Abstract
Social status has been associated with health consequences, although the mechanisms by which status affects health are relatively unknown. At the physiological level, many studies have investigated the potential relationship between social behaviour/rank and physiological stress, with a particular focus on glucocorticoid (GC) production. GCs are of interest because of their experimentally established influence on health-related processes such as metabolism and immune function. Studies in a variety of species, in both naturalistic and laboratory settings, have led to complex outcomes. This paper reviews findings from primates and rodents and proposes a psychologically and physiologically relevant framework in which to study the relationship between social status and GC function. We (i) compare status-specific GC production between male and female primates, (ii) review the functional significance of different temporal patterns of GC production, (iii) propose ways to assess these temporal dynamics, and (iv) present novel hypotheses about the relationship between social status and GC temporal dynamics, and potential fitness and health implications. To understand whether GC production mediates social status-related fitness disparities, we must consider social contest conditions and the temporal dynamics of GC production. This framework will provide greater insights into the relationship between social status, physiological stress and health.
Keywords: dominance, social status, cortisol, corticosterone, chronic stress, acute stress
1. Introduction
Individuals living in groups often develop social hierarchies [1,2]. The associations between social status and developmental, physiological, behavioural and health processes have been documented in a variety of species and settings (e.g. [3–8]). However, the proximate mechanisms by which social status has any causal influence on development, physiology, behaviour and/or health are not clear. In this paper, we examine the relationship between social status and one aspect of physiological stress, production of glucocorticoid (GC) hormones, which can have a significant influence on health, ageing, behaviour and development. We focus this review on free-ranging primates because of the abundance of studies on social status and GC production in this taxon. Studies that have investigated the relationship between social status and health and/or stress physiology have led to complex findings. We show that these findings can be clarified by comparing primate male- and female-typical social competition and by adopting a nuanced understanding of hypothalamic–pituitary–adrenal (HPA) axis regulation and the functional significance of GC production temporal dynamics. To provide insights into the functional significance of GC temporal dynamics, we examine results from studies with laboratory rodents and humans. By understanding GC temporal patterns in high- and low-ranking individuals, we will achieve a better understanding of how GC production may mediate the relationship between social status and long-term health outcomes.
In this review, we investigate the relationship between social status and GC production by considering two important functional observations: (i) the sexes often differ in hierarchy formation processes [9] and (ii) long- versus short-term elevations in GC production have different influences on physiology and health [10,11]. We review these two phenomena, and then provide specific predictions of how social status in different societies could differentially relate to GC temporal dynamics to provide insights into proximate costs and benefits of maintaining specific social rank in different social systems.
Males and females regularly form dominance hierarchies across a variety of primate species. However, the social behaviour and associated costs involved in acquiring and maintaining rank can differ between sexes. Males typically engage in more intense and frequent aggressive interactions than females, whereas females tend to engage in more complex affiliative interactions [12–14], with some exceptions [9]. Male rank is often achieved by violent turnovers of an existing hierarchy and competition is over access to mates, whereas female rank is typically determined by more subtle aggressive and affiliative interactions and competition is often over access to quality food resources ([12–15], cf. [9]). In addition, the immediate costs of aggressive interactions are usually greater for males than females (e.g. wounding) [16,17] given increased male weaponry (teeth, claws, body size) [18–20].
Given broad differences in social and reproductive strategies between males and females, the challenges involved in attaining and maintaining social status differ between sexes. In a ‘typical’ species, in which males are large with significant weaponry, male dominance requires good physical condition and can be dangerous and costly to acquire during discrete periods. In a similar species where females use coalitionary and affiliative strategies, dominance may be less energetically costly and subordinate exclusion from quality resources may be more costly over longer periods. These two methods of attaining and maintaining dominance should lead to different GC production dynamics: acute elevations in typical dominant males versus chronic elevations in typical subordinate females.
In addition to considering broad differences in male versus female sociality, we consider temporal dynamics and costs/benefits of elevated GC production. GC production is a dynamic process that involves complex feedforward and feedback regulatory processes [10]. This complex regulation allows for a range of GC production temporal patterns, with differences in peak and trough amplitudes, rates of recovery to basal production and frequency of elevated production (figure 1). In laboratory studies, these differences in temporal dynamics have been associated with differences in behaviour, environment, age and genetics (reviewed below) [21–28].
Figure 1.
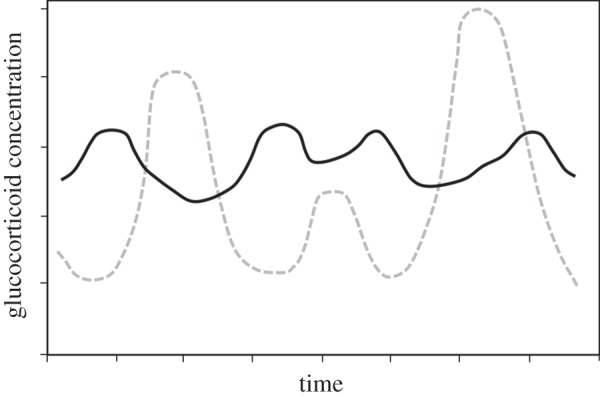
Stylized examples of two different circulating GC temporal profiles that would produce similar mean GC levels.
Differences in GC temporal dynamics are particularly important because they may confer different health consequences. Short- versus long-term elevations in circulating GC levels may have seemingly opposite influences on metabolic, cardiovascular and immune processes. For example, short-term elevations in circulating GC levels can increase certain aspects of cellular immunity, have a negligible influence on cardiovascular function and lead to temporary weight loss; whereas long-term elevations can cause decreased cellular immune responses, increased arterial blood pressure and heart rate and increased weight gain [29–32]. These bidirectional influences of GC production are important to consider in terms of fitness consequences. If GC production mediates differential health outcomes among social ranks, then it is important to study status-specific GC production temporal patterns. To predict fitness-related outcomes, we need information on timing and duration of elevated and dampened GC production.
2. Sex differences in social status and glucocorticoid production
A decade ago, several reviews were published on social status and GC production [33–36]. These concluded that GCs are elevated in group-living individuals that have heightened metabolic demands, and that these demands are greatest during periods of social instability. The ‘stress of subordination’ and ‘stress of dominance’ hypotheses were coined, based on the realization that both high- and low-rank are associated with specific challenges [33–35,37]. Importantly, challenges associated with subordination and dominance can trigger increased GC production that fuels specific behavioural and physiological responses. These reviews, and the refinement of non-invasive faecal steroid methods, stimulated further investigation of social status and GC production in a variety of free-ranging species, particularly primates.
Primates are a useful biological system in which to investigate these questions because many species maintain relatively large and complex social groups, where quantification of social interactions is relatively easy. We focus on studies of free-ranging primates, which provide a good basis to assess the relationship between physiological stress and social status and the selection pressures associated with systematic covariation of social behaviour and physiology. We compare results according to the sex of study subjects and methods of rank attainment/maintenance (whether rank is inherited or not). Given prior results ([36,38,39], cf. [40]), we focus on studies conducted during social instability when rank acquisition or maintenance is presumably most costly (for both subordinate and dominant individuals; table 1).
Table 1.
Summary of studies that have measured GC levels among high- and low-ranking individuals in free-ranging primate groups. The table is organized by sex of study subjects and whether rank is inherited from the mother or not. Results are focused on study periods that were identified as socially unstable (e.g. mating season, social disruptions such as rank-reversals, immigration events, etc.). In studies that included both stable and unstable periods, we only present results from unstable periods. For studies that only included stable periods, we include those data in the table (for a summary that includes other species and a comparison of stable versus unstable periods, refer to [41]). The footnotes provide an explanation of the information in each column. Text provides information on literature review criteria.
| sex—rank inheritance species | animals/groupsa | sample mediumb | months of samplingc | mean samples per Ind'ld | social stabilitye | CORT relative to rankf | ref. |
|---|---|---|---|---|---|---|---|
| FEMALES—inherited | |||||||
| chacma baboons | 10/1 | faeces | 17 | 26 | ? | = | [42] |
| chacma baboons | 21/1 | faeces | 16 | 30 | unstable | = | [43] |
| chacma baboons | 18/1 | faeces | 8 | 31 | unstable | = | [41] |
| chacma baboons | 22/1 | faeces | 1.5 | 24 | unstable | S | [44] |
| chacma baboons | 45/1 | faeces | 48 | ? | stable | S | [45] |
| mandrills | 19/1 | faeces | 12 | 18 | stable | = | [46] |
| rhesus macaques | 70/6 | serum (reactivity) | 5 (across 2 years) | 1–2 | ? | = | [47] |
| long-tailed macaques | 34/4 | urine | 2 | ∼3 | stable? | = | [48] |
| barbary macaques | 8/1 | faeces | 5–7 | 16 | stable | I | [49] |
| FEMALES—not inherited | |||||||
| chimpanzees | 18/1 | urine | 60 | 67 | unstable | S | [50] |
| sykes’ monkeys | 11/1 | faeces | 16 | 165 | unstable | S | [51] |
| common marmoset | 6/3 | faeces | 10–16 | 69 | unstable | S | [52] |
| ring-tailed lemurs | 39/8 | faeces | 0.6 | 6 | unstable | D | [53] |
| ring-tailed lemurs | 45/7 | faeces | 4–12 | ∼10 | ? | = | [54] |
| ring-tailed lemurs | 32/3 | faeces | 51 (across 7 years) | 43 | unstable | I | [55] |
| MALES—not inherited | |||||||
| chimpanzees | 11/1 | urine | 12 | AM: 31 PM: 15 |
stable | AM: = PM:D | [56] |
| gorilla | 10/3 | urine | 15 | 21 | unstable | = | [57] |
| olive baboons | 13/1 | serum (reactivity) | 0.5 | 6–9 ? sequential |
stable unstable |
=, D? = |
[58] [59] |
| savannah baboons | 125/5 | faeces | 108 | 36 | unstable | D,S | [40] |
| chacma baboons | 13/1 | faeces | 14 | 37 | unstable | D | [60] |
| mandrills | 16/2 | faeces | 1–3 | 20 | unstable | D | [61] |
| long-tailed macaques | 24/4 | urine | 2 | 3 | unstable | = D? | [48] |
| long-tailed macaques | 16/1 | faeces | 4.5 | 15 | unstable | = | [62] |
| Japanese macaques | 6/1 | faeces | 6 | 42 | stable | D | [63] |
| Assamese macaques | 6/1 | faeces | 5 | 35 | unstable | S | [64] |
| tufted capuchins | 6/1 | faeces | 12 | >14 | unstable | = | [65] |
| white-faced capuchins | 8/3 | faeces | 6 | 24 | unstable? | D | [66] |
| bearded capuchins | 3/1 | faeces | 3 | 15 | unstable | D | [67] |
| golden lion tamarins | 24/14 | faeces | 16 | 8 | ? | = | [68] |
| Verreaux's sifakas | 10/5 | faeces | 5 | 32 | unstable | D | [69] |
| ring-tailed lemurs | 13/3 | faeces | 4–5 (across 3 years) | 5–6 | unstable | = | [70] |
aTotal number of animals/total number of social groups.
bBiological sample used to measure GC production.
cNumber of months that samples were collected to measure GC production.
dMean number of samples collected from each individual.
eEstimate of social stability based on description of social conditions in paper. Mating periods in seasonally breeding species with multiple breeding males were considered unstable. New group formation and increased aggression were considered unstable.
fDominance rank with greatest GC production: ‘D’ indicates dominants greater than subordinates, ‘S’ indicates subordinates greater than dominants, ‘I’ indicates intermediate rank greater than subordinates/dominants, ‘ = ’ indicates no rank effects.
The majority of primate studies indicate that dominant males produce levels of GCs that are more than or equal to subordinate males, whereas only one study has found that subordinate males excrete more GCs than dominant males (table 1—see ‘MALES—not inherited’) [40,48,56–70]. The opposite pattern emerges for females, where subordinate females often have greater GC production than dominant individuals, particularly when rank is not inherited (table 1—see ‘FEMALES—inherited’ and ‘FEMALES—not inherited’) ([24,35,41–52), cf. [53,54,55]). Given the role of GCs in metabolism, these findings support the hypothesis that male attainment and maintenance of high rank are associated with increased metabolic costs relative to subordinates, but that subordinate status in females is associated with greater metabolic costs relative to dominants.
Exceptions to these sex-specific patterns of GC production occur in male Assamese macaques (Macaca assamensis) where dominant males have lower mean GC levels than subordinates [64], and in female ring-tailed lemurs (Lemur catta) where high-ranking females have greater mean GC levels than subordinates [53,55]. In both cases, the social structure does not follow ‘sex-typical’ patterns described above. Male Assamese macaques attain high rank through male–male coalitionary support and high rank does not necessarily confer exclusive access to reproductive females [64,71]. Female ring-tailed lemurs are dominant to males, they have significant weaponry, and they are relatively aggressive within and between social groups [72,73]. These two exceptions may prove the rule that high rates of within-sex aggression for high-rank attainment predict short-term costs and GC elevations in dominant individuals, whereas in social systems with low rates of within-sex aggression, subordinate exclusion from key resources predicts longer term costs and GC elevations in subordinates.
The above summary supports the hypothesis that during periods of social instability, dominant male primates produce more GCs than subordinate males, and that the reverse is true for females. However, there are a several caveats: (i) plenty of studies indicate no relationship between GC production and dominance status, (ii) several studies indicate a nonlinear relationship between dominance rank and GC production and (iii) current studies tell us little about GC production dynamics (e.g. peak versus trough, basal versus reactivity levels) as they relate to dominance rank. In the following section, we expand on the functional significance of basal and reactive HPA activity and delve into the hypothesis that dominance status may be more closely related to the temporal dynamics of GC production than to mean production over time (as assessed by non-invasive urine and faecal sampling methods).
3. Temporal dynamics of glucocorticoid production
(a). Basic neuroendocrine-stress physiology
GCs are produced by the adrenal cortex and released into peripheral circulation in response to endocrine signalling from the anterior pituitary (adrenocorticotrophin, ACTH), mediated by hypothalamic corticotropin-releasing hormone (CRH). Stimulation of this HPA axis occurs in response to external stimulation, physical exertion, cognitive/emotional processes and circadian rhythms. Once in circulation, GCs alter the function of multiple organs simultaneously and in a sustained manner by binding receptors in brain, peripheral organs and immune cells [11,74–77]. Bound intracellular receptors stimulate slow, long-term alterations in cell function through transcriptional and epigenetic processes [78–81], and GC binding to putative membrane receptors also influences HPA axis sensitivity through non-genomic signalling [82,83]. A fine-tuned balance of receptor expression in the central nervous system moderates HPA axis responses to stress and stress coping strategies [84,85]. Based on these complex and long-lasting organism-level changes, the endocrine system may be particularly well-suited to support subtle and sustained responses to social status-related challenges and subsequent health-related outcomes.
Intermediate signalling hormones in the HPA axis have significant effects on cellular function; CRH and ACTH directly alter brain and peripheral cell function in ways that could affect health beyond the influence of GCs (e.g. [86]); these influences are discussed in depth elsewhere [87–93]. Sympathetic activation is also closely related to social behaviour, status and health consequences [94,95], but fewer social behaviour studies have focused on this metabolically relevant system, particularly in free-ranging animals.
(b). Cues that alter glucocorticoid production
Based on laboratory studies with rodents, diverse physical and social stimuli cause significant short-term elevations in circulating GC levels [28]. In these controlled situations, standardized social stressors cause twice as much GC production as physical stressors. In free-ranging animals (reptiles, amphibians, birds and mammals), rapid elevations in GC production have been documented following capture, severe weather conditions and aggressive male immigration, and long-lasting GC alterations have been documented during periods of low food supply and across seasons [58,96–100].
The time course of GC responses to environmental and internal cues is modified by stressor qualities (intensity, frequency, duration, degree of novelty, etc.), organism characteristics (age, genetics, etc.) and time of day or season. For example, in laboratory rodents and primates, repeat exposure to the same stressor leads to habituation and faster return to basal levels [101,102], and different stressor types or intensities lead to different recovery durations [24,25]. Rodent and primate genetic background further influences the duration and intensity of GC production, which are not necessarily linked to behavioural differences [23,103]. Time of day can further influence peak amplitude and duration of GC responses [104], and the sex and age of an organism affect response dynamics in humans and rodents [21,22,26,27,105,106].
These different response dynamics are significant because short- versus long-term elevations in GC production are associated with different influences on biological processes and health. In particular, GC recovery rate (time to return to baseline concentrations after a challenge) has particular functional/health significance (e.g. [107]). Social status, whether high or low, is associated with an array of different challenges, some that stimulate long-term elevations in GC production and others that stimulate short-term elevations. These different challenges may confer different influences on physiology and health.
(c). Metabolic and health effects of short- versus long-term glucocorticoid elevations
Studies with laboratory rodents suggest that short- versus long-term GC elevations have different influences on health-related processes. We review some of these findings and apply these lessons to primates based on the fact that HPA regulatory processes are evolutionarily conserved, with significant homology among vertebrate species [108–110]. Effects of HPA axis hormones on health processes in laboratory rodents may generalize to a wide range of vertebrate organisms.
GCs are widely recognized for their effects on carbohydrate metabolism, gluconeogenesis and subsequent increased blood glucose. In addition, GCs regulate lipid metabolism in adipose tissue, enhance de novo lipid synthesis in the liver, and increase protein catabolism [111,112]. The release of energy-rich substrates supplies an organism with increased metabolic resources to cope with stressors. Over longer periods, these hormones can increase body mass because they tend to increase feeding behaviour and stimulate intake of high-caloric foods in humans and rodents [113–115]. Finally, GCs influence an array of other biological systems such as the cardiovascular, respiratory, immune, visual, metabolic and reproductive systems (reviewed below and in table 2) [119].
Table 2.
Examples of studies in which the duration(s) of GC production and/or stress were manipulated or controlled for to determine the influence of acute versus chronic elevations in GC/stress exposure on fitness- and health-related processes. Methods and specific dose/frequency/duration of experimental manipulations are provided, along with outcomes of acute versus chronic exposure conditions. ADX, adrenalectomy; Pred., prednisolone (synthetic GC/derivative of cortisol); min, minute; d, day; wk, week; m, month; CORT, cortisol in human studies and corticosterone in rodent studies; DEX, dexamethasone (a synthetic glucocorticoid receptor agonist); DTH, delayed-type hypersensitivity (adaptive cell-mediated immune response to a specific antigen); TSST, Trier Social Stress Test (common psychosocial stress challenge for human participants in a laboratory setting).
| species | variable | method | dose/freq./dur. of ‘acute’ manipulation | dose/freq./dur. of ‘chronic’ manipulation | outcome (acute—A versus. chronic—C) | ref. |
|---|---|---|---|---|---|---|
| human | GC | GC (Pred.) pills | 5–10 mg/1 d/7 d | 5 mg/1 d/12 m | protein metabolism A: increased C: no change |
[112] |
| rat | GC | ADX + GC implants | 40 or 80%/1 d/5 d | n.a. | food consumption A: increased weight gain A: decreased |
[113] |
| rat | GC | GC injection | 5 or 20 mg/kg/1 d/1× | 5 or 20 mg/kg/1 d/25 d | anxiety-like behaviour A: decreased C: increased |
[116] |
| rat | GC | ADX + GC implant or injection | 3.7 µg/g DEX/1 d/1× | 238 µg DEX/1 d/3 wk and 9.5 mg CORT/1 d/1 wk | blood glucose/leptin A: increased body weight C: decreased |
[117] |
| rat | GC | ADX + GC injection | 5 mg/kg/1 d/1× | 40 mg/kg/1 d/6 d | DTH response A: enhanced C: supressed |
[29] |
| mouse | GC | GC in drinking water | n.a. | 25 or 100 µg/ml/1 d/4 wk | weight gain/adiposity/food consumption C: increased locomotor activity C: decreased |
[32] |
| human | GC reactors | TSST | TSST/1×/45 min | n.a. | sweet food calorie consumption A: High CORT reactors consumed more than low CORT reactors |
[115] |
| human | life stress | TSST | TSST/1×/20 min | n.a. | telomerase activity C: chronic life stress group lower than low stress group |
[118] |
Duration of elevated GC production is an important determinant of physiological consequences. While many of the acute effects of GCs mobilize energy, chronically elevated circulating GCs enhance energy storage (table 2). For example, in mice, chronic (four-week) exposure to elevated GC caused an initial weight loss followed by significant weight gain [32,117]. Acute GC elevations can activate the adaptive immune system, whereas chronic GC elevations are linked to suppressed cell-mediated leucocyte trafficking [29]. Telomerase, an enzyme that maintains telomere length, is upregulated in human peripheral mononuclear cells 1 h after exposure to an acute laboratory social stressor, but chronic stress is associated with reduced telomere length in these cells [118,120].
Short- versus long-term effects of GCs and stressors are also important in the brain where they influence behavioural responses. Immediately after acute exposure to GC, hippocampal-dependent information processing is impaired, but an hour to days later hippocampal long-term potentiation is enhanced [121]. GC injections 90 min before behavioural testing caused enhanced exploratory behaviour and decreased fear behaviour in rats, but when exposed to repeated GC administration for 25 days, rats were less exploratory and displayed enhanced fear behaviour [116]. Acute actions of GCs may enhance processing of stress-related information, which helps the individual to cope with similar future challenges, but effects of sustained GC elevations may overpower adaptive responses to acute stress.
In regard to the stress of dominance, acute GC elevations support enhanced responding. For example, an aggressive interaction to attain or maintain dominance requires increased energy, and GC-induced carbohydrate, lipid and protein metabolism provides a sustained source of energy. Acute GC elevations also stimulate leucocyte redistribution to the skin, which can fight off infection after potential wounding. While we have discovered a great deal about the role of GCs in health and coping, there is still much to learn. For example, there are few direct comparisons of the effects of short- versus long-term GC elevations in controlled experiments (table 2), and this experimental work is primarily performed in rodents. Further research is needed to clarify open questions with regard to chronic GC elevations such as what constitutes long-term exposure and at what point detrimental effects occur.
(d). Limitations of current methods
Faecal GC metabolites (FGCMs) measures provide a non-invasive estimate of HPA axis function ideal for field studies. However, there are limitations to this method; several factors influence the amount and rate of GC metabolism and excretion [122–128]. For example, GC metabolism differs between sexes in mice, rats and other rodents, with males typically excreting higher concentrations than females, with exceptions [123,124,127,128], and the specific FGCMs excreted are sex- and species-specific [123–125]. Quantification of circadian rhythm is difficult: FGCMs can show a diurnal rhythm mirroring that of circulating GCs, but this rhythm can only be documented in animals that defecate multiple times per day [124,126,127]. Intestinal transit time, which regulates FGCM excretion rate, is influenced by physical activity; rodents excrete GCs more rapidly during the active versus passive phases [123]. Total faecal mass influences FGCM concentration, which may not reflect circulating concentrations [126]. Thus, FGCM measures provide a useful estimate of GC production in free-ranging animals with appropriate methodological considerations and biological validation for each species [125].
More relevant to this review is the fact that faeces are excreted in discrete periods, and therefore, faecal steroid measures reflect a physiological average of circulating steroid levels over several hours or days. These measures represent an estimate of overall production during both low- and high-production periods. Also, most studies necessarily use opportunistic sampling and estimate mean steroid production across long periods (weeks, months). Because GC production is affected by many variables and because faecal samples have inherent limitations, accurate quantification of individual differences in GC production requires frequent within-individual sampling over a significant period. From current studies, it is difficult to know whether high-mean FGCM levels reflect consistently high levels of circulating GCs (e.g. chronic stress associated with negative health consequences) or frequent or particularly high elevations in GC production in response to challenges (e.g. a profile that may activate adaptive metabolic processes; see figure 1 for different GC dynamics underlying the same GC mean). If GC production estimates are to provide a mechanism by which social stress/status confers specific health benefits or costs, then it is particularly important to distinguish between these two kinds of GC profiles.
4. Social status, glucocorticoid production dynamics and fitness: the ‘dynamics of stress’ framework
(a). Novel predictions on social dynamics, social status and glucocorticoid production dynamics
We propose two testable predictions that are sex-specific in ‘typical’ species where males engage in aggressive within-sex competition for limited access to mates and females engage in lower levels of aggression for access to physical resources (male challenges are more intense but shorter lived than female challenges). (i) In species that show ‘stress of dominance’ (dominant individuals have greater mean GC production), dominant individuals will have more frequent and/or higher GC elevations than subordinates as a result of acute intense metabolic demands like within-sex fighting, and trough production will not relate to social status (figure 2a: ‘stress dynamics of dominance’). (ii) In species that show ‘stress of subordination’ (subordinate individuals have greater mean GC production), subordinate individuals will have chronically elevated GC production and/or fewer periods of return to expected basal production relative to dominants as a result of chronic low-grade metabolic demands like exclusion from key resources. In this scenario, peak GC production will not differ between subordinate and dominant individuals (figure 2b: ‘stress dynamics of subordination’). These distinct temporal profiles may have different consequences for health and fitness.
Figure 2.
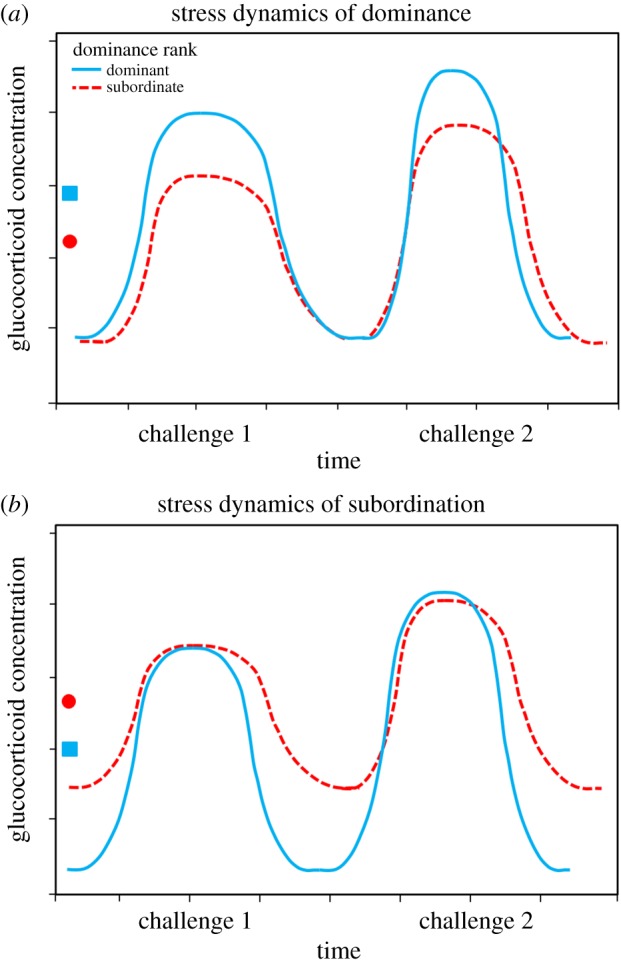
Predictions about adaptive temporal patterns of GC production in a dominant versus subordinate individual in two different social systems: (a) where dominant individuals maintain elevated mean GC production relative to subordinate individuals and (b) where subordinate individuals maintain elevated mean GC production relative to dominant individuals. Square indicates mean GC concentration for dominant individuals, circle indicates mean GC concentration for subordinate individuals. (a) In the ‘stress dynamics of dominance’ scenario, the dominant individual has elevated mean GC production as a result of greater peak production compared with subordinate individuals; this is a pattern that may confer metabolic, reproductive, immune and/or cognitive benefits to the dominant individual and support increased survival/fitness. (b) In the ‘stress dynamics of subordination’ scenario, the subordinate individual has elevated mean GC production as a result of greater trough production and/or slower returns to baseline compared with dominant individuals, and this pattern may incur decreased fitness/survival benefits. (Online version in colour.)
We expect that these status-related GC production patterns will be apparent during periods of instability, in both sexes, and in social groups in which competition more often involves contest than scramble. In addition, we expect the ‘stress dynamics of dominance’ scenario to be more frequent in males in species in which male–male fighting is particularly aggressive and wounding is significant, and the ‘stress dynamics of subordination’ to be frequent in females in species in which female–female aggression and wounding are not frequent and high-quality food supplies are easily monopolized [20]. We expect these patterns to be reversed between the sexes when specific characteristics of dominance rank acquisition and maintenance are not ‘sex-typical’ (e.g. where males compete for access to clumped food resources, and/or females compete in a particularly aggressive manner that involves enhanced weaponry). In the following section, we provide one example of how the above predictions may be tested with existing datasets with frequent GC sampling.
(b). Example of social status-related glucocorticoid production dynamics
This kind of analysis will work best when many samples from the same individual are available. We re-analysed published data from wild female ring-tailed lemurs [129], which live in relatively small social groups (5–30 individuals) in extremely arid environments in Madagascar [130–132]. All females are dominant to all males, and this behavioural adaptation is thought to have evolved as a result of very limited resource availability [72,133].
Faecal samples were analysed from 10 females across two social groups during two months of mid-to-late dry season when females were lactating (mean of 39 samples for each female; range of 32–47 samples/female) [129]. Samples and behaviour were collected during four alternating one-week periods for each group, with approximately 10 samples/female/week, and no differences in sample size between high- and low-ranking females. Dominance rank was quantified using agonistic interaction matrices based on all observed interactions. Prior analyses of these data indicated that the two highest-ranking females in unstable social groups produced more FGCMs than the three lowest-ranking females in these groups, and that FGCM levels were positively related to aggressive behaviour [53]. To determine potential health consequences of elevated GC levels, it is imperative to understand whether elevated faecal levels reflect frequent or intense acute GC elevations (figure 2a) or chronically elevated basal circulating GC levels (figure 2b). Frequent or intense GC elevations in an individual may confer certain health benefits, whereas long-term basal GC elevations or protracted GC responses may have more negative health consequences.
One way to distinguish between the two GC response profiles is to analyse minimum versus maximum FGCM levels from each individual. With enough samples from the same individuals, one can compare individual minimum versus maximum production across time. As the number of samples from an individual increases so too will the range, with greater maximum and lower minimum levels obtained as sample size increases [134]. Therefore, studies of minimum and maximum FGCM levels require many samples from the same individual and comparable sample sizes across individuals or controls for variable sample sizes. With enough samples, it is possible to refine faecal steroid analyses to estimate temporal dynamics, and the potential functional significance of individual GC production profiles. Specifically, if high-mean FGCM levels result from relatively frequent or intense GC spikes in circulation, then minimum FGCM levels should be similar in animals with high- or low-mean FGCM levels, but maximum FGCM levels may be greater in the high- versus low-mean FGCM individual (figure 2a). On the other hand, if high-mean FGCM levels result from chronically elevated circulating GC levels, then minimum FGCM levels will be higher than those from an individual that has chronically lower circulating GC levels (figure 2b).
With the ring-tailed lemurs we were able to determine individual minimum and maximum FGCM values for each one-week observation period. High-ranking females with high mean FGCM levels (ranks 1–2) had similar minimum FGCM levels to low-ranking females (ranks 3–5; 9–19 versus 7–14 ng g−1, repeated measures ANOVA F1,8 = 2.2, n.s.). However, maximum FGCM levels were twice as high in high- versus low-rank females (34–155 versus 27–44 ng g−1, repeated measures ANOVA F1,8 = 10.2, p < 0.05). Results were not affected by sample size (as a covariate), nor by inclusion of more samples from other seasons. These results suggest that high-rank ring-tailed lemur females do not necessarily have chronically elevated GC levels, but rather that they experience higher, longer or more frequent elevations in circulating GC levels with similar trough levels to subordinate females. Further analysis revealed that high-ranking females had greater FGCM levels one day following a significant challenge (territory invasion, predation threat) compared with low-ranking females, suggesting that high-ranking females have a physiological stress response that is more responsive to environmental stressors [1]. Based on the prior review of GC dynamics, heightened GC responses may confer certain fitness advantages.
The analysis of minimum versus maximum FGCM levels in female ring-tailed lemurs supports the prediction that elevated GC production in dominant aggressive versus subordinate less-aggressive individuals probably occurs in a discrete or acute fashion, and may thus confer different fitness consequences from those associated with chronically elevated GC production. This information will be important to collect in future studies of social status-associated GC production. Only by documenting the relative variability in individual GC production can we infer the potential costs or benefits of differential GC production among high- versus low-ranking individuals (e.g. [135,136]). Because social dynamics among ring-tailed lemurs are exceptional [72,73], future studies in species with more prototypical social structures are required.
5. Conclusion and future directions
Quantification of the temporal patterns of GC production among individuals is necessary to understand potential fitness costs and benefits of status-specific GC production. We must distinguish between high-GC individuals with a responsive HPA axis to acute threat and those with more chronically elevated GC production either in the absence of, or as a result of, chronic threat. The former profile of GC production may confer specific fitness benefits (increased coping responses), whereas the latter profile may confer specific health costs (e.g. slower wound healing, decreased memory, decreased energy availability). It is also important to distinguish between low-GC individuals that are hypo-responsive to threat and those with low basal levels and short elevations in GC production at appropriate times. Dissection of individual GC production temporal dynamics as they relate to environmental cues will provide the best estimate of HPA axis regulation efficiency—a key factor in predicting health outcomes. Furthermore, different social structures [137] may help explain different temporal dynamics in stress physiology and predict status-related fitness consequences.
There are several key areas for future work on GC production (and HPA axis regulation) as it relates to health-related outcomes for high- versus low-status individuals. First, it will be important to document individual HPA axis regulation. To this end, frequent measures of GC production within the same individual will facilitate estimation of GC responses to environmental stimuli (e.g. [58,61,129]). Non-invasive GC measures have greatly advanced our understanding of GC production in free-ranging animals and we have suggested a method to assess variability (HPA regulation) within individuals that is feasible with relatively crude estimates of GC production. Moreover, peripheral GC sensitivity assays can provide estimates of HPA axis regulation. Given ethical and logistical constraints for some study populations, one blood sample per study animal could facilitate indirect estimation of GC function. For example, in vitro immune system challenges, with cells from whole blood samples, can be performed to assess GC receptor sensitivity (e.g. [100,138–140]). Second, to understand the specific environmental factors that stimulate stress responses in high- versus low-status individuals, further work is required to document individual GC production in response to specific challenges and benefits associated with high- and low-social status (e.g. physical activity, exposure to aggression, access to social support, access to food/shelter, social stability, costs of reproduction) [33–36,38,40,53,141–144]. Third, the relationship of GC production to specific health outcomes for high- versus low-status individuals will provide information on the moderating role of GC production on status-related outcomes. For example, some studies have related GC production (versus social status) to specific health outcomes like mortality and illness/resilience, and to functional immune measures like wound healing and parasite clearance (e.g. [61,145–148]). The same GC receptor sensitivity assays in cultured immune cells described above can also provide insights into molecular correlates of immune system function in high-GC animals [140]. Finally, experimental work (and taking advantage of naturally occurring shifts in social status) will provide insights into the relative causal relationship between social status costs/benefits and GC production, and between GC production and health-related outcomes [139,144,146,149–152].
Acknowledgements
We thank P. Kappeler, C. Nunn and three anonymous reviewers for substantial and important feedback on this manuscript. S.A.C. would like to dedicate this review to Alison Jolly for her years of dedicated and insightful research on primate behaviour and social dynamics, astute and creative training of students, and tireless support of students and researchers from a variety of locations and backgrounds.
Ethics statement
Institutional Animal Care and Use Committee approval was obtained from Duke University and approval from the Madagascar government was obtained prior to data collection with ring-tailed lemurs in Madagascar.
Author contributions
S.A.C. conceived of and drafted the manuscript; M.J.C. contributed specific sections, developed figures, edited the manuscript. Both authors give final approval for publication.
Funding statement
Data collection from lemurs was supported by the Sigma Xi Scientific Research Society and the Center for International Studies at Duke University.
Competing interests
The authors have no competing interests.
References
- 1.Rowell TE. 1974. The concept of social dominance. Behav. Biol. 11, 131–154. ( 10.1016/S0091-6773(74)90289-2) [DOI] [PubMed] [Google Scholar]
- 2.Bernstein IS. 1981. Dominance: the baby and the bathwater. Behav. Brain Sci. 4, 419–457. ( 10.1017/S0140525X00009614) [DOI] [Google Scholar]
- 3.Pusey A, Williams J, Goodall J. 1997. The influence of dominance rank on the reproductive success of female chimpanzees. Science 277, 828–831. ( 10.1126/science.277.5327.828) [DOI] [PubMed] [Google Scholar]
- 4.Steptoe A, Marmot M. 2004. Socioeconomic status and coronary heart disease: a psychobiological perspective. Popul. Council 30, 133–150. [Google Scholar]
- 5.Wroblewski EE, Murray CM, Keele F, Schumacher-Stankey JC, Hahn BH, Pusey AE. 2009. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Anim. Behav. 77, 873–885. ( 10.1016/j.anbehav.2008.12.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filby AL, Paull GC, Bartlett EJ, Van Look KJW, Tyler CR. 2010. Physiological and health consequences of social status in zebrafish (Danio rerio). Physiol. Behav. 101, 576–587. ( 10.1016/j.physbeh.2010.09.004) [DOI] [PubMed] [Google Scholar]
- 7.Archie EA, Altmann J, Alberts SC. 2012. Social status predicts wound healing in wild baboons. Proc. Natl Acad. Sci. USA 109, 9017–9022. ( 10.1073/pnas.1206391109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen E, Miller GE. 2013. Socioeconomic status and health: mediating and moderating factors. Annu. Rev. Clin. Psychol. 9, 723–749. ( 10.1146/annurev-clinpsy-050212-185634) [DOI] [PubMed] [Google Scholar]
- 9.Clutton-Brock TH, Huchard E. 2013. Social competition and selection in males and females. Phil. Trans. R. Soc. B 368, 20130074 ( 10.1098/rstb.2013.0074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sapolsky R, Romero LM, Munck A. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 56–89. [DOI] [PubMed] [Google Scholar]
- 11.McEwen BS. 2004. Protection and damage from acute and chronic stress. Ann. NY Acad. Sci. 1032, 1–7. ( 10.1196/annals.1314.001) [DOI] [PubMed] [Google Scholar]
- 12.Trivers R. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man (ed. Campbell B.), pp. 139–179. Chicago, IL: Aldine Press. [Google Scholar]
- 13.Wrangham RW. 1980. An ecological model of female-bonded primate groups. Behaviour 75, 262–300. ( 10.1163/156853980X00447) [DOI] [Google Scholar]
- 14.van Schaik CP. 1989. The ecology of social relationships among female primates. In Comparative socioecology: the behavioural ecology of humans and other animals (eds Standen V, Foley FA.), pp. 195–218. Oxford, UK: Blackwell Scientific Press. [Google Scholar]
- 15.Bernstein IS. 1976. Dominance, aggression and reproduction in primate societies. J. Theor. Biol. 69, 459–472. ( 10.1016/0022-5193(76)90072-2) [DOI] [PubMed] [Google Scholar]
- 16.Drews C. 1996. Contexts and patterns of injuries in free-ranging male baboons (Papio cynocephalus). Behavior 133, 443–474. ( 10.1163/156853996X00530) [DOI] [Google Scholar]
- 17.MacCormick HA, MacNulty DR, Bosacker AL, Lehman C, Bailey A, Collins DA, Packer C. 2012. Male and female aggression: lessons from sex, rank, age, and injury in olive baboons. Behav. Ecol. 23, 684–691. ( 10.1093/beheco/ars021) [DOI] [Google Scholar]
- 18.Alexander RD. 1974. The evolution of social behavior. Ann. Rev. Ecol. System. 5, 325–383. ( 10.1146/annurev.es.05.110174.001545) [DOI] [Google Scholar]
- 19.Harvey PH, Kavanaugh M, Clutton-Brock TH. 1978. Sexual dimorphism in primate teeth. J. Zool. London 186, 475–485. ( 10.1111/j.1469-7998.1978.tb03934.x) [DOI] [Google Scholar]
- 20.Plavcan JM, van Schaik CP, Kappeler PM. 1995. Competition, coalitions and canine size in primates. J. Hum. Evol. 28, 245–276. ( 10.1006/jhev.1995.1019) [DOI] [Google Scholar]
- 21.Sapolsky R, Krey L, McEwen B. 1983. The adrenocortical stress-response in the aged male rat: impairment of recovery from stress. Exp. Gerontol. 18, 55–64. ( 10.1016/0531-5565(83)90051-7) [DOI] [PubMed] [Google Scholar]
- 22.Handa RJ, Burgess LH, Kerr JE, Keefe JA. 1994. Gonadal steroid hormone receptors and sex differences in the hypothalamic–pituitary–adrenal axis. Horm. Behav. 28, 464–476. ( 10.1006/hbeh.1994.1044) [DOI] [PubMed] [Google Scholar]
- 23.Armario A, Gavaldà A, Martí J. 1995. Comparison of the behavioural and endocrine response to forced swimming stress in five inbred strains of rats. Psychoneuroendocrinology 20, 879–890. ( 10.1016/0306-4530(95)00018-6) [DOI] [PubMed] [Google Scholar]
- 24.García A, Martí O, Vallès A, Dal-Zotto S, Armario A. 2000. Recovery of the hypothalamic–pituitary–adrenal response to stress: effects of stress intensity, stress duration and previous stress exposure. Neuroendocrinology 72, 114–125. ( 10.1159/000054578) [DOI] [PubMed] [Google Scholar]
- 25.Márquez C, Belda X, Armario A. 2002. Post-stress recovery of pituitary–adrenal hormones and glucose but not the response during exposure to the stressor, is a marker of stress intensity in highly stressful situations. Brain Res. 926, 181–185. ( 10.1016/S0006-8993(01)03112-2) [DOI] [PubMed] [Google Scholar]
- 26.Kudielka BM, Kirschbaum C. 2005. Sex differences in HPA axis responses to stress: a review. Biol. Psychol. 69, 113–132. ( 10.1016/j.biopsycho.2004.11.009) [DOI] [PubMed] [Google Scholar]
- 27.Romeo RD. 2010. Pubertal maturation and programming of hypothalamic–pituitary–adrenal reactivity. Front. Neuroendocrinol. 31, 232–240. ( 10.1016/j.yfrne.2010.02.004) [DOI] [PubMed] [Google Scholar]
- 28.Koolhaas JM, et al. 2011. Stress revisited: a critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 35, 1291–1301. ( 10.1016/j.neubiorev.2011.02.003) [DOI] [PubMed] [Google Scholar]
- 29.Dhabhar FS, McEwen BS. 1999. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc. Natl Acad. Sci. USA 96, 1059–1064. ( 10.1073/pnas.96.3.1059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosmond R, Chagnon YC, Holm G, Chagnon M, Perusse L, Lindell K, Carlsson B, Bouchard C, Bjorntorp P. 2000. A glucocorticoid receptor gene marker is associated with abdominal obesity, leptin, and dysregulation of the hypothalamic–pituitary–adrenal axis. Obesity Res. 8, 211–218. ( 10.1038/oby.2000.24) [DOI] [PubMed] [Google Scholar]
- 31.Scheuer DA, Bechtold AG, Shank SS, Akana SF. 2004. Glucocorticoids act in the dorsal hindbrain to increase arterial pressure. Am. J. Physiol. Heart Circ. Physiol. 286, H458–H467. ( 10.1152/ajpheart.00824.2003) [DOI] [PubMed] [Google Scholar]
- 32.Karatsoreos IN, Bhagat SM, Bowles NP, Weil ZM, Pfaff DW, McEwen BS. 2010. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology 151, 2117–2127. ( 10.1210/en.2009-1436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Creel S. 2001. Social dominance and stress hormones. Trends Ecol. Evol. 16, 491–497. ( 10.1016/S0169-5347(01)02227-3) [DOI] [Google Scholar]
- 34.Abbott DH, et al. 2003. Are subordinates always stressed? A comparative canalysis of rank differences in cortisol levels among primates. Horm. Behav. 43, 67–82. ( 10.1016/S0018-506X(02)00037-5) [DOI] [PubMed] [Google Scholar]
- 35.Goymann W, Wingfield JC. 2004. Allostatic load, social status and stress hormones: the costs of social status matter. Anim. Behav. 67, 591–602. ( 10.1016/j.anbehav.2003.08.007) [DOI] [Google Scholar]
- 36.Sapolsky RM. 2005. The influence of social hierarchy on primate health. Science 308, 648–652. ( 10.1126/science.1106477) [DOI] [PubMed] [Google Scholar]
- 37.Habig B, Archie EA. 2015. Social status, immune response and parasitism in males: a meta-analysis. Phil. Trans. R. Soc. B 370, 20140109 ( 10.1098/rstb.2014.0109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cavigelli SA, Chaudhry H. 2012. Social status, glucocorticoids, immune function, and health: can animal studies help us understand human socioeconomic-status-related health disparities? Horm. Behav. 62, 295–313. ( 10.1016/j.yhbeh.2012.07.006) [DOI] [PubMed] [Google Scholar]
- 39.Creel S, Dantzer B, Goymann W, Rubenstein DR. 2013. The ecology of stress: effects of the social environment. Func. Ecol. 27, 66–80. ( 10.1111/j.1365-2435.2012.02029.x) [DOI] [Google Scholar]
- 40.Gesquiere LR, Learn NH, Simao MCM, Onyango PO, Alberts SC, Altmann J. 2011. Life at the top: rank and stress in wild male baboons. Science 333, 357–360. ( 10.1126/science.1207120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crockford C, Wittig RM, Whitten PL, Seyfarth RM, Cheney DL. 2008. Social stressors and coping mechanisms in wild female baboons (Papio hamadryas ursinus). Horm. Behav. 53, 254–265. ( 10.1016/j.yhbeh.2007.10.007) [DOI] [PubMed] [Google Scholar]
- 42.Weingrill T, Gray DA, Barrett L, Henzi SP. 2004. Fecal cortisol levels in free-ranging female chacma baboons: relationship to dominance, reproductive state and environmental factors. Horm. Behav. 45, 259–269. ( 10.1016/j.yhbeh.2003.12.004) [DOI] [PubMed] [Google Scholar]
- 43.Engh AL, Beehner JC, Bergman TJ, Whitten PL, Hoffmeier RR, Seyfarth RM, Cheney DL. 2006. Female hierarchy instability, male immigration and infanticide increase glucocorticoid levels in female chacma baboons. Anim. Behav. 71, 1227–1237. ( 10.1016/j.anbehav.2005.11.009) [DOI] [Google Scholar]
- 44.Wittig RM, Crockford C, Lehmann J, Whitten PL, Seyfarth RM, Cheney DL. 2008. Focused grooming networks and stress alleviation in wild female baboons. Horm. Behav. 54, 170–177. ( 10.1016/j.yhbeh.2008.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seyfarth RM, Silk JB, Cheney DL. 2012. Variation in personality and fitness in wild female baboons. Proc. Natl Acad. Sci. USA 109, 16 980–16 985. ( 10.1073/pnas.1210780109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Setchell JM, Smith T, Wickings EJ, Knapp LA. 2008. Factors affecting fecal glucocorticoid levels in semi-free-ranging female mandrills (Mandrillus sphinx). Am. J. Primatol. 70, 1023–1032. ( 10.1002/ajp.20594) [DOI] [PubMed] [Google Scholar]
- 47.Hoffman CL, Ayala JE, Mas-Rivera A, Maestripieri D. 2010. Effects of reproductive condition and dominance rank on cortisol responsiveness to stress in free-ranging female rhesus macaques. Am. J. Primatol. 72, 559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Schaik CP, van Noordwijk MA, van Bragt T, Blankenstein MA. 1991. A pilot study of the social correlates of levels of urinary cortisol, prolactin, and testosterone in wild long-tailed macaques (Macaca fascicularis). Primates 32, 345–356. ( 10.1007/BF02382675) [DOI] [Google Scholar]
- 49.Edwards KL, Walker SL, Bodenham RF, Ritchie H, Shultz S. 2013. Associations between social behaviour and adrenal activity in female Barbary macaques: consequences of study design. Gen. Comp. Endocrinol. 186, 72–79. ( 10.1016/j.ygcen.2013.02.023) [DOI] [PubMed] [Google Scholar]
- 50.Thompson ME, Muller MN, Kahlenberg SM, Wrangham RW. 2010. Dynamics of social and energetic stress in wild female chimpanzees. Horm. Behav. 58, 440–449. ( 10.1016/j.yhbeh.2010.05.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foerster S, Monfort SL. 2010. Fecal glucocorticoids as indicators of metabolic stress in female Sykes’ monkeys (Cercopithecus mitis albogularis). Horm. Behav. 58, 685–697. ( 10.1016/j.yhbeh.2010.06.002) [DOI] [PubMed] [Google Scholar]
- 52.Sousa MBC, Da Rocha Albuquerque ACS, Da Silva AF, Araujo A, Yamamoto ME, De Fatima AM. 2005. Behavioral strategies and hormonal profiles of dominant and subordinate common marmoset (Callithrix jacchus) females in wild monogamous groups. Am. J. Primatol. 67, 37–50. ( 10.1002/ajp.20168) [DOI] [PubMed] [Google Scholar]
- 53.Cavigelli SA, Dubovick T, Levash W, Jolly A, Pitts A. 2003. Female dominance status and fecal corticoids in a cooperative breeder with low reproductive skew: ring-tailed lemurs (Lemur catta). Horm. Behav. 43, 166–179. ( 10.1016/S0018-506X(02)00031-4) [DOI] [PubMed] [Google Scholar]
- 54.Pride RE. 2005. Foraging success, agonism, and predator alarms: behavioral predictors of cortisol in Lemur catta. Int. J. Primatol. 26, 295–319. ( 10.1007/s10764-005-2926-9) [DOI] [Google Scholar]
- 55.Starling AP, Charpentier MJE, Fitzpatrick C, Scordato ES, Drea CM. 2010. Seasonality, sociality, and reproduction: long-term stressors of ring-tailed lemurs (Lemur catta). Horm. Behav. 57, 76–85. ( 10.1016/j.yhbeh.2009.09.016) [DOI] [PubMed] [Google Scholar]
- 56.Muller MN, Wrangham RW. 2004. Dominance, cortisol and stress in wild chimpanzees (Pan troglodytes schweinfurthii). Behav. Ecol. Sociobiol. 55, 332–340. ( 10.1007/s00265-003-0713-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robbins MM, Czekala NM. 1997. A preliminary investigation of urinary testosterone and cortisol levels in wild male mountain gorillas. Am. J. Primatol. 43, 51–64. () [DOI] [PubMed] [Google Scholar]
- 58.Sapolsky RM. 1982. The endocrine stress-response and social status in the wild baboon. Horm. Behav. 16, 279–292. ( 10.1016/0018-506X(82)90027-7) [DOI] [PubMed] [Google Scholar]
- 59.Sapolsky RM. 1983. Endocrine aspects of social instability in the olive baboon (Papio anubis). Am. J. Primatol. 5, 365–379. ( 10.1002/ajp.1350050406) [DOI] [PubMed] [Google Scholar]
- 60.Bergman TJ, Beehner JC, Cheney DL, Seyfarth RM, Whitten PL. 2005. Correlates of stress in free-ranging male chacma baboons, Papio hamadryas ursinus. Anim. Behav. 70, 703–713. ( 10.1016/j.anbehav.2004.12.017) [DOI] [Google Scholar]
- 61.Setchell JM, Smith T, Wickings EJ, Knapp LA. 2010. Stress, social behaviour, and secondary sexual traits in a male primate. Horm. Behav. 58, 720–728. ( 10.1016/j.yhbeh.2010.07.004) [DOI] [PubMed] [Google Scholar]
- 62.Girard-Buttoz C, Heistermann M, Krummel S, Engelhardt A. 2009. Seasonal and social influences on fecal androgen and glucocorticoid excretion in wild male long-tailed macaques (Macaca fascicularis). Physiol. Behav. 98, 168–175. ( 10.1016/j.physbeh.2009.05.005) [DOI] [PubMed] [Google Scholar]
- 63.Barrett GM, Shimizu K, Bardi M, Asaba S, Mori A. 2002. Endocrine correlates of rank, reproduction, and female-directed aggression in male Japanese macaques (Macaca fuscata). Horm. Behav. 42, 85–96. ( 10.1006/hbeh.2002.1804) [DOI] [PubMed] [Google Scholar]
- 64.Ostner J, Heistermann M, Schülke O. 2008. Dominance, aggression and physiological stress in wild male Assamese macaques (Macaca assamensis). Horm. Behav. 54, 613–619. ( 10.1016/j.yhbeh.2008.05.020) [DOI] [PubMed] [Google Scholar]
- 65.Lynch JW, Ziegler TE, Strier KB. 2002. Individual and seasonal variation in fecal testosterone and cortisol levels of wild male tufted capuchin monkeys, Cebus paella nigritus. Horm. Behav. 41, 275–287. ( 10.1006/hbeh.2002.1772) [DOI] [PubMed] [Google Scholar]
- 66.Schoof VAM, Jack KM. 2013. The association of intergroup encounters, dominance status, and fecal androgen and glucocorticoid profiles in wild male white-faced capuchins (Cebus capucinus). Am. J. Primatol. 75, 107–115. ( 10.1002/ajp.22089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mendonça-Furtado O, Edaes M, Palme R, Rodrigues A, Siqueira J, Izar P. 2014. Does hierarchy stability influence testosterone and cortisol levels of bearded capuchin monkeys (Sapajus libidinosus) adult males? A comparison between two wild groups. Behav. Proc. 109, 79–88. ( 10.1016/j.beproc.2014.09.010) [DOI] [PubMed] [Google Scholar]
- 68.Bales KL, French JA, McWilliams J, Lake RA, Dietz JM. 2006. Effects of social status, age, and season on androgen and cortisol levels in wild male golden lion tamarins (Leontopithecus rosalia). Horm. Behav. 49, 88–95. ( 10.1016/j.yhbeh.2005.05.006) [DOI] [PubMed] [Google Scholar]
- 69.Fichtel C, Kraus C, Ganswindt A, Heistermann M. 2007. Influence of reproductive season and rank on fecal glucocorticoid levels in free-ranging male Verreaux's sifakas (Propithecus verreauxi). Horm. Behav. 51, 640–648. ( 10.1016/j.yhbeh.2007.03.005) [DOI] [PubMed] [Google Scholar]
- 70.Gould L, Ziegler TE, Wittwer DJ. 2005. Effects of reproductive and social variables on fecal glucococorticoid levels in a sample of adult male ring-tailed lemurs (Lemur catta) at the Beza Mahafaly Reserve, Madagascar. Am. J. Primatol. 67, 5–23. ( 10.1002/ajp.20166) [DOI] [PubMed] [Google Scholar]
- 71.Schülke O, Bhagavatula J, Vigilant L, Ostner J. 2010. Social bonds enhance reproductive success in male macaques. Curr. Biol. 20, 2207–2210. ( 10.1016/j.cub.2010.10.058) [DOI] [PubMed] [Google Scholar]
- 72.Jolly A. 1967. Lemur behavior. A Madagascar field study. Chicago, IL: University of Chicago Press. [Google Scholar]
- 73.Vick LG, Pereira ME. 1989. Episodic targeting aggression and the histories of Lemur social groups. Behav. Ecol. Sociobiol. 25, 3–12. ( 10.1007/BF00299705) [DOI] [Google Scholar]
- 74.Arriza JL, Weinberger C, Cerelli G, Glaser TM, Hendelin BL, Houseman DE, Evans RM. 1987. Cloning of the human mineralocorticoid receptor complementary cDNA: structural and functional kinship with the glucocorticoid receptor. Science 237, 268–274. ( 10.1126/science.3037703) [DOI] [PubMed] [Google Scholar]
- 75.de Kloet ER, Oitzl MS, Joels M. 1993. Functional implications of brain corticosteroid receptor diversity. Cell Mol. Neurobiol. 13, 433–455. ( 10.1007/BF00711582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rupprecht R, Arriza JL, Sprengler D, Reul JMHM, Evans RM, Holsboer F, Damm K. 1993. Transactivation and synergistic properties of the mineralocorticoid receptor: relations to the glucocorticoid receptor. Mol. Endocrinol. 7, 597–603. [DOI] [PubMed] [Google Scholar]
- 77.Miller AH, Spencer RL, Pearce BD, Pisell TL, Azrieli Y, Tanapat P, Moday H, Rhee R, McEwen BS. 1998. Glucocorticoid receptors are differentially expressed in the cells and tissues of the immune system. Cell. Immunol. 186, 45–54. ( 10.1006/cimm.1998.1293) [DOI] [PubMed] [Google Scholar]
- 78.Joëls M, Sarabdjitsingh A, Karst H. 2012. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacol. Rev. 64, 901–938. ( 10.1124/pr.112.005892) [DOI] [PubMed] [Google Scholar]
- 79.Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, Haettig J, Wood MA. 2010. Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. J. Neurosci. 30, 5037–5046. ( 10.1523/JNEUROSCI.5717-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gutièrrez-Mecinas M, Trollope AF, Collins A, Morfett H, Hesketh SA, Kersanté F, Reul JM. 2011. Long-lasting behavioral responses to stress involve a direct interaction of glucocorticoid receptors with ERK1/2-MSK1-Elk-1 signaling. Proc. Natl Acad. Sci. USA 108, 13 806–13 811. ( 10.1073/pnas.1104383108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hunter RG, Murakami G, Dewell S, Seligsohn M, Baker ME, Datson NA, McEwen BS, Pfaff DW. 2012. Acute stress and hippocampal histone H3 lysine 9 trimethylation, a retrotransposon silencing response. Proc. Natl Acad. Sci. USA 109, 17 657–17 662. ( 10.1073/pnas.1215810109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. 2003. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J. Neurosci. 23, 4850–4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Di S, Malcher-Lopes R, Marcheselli VL, Bazan NG, Tasker JG. 2005. Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and γ-aminobutyric acid inputs to hypothalamic magnocellular neurons. Endocrinology 146, 4292–4301. ( 10.1210/en.2005-0610) [DOI] [PubMed] [Google Scholar]
- 84.Le Menuet D, Lombes M. 2014. The neuronal mineralocorticoid receptor: from cell survival to neurogenesis. Steroid 99, 11–19. ( 10.1016/j.steroids.2014.05.018) [DOI] [PubMed] [Google Scholar]
- 85.Harris AP, Holmes MC, de Kloet ER, Chapman KE, Seckl JR. 2013. Mineralocorticoid and glucocorticoid receptor balance in control of HPA axis and behavior. Psychoneuroendocrinology 38, 648–658. ( 10.1016/j.psyneuen.2012.08.007) [DOI] [PubMed] [Google Scholar]
- 86.Blank T, Nijholt I, Eckart K, Spiess J. 2002. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J. Neurosci. 22, 3788–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reul JM, Holsboer F. 2002. On the role of corticotropin-releasing hormone receptors in anxiety and depression. Dialogues Clin. Neurosci. 4, 31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Starowicz K, Przewlocka B. 2003. The role of melanocortins and their receptors in inflammatory processes, nerve regeneration and nociception. Life Sci. 73, 823–847. ( 10.1016/S0024-3205(03)00349-7) [DOI] [PubMed] [Google Scholar]
- 89.Bale TL, Vale WW. 2004. CRF and CRF receptors: role in stress responsivity and other behaviors. Ann. Rev. Pharmacol. Toxicol. 44, 525–557. ( 10.1146/annurev.pharmtox.44.101802.121410) [DOI] [PubMed] [Google Scholar]
- 90.Charles CJ, Rademaker MT, Richards AM. 2004. Urocortins: putative role in cardiovascular disease. Curr. Med. Chem. Cardiovasc. Hematol. Agents 2, 43–47. ( 10.2174/1568016043477341) [DOI] [PubMed] [Google Scholar]
- 91.Getting S. 2006. Targeting melanocortin receptors as potential novel therapeutics. Pharmacol. Therapeut. 111, 1–15. ( 10.1016/j.pharmthera.2005.06.022) [DOI] [PubMed] [Google Scholar]
- 92.Aguilera G, Subburaju S, Young S, Chen J. 2008. The parvocellular vassopressinergic system and responsiveness of the hypothalamic pituitary adrenal axis during chronic stress. Prog. Brain Res. 170, 29–39. ( 10.1016/S0079-6123(08)00403-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Catania A. 2008. Neuroprotective actions of melanocortins: a therapeutic opportunity. Trends Neurosci. 31, 353–360. ( 10.1016/j.tins.2008.04.002) [DOI] [PubMed] [Google Scholar]
- 94.Capitanio JP, Cole SW. 2015. Social instability and immunity in rhesus monkeys: the role of the sympathetic nervous system. Phil. Trans. R. Soc. B 370, 20140104 ( 10.1098/rstb.2014.0104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wong DL, Tai TC, Wong-Faull DC, Claycomb R, Meloni EG, Myers KM, Carlezon WA, Kvetnansky R. 2012. Epinephrine: a short- and long-term regulator of stress and developmental illness: a potential new role for epinephrine in stress. Cell. Mol. Neurobiol. 32, 737–748. ( 10.1007/s10571-011-9768-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alberts SC, Sapolsky RM, Altmann J. 1992. Behavioral, endocrine, and immunological correlates of immigration by an aggressive male into a natural primate group. Horm. Behav. 26, 167–178. ( 10.1016/0018-506X(92)90040-3) [DOI] [PubMed] [Google Scholar]
- 97.Romero LM, Reed JM, Wingfield JC. 2000. Effects of weather on corticosterone responses in wild free-living passerine birds. Gen. Comp. Endocrinol. 118, 113–122. ( 10.1006/gcen.1999.7446) [DOI] [PubMed] [Google Scholar]
- 98.Sapolsky RM. 1986. Endocrine and behavioral correlates of drought in the wild baboons. Am. J. Primatol. 11, 217–226. ( 10.1002/ajp.1350110303) [DOI] [PubMed] [Google Scholar]
- 99.Romero LM. 2002. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 128, 1–24. ( 10.1016/S0016-6480(02)00064-3) [DOI] [PubMed] [Google Scholar]
- 100.Landys MM, Ramenofsky M, Wingfield JC. 2006. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of period life processes. Gen. Comp. Endocrinol. 148, 132–149. ( 10.1016/j.ygcen.2006.02.013) [DOI] [PubMed] [Google Scholar]
- 101.Ruys JD, Mendoza SP, Capitanio JP, Mason WA. 2004. Behavioral and physiological adaptation to repeated chair restraint in rhesus macaques. Physiol. Behav. 82, 205–213. ( 10.1016/j.physbeh.2004.02.031) [DOI] [PubMed] [Google Scholar]
- 102.Dal-Zotto S, Martí O, Armario A. 2002. Is repeated exposure to immobilization needed to induce adaptation of the hypothalamic–pituitary–adrenal axis? Influence of adrenal factors. Behav. Brain Res. 129, 187–195. ( 10.1016/S0166-4328(01)00340-0) [DOI] [PubMed] [Google Scholar]
- 103.Marissal-Arvy N, Gaumont A, Langlois A, Dabertrand F, Bouchecareilh M, Tridon C, Mormède P. 2007. Strain differences in hypothalamic pituitary adrenocortical axis function and adipogenic effects of corticosterone in rats. J. Endocrinol. 195, 473–484. ( 10.1677/JOE-07-0077) [DOI] [PubMed] [Google Scholar]
- 104.Atkinson H, Wood S, Kershaw YM, Bate E, Lightman SL. 2006. Diurnal variation in the responsiveness of the hypothalamic–pituitary–adrenal axis of the male rat to noise stress. J. Neuroendocrinol. 18, 526–533. ( 10.1111/j.1365-2826.2006.01444.x) [DOI] [PubMed] [Google Scholar]
- 105.Seeman TE, Robbins RJ. 1994. Aging and hypothalamic–pituitary–adrenal response to challenge in humans. Endocr. Rev. 15, 233–260. [DOI] [PubMed] [Google Scholar]
- 106.Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. 2009. Developmental changes in hypothalamus–pituitary–adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev. Psychopath. 21, 69–85. ( 10.1017/S0954579409000054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stewart JG, Mazurka R, Bond L, Wynne-Edwards KE, Harkness KL. 2013. Rumination and impaired cortisol recovery following a social stressor in adolescent depression. J. Abnorm. Child Psychol. 41, 1015–1026. ( 10.1007/s10802-013-9740-1) [DOI] [PubMed] [Google Scholar]
- 108.Stolte EH, Verbug van Kemenade BML, Savelkoul HFJ, Flik G. 2006. Evolution of glucocorticoid receptors with different glucocorticoid sensitivity. J. Endocrinol. 190, 17–28. ( 10.1677/joe.1.06703) [DOI] [PubMed] [Google Scholar]
- 109.Westphal NJ, Seasholtz AF. 2006. CRH-BP: The regulation and function of a phylogenetically conserved binding protein. Front. Biosci. 11, 1878–1891. ( 10.2741/1931) [DOI] [PubMed] [Google Scholar]
- 110.Yao M, Denver RJ. 2007. Regulation of vertebrate corticotropin-releasing factor genes. Gen. Comp. Endocrinol. 153, 200–216. ( 10.1016/j.ygcen.2007.01.046) [DOI] [PubMed] [Google Scholar]
- 111.Peckett AJ, Wright DC, Riddell MC. 2012. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism 60, 1500–1510. ( 10.1016/j.metabol.2011.06.012) [DOI] [PubMed] [Google Scholar]
- 112.Burt MG, Johannsson G, Umpleby AM, Chisholm DJ, Ho KKY. 2007. Impact of acute and chronic low-dose glucocorticoids on protein metabolism. J. Clin. Endicrinol. Metab. 92, 3923–3929. ( 10.1210/jc.2007-0951) [DOI] [PubMed] [Google Scholar]
- 113.Strack AM, Sebastian RJ, Schwartz MW, Dallman MF. 1995. Glucocorticoids and insulin: reciprocal signals for energy balance. Am. J. Physiol. 268, R142–R149. [DOI] [PubMed] [Google Scholar]
- 114.Dallman MF, Pecoraro NC, la Fleur SE. 2005. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav. Immun. 9, 275–280. ( 10.1016/j.bbi.2004.11.004) [DOI] [PubMed] [Google Scholar]
- 115.Epel E, Lapidus R, McEwen B, Brownell K. 2001. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 26, 37–49. ( 10.1016/S0306-4530(00)00035-4) [DOI] [PubMed] [Google Scholar]
- 116.Skórzewska A, et al. 2006. The effects of acute and chronic administration of corticosterone on rat behavior in two models of fear responses, plasma corticosterone concentration, and c-Fos expression in the brain structures. Pharmacol. Biochem. Behav. 85, 522–534. ( 10.1016/j.pbb.2006.10.001) [DOI] [PubMed] [Google Scholar]
- 117.Tan JTT, Patel BK, Kaplan LM, Koenig JI, Hooi SC. 1998. Regulation of leptin expression and secretion by corticosteroids and insulin. Endocrine 8, 85–92. ( 10.1385/ENDO:8:1:85) [DOI] [PubMed] [Google Scholar]
- 118.Epel ES, Lin J, Dhabhar FS, Wolkowitz OM, Puterman E, Karan L, Blackburn EH. 2010. Dynamics of telomerase activity in response to acute psychological stress. Brain Behav. Immun. 24, 531–539. ( 10.1016/j.bbi.2009.11.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kadmiel M, Cidlowski JA. 2013. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol. Sci. 34, 518–530. ( 10.1016/j.tips.2013.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shalev I, Entrigner S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, Epel ES. 2013. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology 38, 1835–1842. ( 10.1016/j.psyneuen.2013.03.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Joëls M, Krugers H, Karst H. 2014. Regulation of excitatory synapses by stress hormones. In Synaptic stress and pathogenesis of neuropsychiatric disorders (eds Popoli M, Diamond D, Sanacora G.), pp. 19–32. New York, NY: Springer. [Google Scholar]
- 122.Monfort SL. 2003. Non-invasive endocrine measures of reproduction and stress in wild populations. In Reproduction and integrated conservation science (eds Wildt DE, Holt W, Pickard A.), pp. 147–165. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 123.Touma C, Sachser N, Möstl E, Palme R. 2003. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen. Comp. Endocrinol. 130, 267–278. ( 10.1016/S0016-6480(02)00620-2) [DOI] [PubMed] [Google Scholar]
- 124.Cavigelli SA, Monfort SL, Whitney TW, Mechref YS, Novotny M, McClintock MK. 2005. Frequent serial rat fecal corticoid measures reflect circadian and ovarian corticosterone rhythms. J. Endocrinol. 184, 153–163. ( 10.1677/joe.1.05935) [DOI] [PubMed] [Google Scholar]
- 125.Palme R. 2005. Measuring fecal steroids: guidelines for practical application. Ann. NY Acad. Sci. 1046, 75–80. ( 10.1196/annals.1343.007) [DOI] [PubMed] [Google Scholar]
- 126.Goymann W, Trappschuh M, Jensen W, Schwabl I. 2006. Low ambient temperature increases food intake and dropping production, leading to incorrect estimates of hormone metabolite concentrations in European stonechats. Horm. Behav. 49, 644–653. ( 10.1016/j.yhbeh.2005.12.006) [DOI] [PubMed] [Google Scholar]
- 127.Rimbach R, Heymann EW, Link A, Heistermann M. 2013. Validation of an enzyme immunoassay for assessing adrenocortical activity and evaluation of factors that affect levels of fecal glucocorticoid metabolites in two New World primates. Gen. Comp. Endocrinol. 191, 13–23. ( 10.1016/j.ygcen.2013.05.010) [DOI] [PubMed] [Google Scholar]
- 128.Smith JE, Monclus R, Wantuck D, Florant GL, Blumstein DT. 2012. Fecal glucocorticoid metabolites in wild yellow-bellied marmots: experimental validation, individual differences and ecological correlates. Gen. Comp. Endocrinol. 17, 417–426. ( 10.1016/j.ygcen.2012.06.015) [DOI] [PubMed] [Google Scholar]
- 129.Cavigelli SA. 1999. Behavioural patterns associated with faecal cortisol levels in free-ranging female ring-tailed lemurs, Lemur catta. Anim. Behav. 57, 935–944. ( 10.1006/anbe.1998.1054) [DOI] [PubMed] [Google Scholar]
- 130.Sussman RW. 1991. Demography and social organization of free-ranging Lemur catta in the Beza Mahafaly Reserve, Madagascar. Am. J. Phys. Anthropol. 84, 43–58. ( 10.1002/ajpa.1330840105) [DOI] [Google Scholar]
- 131.Sauther ML, Sussman RW, Gould L. 1999. The socioecology of the ringtailed lemur: thirty-five years of research. Evol. Anthropol. 8, 120–132. () [DOI] [Google Scholar]
- 132.Hood LC, Jolly A. 1995. Troop fission in female Lemur catta at Berenty Reserve, Madagascar. Int. J. Primatol. 16, 997–1015. ( 10.1007/BF02696113) [DOI] [Google Scholar]
- 133.Kappeler PM. 1993. Female dominance in primates and other mammals. In Perspectives in ethology, behaviour and evolution, vol. 10 (eds Bateson PPG, Klopfer PH, Thompson NS.), pp. 143–158. New York, NY: Plenum Press. [Google Scholar]
- 134.Sokal RR, Rohlf FJ. 1995. Biometry: the principles and practice of statistics in biological research, 3rd edn New York, NY: W.H. Freeman and Company. [Google Scholar]
- 135.Eriksen HR, Olff M, Murison R, Ursin H. 1999. The time dimension in stress responses: relevance for survival and health. Psychiat. Res. 85, 39–50. ( 10.1016/S0165-1781(98)00141-3) [DOI] [PubMed] [Google Scholar]
- 136.Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. 2006. Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proc. Natl Acad. Sci. USA 103, 17 058–17 063. ( 10.1073/pnas.0605053103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kappeler PM, van Schaik CP. 2002. Evolution of primate social systems. Int. J. Primatol. 23, 707–740. ( 10.1023/A:1015520830318) [DOI] [Google Scholar]
- 138.Avitsur R, Stark JL, Sheridan JF. 2001. Social stress induces glucocorticoid resistance in subordinate animals. Horm. Behav. 39, 247–257. ( 10.1006/hbeh.2001.1653) [DOI] [PubMed] [Google Scholar]
- 139.Korzan WJ, Fernald RD, Grone BP. 2014. Social regulation of cortisol receptor gene expression. J. Exp. Biol. 217, 3221–3228. ( 10.1242/jeb.104430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Miller GE, Cohen S, Ritchey AK. 2002. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 21, 531–541. ( 10.1037/0278-6133.21.6.531) [DOI] [PubMed] [Google Scholar]
- 141.Sachser N. 1987. Short-term responses of plasma norepinephrine, epinephrine, glucocorticoid and testosterone titers to social and non-social stressors in male guinea pigs of different social status. Physiol. Behav. 39, 11–20. ( 10.1016/0031-9384(87)90338-6) [DOI] [PubMed] [Google Scholar]
- 142.Stringhini S, Sabia S, Shipley M, Brunner E, Nabi H, Kivimaki M, Singh-Manoux A. 2010. Association of socioeconomic position with health behaviors and mortality. J. Am. Med. Assoc. 303, 1159–1166. ( 10.1001/jama.2010.297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Grobler JMB, Wood CM. 2013. The physiology of rainbow trout in social hierarchies: two ways of looking at the same data. J. Comp. Physiol. B 183, 787–799. ( 10.1007/s00360-013-0752-5) [DOI] [PubMed] [Google Scholar]
- 144.Corlatti L, Palme R, Lovari S. 2014. Physiological response to etho-ecological stressors in male Alpine chamois: timescale matters! Naturwissenschaften 101, 577–586. ( 10.1007/s00114-014-1195-x) [DOI] [PubMed] [Google Scholar]
- 145.Pride RE. 2005. High faecal glucocorticoid levels predict mortality in ring-tailed lemurs (Lemur catta). Biol. Lett. 1, 60–63. ( 10.1098/rsbl.2004.0245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cote J, Clobert J, Meylan S, Fitze PS. 2006. Experimental enhancement of corticosterone levels positively affects subsequent male survival. Horm. Behav. 49, 320–327. ( 10.1016/j.yhbeh.2005.08.004) [DOI] [PubMed] [Google Scholar]
- 147.Cabezas S, Blas J, Marchant TA, Moreno S. 2007. Physiological stress levels predict survival probabilities in wild rabbits. Horm. Behav. 51, 313–320. ( 10.1016/j.yhbeh.2006.11.004) [DOI] [PubMed] [Google Scholar]
- 148.Romero LM, Wikelski M. 2010. Stress physiology as a predictor of survival in Galapagos marine iguanas. Proc. R. Soc. B 277, 3157–3162. ( 10.1098/rspb.2010.0678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Roberts ML, Buchanan KL, Hasselquist D, Bennett ATD, Evans MR. 2007. Physiological, morphological and behavioural effects of selecting zebra finches for divergent levels of corticosetrone. J. Exp. Biol. 210, 4368–4378. ( 10.1242/jeb.007104) [DOI] [PubMed] [Google Scholar]
- 150.Tung J, Barreiro LB, Johnson ZP, Hansen KD, Michopoulos V, Toufexis D, Michelini K, Wilson ME, Gilad Y. 2012. Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proc. Natl Acad. Sci. USA 109, 6490–6495. ( 10.1073/pnas.1202734109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Maruska KP, Becker L, Neboori A, Fernald RD. 2013. Social descent with territory loss causes rapid behavioral, endocrine and transcriptional changes in the brain. J. Exp. Biol. 216, 3656–3666. ( 10.1242/jeb.088617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Saltzmann W, Schultz-Darken NJ, Wegner FH, Wittwer DJ, Abbott DH. 1998. Suppression of cortisol levels in subordinate female marmosets: reproductive and social contributions. Horm. Behav. 33, 58–74. ( 10.1006/hbeh.1998.1436) [DOI] [PubMed] [Google Scholar]


