Abstract
Despite strong links between sociality and fitness that ultimately affect the size of animal populations, the particular social and ecological factors that lead to endangerment are not well understood. Here, we synthesize approximately 25 years of data and present new analyses that highlight dynamics in forest composition, food availability, the nutritional quality of food, disease, physiological stress and population size of endangered folivorous red colobus monkeys (Procolobus rufomitratus). There is a decline in the quality of leaves 15 and 30 years following two previous studies in an undisturbed area of forest. The consumption of a low-quality diet in one month was associated with higher glucocorticoid levels in the subsequent month and stress levels in groups living in degraded forest fragments where diet was poor was more than twice those in forest groups. In contrast, forest composition has changed and when red colobus food availability was weighted by the protein-to-fibre ratio, which we have shown positively predicts folivore biomass, there was an increase in the availability of high-quality trees. Despite these changing social and ecological factors, the abundance of red colobus has remained stable, possibly through a combination of increasing group size and behavioural flexibility.
Keywords: endocrinology, glucocorticoids, non-equilibrium dynamics, nutritional ecology, endangered populations
1. Introduction
Threatened animal species frequently share many common characteristics such as decreasing habitat size and quality, poor nutrition, physiological stress, low fecundity and long generation times. In addition, their small population sizes put them at a high risk of declines owing to stochastic events, such as extreme weather events or disease outbreaks [1,2]. For example, the African rinderpest epidemic in the late 1880s that caused the devastating loss of cattle and wildlife [3], and the distressing effects of Ebola on great ape populations [4–6] underscore the need to investigate the impact of infectious diseases [7–9]. Also, the loss of co-evolved endemic parasites in small and isolated threatened host populations may make them susceptible to gaining novel infectious agents that can cause declines [10].
Rates of discovery [11] and emergence of infectious diseases appear to be accelerating [12–14], though some of this is likely based on increased sampling effort and improved diagnostics techniques; also, some of these diseases may cause pathology, whereas others may not. For example, within the past decade in one small area of Kibale National Park, Uganda, our research group has discovered distinct simian pegiviruses in three species of wild monkeys [15], a new SIV lineage in red-tailed guenons (Cercopithecus ascanius) [16], and novel delta-lenti-and spuma-retroviruses [17], just to name a few. If diseases are emerging at a faster rate and/or their role as stochastic events causing population declines are more important than previously recognized, then the small population sizes of endangered species may place them at greater risk from disease outbreaks than previously thought.
While not all pathogens are necessarily lethal, some can clearly devastate plant and animal populations [3,4,9]. For example, a number of tree populations have been decimated by emerging infections, including chestnut, elm, pine and oak trees [18–21]. Fungal infections have been associated with the loss or reduction in populations of amphibians, snakes, bees, crayfish and bats [18,21,22]. Some Hawaiian bird populations have been driven to extinction or near extinction by avian malaria, and this pathogen is predicted to endanger additional populations as a result of climate change [23,24]. An infectious cancer has caused the near extinction of the Tasmanian devil (Sarcophilus harrisii) [22,25].
However, pathogens do not act in isolation in their impact on populations of endangered species. There is ample evidence that single-factor explanations for complex biological phenomena, such as animal abundance, are uncommon. Rather, studies have highlighted the importance of multifactorial explanations, with disease being one of the factors interacting with other important variables. For example, high stress levels or poor nutrition could lead to a greater susceptibility to disease, which together could have a negative influence on population size. Gulland [26] presented an excellent experiment in wild sheep (Ovis aries), where she studied the interacting effects of nutrition and parasites on survival. Gulland found that an observed population crash coincided with both malnourishment and high nematode loads. However, sheep mortality rates were reduced if sheep were left in the field with low-quality diets but were treated for gastrointestinal parasites, or if they had high parasite loads but were fed high-quality diets. Thus, while the independent negative effects of nutrition and disease were negligible, their simultaneous occurrence had an additive or synergistic effect that influenced fitness and population abundance.
Physiological mechanisms, such as hormonal responses to changing social and environmental conditions, may mediate the impact of nutrition and disease on a population's abundance [27]. Although few field studies confirm a direct causal relationship between activation of the hypothalamic–pituitary–adrenal (HPA) axis (i.e. secretion of glucocorticoids, such as cortisol) and fitness, chronically elevated glucocorticoid levels may suppress reproduction, immune response and ultimately reduce survival [28–34]. Pride [35] found that glucocorticoid levels were positively associated with mortality in female ring-tailed lemurs (Lemur catta), and similar results have been reported in marine iguanas (Amblyrhynchus cristatus: [36]). Pride [37] also demonstrated that lemurs with low food intake had elevated glucocorticoid levels, highlighting nutritional stress as a probable factor influencing survival as a result of HPA axis activation. While these studies suggest that glucocorticoid levels are useful as a proxy for individual or population health, and are thus useful for conservation studies, the relationship between glucocorticoids and fitness is not always clear. Two recent reviews have found that variation in glucocorticoid levels were positively, negatively, or non-significantly related to fitness [38,39]. For example, Ebensperger et al. [40] found no relationship between faecal cortisol and survival of Octodon debus, a social rodent, but faecal cortisol was associated with reduced female reproduction, with females having high cortisol levels being less likely to produce a second litter in the same season. This study highlights that any relationship that is found between glucocorticoid levels and fitness can be influenced by the exact fitness estimate considered (e.g. survival, seasonal versus lifetime reproductive output). Therefore, glucocorticoid measurements cannot be assumed to be a proxy of fitness for conservation purposes; rather, levels must be related to relevant conservation variables and the measure of fitness clearly defined.
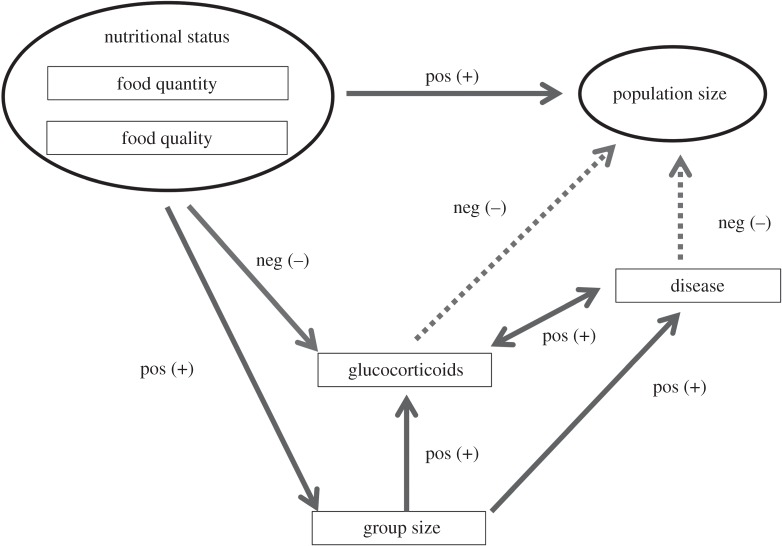
Over the past approximately 25 years, various research teams have examined factors influencing the abundance of one endangered primate species, the red colobus monkey (Procolobus rufomitratus), a gregarious, long-lived primate with a largely folivorous diet. Research on the red colobus of Uganda was initiated by Struhsaker (1970–1987) and further intensive research has been led by Chapman since 1989 [41]. We examine and summarize changes in population size with respect to changes in (i) forest composition, (ii) quality of food items, (iii) parasite levels and (iv) glucocorticoids over various time periods (figure 1), with the aim of investigating what is driving population change in this species.
Figure 1.
Theoretical relationships among examined factors predicted to influence population size of red colobus (Procolobus rufomitratus) in Kibale National Park, Uganda. Ovals indicate long-term data, whereas rectangles indicate cross-sectional data. Solid lines indicate relationships that were examined, whereas dotted lines represent theoretical relationships that were not examined. Positives (+) and negatives (–) indicate predicted (not necessarily observed) relationships.
2. Methods and results
(a). Study site
This research was conducted in and around Kibale National Park (hereafter Kibale), Uganda, a 795 km2 area composed predominantly of mature moist semi-deciduous and evergreen forest that also includes a variety of other habitats, including grassland, woodland, lakes, wetland and colonizing forest [42,43]. Kibale receives an average of 1691 mm rainfall annually (C. A. Chapman 1990–2013, unpublished data) during two rainy seasons, with daily temperatures ranging from 15.5°C to 23.7°C. Kibale is divided into forestry compartments that were previously subjected to varying degrees of logging and restoration efforts [44,45]. Research focused on the old-growth forest compartment of K-30, but we supplement this with data from other variably logged park compartments within the park (i.e. K-14 and K-15) and from nearby forest fragments outside the park.
(b). Red colobus diet and changes in forest composition and food quantity
In 1989, 26 permanent vegetation plots (200 × 10 m; 22 analysed here, excluding four with equivocal logging history) were established and randomly distributed within an existing trail system in all three forest compartments (K-30, K-14, K-15). Following the initial survey in 1989, all plots were resurveyed in May 2000, September–November 2006 and January 2013; during each survey, tree species was identified (less than 95% to species level) and the diameter at breast height (DBH) was recorded for all tree stems with DBH > 10 cm [44,46,47].
To determine the diet and general activity of red colobus, animals were observed for 181 months between May 1994 and November 2013, typically beginning at 08.00 and ending at approximately 16.00. At 15-min intervals, instantaneous behavioural samples were made of five different individuals [47]. If the individual was feeding, the species and plant part (e.g. fruit, young leaf, leaf petiole) were recorded, and these data were used to determine which tree species constituted important foods.
(i). Results
A linear-mixed effects model (fixed effect = sampling period, random effect = vegetation plot identity) detected a significant increase in the cumulative log10 DBH of all trees larger than 10 cm in both the 2006 and 2013 tree surveys relative to the initial 1989 survey (table 1a; [47]). In addition, when only those species consumed regularly by red colobus were included in the analysis, the linear-mixed effects model indicated that the availability of red colobus food (i.e. cumulative log10 DBH) was significantly higher in both 2006 and 2013 than in 1989 (table 1b; [47]).
Table 1.
Results of the linear-mixed effects models to test for changes in food availability (cumulative log10 DBH) in 22 plots between four survey periods in K-30, K-15 and K-14. Means ± 95% confidence intervals (CI95 = 1.96 × s.e.) and t-values of fixed effects (survey periods) are presented. Significant values at the 5% level are in italics and marked with an asterisk. The baseline year to test for changes in food availability over time is 1989 for (a,b), and 1996 for (c,d). We included the sampling period as the fixed effect, and vegetation plot identity as a random effect. We summarize model results using the ANOVA function in the R package ‘car’ [48], which calculates p-values for the predictor variable using Wald tests, and calculated R2 for the model by dividing the sum of squares for the sampling period divided by the residual sum of squares. RC, red colobus; YL, young leaves, ML, mature leaves (data from [47]; however, R2 and p-values represent novel analyses).
| food abundance (cumulative logDBH) | intercept (mean ± CI95) | t | 2000 (mean ± CI95) | t | 2006 (mean ± CI95) | t | 2013 (mean ± CI95) | t | R2 | F-statistic (d.f. = 3,63) |
|---|---|---|---|---|---|---|---|---|---|---|
| (a) all trees | 111.7 ± 13.7 | 16.66* | –1.9 ± 5.1 | –0.72 | 7.3 ± 5.1 | 2.83* | 12.4 ± 5.1 | 4.78* | 0.393 | 13.03* |
| (b) RC food species | 46.6 ± 9.7 | 9.44* | 1.1 ± 3.3 | 0.65 | 5.4 ± 3.3 | 3.20* | 8.6 ± 3.3 | 5.09* | 0.346 | 11.10* |
| (c) RC food species weighted by protein : fibre of YL | 46.4 ± 7.6 | 12.03* | 0.0 ± 2.8 | 0.01 | 2.0 ± 2.8 | 1.39 | 3.2 ± 2.8 | 2.21* | 0.102 | 2.38 (p = 0.078) |
| (d) RC food species weighted by protein : fibre of ML | 30.8 ± 5.9 | 10.27* | 1.6 ± 1.9 | 1.70 | 4.5 ± 1.9 | 4.68* | 6.9 ± 1.9 | 7.12* | 0.487 | 19.93* |
(c). Food quality
Building on previous research by Milton [49] demonstrating the importance of the protein-to-fibre ratio as a criterion for leaf selection by folivorous primates, we have accumulated data to examine if this factor predicts the biomass of arboreal folivores [50]. High fibre content is often considered to deter feeding on foods as it requires time-consuming fermentation by symbiotic microbes [51]. Furthermore, because nitrogen is a limiting nutrient in many food webs and is predominantly found in protein, herbivores should compensate for this limitation by choosing food with higher protein-to-fibre ratios [52]. Milton's seminal idea has since been applied at the population level to red colobus populations and other species [53–57]. The protein-to-fibre ratio as an index of dietary quality has generally been successfully applied to predict the biomass of small-bodied folivorous monkeys at both local [58,59] and regional scales [50,54,55,60,61], but some results are mixed [62]. As previously reported, this dataset is able to accurately (r2 = 0.87, p < 0.001) predict biomass of folivorous primates, supporting the idea that this ratio accurately reflects food quality [50].
Based on published greenhouse experiments, increases in temperature, rainfall and CO2 are all predicted to negatively impact the nutritional quality of leaves (i.e. decrease protein and increase fibre [63]); this is the direction in which climate is changing in Kibale.
(i). Results
Red colobus food availability was weighted by the protein-to-fibre ratio to obtain a measure of food abundance in relation to food quality [44]. Mixed effect linear models indicated there was no difference in the availability of high-quality (i.e. high protein-to-fibre) young leaves (the primary food part consumed by red colobus) in 2006, but an increase was observed in 2013 relative to 1996, the first year nutrient content was measured (table 1c). There was an increase in the availability of lower quality (i.e. low protein-to-fibre) mature leaves in 2006 and 2013 relative to the baseline year (table 1d).
All predictions based on greenhouse experiments were supported and showed a decline in the quality of tree leaves 15 and 30 years after two previous studies in an undisturbed area of Kibale [64]. With a sample of individuals of 10 tree species, after 30 years, there was a mean increase in the fibre content of mature leaves of 10%. In eight tree species, the fibre content of young leaves increased 15%, and protein decreased 6% after 15 years. A 31% decline in monkey abundance was forecast when these nutrient values were placed into the model predicting folivorous primate biomass [64]; however, these results did not take into account the increasing habitat-wide food availability found by Gogarten et al. [47] over a different time period. Thus, the observed increase in food availability and decrease in quality of food predict contrary effects on abundance.
(d). Infectious disease
Freeland [65–68] sampled protozoan infections of Kibale primates between June 1974 and May 1975, providing valuable baseline data. Following Freeland's methods [65] as closely as possible, between February 2008 and July 2010, Chapman et al. resampled primates in the same population to determine their protozoan infections. They collected faecal samples from adults and juveniles in multiple groups (n = 5–9) for each of five species [69]. Samples were collected from groups inhabiting the same area in Kibale that were originally sampled. Entamoeba histolytica and Entamoeba dispar have cysts that are morphologically indistinguishable and it was only recently that E. dispar was considered a distinct species [70]. While E. histolytica is pathogenic and Entamoeba dispar is not, we consider them as a complex (E. histolytica/dispar).
(i). Results
Samples collected by Freeland indicated that red colobus were uninfected by protozoans in 1974–1975 [67]. This is in accord with Kuhn [71], who did not find protzoans in the stomachs of colobines in general. In marked contrast, the more recent data indicate that red colobus groups have protozoan infections; Chilomastix mesnili, Dientamoeba fragilis, Endolimax nana, Entamoeba coli, Entamoeba hartmanni, E. histolytica/dispar, Iodamoeba bütschlii and Isospora sp. were identified. Thus, species richness of protozoan infections appears to have gone from zero in 1974–1975 to eight in 2008–2010, and for many of these protozoans, prevalence (i.e. proportion of infected faecal samples) was high during this last sampling period. However, this apparent change should be interpreted with caution for a number of reasons. First, Freeland did not report his sample size, making it difficult to evaluate how robust the initial results are. Second, Gillespie et al. [72] analysed over 1600 red colobus faecal samples collected between 1997 and 2003 and found that individuals were infected with up to five protozoan parasite species, but at a low prevalence (a species maximum of 4.4% of the samples were infected with given protozoan species). Thus, if Freeland's sample size was small, it is possible that he concluded the population did not harbour protozoans when in fact it did (i.e. a false negative). Long-term monitoring to determine if there are cycles in protozoan prevalence levels would help clarify if there has been a change, as would monitoring other isolated red colobus populations. However, without knowing the original sample size examined by Freeland, we view it as premature to state conclusively that a change in disease state has occurred.
There is recently an abundance of data on the viruses of red colobus [17,73]. Unfortunately, no long-term comparative data are available, so we cannot evaluate if there has been a change in the nature of the viral infections in this population of red colobus.
(e). Glucocorticoids
To examine the possible role of stress in determining red colobus population numbers, Chapman et al. quantified patterns of faecal glucocorticoid excretion in relation to food availability and quality, species richness/prevalence of some parasites (nematodes and protozoa), and group size in one large group (92 individuals; June 2003–April 2005) and one small group of red colobus (36 individuals; March 2004–April 2005) [74,75]. Poor nutrition and parasite infection were expected to lead to elevated glucocorticoid levels, and a time lag was expected before this became apparent. Larger group size was also predicted to be associated with higher glucocorticoid levels because of elevated within-group food competition. Additionally, group size could influence parasitism via increased encounter and transmission risk, or via increased susceptibility to parasites resulting from nutritional stress [76].
Each group was observed for six days per month and 1036 faecal samples (n = 759 from the large group; n = 277 from the small group) were collected. Faecal steroid levels can vary along a number of dimensions, including time of day, age and reproductive state. To reduce diurnal variation, sample collection time was restricted to 08.00–13.00 [77]. Sample collection was also limited to adult males and females with nursing infant, as it was not possible to determine if females were in the early stages of pregnancy and glucocorticoid levels are known to vary over pregnancy in a number of species [78,79]. Methods for sample processing and analyses can be found elsewhere [74,75,80]. Glucocorticoid levels from the two groups in Kibale were contrasted to levels from red colobus inhabiting eight nearby forest fragments [74].
(i). Results
There was considerable variation in monthly diet quality, with a fourfold difference between months with the highest and lowest mean monthly protein-to-fibre ratios. Mean monthly glucocorticoid level also varied (range: 72–133 ng g−1, n = 23 months; [75]). As predicted, consumption of a low-quality diet in one month was associated with elevated glucocorticoid levels in the subsequent month (r = –0.415, p = 0.027; [75]). Furthermore, a high proportion of samples with multiple parasite infections was associated with high glucocorticoid levels (r = 0.484, p = 0.011). Moreover, mean monthly glucocorticoid levels for groups in forest fragments were more than twice those in the large forest group (fragment groups: 267.2 ng g−1; forest group: 104.4 ng g−1), indicating much higher stress levels in the fragments where high-quality foods are limited. As predicted, there was a positive relationship between group size and mean glucocorticoid levels (large forest group: 99.8 ng g−1; small forest group: 85.4 ng g−1; t = –1.930, p = 0.039) [75], but there was no relationship between group size and the proportion of samples with multiple parasite infections (p > 0.10).
(f). Primate density
Estimating primate population size presents many challenges [81–84]. For this reason, our research group has used four different methods to estimate the number of red colobus in Kibale: (i) group density (groups km−2), (ii) individual density (individuals km−2), (iii) group encounter rate (groups observed per km of trail walked) and (iv) individual encounter rate (groups observed per km of trail walked, multiplied by group size determined after the census walk). See [85] for description of these estimators. All methods are based on line-transect census methodologies, considered the most appropriate methods for estimating densities of large-bodied diurnal species such as red colobus [86]. Primate censuses were conducted in the old-growth K-30 forestry compartment from August 1970 to October 1976 (by Struhsaker), and in the three study forestry compartments (K-30, K-14, K-15) from February 1980 to December 1981 (by Skorupa), from July 1996 to June 1997 (by Chapman, Balcomb and field assistants), and from July 2005 to June 2006 (by Chapman and field assistants). Using these techniques, identical routes were walked typically bi-weekly in each compartment (table 2). In total, 283 transects covering 1104 km were walked (see [85] for detailed methods).
Table 2.
Characteristics of census routes used at Kibale National Park, Uganda and the results of the censuses conducted over four periods in old-growth forest (statistics provided in the original studies) [47,85].
| 1970–1976 | 1980–1981 | 1996–1997 | 2005–2006 | |
|---|---|---|---|---|
| route length (km) | 4.0 | 4.0 | 4.0 | 4.0 |
| number of census walks | 61 | 28 | 25 | 22 |
| distance censused (km) | 244 | 112 | 100 | 88 |
| group density | 5.36 | 5.46 | 5.50 | 4.21 |
| mean group size | — | — | 37.17 | 52.07 |
| individual density | — | — | 204 | 219 |
| groups per kilometre walked | 0.824 | 1.144 | 0.636 | 0.465 |
| individuals per kilometre walked | — | — | 24 | 24 |
Group encounter rate does not take into account interobserver variation in the ability to detect animals (although the same observers conducted the last two censuses), differences in group size over time, nor does it correct for visibility differences between areas or over time (e.g. owing to forest regeneration), but it does avoid difficulties in assessing strip width. Each method has its advantages and disadvantages, but when they each indicate the same trend (e.g. a decline), it suggests the trend is real.
Large, widely dispersed forest primate groups are extremely difficult to accurately count and cannot be counted without disrupting the census for an unsuitable period (i.e. a group count takes up to a day to complete for some species). To obtain accurate estimates of group size, three observers selected an area of forest for eight days per month. To count all individuals, a specific group was often followed for up to 10 h and observers waited until the group made a single-file movement across a canopy opening, such as a treefall gap or road, where it is easier to count individuals. To ensure accuracy, repeated counts were made of the same group. Group sizes were quantified in two periods (July 1996—May 1998 and July 2010—May 2011; n = 268 group counts) at three nested spatial scales: (i) unhabituated groups throughout the park (broad scale), (ii) unhabituated and habituated groups in adjacent logged (K-14 and K-15) and old-growth (K-30) forest (intermediate scale), and (iii) habituated groups occurring only in old-growth forest (K-30, fine scale). Given that all three nested scales document the same trends [47], we report data only from the intermediate scale because these represent the areas of intensive red colobus and tree data collection.
(i). Results
There was no indication of any change in red colobus population size (table 2). While the number of groups (group density and number of groups per km walked) decreased over time, this decline is offset by increased group size, resulting in a stable number of individuals [47].
3. Discussion
Understanding and interpreting the influence of multiple factors on biological phenomena is rarely straightforward. The large body of research conducted at Kibale has found that over the past several decades, the population size of red colobus monkeys in Kibale has remained stable in spite of changing ecological conditions. Thus, our examination of the factors influencing red colobus population dynamics warrants careful consideration given the potential ecological pressures examined and conflicting predictions of how population size might be impacted (figure 1).
On the one hand, our results indicate that habitat-wide red colobus food availability and quality have recently increased, despite a decline in the quality of specific tree species (i.e. the study done in response to the greenhouse experiments), suggesting that we might expect an increase in population size. There was an increase in group size, possibly as a result of increased food availability and decreased food competition within groups; however, this has not resulted in a concomitant increase in population size because it appears that the overall number of groups has decreased, resulting in stable population numbers. It is unclear why such a change in the structure of the population might occur. Furthermore, short-term analyses indicate that food availability and quality are negatively correlated to faecal glucocorticoids [87], such that the absence of a change in population size is likely not the result of increased nutritional stress. However, the cross-sectional analyses indicate that larger groups have higher stress levels, though it is not known whether this is a result of nutritional and/or social stress (i.e. increased opportunity for conflict ([75], but see [88])).
Parasites may also have a negative impact on population size, but it remains unclear if there were any real increases in the red colobus protozoan community in the past three decades, nor if such changes would have a negative fitness effect. While new viruses have been documented in our red colobus study population, it is more likely that these viruses represent new discoveries rather than new infections. Nonetheless, it has been proposed that increased group size may lead to increased disease transmission, because sociality can increase parasite encounter probability [66,89]. Furthermore, larger groups may be more susceptible to infection given that potentially immunosuppressive glucocorticoid levels are elevated compared with smaller groups [32]. However, the short-term analysis of parasites does not support an effect of group-size on parasitism (C. A. Chapman 2006–2014, unpublished data). Larger groups were associated with fewer parasites, perhaps an effect of behavioural mechanisms such as cliques in social networks that reduce parasite transmission in larger groups [90–93].
Habitat-wide food availability appears to have recently increased, such that all else being equal, we would expect stress levels to decline. Both these parameters are speculated to result in population growth [28–32]. Although glucocorticoid levels are on average twice as high in forest fragments compared with those in Kibale [74], birth and early infant survival rates are comparable between fragments and the national park [94]. Thus, food availability and stress do not appear to influence red colobus population growth, at least where these early life stages are concerned.
In spite of all of these changes, red colobus population size has remained constant. This begs the questions: is disease/parasitism offsetting the benefits of increased habitat-wide food (quantity and quality as indicated by the habitat-wide assessment), or is the population responding in compensatory ways to the increased risk of disease, possibly via behavioural flexibility (e.g. avoidance of infected individuals or increased clique formation within social networks) [90,92,93,95]? As the number of studies on different populations of the same species accumulate, there is increasing recognition that there can be considerable flexibility in behaviour and demographic structure, which presumably can influence the numeric response of a species to a given environmental change [96–99]. If individuals are able to respond to environmental change rapidly by altering their behaviour, the population may have a large scope to adapt to environmental change without showing a change in size. It seems likely that for long-lived species, such as primates, and for species that have large geographical ranges within the same breeding population (see evidence of this for red colobus [100]), a premium would be placed on the ability to respond behaviourally to the nature of the food that is available or aspects of disease risk (e.g. change group size, dispersal rates, intergroup contact, movement patterns). Being able to respond behaviourally to environmental change is more likely to be found in species living in environments considered to be in a non-equilibrium state, now accepted as a fundamental property of many ecosystems [101–103].
Our summary of long-term research in and around Kibale revealed changes in social factors (i.e. increase in group size and corresponding changes in behaviour [47,104]), ecological conditions (i.e. increases in food availability, declines in nutritional quality per tree, but increases in the number of tree species providing nutritional foods [47,64]), and an interaction between stress levels and the quality of the diet, but it is premature to conclude whether there has been a change in disease state. Whatever the explanation for the stability of the red colobus population in spite of changes in the environment, our review draws attention to the importance of continuous, long-term data collection on a variety of parameters to start to unravel complex questions, such as the determinants of animal population abundance.
Acknowledgements
We thank Steve Dobson, Peter Kappeler, Charles Nunn, Patrick Omeja, Jessica Rothman, Tom Struhsaker, Dennis Twinomugisha, Eric Vander Wal, Peter Waterman, Richard Wrangham and an anonymous reviewer for helpful comments on this project. Richard Wrangham was essential in establishing the vegetation plots.
Ethics statement
Ethical approval was received from the Office of the President, Uganda, the Uganda National Council for Science and Technology, the Uganda Wildlife Authority and McGill Animal Care Committee.
Authors' contributions
C.A.C. contributed to all aspects of the research and manuscript preparation. V.A.M.S., T.R.B., J.F.G. and S.C. contributed substantially to data analysis, interpretation, manuscript preparation, revisions and final approval of the manuscript.
Funding statement
Funding to C.A.C. for research was provided by the Canada Research Chairs Programme, Natural Science and Engineering Research Council of Canada, Fonds Québécois de la Recherche sur la Nature et les Technologies, Wildlife Conservation Society, Canadian Foundation for Innovation, Killam Foundation, Primate Conservation Inc., Morris Animal Fund, Leakey Foundation, Ford Foundation, McGill University, National Geographic Society, and by NIH grant TW009237 as part of the joint NIH–NSF Ecology of Infectious Disease programme and the UK Economic and Social Research Council. T.R.B. was supported by an FQRNT Fellowship. J.F.G. was supported by an NSF Graduate Research Fellowship (DGE-1142336), the Canadian Institutes of Health Research's Strategic Training Initiative in Health Research's Systems Biology Training Programme, an NSERC Vanier Canada Graduate Scholarship (CGS), and a long-term Research Grant from the German Academic Exchange Service (DAAD-91525837-57048249).
Competing interests
We have no competing interests.
References
- 1.Pavelka MSM, Behie AM. 2005. The effect of hurricane iris on the food supply of black howlers (Alouatta pigra) in southern Belize. Biotropica 37, 102–108. ( 10.1111/j.1744-7429.2005.03102.x) [DOI] [Google Scholar]
- 2.Young TP. 1994. Natural die-offs of large mammals: implications for conservation. Conserv. Biol. 8, 410–418. ( 10.1046/j.1523-1739.1994.08020410.x) [DOI] [Google Scholar]
- 3.Morens DM, Holmes EC, Davis AS, Tubenberger JK. 2011. Global rinderpest eradication: lessons learned and why humans should celebrate too. J. Infect. Dis. 204, 502–505. ( 10.1093/infdis/jir327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh PD, et al. 2003. Catastrophic ape decline in western equatorial Africa. Nature 422, 611–614. ( 10.1038/nature01566) [DOI] [PubMed] [Google Scholar]
- 5.Leroy EM, et al. 2004. Multiple Ebola virus transmission events and rapid decline of Central African Wildlife. Science 303, 387–390. ( 10.1126/science.1092528) [DOI] [PubMed] [Google Scholar]
- 6.Huijbergts D, Wachter D. 2003. Ebola and the decline of gorilla (Gorilla gorilla) and chimpanzee (Pan troglodytes) populations in the Minkebe Forest, north-eastern Gabon. Oryx 37, 437–443. [Google Scholar]
- 7.Vander Wal E, Edye I, Paquet PC, Coltman DW, Bayner R, Brook RK, Andres JA. 2013. Juxtaposition between host population structures: implication for disease transmission in a sympatric cervid community. Evol. Appl. 6, 1001–1011. ( 10.1111/eva.12065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vander Wal E, Garant D, Fest-Bianchet M, Pelletier F. 2013. Evolutionary rescue in vertebrates: evidence, application and uncertainty. Phil. Trans. R. Soc. B 368, 20120090 ( 10.1098/rstb.2012.0090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman CA, Gillespie TR, Goldberg TL. 2005. Primates and the ecology of their infectious diseases: how will anthropogenic change affect host–parasite interactions? Evol. Anthropol. 14, 134–144. ( 10.1002/evan.20068) [DOI] [Google Scholar]
- 10.Altizer S, Nunn CL, Lindenfors P. 2007. Do threatened hosts have fewer parasites? A comparative study in primates. J. Anim. Ecol. 76, 304–314. ( 10.1111/j.1365-2656.2007.01214.x) [DOI] [PubMed] [Google Scholar]
- 11.Patz JA, Graczyk TK, Geller N, Vittor AY. 2000. Effects of environmental change on emerging parasitic diseases. Int. J. Parasitol. 30, 1395–1405. ( 10.1016/S0020-7519(00)00141-7) [DOI] [PubMed] [Google Scholar]
- 12.Daszak P, Cunningham AA, Hyatt AD. 2000. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287, 443–449. ( 10.1126/science.287.5452.443) [DOI] [PubMed] [Google Scholar]
- 13.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451, 990–994. ( 10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nunn CL, Thrall PH, Stewart K, Harcourt AH. 2008. Emerging infectious diseases and animal social systems. Evol. Ecol. 22, 519–543. ( 10.1007/s10682-007-9180-x) [DOI] [Google Scholar]
- 15.Sibley SL, et al. 2014. Discovery and characterization of distinct simian pegiviruses in three wild African Old World monkey species. PLoS ONE 9, e98569 ( 10.1371/journal.pone.0098569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauck M, et al. 2015. Discovery and full genome characterization of a new SIV lineage infecting red-tailed guenons (Cercopithecus ascanius schmidti) in Kibale National Park, Uganda. Retrovirology 11, 55 ( 10.1186/1742-4690-11-55) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg TL, et al. 2009. Co-infection of Ugandan red colobus (Procolobus [Piliocolobus] rufomitratus tephrosceles) with novel, divergent delta-, lenti- and spumaretroviruses. J. Virol. 83, 11 318–11 329. ( 10.1128/JVI.02616-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rohr JR, Raffel TR, Blaustein AR, Johnson PTJ, Paull SH, Young S. 2013. Using physiology to understand climate-driven changes in disease and their implications for conservation. Conserv. Physiol. 1, 1–15. ( 10.1093/conphys/cot022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anagnostakis SL. 1987. Chestnut blight: the classical problem of an introduced pathogen. Mycologia 79, 23–37. ( 10.2307/3807741) [DOI] [Google Scholar]
- 20.Mamiya Y. 1988. History of pine wilt disease in Japan. J. Nematol. 20, 219–226. [PMC free article] [PubMed] [Google Scholar]
- 21.Rohr JR, Raffel TR, Romansic JM, McCallum H, Hudson PJ. 2008. Evaluating the links between climate, disease spread, and amphibian declines. Proc. Natl Acad. Sci. USA 105, 17 436–17 441. ( 10.1073/pnas.0806368105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ. 2012. Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194. ( 10.1038/nature10947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Riper C, Van Riper SG, Goff ML, Laird M. 1986. The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol. Monogr. 56, 327–344. ( 10.2307/1942550) [DOI] [Google Scholar]
- 24.Garamszegi LZ. 2011. Climate change increases the risk of malaria in birds. Glob. Change Biol. 17, 1751–1759. ( 10.1111/j.1365-2486.2010.02346.x) [DOI] [Google Scholar]
- 25.McCallum H, Jones M, Hawkins C, Hamede R, Lachish S, Sinn DL, Beeton N, Lazenby B. 2009. Transmission dynamics of Tasmanian devil facial tumor disease may lead to disease-induced extinction. Ecology 90, 3379–3392. ( 10.1890/08-1763.1) [DOI] [PubMed] [Google Scholar]
- 26.Gulland FMD. 1992. The role of nematode parasites in Soay sheep (Ovis aries L.) mortality during a population crash. Parasitol. Res. 105, 493–503. ( 10.1017/S0031182000074679) [DOI] [PubMed] [Google Scholar]
- 27.Habig B, Archie EA. 2015. Social status, immune response and parasitism in males: a meta-analysis. Phil. Trans. R. Soc. B 370, 20140109 ( 10.1098/rstb.2014.0109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wasser SK, Bevis K, King G, Hanson E. 1997. Noninvasive physiological measures of disturbance in the Northern spotted owl. Conserv. Biol. 11, 1019–1022. ( 10.1046/j.1523-1739.1997.96240.x) [DOI] [Google Scholar]
- 29.Creel S. 2001. Social dominance and stress hormones. Trends Ecol. Evol. 16, 491–497. ( 10.1016/S0169-5347(01)02227-3) [DOI] [Google Scholar]
- 30.Bercovitch FB, Ziegler TE. 2002. Current topics in primate socioendocrinology. Annu. Rev. Anthropol. 31, 45–67. ( 10.1146/annurev.anthro.31.040202.105553) [DOI] [Google Scholar]
- 31.Creel S, Fox JE, Hardy J, Sands B, Garrot B, Peterson RO. 2002. Snowmobile activity and glucocorticoid stress responses in wolves and elk. Conserv. Biol. 16, 809–814. ( 10.1046/j.1523-1739.2002.00554.x) [DOI] [Google Scholar]
- 32.Boonstra R, Singleton GR. 1993. Population declines in the snowshoe hare and the role of stress. Gen. Comp. Endocrinol. 91, 126–143. ( 10.1006/gcen.1993.1113) [DOI] [PubMed] [Google Scholar]
- 33.Sapolsky RM. 2004. Social status and health in humans and other animals. Annu. Rev. Anthropol. 33, 393–418. ( 10.1146/annurev.anthro.33.070203.144000) [DOI] [Google Scholar]
- 34.Cavigelli SA, Caruso MJ. 2015. Sex, social status and physiological stress in primates: the importance of social and glucocorticoid dynamics. Phil. Trans. R. Soc. B 370, 20140103 ( 10.1098/rstb.2014.0103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pride RE. 2005. High faecal glucocorticoid levels predict mortality in ring-tailed lemurs (Lemur catta). Biol. Lett. 1, 60–63. ( 10.1098/rsbl.2004.0245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romero LM, Wikelski M. 2001. Corticosterone levels predict survival probabilities of Galapagos marine iguanas during El Nino events. Proc. Natl Acad. Sci. USA 98, 7366–7370. ( 10.1073/pnas.131091498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pride RE. 2005. Foraging success, agonism, and predator alarms: behavioral predictors of cortisol in Lemur catta. Int. J. Primatol. 26, 295–319. ( 10.1007/s10764-005-2926-9) [DOI] [Google Scholar]
- 38.Busch DS, Hayward LS. 2009. Stress in a conservation context: a discussion of glucocorticoid actions and how levels change with conservation-relevant variables. Biol. Conserv. 142, 2844–2853. ( 10.1016/j.biocon.2009.08.013) [DOI] [Google Scholar]
- 39.Bonier F, Martin PR, Moore IT, Wingfield JC. 2009. Do baseline glucocorticoids predict fitness? Trends Ecol. Evol. 24, 634–642. ( 10.1016/j.tree.2009.04.013) [DOI] [PubMed] [Google Scholar]
- 40.Ebensperger LA, Tapia D, Ramirez-Estrada J, Leon C, Soto-Gamboa M, Hayes LD. 2013. Fecal cortisol levels predict breeding but not survival of females in the short-lived rodent, Octodon degus. Gen. Comp. Endocrinol. 186, 164–171. ( 10.1016/j.ygcen.2013.02.044) [DOI] [PubMed] [Google Scholar]
- 41.Chapman CA, Struhsaker TT, Lambert JE. 2005. Thirty years of research in Kibale National Park, Uganda, reveals a complex picture for Conservation. Int. J. Primatol. 26, 539–555. ( 10.1007/s10764-005-4365-z) [DOI] [Google Scholar]
- 42.Chapman CA, Lambert JE. 2000. Habitat alteration and the conservation of African primates: case study of Kibale National Park, Uganda. Am. J. Primatol. 50, 169–185. () [DOI] [PubMed] [Google Scholar]
- 43.Omeja PA, Chapman CA, Obua J, Lwanga JS, Jacob AL, Wanyama F, Mugenyi R. 2011. Intensive tree planting facilitates tropical forest biodiversity and biomass accumulation. For. Ecol. Manage. 261, 703–709. ( 10.1016/j.foreco.2010.11.029) [DOI] [Google Scholar]
- 44.Chapman CA, Chapman LJ, Jacob AL, Rothman JM, Omeja PA, Reyna-Hurtado R, Hartter J, Lawes MJ. 2010. Tropical tree community shifts: implications for wildlife conservation. Biol. Conserv. 143, 366–374. ( 10.1016/j.biocon.2009.10.023) [DOI] [Google Scholar]
- 45.Omeja PA, Obua J, Rwetsiba A, Chapman CA. 2012. Biomass accumulation in tropical lands with different disturbance histories: contrasts within one landscape and across regions. For. Ecol. Manage. 269, 293–300. ( 10.1016/j.foreco.2011.12.044) [DOI] [Google Scholar]
- 46.Chapman CA, Chapman LJ, Wrangham RW, Hunt K, Gebo D, Gardner L. 1992. Estimators of fruit abundance of tropical trees. Biotropica 24, 527–531. ( 10.2307/2389015) [DOI] [Google Scholar]
- 47.Gogarten JF, Jacob AL, Ghai RR, Rothman JM, Twinomugisha D, Wasserman MD, Chapman CA. 2015. Group size dynamics over 15+years in an African forest primate community. Biotropica 47, 101–112. ( 10.1111/btp.12177) [DOI] [Google Scholar]
- 48.Fox J, Weisberg S. 2011. An {R} companion to applied regression. Thousand Oaks, CA: Sage. [Google Scholar]
- 49.Milton K. 1979. Factors influencing leaf choice by howler monkeys: a test of some hypotheses of food selection by generalist herbivores. Am. Nat. 114, 363–378. ( 10.1086/283485) [DOI] [Google Scholar]
- 50.Chapman CA, Chapman LJ, Naughton-Treves L, Lawes MJ, McDowell LR. 2004. Predicting folivorous primate abundance: validation of a nutritional model. Am. J. Primatol. 62, 55–69. ( 10.1002/ajp.20006) [DOI] [PubMed] [Google Scholar]
- 51.McNab BK. 2002. The physiological ecology of vertebrates: a view from energetics. Cornell, NY: Cornell University Press. [Google Scholar]
- 52.White TCR. 1993. The inadequate environment: nitrogen and the abundance of animals, p. 425 Berlin, Germany: Springer. [Google Scholar]
- 53.McKey DB, Gartlan JS, Waterman PG, Choo GM. 1981. Food selection by black colobus monkeys (Colobus satanas) in relation to plant chemistry. Biol. J. Linn. Soc. 16, 115–146. ( 10.1111/j.1095-8312.1981.tb01646.x) [DOI] [Google Scholar]
- 54.Oates JF, Whitesides GH, Davies AG, Waterman PG, Green SM, DaSilva GL, Mole S. 1990. Determinants of variation in tropical forest primate biomass: new evidence from West Africa. Ecology 71, 328–343. ( 10.2307/1940272) [DOI] [Google Scholar]
- 55.Waterman PG, Ross JAM, Bennet EL, Davies AG. 1988. A comparison of the floristics and leaf chemistry of the tree flora in two Malaysian rain forests and the influence of leaf chemistry on populations of colobine monkeys in the old world. Biol. J. Linn. Soc. 34, 1–32. ( 10.1111/j.1095-8312.1988.tb01946.x) [DOI] [Google Scholar]
- 56.Chapman CA, Chapman LJ. 2002. Foraging challenges of red colobus monkeys: influence of nutrients and secondary compounds. Comp. Biochem. Physiol. A, Physiol. 133, 861–875. ( 10.1016/S1095-6433(02)00209-X) [DOI] [PubMed] [Google Scholar]
- 57.DeGabriel JL, Moore BD, Felton AM, Ganzhorn JU, Stolter C, Wallis IR, Johnson CN, Foley WJ. 2014. Translating nutritional ecology from the laboratory to the field: milestones in linking plant chemistry to population regulation in mammalian browsers. Oikos 123, 298–308. ( 10.1111/j.1600-0706.2013.00727.x) [DOI] [Google Scholar]
- 58.Chapman CA, Chapman LJ, Bjorndal KA, Onderdonk DA. 2002. Application of protein-to-fiber ratios to predict colobine abundance on different spatial scales. Int. J. Primatol. 23, 283–310. ( 10.1023/A:1013831511405) [DOI] [Google Scholar]
- 59.Ganzhorn JU. 2002. Distribution of a folivorous lemur in relation to seasonally varying food resources: integrating quantitative and qualitative aspects of food characteristics. Oecologia 131, 427–435. ( 10.1007/s00442-002-0891-y) [DOI] [PubMed] [Google Scholar]
- 60.Davies AG. 1994. Colobine populations. In Colobine monkeys. Their ecology, behaviour and evolution (eds Davies AG, Oates JF.), pp. 285–310. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 61.Fashing PJ, Dierenfeld E, Mowry CB. 2007. Influence of plant and soil chemistry on food selection, ranging patterns, and biomass of Colobus guereza in Kakamega Forest, Kenya. Int. J. Primatol. 28, 673–703. ( 10.1007/s10764-006-9096-2) [DOI] [Google Scholar]
- 62.Gogarten JF, Guzman M, Chapman CA, Jacob AL, Omeja PA, Rothman JM. 2012. What is the predictive power of the colobine protein-to-fiber model and its conservation value? Trop. Conserv. Sci. 5, 381–393. [Google Scholar]
- 63.Coley PD, Massa M, Lovelock CE, Winter K. 2002. Effects of elevated CO2 on foliar chemistry of saplings of nine species of tropical tree. Oecologia 133, 62–69. ( 10.1007/s00442-002-1005-6) [DOI] [PubMed] [Google Scholar]
- 64.Rothman JM, Chapman CA, Struhsaker TT, Raubenheimer D, Twinomugisha D, Waterman PG. In press. Cascading effects of global change: decline in nutritional quality of tropical leaves. Ecology. [DOI] [PubMed] [Google Scholar]
- 65.Freeland WJ. 1977. The dynamics of primate parasites. Ann Arbor, MI: University of Michigan. [Google Scholar]
- 66.Freeland WJ. 1976. Pathogens and the evolution of primate sociality. Biotropica 8, 12–24. ( 10.2307/2387816) [DOI] [Google Scholar]
- 67.Freeland WJ. 1979. Primate social groups as biological islands. Ecology 60, 719–728. ( 10.2307/1936609) [DOI] [Google Scholar]
- 68.Freeland WJ. 1980. Mangabey (Cerocebus albigena) movement patterns in relation to food availability and fecal contamination. Ecology 61, 1297–1303. ( 10.2307/1939037) [DOI] [Google Scholar]
- 69.Chapman CA, Bowman DD, Ghai RR, Goldberg TL, Gogarten JF, Rothman JM, Twinomugisha D, Walsh C. 2012. Protozoan parasites in group-living primates: testing the biological island hypothesis. Am. J. Primatol. 74, 510–517. ( 10.1002/ajp.20992) [DOI] [PubMed] [Google Scholar]
- 70.Gatti S, et al. 2002. Amoebic infections due to the Entamoeba histolytica–Entamoeba dispar complex: a study of the incidence in a remote rural area of Ecuador. Am. J. Trop. Med. Hyg. 67, 123–127. [DOI] [PubMed] [Google Scholar]
- 71.Kuhn H-J. 1964. Zur Kenntniss von Bau und Funktion des Magens der Schlankaffen (Colobinae). Folia Primatol. 2, 193–221. ( 10.1159/000155017) [DOI] [Google Scholar]
- 72.Gillespie TR, Greiner EC, Chapman CA. 2005. Gastrointestinal parasites of the colobus monkeys of Uganda. J. Parasitol. 91, 569–573. ( 10.1645/GE-434R) [DOI] [PubMed] [Google Scholar]
- 73.Lauck M, et al. 2013. Exceptional simian hemorrhagic fever virus diversity in a wild African primate community. J. Virol. 87, 688–691. ( 10.1128/JVI.02433-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chapman CA, Wasserman MD, Gillespie TR, Speirs ML, Lawes MJ, Saj TL, Ziegler TE. 2006. Do nutrition, parasitism, and stress have synergistic effects on red colobus populations living in forest fragments? Am. J. Phys. Anthropol. 131, 525–534. ( 10.1002/ajpa.20477) [DOI] [PubMed] [Google Scholar]
- 75.Chapman CA, Saj TL, Snaith TV. 2007. Temporal dynamics of nutrition, parasitism, and stress in colobus monkeys: implications for population regulation and conservation. Am. J. Phys. Anthropol. 134, 240–250. ( 10.1002/ajpa.20664) [DOI] [PubMed] [Google Scholar]
- 76.Nunn CL, Altizer S. 2006. Infectious diseases in primates: behavior, ecology and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 77.Sousa MBC, Ziegler TE. 1998. Diurnal variation on the excretion patterns of fecal steroids in common marmoset (Callithrix jacchus) females. Am. J. Primatol. 46, 105–117. () [DOI] [PubMed] [Google Scholar]
- 78.Setchell JM, Smith T, Wickings EJ, Knapp LA. 2008. Factors affecting fecal glucocorticoids in semi-free-ranging female mandrills (Mandrills sphinx). Am. J. Primatol. 70, 1023–1032. ( 10.1002/ajp.20594) [DOI] [PubMed] [Google Scholar]
- 79.Carnegie SD, Fedigan LM, Ziegler TE. 2011. Social and environmental factors affecting fecal glucocorticoids in wild, female white-faced capuchins (Cebus capucinus). Am. J. Primatol. 73, 1–9. ( 10.1002/ajp.20954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ziegler TE, Scheffler G, Snowdon CT. 1995. The relationship of cortisol levels to social environment and reproductive functioning in female cotton-top tamarins, Saguinus oedipus. Horm. Behav. 29, 407–424. ( 10.1006/hbeh.1995.1028) [DOI] [PubMed] [Google Scholar]
- 81.Chapman CA, Balcomb SR, Gillespie T, Skorupa J, Struhsaker TT. 2000. Long-term effects of logging on African primate communities: a 28-year comparison from Kibale National Park, Uganda. Conserv. Biol. 14, 207–217. ( 10.1046/j.1523-1739.2000.98592.x) [DOI] [Google Scholar]
- 82.Mitani JC, Struhsaker TT, Lwanga JS. 2000. Primate community dynamics in old growth forest over 23.5 years at Ngogo, Kibale National Park, Uganda: implications for conservation and census methods. Int. J. Primatol. 21, 269–286. ( 10.1023/A:1005477504728) [DOI] [Google Scholar]
- 83.Teelen S. 2007. Primate abundance along five transect lines at Ngogo, Kibale National Park, Uganda. Am. J. Primatol. 69, 1–15. ( 10.1002/ajp.20417) [DOI] [PubMed] [Google Scholar]
- 84.Plumptre AJ. 2000. Monitoring mammal populations with line transect techniques in African forests. J. Appl. Ecol. 37, 356–368. ( 10.1046/j.1365-2664.2000.00499.x) [DOI] [Google Scholar]
- 85.Chapman CA, Struhsaker TT, Skorupa JP, Snaith TV, Rothman JM. 2010. Understanding long-term primate community dynamics: implications of forest change. Ecol. Appl. 20, 179–191. ( 10.1890/09-0128.1) [DOI] [PubMed] [Google Scholar]
- 86.National Research Council. 1981. Techniques for the study of primate population ecology. Washington, DC: National Academy Press. [Google Scholar]
- 87.Pride RE. 2005. Optimal group size and seasonal stress in ring-tailed lemurs (Lemur catta). Behav. Ecol. 16, 550–560. ( 10.1093/beheco/ari025) [DOI] [Google Scholar]
- 88.Snaith TV, Chapman CA, Rothman JM, Wasserman MD. 2008. Bigger groups have fewer parasites and similar cortisol levels: a multi-group analysis in red colobus monkeys. Am. J. Primatol. 70, 1–9. ( 10.1002/ajp.20601) [DOI] [PubMed] [Google Scholar]
- 89.Chapman CA, Rothman JM, Hodder SAM. 2009. Can parasites limit primate group size?: a test with red colobus. In Primate parasite ecology: the dynamics and study of host–parasite relationships (eds Huffman MA, Chapman CA.), pp. 403–422. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 90.Griffin R, Nunn C. 2012. Community structure and the spread of infectious disease in primate social networks. Evol. Ecol. 26, 779–800. ( 10.1007/s10682-011-9526-2) [DOI] [Google Scholar]
- 91.Rimbach R, Bisanzio D, Galvis N, Link A, Di Fiore A, Gillespie TR. 2015. Brown spider monkeys (Ateles hybridus): a model for differentiating the role of social networks and physical contact on parasite transmission dynamics. Phil. Trans. R. Soc. B 370, 20140110 ( 10.1098/rstb.2014.0110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nunn CL, Craft ME, Gillespie TR, Schaller M, Kappeler PM. 2015. The sociality–health–fitness nexus: synthesis, conclusions and future directions. Phil. Trans. R. Soc. B 370, 20140115 ( 10.1098/rstb.2014.0115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Craft ME. 2015. Infectious disease transmission and contact networks in wildlife and livestock. Phil. Trans. R. Soc. B 370, 20140107 ( 10.1098/rstb.2014.0107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Onderdonk DA, Chapman CA. 2000. Coping with forest fragmentation: the primates of Kibale National Park, Uganda. Int. J. Primatol. 21, 587–611. ( 10.1023/A:1005509119693) [DOI] [Google Scholar]
- 95.Kappeler PM, Barrett L, Blumstein DT, Clutton-Brock TH. 2013. Constraints and flexibility in mammalian social behaviour: introduction and synthesis. Phil. Trans. R. Soc. B 368, 20120337 ( 10.1098/rstb.2012.0337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chapman CA, Rothman JM. 2009. Within-species differences in primate social structure: evolution of plasticity and phylogenetic constraints. Primates 50, 12–22. ( 10.1007/s10329-008-0123-0) [DOI] [PubMed] [Google Scholar]
- 97.Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA. 2005. Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 20, 685–692. ( 10.1016/j.tree.2005.08.002) [DOI] [PubMed] [Google Scholar]
- 98.Sih A, Ferrari MCO, Harris DJ. 2011. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 4, 367–387. ( 10.1111/j.1752-4571.2010.00166.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hanya G, Fuse M, Aiba S-I, Takafumi H, Tsujino R, Agetsuma N, Chapman CA. 2014. Ecosystem impacts of folivory and frugivory by Japanese macaques in two temperate forests in Takushima. Am. J. Primatol. 76, 596–607. ( 10.1002/ajp.22253) [DOI] [PubMed] [Google Scholar]
- 100.Miyamoto MM, Allen JA, Ting N, Gogarten JF, Chapman CA. 2013. Microsatellite DNA suggests that group size affects sex-biased dispersal patterns in red colobus monkeys. Am. J. Primatol. 75, 478–790. ( 10.1002/ajp.22124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Turner MG, Romme WH, Gardner RH, O'Neill RV, Kratz TK. 1993. A revised concept of landscape equilibrium: disturbance and stability on scaled landscapes. Landsc. Ecol. 8, 213–227. ( 10.1007/BF00125352) [DOI] [Google Scholar]
- 102.Turkington R. 2009. Top-down and bottom-up forces in mammalian herbivore–vegetation systems: an essay review. Botany 87, 723–739. ( 10.1139/B09-035) [DOI] [Google Scholar]
- 103.Mori AS. 2011. Ecosystem management based on natural disturbances: hierarchical context and non-equilibrium paradigm. J. Appl. Ecol. 48, 280–292. ( 10.1111/j.1365-2664.2010.01956.x) [DOI] [Google Scholar]
- 104.Gogarten JF, Bonnell TR, Brown LM, Campenni M, Wasserman MD, Chapman CA. 2014. Increasing group size alters behavior of a folivorous primate. Int. J. Primatol. 35, 590–608. ( 10.1007/s10764-014-9770-8) [DOI] [Google Scholar]