Abstract
Background
Pulmonary contusion (PC) is a common, potentially lethal injury that results in priming for exaggerated inflammatory responses to subsequent immune challenge like infection (2nd hit). The molecular mechanism of priming and the 2nd hit phenomenon after PC remain obscure. Using a mouse model of PC, this study explores the role of Sirtuin 1 (SIRT1), an NAD+ dependent deacetylase, in priming for a 2nd hit after injury.
Methods
Using a mouse model of PC, injury-primed 2nd hit host responses were tested at 24H after PC by (1) in vivo infectious challenge of injured mice or (2) ex vivo inflammatory challenge of isolated immune cells from injured mice. SIRT activators or repressors were used to test for SIRT1 participation in these 2nd hit responses.
Results
PC injured mice given an in vivo infectious challenge by cecal ligation and puncture (CLP) had significantly increased mortality compared injury or infectious challenge alone. Isolated broncho-alveolar lavage (BAL) cells from injured mice given an ex vivo inflammatory challenge with bacterial lipopolysaccharide (LPS) had increased levels of TNF-α mRNA compared to uninjured mice. We found that PC reduced SIRT1 protein, mRNA, and SIRT1 enzymatic activity in injured lung tissue. We also found decreased SIRT1 protein levels in BAL cells from injured mice. We further found that injured mice treated with a SIRT1 activator, resveratrol, showed significantly decreased PMN in the BAL in response to intra-tracheal LPS and increased survival from CLP.
Conclusions
These results showed that PC decreased SIRT 1 levels in the lung correlated with enhanced responses to infectious or inflammatory stimuli in injured mice. Treatment of injured mice with a SIRT1 activator, resveratrol, decreased LPS inflammatory response and increased survival after CLP. Our results suggest that SIRT1 participates in the 2nd hit response after injury.
Keywords: pulmonary contusion, second hit, inflammation, infection, SIRT1, immune priming
Introduction
Significant traumatic injury induces a prevailing period of systemic inflammation that can prime the immune system for an exaggerated response to subsequent immune challenge (the second hit) with the lung as a particularly vulnerable organ.(1) This “second hit” response is linked multiple organ dysfunction and death; however, injury induced mediators of these enhanced or primed responses are not yet well defined.(2) In addition to patients with acute injuries, the propensity to enhanced immune/inflammatory response extends to elderly patients or patients with other chronic inflammatory conditions like diabetes and obesity.(3)
Sirtuins are conserved family of NAD+ dependent deacetylases that regulate cellular metabolism.(4) SIRT1 (Sirtuin 1), the mammalian homologue of Sir2, has been implicated in a variety of physiological conditions including cancer, aging, and obesity. In addition to its metabolic role, we (5) and others (6) have implicated SIRT1 as a key regulator of immune responses. This new concept, “immunometabolism”, recognizes the significance of immunologic-metabolic cross talk and that host metabolism informs immune responses.(7) A recent review of mouse models suggests that SIRT1 variably impacts the function of many organs and tissues, in addition to the overall metabolism.(8)
Given the enhanced immune response after traumatic injury, we sought to examine a potential role for SIRT1 in the second hit. In our previous studies in a mouse model of isolated blunt chest trauma (pulmonary contusion, PC), we showed that this injury primed the mouse inflammatory response to LPS.(9) In this report, we use this PC model to study the injury primed exaggerated response to subsequent infectious challenge using a mouse model of sepsis, CLP. We provide data suggesting that decreased SIRT1 participates in priming the host response to a second hit, in the whole animal, in the lung, and in cells isolated from the injured mouse. Treatment of injured mice with a SIRT1 activator, resveratrol, decreases inflammation and increases survival in second hit responses. These results suggest that injured patients at risk for a second hit may benefit from treatment with SIRT1 activator.
Methods
Animals
Male, age matched (8–9 weeks) C57BL/6 mice obtained from Jackson Laboratories (Bar Harbor, ME). Study protocols were approved by the WFUHS Animal Care and Use Committee.(2)
Animal injury model
Pulmonary contusion (PC) was induced using the Cortical Contusion Impactor (CCI) as described previously.(9) Briefly, mice were anesthetized, positioned left lateral decubitus and during inspiration, the right chest was struck with the CCI along the posterior axillary line, 1cm above the costal margin.
Second hit mouse model (CLP)
The sepsis mouse model (CLP) used was previously described.(10) Briefly, at 24 hours following PC, mouse cecum was ligated, perforated twice with a 22-gauge needle, returned to the abdomen and incision closed (PC+CLP). Fluid resuscitation (normal saline 1ml subcutaneously) was given to each mouse. For comparison, mice received injury (PC alone) or sepsis (CLP alone). For survival studies, mice were followed for 7 days. Resveratrol treatment: At 6H after injury, mice were treated with resveratrol (30mg/kg, IP) followed 18H later by CLP (PC+RES+CLP). Uninjured (without PC) mice were treated with resveratrol for 18H before CLP (RES+CLP). For survival studies, mice were followed for 7 days.
Second hit cell model (LPS)
For ex vivo cell experiments, BAL cells from uninjured or injured mice were isolated at 24H after injury, counted, resuspended (2×106 cells/ml) in RPMI media (Gibco) supplemented with 10% FBS, and stimulated with LPS (1ug/ml, E. coli O111:B4) for 2H. Total RNA was isolated, purified, quantitated and TNFa mRNA levels measured by qPCR (TaqMan gene expression assay, Applied Biosystems) as previously described.(11) Resveratrol treatment: Injured mice were treated with resveratrol (30mg/kg, IP) at 2H prior to a 4H LPS challenge (50ug, IT). PMN in the BAL were quantitated (PC+RES+LPS) and compared to PMN in the BAL of untreated injured mice (PC+LPS).(2)
Bronchoalveolar lavage (BAL)
BAL was performed at 3H or 24H after injury and BAL cell pellet was counted and differentiated as described previously.(2, 9)
SIRT1 analysis
Uninjured and injured lungs at 24H after injury were harvested and analyzed for SIRT1. Immunohistochemistry: 10% formalin fixed, paraffin embedded sections of lung were stained using SIRT1 antibodies (Santa Cruz Biotechnology, Inc.) and Cy™ 3-conjugated labeled secondary antibodies (Jackson Immuno Research Laboratories, Inc. West Grove, PA). Gene expression: Lung homogenates were treated with STAT60™ (Tel-Test, Inc.) and SIRT1 mRNA levels determined by qPCR (TaqMan gene expression assay, Applied Biosystems). SIRT1 activity: Lung homogenates were treated with T-PER Tissue protein extraction reagent (Thermo Scientific) and SIRT1 activity in lung tissue determined using SensoLyte Green SIRT1 Assay kit according to manufacturer’s instructions (Anaspec). Immunoblot: BAL cells from uninjured and injured lungs at 3H or 24H were isolated and SIRT1 protein levels visualized and quanititated by immunoblot (anti-SIRT1 antibodies, EMD Millipore) as previously described.(12)
Statistical analysis
At the indicated times, tissue and BAL samples were collected after death. Data are reported using GraphPad Prism (v 4.03, San Diego, CA) and expressed as the mean ± SEM of independent observations as indicated in the Figures. Student’s t-test and/or one-way analysis of variance (1way ANOVA) with multiple comparison post-test (Bonferroni) was used to compare the means between experimental groups as indicated. A p-value ≤0.05 was considered to be significant.(2)
RESULTS
Pulmonary contusion primes second hit responses both in vivo by increased death in a sepsis model, and ex vivo, by increased inflammatory response to LPS
As shown in Figure 1A, uninjured and injured mice received an infectious challenge and survival followed for 7 days (Fig. 1B). We found a significant decrease in survival in injured mice with sepsis (20% survival) compared to sepsis alone (40% survival) or injury alone (100%). As shown in Figure 2, isolated BAL cells from uninjured and injured mice were given an inflammatory challenge with LPS. We found that cells from injured mice had a significant increase in TNFa gene expression compared to uninjured mice. These results are consistent with primed second hit responses both in the injured animal and in cells isolated from the injured lung.
Figure 1. Injured mice show decrease survival after infectious challenge.
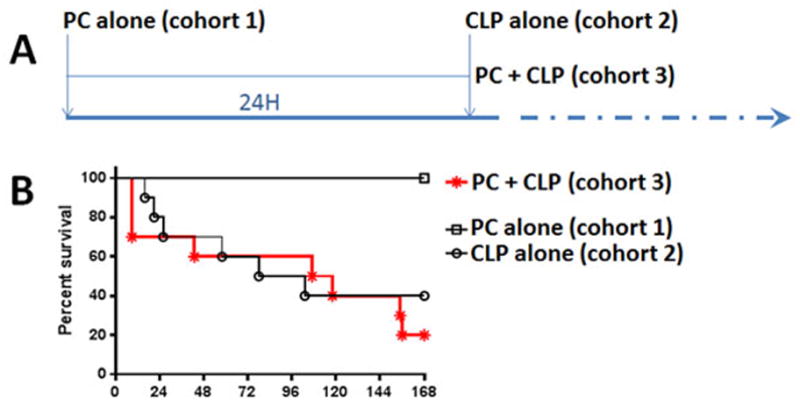
Survival studies were performed on 3 cohorts as shown in (A). Mice received blunt chest trauma resulting in PC (PC alone, cohort 1), infectious challenge by CLP (CLP alone, cohort 2), or PC followed 24H later by CLP (PC+CLP, cohort 3). n = 10 mice/cohort. Survival was followed for 7 days (168H) after CLP (B). Survival was significantly decreased (p=0.001) for injured mice with infection (20%) compared to injury (100%) or infection (40%) alone.
Figure 2. Isolated BAL cells from injured mice show increased inflammatory response after LPS challenge.
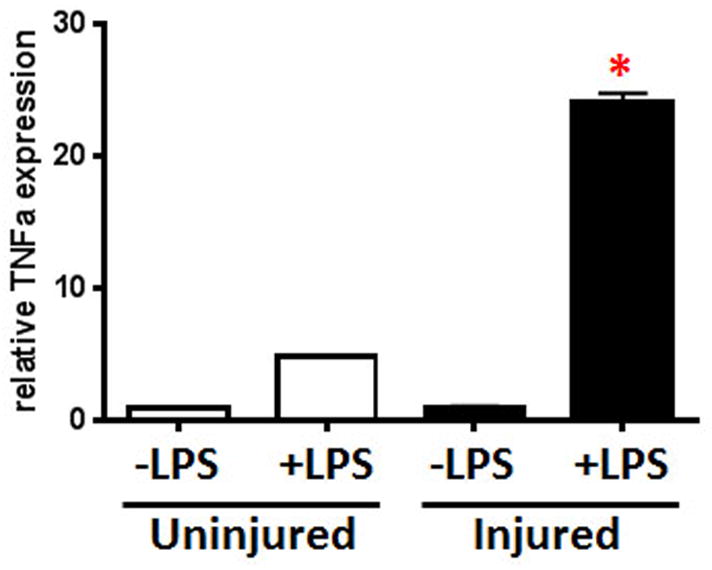
BAL cells were isolated from Uninjured and Injured mice (24H) and inflammatory response to LPS (−/+LPS, 2H) determined by TNFa expression. Results shown are reported as relative TNFa expression using GAPDH mRNA as the internal control. n=2 for all groups except Injured+LPS where n=4; qPCR analyses were performed in duplicate; *p<0.0001 for Injured+LPS compared to Uninjured+LPS.
Pulmonary contusion decreases SIRT1 levels in the lung and in isolated BAL cells
To evaluate the impact of injury on SIRT1, we assessed SIRT1 levels in lung. As shown in Figure 3, we found reduced SIRT1 in lung tissue (Fig. 3A) and in BAL cells (Fig. 3B) isolated from injured mice. Immunohistochemical analysis of injured lung tissue shows decreased SIRT1 levels. Consistent with this observation, injured lung tissue shows significantly decreased SIRT1 mRNA and activity, and isolated BAL cells show decreased SIRT1 by immunoblot.
Figure 3. PC reduces SIRT1 levels in the injured lung.
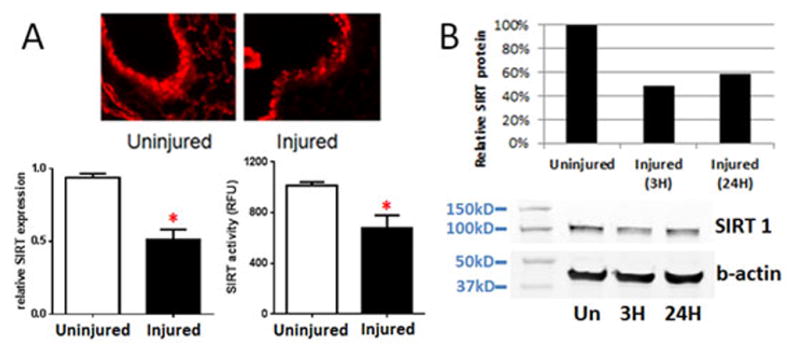
After 24 hours, lung tissue from injured mice was removed and compared to lung tissue from uninjured mice. Lung tissue (A) was fixed and stained for SIRT1 protein (upper panel) or processed for RNA analysis (lower left) or SIRT activity (lower right). A representative section of uninjured (n=1) and injured (n=3) lung is shown. PCR results shown are reported as relative SIRT expression using GAPDH mRNA as the internal control (n=8, *p<0.0001). SIRT activity results are reported as specific SIRT1 activity in RFU (n=3 uninjured; n=4 injured, *p<0.05). At 3 or 24 hours after PC, BAL cells from injured mice (n=4) were harvested, pooled and analyzed for SIRT1 protein (B). SIRT1 or b-actin (loading control) levels were quantitated by densitometric analysis of the immunoblot and reported as SIRT1 protein levels in injured mice relative to uninjured mice.
Increasing SIRT1 activity after PC results in loss of primed response
The observation that injury primed infectious and inflammatory host responses correlated with decreased SIRT1 led us to hypothesize that increasing SIRT1 activity could attenuate the second hit response. To test this, injured mice were treated with resveratrol, a SIRT1 activator, before the second hit. As shown in Figure 4A, we found that resveratrol treatment of injured mice improved survival after CLP (80% survival). Resveratrol treatment did not significantly improve survival (38% survival) from CLP alone. We also found that resveratrol treatment of injured mice had a significant decrease in PMN infiltrate into the lungs in response to LPS (Fig. 4B).
Figure 4. Injured mice treated with SIRT1 activator show improved survival after sepsis and decreased inflammatory responses in the injured lung.

Uninjured (RES+CLP) and injured mice (PC+RES+CLP) were treated with resveratrol prior to CLP (A). Survival was significantly increased in PC+RES+CLP mice (n=10, 80% survival) compared to untreated PC+CLP mice (p=.001, compare with Fig. 1B). Resveratrol treatment did not improve survival in mice with CLP alone (n=8, 38% survival). Injured mice were treated with resveratrol prior to a 4H LPS challenge (B). PMN in the BAL were quantitated and showed a significant decrease (*p=0.001) compared to untreated mice. Results are reported as total BAL PMN (mean±SEM, n=6 mice/cohort).
DISCUSSION
This study shows that pulmonary contusion serves as a priming stimulus for worse outcomes after infectious second hit insults. In addition, our data supports the hypothesis that SIRT1 participates in this priming event as a counter-regulator of acute inflammation. Pharmacologic treatment with a SIRT1 activator, resveratrol, increases survival in second hit insults and reduces the inflammatory response to a LPS challenge.
Of note, injured animals demonstrate greater susceptibility to acute inflammation, higher TNFa gene expression in lung BAL cells after LPS challenge, and worse survival after CLP (Figs.1 and 2). This couples a molecular mechanism for priming to a phenotype of increase susceptibility to infection. In addition, the infectious second hit need not occur at the original site of injury in the lung since priming is a whole body phenomenon. So far, we have been unable to demonstrate consistently low SIRT1 serum levels after pulmonary contusion. Thus, further studies are needed to determine to what extent priming is due to a hyperinflammatory environment within the lung or a more global reset in immune function.
The mechanism of SIRT1’s hypoinflammatory effect involves the deacetylation of NF-κB p65 to generate silent heterochromatin at the promoters of proinflammatory genes such as TNFa.(11) We have shown in Figure 3 that pulmonary contusion results in a decrease in SIRT1 mRNA, relative protein, and enzyme activity for the first 24 hours after injury, which suggests pre- and post-translational mechanisms to this priming. This provides a window of opportunity for second hit infectious stimuli as the animal is sensitized to TLR4 stimulation and susceptible to the hyperinflammatory phase of sepsis. Our observations that priming occurs in the first 24 hours are consistent with previous observations that serum pro-inflammatory cytokines such as IL-6 levels reach a peak within this period.(13, 14)
Derived from grape products, resveratrol is a polyphenol that is thought to induce a conformational change in SIRT1 and enhance enzyme activity.(15) This is supported by other studies that show resveratrol directly binding to SIRT1 and increasing its activity without changing the turnover rate of the enzyme.(16) We found that injured animals treated with resveratrol had better outcomes, less inflammation and increased survival, compared to their untreated cohorts. The beneficial effects of resveratrol were not seen in animals subjected to an infectious insult alone via CLP, which suggests a drop in SIRT1 activity after pulmonary contusions that is rescued by the SIRT1 activator. Our own studies have failed to show an increase in SIRT1 mRNA or protein after resveratrol treatment, which supports the notion that resveratrol acts in vivo to increase SIRT activity.
SIRT1 catalyzes the deacetylation of multiple transcription factors important in the regulation of metabolism.(10) For example, SIRT1 interacts with peroxisome proliferator-activated receptor γ and peroxisome proliferator-activated receptor γ coactivator 1α to regulate mitochondrial oxygen consumption, hepatic glucose output, and fatty acid beta oxidation.(17–19) We have also shown that the sequential actions of nuclear SIRT1, RelB, and mitochondrial SIRT3 reprogram cellular metabolism from glycolysis in the acute phase of sepsis to fatty acid oxidation and mitochondrial biogenesis during the adaptive phase of sepsis.(20) In addition, persistent activation of SIRT1 delays sepsis resolution by altering mitochondrial bioenergetics. Thus, there appears to be a critical period around the time of a priming injury and acute sepsis where SIRT1 activation is beneficial, but continued activation into the adaptive phase of sepsis results in dysregulated bioenergetics and poor outcomes. This provides a plausible explanation for the improved early mortality seen in animals treated with resveratrol prior to a second hit septic insult via CLP (Figs. 1 and 4) as the acute phase of sepsis is attenuated by SIRT1 activation.
Finally, SIRT1 activity is degraded by phosphorylation, oxidation by reactive oxygen species, nitrosylation, and glutathionylation. This is seen in situations of chronic oxidative stress such as cigarette smoke exposure.(21, 22) This raises the possibility that metabolic diseases such as diabetes and smoking play a role through SIRT1 in outcomes from second hit insults. These metabolically stressed individuals may represent a special population who are at particular risk from second hit injuries. Further studies are necessary to determine the extent chronic metabolic disease predispose the host to second hit injuries, and how treatments aimed at modulating SIRT1 activity might benefit those at risk populations.
In summary, our study supports the hypothesis that SIRT1 participates in priming by counteracting the acute inflammation that results from pulmonary contusion. The SIRT1 activator resveratrol has a beneficial effect on mortality in our second hit model of sepsis. Further studies are needed to determine the usefulness of SIRT1 activators as therapeutic targets in patients suffering from chronic inflammatory states.
Acknowledgments
This work was supported, in part, by the Clowes/ACS/AAST Award, and GM083154 (JH), AI065791 and AI079144 (CM, BY) and GM099807 (VV).
Footnotes
All authors declare no conflicts of interest.
Portions presented at the 73nd Annual AAST meeting, Sept. 10–13, 2014, Philadelphia, PA.
AUTHOR CONTRIBUTIONS
LM Smith, JD Wells, VT Vachharajani: study design, data collection, data analysis, manuscript preparation; JJ Hoth and BK Yoza: study design, data analysis and interpretation, study reporting; CE McCall: study design, data interpretation.
Contributor Information
Lane M. Smith, Email: lmsmith@wakehealth.edu.
Jonathan D. Wells, Email: jwells@wakehealth.edu.
Vidula T. Vachharajani, Email: vvachar@wakehealth.edu.
Barbara K. Yoza, Email: byoza@wakehealth.edu.
Charles E. McCall, Email: chmccall@wakehealth.edu.
References
- 1.Watkins TR, Nathens AB, Cooke CR, Psaty BM, Maier RV, Cuschieri J, Rubenfeld GD. Acute respiratory distress syndrome after trauma: development and validation of a predictive model. Critical care medicine. 2012;40:2295–2303. doi: 10.1097/CCM.0b013e3182544f6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoth JJ, Wells JD, Jones SE, Yoza BK, McCall CE. Complement mediates a primed inflammatory response after traumatic lung injury. The journal of trauma and acute care surgery. 2014;76:601–608. doi: 10.1097/TA.0000000000000129. discussion 608–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lasanianos NG, Kanakaris NK, Dimitriou R, Pape HC, Giannoudis PV. Second hit phenomenon: existing evidence of clinical implications. Injury. 2011;42:617–629. doi: 10.1016/j.injury.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends in endocrinology and metabolism: TEM. 2014;25:138–145. doi: 10.1016/j.tem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu TF, Brown CM, El Gazzar M, McPhail L, Millet P, Rao A, Vachharajani VT, Yoza BK, McCall CE. Fueling the flame: bioenergy couples metabolism and inflammation. Journal of leukocyte biology. 2012;92:499–507. doi: 10.1189/jlb.0212078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong S, McBurney MW, Fang D. Sirtuin 1 in immune regulation and autoimmunity. Immunology and cell biology. 2012;90:6–13. doi: 10.1038/icb.2011.102. [DOI] [PubMed] [Google Scholar]
- 7.Granger A, Emambokus N. Focus on immunometabolism. Cell metabolism. 2013;17:807. doi: 10.1016/j.cmet.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Boutant M, Canto C. SIRT1 metabolic actions: Integrating recent advances from mouse models. Molecular metabolism. 2014;3:5–18. doi: 10.1016/j.molmet.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoth JJ, Martin RS, Yoza BK, Wells JD, Meredith JW, McCall CE. Pulmonary contusion primes systemic innate immunity responses. The Journal of trauma. 2009;67:14–21. doi: 10.1097/TA.0b013e31819ea600. discussion 21-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vachharajani VT, Liu T, Brown CM, Wang X, Buechler NL, Wells JD, Yoza BK, McCall CE. SIRT1 inhibition during the hypoinflammatory phenotype of sepsis enhances immunity and improves outcome. Journal of leukocyte biology. 2014 doi: 10.1189/jlb.3MA0114-034RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu TF, Yoza BK, El Gazzar M, Vachharajani VT, McCall CE. NAD+-dependent SIRT1 deacetylase participates in epigenetic reprogramming during endotoxin tolerance. The Journal of biological chemistry. 2011;286:9856–9864. doi: 10.1074/jbc.M110.196790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoth JJ, Wells JD, Yoza BK, McCall CE. Innate immune response to pulmonary contusion: identification of cell type-specific inflammatory responses. Shock. 2012;37:385–391. doi: 10.1097/SHK.0b013e3182478478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoth JJ, Wells JD, Brownlee NA, Hiltbold EM, Meredith JW, McCall CE, Yoza BK. Toll-like receptor 4-dependent responses to lung injury in a murine model of pulmonary contusion. Shock. 2009;31:376–381. doi: 10.1097/SHK.0b013e3181862279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoth JJ, Wells JD, Hiltbold EM, McCall CE, Yoza BK. Mechanism of neutrophil recruitment to the lung after pulmonary contusion. Shock. 2011;35:604–609. doi: 10.1097/SHK.0b013e3182144a50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. The Journal of biological chemistry. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, ESY, Lamming DW, Pentelute BL, Schuman ER, Stevens LA, Ling AJ, Armour SM, Michan S, Zhao H, Jiang Y, Sweitzer SM, Blum CA, Disch JS, Ng PY, Howitz KT, Rolo AP, Hamuro Y, Moss J, Perni RB, Ellis JL, Vlasuk GP, Sinclair DA. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013;339:1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu TF, Vachharajani VT, Yoza BK, McCall CE. NAD+-dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. The Journal of biological chemistry. 2012;287:25758–25769. doi: 10.1074/jbc.M112.362343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Lowry SF, Guarente L, Haimovich B. Roles of SIRT1 in the acute and restorative phases following induction of inflammation. The Journal of biological chemistry. 2010;285:41391–41401. doi: 10.1074/jbc.M110.174482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu TF, Vachharajani V, Millet P, Bharadwaj MS, Molina AJ, McCall CE. Sequential Actions of SIRT1-RELB-SIRT3 Coordinate Nuclear-Mitochondrial Communication during Immunometabolic Adaptation to Acute Inflammation and Sepsis. J Biol Chem. 2015;290:396–408. doi: 10.1074/jbc.M114.566349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2008;177:861–870. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. American journal of physiology Lung cellular and molecular physiology. 2007;292:L567–576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]


