profile of schizophrenia. Neuropsychopharmacology. 2001a;25(5):777–788. doi: 10.1016/S0893-133X(01)00279-2. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001b;25(5):766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Loughead J, Connolly JJ, Qiu H, Mentch FD, Abou-Sleiman PM, Hakonarson H, Gur RE. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26(2):251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 2010;187(2):254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Calkins ME, Gur RC, Horan WP, Nuechterlein KH, Seidman LJ, Stone WS. The Consortium on the Genetics of Schizophrenia: neurocognitive endophenotypes. Schizophr Bull. 2007;33(1):49–68. doi: 10.1093/schbul/sbl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, Maier SE, Schroth G, Lovblad K, Dierks T. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61(7):658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci. 2014;111(2):823–828. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalhalikar M, Yang J, Davatzikos C, Verma R. DTI – DROID: Diffusion tensor imaging – deformable registration using orientation and intensity descriptors. Int J Imag Syst Tech. 2010;20(2):99–107. [Google Scholar]
- Kanaan R, Barker G, Brammer M, Giampietro V, Shergill S, Woolley J, Picchioni M, Toulopoulou T, McGuire P. White matter microstructure in schizophrenia: effects of disorder, duration and medication. Br J Psychiatry. 2009;194(3):236–242. doi: 10.1192/bjp.bp.108.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41(1):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, Niznikiewicz M, Connor EE, Levitt JJ, Frumin M. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26(4):1109–1118. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakopoulos M, Bargiotas T, Barker GJ, Frangou S. Diffusion tensor imaging in schizophrenia. Eur Psychiatry. 2008;23(4):255–273. doi: 10.1016/j.eurpsy.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kubicki M, Asami T, Seidman LJ, Goldstein JM, Mesholam-Gately RI, McCarley RW, Shenton ME. Extensive white matter abnormalities in patients with first-episode schizophrenia: a diffusion tensor imaging (DTI) study. Schizophr Res. 2013;143(2):231–238. doi: 10.1016/j.schres.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RSE, Green AI, Gur RE, McEvoy J. Antipsychotic drug effects on brain morphology in first–episode psychosis. Arch Gen Psychiatry. 2005;62(4):361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- Lim KO, Hedehus M, Moseley M, de Crespigny A, Sullivan EV, Pfefferbaum A. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry. 1999;56(4):367–374. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]
- Liu X, Lai Y, Wang X, Hao C, Chen L, Zhou Z, Yu X, Hong N. Reduced white matter integrity and cognitive deficit in never-medicated chronic schizophrenia: A diffusion tensor study using TBSS. Behav Brain Res. 2013;252:157–163. doi: 10.1016/j.bbr.2013.05.061. [DOI] [PubMed] [Google Scholar]
- Marques TR, Taylor H, Chaddock C, Dell’Acqua F, Handley R, Reinders AATS, Mondelli V, Bonaccorso S, DiForti M, Simmons A. White matter integrity as a predictor of response to treatment in first episode psychosis. Brain. 2014;137(1):172–182. doi: 10.1093/brain/awt310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melonakos ED, Shenton ME, Rathi Y, Terry DP, Bouix S, Kubicki M. Voxel-based morphometry (VBM) studies in schizophrenia—can white matter changes be reliably detected with VBM? Psychiatry Res: Neuroimaging. 2011;193(2):65–70. doi: 10.1016/j.pscychresns.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami T, Nobuhara K, Okugawa G, Takase K, Yoshida T, Sawada S, Ha-Kawa S, Ikeda K, Kinoshita T. Diffusion tensor magnetic resonance imaging of disruption of regional white matter in schizophrenia. Neuropsychobiology. 2003;47(3):141–145. doi: 10.1159/000070583. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Brickman AM, Shihabuddin L, Newmark RE, Hazlett EA, Haznedar MM, Buchsbaum MS. A comprehensive assessment of gray and white matter volumes and their relationship to outcome and severity in schizophrenia. Neuroimage. 2007;37(2):449–462. doi: 10.1016/j.neuroimage.2007.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman SA, Canfield EL, Newmark RE, Brickman AM, Torosjan Y, Chu KW, Hazlett EA, Haznedar MM, Shihabuddin L, Buchsbaum MS. Longitudinal assessment of gray and white matter in chronic schizophrenia: a combined diffusion-tensor and structural magnetic resonance imaging study. Open Neuroimag J. 2009;3:31. doi: 10.2174/1874440000903010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman SA, Newmark RE, Torosjan Y, Chu KW, Brickman AM, Haznedar MM, Hazlett EA, Tang CY, Shihabuddin L, Buchsbaum MS. White matter fractional anisotropy and outcome in schizophrenia. Schizophr Res. 2006;87(1–3):138–159. doi: 10.1016/j.schres.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, van Zijl PCM, Nagae-Poetscher LM. MRI Atlas of Human White Matter. 1. Elsevier; Amsterdam, The Netherlands: 2005. [Google Scholar]
- Murtaugh PA. Methods of variable selection in regression modeling. Commun Stat Simulat. 1998;27:711–734. [Google Scholar]
- Nazeri A, Chakravarty MM, Felsky D, Lobaugh NJ, Rajji TK, Mulsant BH, Voineskos AN. Alterations of superficial white matter in schizophrenia and relationship to cognitive performance. Neuropsychopharmacology. 2013;38(10):1954–1962. doi: 10.1038/npp.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Niznikiewicz M, Gurrera RJ, McCarley RW, Shenton ME. Neuropsychological disturbance in schizophrenia: a diffusion tensor imaging study. Neuropsychology. 2008;22(2):246–254. doi: 10.1037/0894-4105.22.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Spencer KM, Niznikiewicz M, McCarley RW, Shenton ME. Attentional networks and cingulum bundle in chronic schizophrenia. Schizophr Res. 2007;90(1–3):308–315. doi: 10.1016/j.schres.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oouchi H, Yamada K, Sakai K, Kizu O, Kubota T, Ito H, Nishimura T. Diffusion anisotropy measurement of brain white matter is affected by voxel size: underestimation occurs in areas with crossing fibers. Am J Neuroradiol. 2007;28(6):1102–1106. doi: 10.3174/ajnr.A0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillere-Martinot ML, Caclin A, Artiges E, Poline JB, Joliot M, Mallet L, Recasens C, Attar-Levy D, Martinot JL. Cerebral gray and white matter reductions and clinical correlates in patients with early onset schizophrenia. Schizophr Res. 2001;50(1):19–26. doi: 10.1016/s0920-9964(00)00137-7. [DOI] [PubMed] [Google Scholar]
- Perez-Iglesias R, Tordesillas-Gutierrez D, McGuire PK, Barker GJ, Roiz-Santianez R, Mata I, de Lucas EM, Rodriguez-Sanchez JM, Ayesa-Arriola R, Vazquez-Barquero JL, Crespo-Facorro B. White matter integrity and cognitive impairment in first-episode psychosis. Am J Psychiatry. 2010;167(4):451–458. doi: 10.1176/appi.ajp.2009.09050716. [DOI] [PubMed] [Google Scholar]
- Phillips OR, Nuechterlein KH, Clark KA, Hamilton LS, Asarnow RF, Hageman NS, Toga AW, Narr KL. Fiber tractography reveals disruption of temporal lobe white matter tracts in schizophrenia. Schizophr Res. 2009;107(1):30–38. doi: 10.1016/j.schres.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Roalf DR, Gur RE, Ruparel K, Calkins ME, Satterthwaite TD, Bilker W, Hakonarson H, Harris LJ, Gur RC. Within-Individual Variability in Neurocognitive Performance: Age and Sex-Related Differences in Children and Youths From Ages 8 to 21. Neuropsychology. doi: 10.1037/neu0000067. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Ruparel K, Gur RE, Bilker W, Gerraty R, Elliott MA, Gallagher RS, Almasy L, Pogue-Geile MF, Prasad K. Neuroimaging Predictors of Cognitive Performance Across a Standardized Neurocognitive Battery. Neuropsychology. 2014;28(2):161–176. doi: 10.1037/neu0000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Ruparel K, Verma R, Elliott MA, Gur RE, Gur RC. White matter organization and neurocognitive performance variability in schizophrenia. Schizophr Res. 2013;143(1):172–178. doi: 10.1016/j.schres.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotarska-Jagiela A, Oertel-Knoechel V, DeMartino F, van de Ven V, Formisano E, Roebroeck A, Rami A, Schoenmeyer R, Haenschel C, Hendler T. Anatomical brain connectivity and positive symptoms of schizophrenia: a diffusion tensor imaging study. Psychiatry res Neuroimaging. 2009;174(1):9–16. doi: 10.1016/j.pscychresns.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Seok JH, Park HJ, Chun JW, Lee SK, Cho HS, Kwon JS, Kim JJ. White matter abnormalities associated with auditory hallucinations in schizophrenia: a combined study of voxel-based analyses of diffusion tensor imaging and structural magnetic resonance imaging. Psychiatry Res: Neuroimaging. 2007;156(2):93–104. doi: 10.1016/j.pscychresns.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Sigmundsson T, Suckling J, Maier M, Williams SCR, Bullmore ET, Greenwood KE, Fukuda R, Ron MA, Toone BK. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry. 2001;158(2):234–243. doi: 10.1176/appi.ajp.158.2.234. [DOI] [PubMed] [Google Scholar]
- Skelly LR, Calhoun V, Meda SA, Kim J, Mathalon DH, Pearlson GD. Diffusion tensor imaging in schizophrenia: relationship to symptoms. Schizophr Res. 2008;98(1):157–162. doi: 10.1016/j.schres.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P, Schretlen DJ, Thaker GK, Stevens MC, Keshavan MS, Sweeney JA, Tamminga CA, Clementz BA, O’Neil K, Pearlson GD. Diffusion tensor imaging white matter endophenotypes in patients with schizophrenia or psychotic bipolar disorder and their relatives. Am J Psychiatry. 2013;170(8):886–898. doi: 10.1176/appi.ajp.2013.12111448. [DOI] [PubMed] [Google Scholar]
- Sugranyes G, Kyriakopoulos M, Dima D, O'Muircheartaigh J, Corrigall R, Pendelbury G, Hayes D, Calhoun VD, Frangou S. Multimodal analyses identify linked functional and white matter abnormalities within the working memory network in schizophrenia. Schizophr Res. 2012;138(2):136–142. doi: 10.1016/j.schres.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Robinson DG, Ashtari M, Vogel J, Betensky J, Sevy S, Ardekani BA, Lencz T, Malhotra AK, McCormack J, Miller R, Lim KO, Gunduz-Bruce H, Kane JM, Bilder RM. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology. 2008;33(5):976–984. doi: 10.1038/sj.npp.1301480. [DOI] [PubMed] [Google Scholar]
- Takei K, Yamasue H, Abe O, Yamada H, Inoue H, Suga M, Muroi M, Sasaki H, Aoki S, Kasai K. Structural disruption of the dorsal cingulum bundle is associated with impaired Stroop performance in patients with schizophrenia. Schizophr Res. 2009;114(1–3):119–127. doi: 10.1016/j.schres.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Thompson PM. Diffusion imaging, white matter, and psychopathology. Clin Psychol (New York) 2011;7(1):63. doi: 10.1146/annurev-clinpsy-032210-104507. [DOI] [PubMed] [Google Scholar]
- Tibshirani R. Regression Shrinkage and Selection via the Lasso. J Roy Stat Soc B. 1996;58(1):267–288. [Google Scholar]
- Westin CF, Maier SE, Mamata H, Nabavi A, Jolesz FA, Kikinis R. Processing and visualization for diffusion tensor MRI. Med Image Anal. 2002;6(2):93–108. doi: 10.1016/s1361-8415(02)00053-1. [DOI] [PubMed] [Google Scholar]
- Wheeler AL, Voineskos AN. A review of structural neuroimaging in schizophrenia: from connectivity to connectomics. Front Hum Neurosci. 2014;8:653. doi: 10.3389/fnhum.2014.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Kendi ATK, Lehéricy S, Kendi M, Karatekin C, Guimaraes A, Davenport N, Schulz SC, Lim KO. Disruption of hippocampal connectivity in children and adolescents with schizophrenia—a voxel-based diffusion tensor imaging study. Schizophr Res. 2007;90(1):302–307. doi: 10.1016/j.schres.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Kubicki M, Schneiderman JS, O'Donnell LJ, King R, Alvarado JL, Khan U, Markant D, Nestor PG, Niznikiewicz M. Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biol Psychiatry. 2010;68(1):70–77. doi: 10.1016/j.biopsych.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wible CG, Anderson J, Shenton ME, Kricun A, Hirayasu Y, Tanaka S, Levitt JJ, O'Donnell BF, Kikinis R, Jolesz FA. Prefrontal cortex, negative symptoms, and schizophrenia: an MRI study. Psychiatry Res: Neuroimaging. 2001;108(2):65–78. doi: 10.1016/s0925-4927(01)00109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkin A, Choi SJ, Szilagyi S, Sanfilipo M, Rotrosen JP, Lim KO. Inferior frontal white matter anisotropy and negative symptoms of schizophrenia: a diffusion tensor imaging study. Am J Psychiatry. 2003;160(3):572–574. doi: 10.1176/appi.ajp.160.3.572. [DOI] [PubMed] [Google Scholar]
- Yang J, Shen D, Davatzikos C, Verma R. Diffusion tensor image registration using tensor geometry and orientation features. Medical Image Computing and Computer-Assisted Intervention–MICCAI; 2008; Springer; 2008. pp. 905–913. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Vercauteren T, Fillard P, Pennec X, Golland P, Ayache N, Clatz O. DTI registration with exact finite-strain differential, Biomedical Imaging: From Nano to Macro, 2008. ISBI 2008. 5th IEEE International Symposium on. IEEE; 2008. pp. 700–703. [Google Scholar]
- Zhang H, Yushkevich PA, Alexander DC, Gee JC. Deformable registration of diffusion tensor MR images with explicit orientation optimization. Med Image Anal. 2006;10(5):764–785. doi: 10.1016/j.media.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yushkevich PA, Alexander DC, Gee JC. Deformable registration of diffusion tensor MR images with explicit orientation optimization. Med Image Anal. 2006;10(5):764–785. doi: 10.1016/j.media.2006.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.