Abstract
Several risk factors for atherosclerotic peripheral arterial disease (PAD) such as dyslipidemia, diabetes and hypertension, are heritable. However, predisposition to PAD may be influenced by genetic variants acting independently of these risk factors. Identification of such genetic variants will provide insights into underlying pathophysiologic mechanisms and facilitate the development of novel diagnostic and therapeutic approaches. In contrast to coronary heart disease, relatively few genetic variants that influence susceptibility to PAD have been discovered. This may be in part due to greater clinical and genetic heterogeneity in PAD. In this review, we a) provide an update on the current state of knowledge about the genetic basis of PAD including results of family studies and candidate gene, linkage as well as genome-wide association studies; b) highlight the challenges in investigating the genetic basis of PAD and possible strategies to overcome these challenges; and c) discuss the potential of genome sequencing, RNA sequencing, differential gene expression, epigenetic profiling and systems biology in increasing our understanding of the molecular genetics of PAD.
Keywords: genetic epidemiology, genome wide association studies, genome sequencing, epigenetics, peripheral arterial disease
Introduction
The most common cause of peripheral arterial disease (PAD) is atherosclerotic vascular disease. PAD due to atherosclerosis is relatively highly prevalent, affecting more than 200 million people worldwide1 including an estimated 8-10 million persons in the United States alone.2, 3 Atherosclerotic PAD is typically identified in the clinical setting when patients present with claudication or critical limb ischemia. PAD may also be ascertained on the basis of an abnormal ankle brachial index (ABI) in patients referred to the noninvasive vascular laboratory or based on lower extremity imaging studies (Table 1). PAD is associated with considerable morbidity4 including impaired functional capacity, frailty, poor quality of life, as well as high medical care costs.5, 6 The Institute of Medicine has listed PAD as a high priority research area to reduce mortality and morbidity from this condition.7
PAD is a distinct subtype of atherosclerotic vascular disease that differs from coronary artery and cerebrovascular disease in its clinical presentation. The phenomenon of ‘plaque instability’ in the coronary or cerebral arterial beds leads to acute ‘events’ such as myocardial infarction or ischemic stroke. For reasons that are not fully understood, such acute ‘events’ are relatively uncommon in PAD and symptoms most often result from progressive arterial narrowing due to ongoing atherogenesis. It is therefore likely that risk factors, both genetic and environmental, and the intermediate biochemical pathways through which they act, contribute differently to PAD than to coronary heart disease (CHD) or cerebrovascular disease.
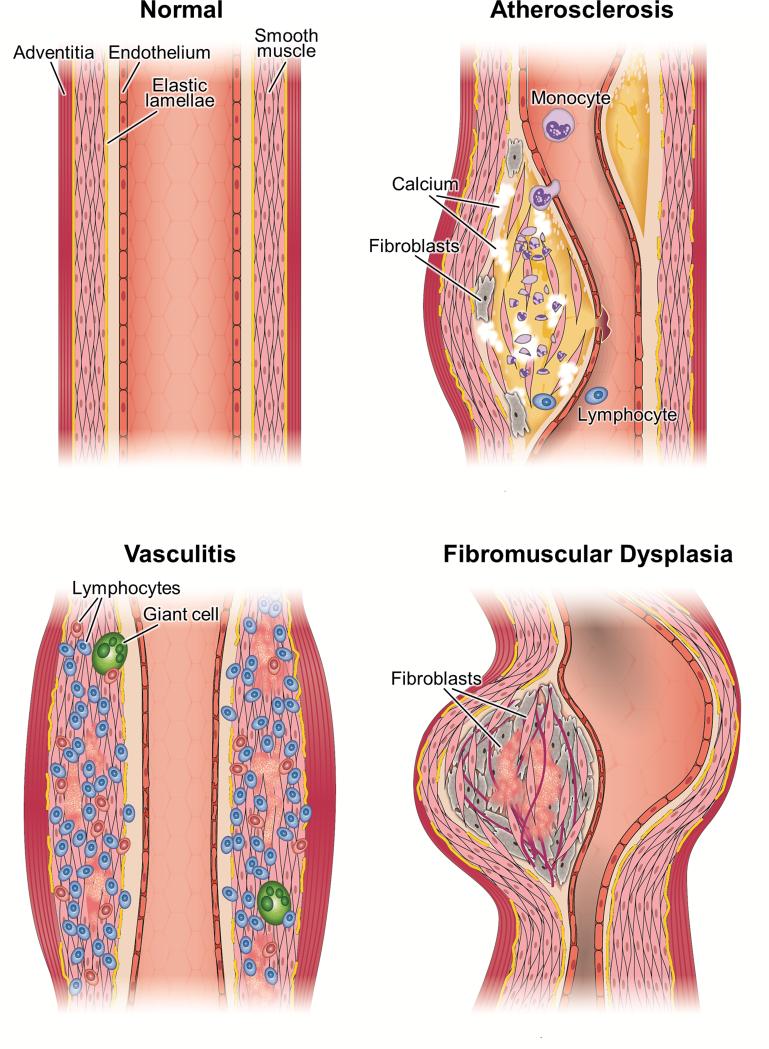
Several risk factors for PAD (such as dyslipidemia, diabetes and hypertension) are heritable. However, predisposition to PAD may be influenced by genetic factors acting independently of these risk factors. Identification of such genetic factors will provide insights into underlying pathophysiologic mechanisms and facilitate the development of novel diagnostic and therapeutic approaches.12, 13 In contrast to CHD,14 relatively few genetic variants that influence susceptibility to PAD have been discovered. This may be in part due to greater clinical and genetic heterogeneity in PAD.12 In this review, we provide an update on the current state of knowledge about the genetic basis of atherosclerotic PAD and discuss challenges and future directions. The study of the genetic basis of non-atherosclerotic forms of PAD such as vasculitides and fibromuscular dysplasia may provide insights into the pathogenesis of atherosclerotic PAD, given shared features such as inflammation, vascular remodeling, aneurysm formation, and smooth muscle cell proliferation (Fig. 1). A detailed discussion of the genetic basis of non-atherosclerotic forms of PAD, however, is beyond the scope of this review.
Fig. 1.
Arterial pathology in atherosclerotic, inflammatory, and non-atherosclerotic non-inflammatory arteriopathies. Atherosclerosis is characterized by plaques with varying amounts of inflammatory cells, lipid deposition, fibrosis, calcification, cellular necrosis, smooth muscle proliferation and necrosis, disruption of internal elastic lamina. Inflammatory arterial diseases are characterized by marked inflammatory cell infiltration and in the case of large vessel vasculitis, by giant cell formation. Features of non-inflammatory non-atherosclerotic arterial disease vary. In fibromuscular dysplasia, the prominent features are excessive fibroblasts, deposition of increased amounts of extra cellular matrix and relative paucity of inflammatory cells.
CURRENT KNOWLEDGE
In this section we provide an update on the current state of knowledge about the genetic basis of PAD including ethnic differences in the prevalence of PAD, familial clustering, early-onset PAD and results of candidate gene, linkage as well as genome-wide association studies (GWAS).
Ethnic differences in PAD
Ethnic differences in disease prevalence may be in part due to genetic factors as well as differences in socioeconomic status and access to care.15 In several population-based studies, African American ethnicity has been associated with a lower ABI and higher prevalence of PAD in both men and women, independent of age and other conventional risk factors.15-18 Moreover, in non-white adults (predominantly African Americans) symptomatic PAD was associated with worse clinical outcomes than in non-Hispanic white adults.19 In the Bogalusa Heart Study,20 during adolescence and early adulthood, African Americans had approximately 1.5 times as much aortic surface involvement of fatty streaks as did non-Hispanic whites, independent of ante-mortem lipid levels, blood pressure, or obesity. Analysis of data from National Health and Nutrition Examination Survey (NHANES) revealed that ABI is lower in African Americans than in non-Hispanic whites even among younger individuals without cardiovascular risk factors, raising the possibility that ethnic differences in ABI may not be due to differences in atherosclerotic burden.21
Family history
Family history is a simple and inexpensive yet powerful clinical tool for improving risk assessment and thereby reducing the burden of common chronic diseases.22, 23 The American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend screening for abdominal aortic aneurysm in patients with family history of abdominal aortic aneurysm.24 Given that screening for PAD is relatively inexpensive and non-invasive, similar screening in asymptomatic patients with family history of PAD may be useful for early detection and treatment. However, relatively few studies (discussed below) have assessed whether family history of PAD is a risk for factor for PAD.
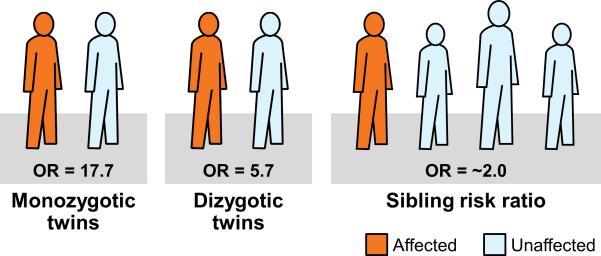
In the population-based Swedish Twin Registry,25 the odds ratio of having PAD in persons whose twin had PAD compared with persons whose twin did not have PAD was 17.7 (95% CI, 11.7 to 26.6) for monozygotic twins and 5.7 (95% CI, 4.1 to 7.9) for dizygotic twins (Fig. 2). In the San Diego Population study,26 any family history of PAD or parental history of PAD was only marginally associated with presence of PAD and family history of cardiovascular disease was not associated with presence of PAD. This study was likely underpowered to detect significant associations as only 87 patients with PAD were included. In a study27 that elicited detailed family history in 2296 PAD cases and 4390 controls, prevalence of family history of PAD was significantly higher in patients with PAD than in controls (10.4% vs. 5.0%, P <0.0001) resulting in a doubling of the odds of the presence of PAD in those with family history of PAD (Fig. 2). The association of family history of PAD with prevalent PAD was only modestly attenuated after adjustment for conventional risk factors: OR 1.97 (1.60-2.42). The association was stronger in individuals younger than 68 years of age and in those with greater number of affected relatives. These results suggest that shared environmental and genetic factors are associated with PAD and motivate the search for genetic susceptibility variants.
Fig. 2.
Family history as a risk factor for PAD. Shown are odds ratios when the affected family member is a monozygotic twin, a dizygotic twin or a sibling.25,27
Early-onset PAD
In the Western world atherosclerosis is the major cause of occlusive disease of the lower extremities in young adults.28, 29 Genetic factors likely have an important role in premature PAD including those acting through pathways of thrombosis, inflammation, and lipid and homocystine metabolism.30 Men and women appear to be equally affected, in contrast to early-onset CHD where men are more commonly affected.31 Similar to CHD, several Mendelian disorders are associated with PAD. These include familial lipoprotein disorders such as chylomicronemia as a result of mutations in the lipoprotein lipase gene and familial hypercholesterolaemia,32-34 hyperhomocysteinemia 35 and pseudoxanthoma elasticum.36
Linkage studies
Linkage analyses for complex diseases have the potential to identify new disease susceptibility genes that may have been unsuspected based on a priori knowledge of disease mechanisms. However such an approach has been largely unsuccessful in identifying specific disease susceptibility variants. Gudmundson and colleagues 37 performed a 10 cM genome-wide scan in 272 patients from 116 extended families who had undergone angiography and/or revascularization procedures for symptomatic PAD.37 Significant linkage to a region on chromosome 1 between 100 and 110 cM was found (LOD score = 3.93; P = 1.04 × 10−5). Several candidate genes (in pathways of inflammation, coagulation, lipid metabolism, blood pressure regulation and vascular matrix regulation) for atherosclerosis were present under the linkage signals, but the causal variants could not be identified. Linkage analyses for ankle brachial index (ABI) as a continuous trait did not reveal any regions of LOD scores ≥3, although several regions with tentative evidence of linkage (multipoint LOD = 1.3-2.0) were detected.38
Candidate gene association studies
In contrast to hundreds of candidate gene association studies for CHD, relatively few have been reported for PAD. The candidate genes studied include β-fibrinogen,39 apo B,40 eNOS,41, 42 MTHFR,41 G-protein beta unit 3 and alpha-adducin,43 interleukin-6,44 and glutathione S-transferase.45 However any reported associations between variants in these genes and PAD have not been confirmed in independent cohorts or in GWAS. Kardia et al46 investigated the association of 435 single nucleotide polymorphisms (SNPs) in 112 positional and biological candidate genes with the ABI in 1046 non-Hispanic whites belonging to hypertensive sibships. The contributions of each SNP, as well as SNP-covariate and SNP-SNP interactions, to the overall genetic architecture of ABI were assessed. Significant associations were corrected for multiple testing and replicated by four-fold cross validation. The following associations were significant, replicated, and cross-validated: two SNP main effects in NOS3, three SNP-covariate interactions (ADRB2 Gly 16 – lipoprotein (a) and SLC4A5 - diabetes interactions), and 25 SNP-SNP interactions (involving SNPs from 29 different genes). The Candidate Gene Association Resource (CARe) consortium performed a meta-analysis of ~ 50,000 SNPs in ~ 2000 cardiovascular candidate genes, but was unable to confidently identify any variants associated with the ABI.47
Genome-wide association studies (GWAS)
The GWAS approach, made possible by knowledge of linkage disequilibrium across the genome as well as the availability of high-density genotyping platforms, is unbiased in nature and has the potential to discover novel disease susceptibility genes. Whereas multiple genetic loci have been associated with inflammatory forms of arterial disease (Table 2), fewer loci with weaker associations have been implicated in atherosclerotic PAD (Table 3). Helgadottir found that the 9p21 locus was associated not only with CHD but also with PAD, abdominal aortic aneurysm (AAA) and intracranial aneurysm.48 Thorgeirsson et al49 found a synonymous SNP (rs1051730) within the cholinergic receptor nicotinic alpha 3 gene (CHRNA3) on chromosome 15q24 to affect nicotine dependence, smoking quantity, and the risk of PAD and lung cancer. In a GWAS for AAA, the variant DAB12 was identified as being associated with both AAA and PAD.50 Koriyama et al51 found the OSBPL10 locus to be associated with PAD in a Japanese cohort. In a meta-analysis52 of 21 population-based cohort studies that included 41,692 participants of European ancestry among whom 3409 participants had PAD (defined as an ABI <0.90), six SNPs were associated (P≤1×10−6) with PAD, but none was significant at a genome-wide significance level. The top SNP associated with PAD was near the PAX gene. In this study, however, a variant at the 9p21 locus was associated with ABI (as a continuous variable) at the genome-wide significance level. Potential mechanisms by which this locus is thought to promote atherosclerosis include cell proliferation, inflammation and impaired efferocytosis (phagocytic clearance) of apoptotic debris in atherosclerotic plaque.53-55
In a recent electronic medical record (EMR)-based GWAS of PAD, the allele C of the intronic SNP rs653178 at the ATXN2-SH2B3 locus on chromosome 12 was present more frequently in PAD cases (52%) than in controls (47%) with a resulting odds ratio (OR) of 1.23 (P= 5.6×10−4) in the discovery cohort.56 In the replication cohort of 740 PAD cases and 1051 controls, the OR was 1.25 (P= 8.9×10−4) and in the combined sample, the OR was 1.22 (P=6.5×10−7). The strength of association of previous GWAS ‘hits’ was tested, but neither the 9p21 variant nor the OSBPL10 variants were associated, whereas the CHRNA3 variant was weakly (P=0.001) associated with PAD. The lead SNP rs653178 is in strong LD (r2=0.99) with a missense SNP (rs3184504) in SH2B3, an adapter protein that plays a key role in immune and inflammatory response pathways and vascular homeostasis.57, 58 The SNP results in substitution of tryptophan (aromatic side chain) by arginine (basic side chain) that may result in altered lipid binding and protein-protein interactions. The SNP may have been protective against bacterial infection in the past allowing it to rise to a relatively high frequency due to natural selection.59-61
CHALLENGES
To date, the search for genetic susceptibility variants for PAD has been less successful than for CHD, likely due to multiple reasons including a potentially stronger environmental contribution to PAD, for example from smoking. Additional challenges in investigating the genetic basis of PAD are summarized in Table 4. Given the results of GWAS so far, it is clear that large numbers of PAD cases and controls are needed to identify genetic susceptibility variants. Investigators will need to collaborate to conduct meta-analyses of GWAS for PAD, similar to those for CHD. Another option is to leverage repositories of DNA linked to EMR systems to conduct genotyping or sequencing studies. Such an approach 71 can reduce the time, effort, and cost involved in conducting genomic association studies. The Electronic Medical Records and Genomics (eMERGE) consortium72 is leveraging biorepositories linked to the EMR for large-scale genomic research.73
Phenotypic heterogeneity appears to be a major challenge in investigating the genetic basis for PAD. PAD is complex and heterogeneous and not a uniform entity. Two broad subtypes of PAD, proximal’ and ‘distal’, are associated with distinct risk factor and comorbidity profiles.74 Female sex, smoking, hypertension, and dyslipidemia are more significantly associated with proximal disease, whereas older age, male sex, and diabetes, are more significantly associated with distal disease. Subtyping of PAD based on location is possible using noninvasive arterial Doppler; an alternative is to stratify patients based on whether or not they have diabetes since diabetic PAD is often distal.
FUTURE DIRECTIONS
The field of complex disease genetics has advanced considerably in the last several years, primarily due to assembly of large case control cohorts and availability of newer genomic technologies. In this section we highlight how these advances might be leveraged in to increase our understanding of the genetic basis of PAD and where possible give examples of early illustrative studies.
Gene-environment and gene-gene interactions
Smoking is the major environmental risk factor for PAD but variability in the susceptibility of smokers to PAD suggests that novel genetic factors may interact with smoking to influence the development and progression of PAD.45 Since PAD results from alterations in multiple atherogenic pathways, large single gene effects are unlikely,75 multiple loci are likely involved and candidate genes may express themselves only through interaction with other genes or with at-risk lifestyles. Identifying the combinations of multilocus genotypes predictive of disease (epistasis) is a daunting task.76 Several statistical methods have been proposed to assess gene-gene and gene-environment interactions but few such interactions have been identified or replicated.
Whole genome/exome sequencing
Genome/exome sequencing may help in identifying causal mutations when PAD clusters in families.77, 78 Exome sequencing in members of three families with symptomatic PAD and arterial and joint calcifications implicated mutations in NT5E, a gene encoding a protein that converts adenosine monophosphate to adenosine. Adenosine inhibits ectopic tissue calcification 77 and adenosine treatment of fibroblasts from an affected patient reduced the levels of alkaline phosphatase and calcification. 65 In another study,66 exome sequencing helped identify the underlying mutation in a family where two siblings had aortic hypoplasia, diffuse atherosclerosis, and PAD. The two siblings were homozygous for a non-synonymous mutation in IN080D which leads to disruption in the function of one of the domains of the protein. INO80D encodes a key component of the human IN080 complex, a multi-protein complex involved in DNA binding, chromatin modification, organization of chromosome structure, and ATP-dependent nucleosome sliding.79
Differential gene expression
Arterial tissue is difficult to obtain and circulating peripheral blood mononuclear cells (PBMCs) have been proposed as ‘reporters’ of arterial wall pathology. Several investigators have examined differentially regulated genes in PBMCs to identify relevant molecular mechanisms for vascular diseases80-82 Masud et al84 found genes influencing immune response, inflammation, apoptosis, and various signalling pathways to be differentially expressed in PBMCs from PAD cases and controls. RNA sequencing (RNA-Seq) has emerged as a tool for investigating known and novel transcripts affecting disease mechanisms and progression. In addition to differential expression and differential splicing, it offers researchers the ability to gain greater insight into changes in gene expression during disease initiation, progression, and response to treatment.
Pleiotropic genetic effects
Several lines of evidence suggest shared genetic susceptibility variants between subtypes of ASCVD. Valentine et al83 reported that premature CHD, PAD, and stroke was more common in parents and siblings of individuals with premature PAD or CHD compared to controls, suggesting the presence of shared genetic factors across these subtypes of ASCVD. In the study by Khaleghi et al,27 family history of CHD was also associated with presence of PAD, suggesting the presence of genetic susceptibility variants shared between PAD and CHD. Several GWAS have reported variants, e.g., 9p21 and DAB21P,48, 50 that are associated with more than one subtype of atherosclerotic vascular disease.84, 85 Gretarsdottir et al 50 reported that the A allele of rs7025486 within DAB2IP, which encodes an inhibitor of cell growth and survival, was associated with AAA, with an odds ratio (OR) of 1.21 and P = 4.6 × 10−10. In tests for association with other vascular diseases, the investigators found this allele to be associated with early onset myocardial infarction (OR = 1.18, P = 3.1 × 10−5) and PAD (OR = 1.14, P = 3.9 × 10−5). The SNP was not associated with risk factors such as smoking, lipid levels, obesity, type 2 diabetes and hypertension. Thus variants found to be associated with CHD, AAA and carotid artery disease should be tested for association with PAD.
The SNP rs3184504 at the ATXN2-SH2B3 locus identified as being associated with PAD is a particularly interesting example of pleiotropy. Not only has it been associated with myocardial infarction but also with immunological disorders,86 hematologic traits such as platelet count, mean-platelet volume,87, 88 and eosinophil count and diabetes.89 The pleiotropic nature of SH2B3 may be due to its role in immune and inflammatory signaling pathways including erythropoietin, cytokine receptor-mediated and integrin signaling.57 The protein also regulates hematopoietic cell lineage and endothelial cells, and influences adhesion and migration of platelets by modulating actin cytoskeleton organization.58, 87, 88, 90
Epigenetics
Epigenetics is the study of factors that modify gene expression, exclusive of changes in the DNA sequence.91 Epigenetic factors include structural modifications to the DNA and its surrounding proteins which alter the accessibility of promoters to transcriptional machinery; as well as soluble factors which interfere with mRNA transcription and translation. Classical epigenetic changes such as chromatin remodeling, histone modification and DNA methylation are of great interest as they can be long-lived and even inherited, but also may be modifiable. Efforts to evaluate the methylome of individuals with PAD could prove insights into how environmental exposures or other risk factors regulate genes important for disease progression. For example, childhood smoking is associated with an increased risk of PAD even after controlling for lifetime tobacco exposure, raising the possibility that early smoking-related epigenetic changes may potentiate risk for disease decades later, as has been shown for other tobacco-related conditions.92 Availability of RNAseq, bisulfite sequencing and ChIP technology is likely to shed more light into the role of the epigenome in atherosclerosis and PAD. The latter allows mapping of histone modifications across the genome, thereby providing insights into repressive/activating changes in the chromatin surrounding genes implicated in atherosclerotic vascular disease.
A growing family of non-coding RNAs is now recognized as another major epigenetic regulator of gene expression.93-95 microRNAs (miRs) are small (~22 nucleotides) single-stranded RNAs that inhibit mRNA translation after binding to the 3’ untranslated region of a target gene. Because they do not require perfect base pairing to repress translation, each miR can regulate dozens or hundreds of genes. miRs regulate endothelial cell function and tube formation, SMC plasticity, lipid metabolism and macrophage biology (reviewed in96) as well as angiogenesis in experimental animals. 97 Additionally, miR-503 has been implicated as a putative regulator of diabetic PAD and limb ischemia human tissue samples.98, 99 A panel of 12 miRs measured in the peripheral blood was recently correlated with the presence of PAD.100 Long noncoding RNAs (IncRNAs) guide chromatin modifiers to transcriptional promoters and are thought to regulate more than two-thirds of all protein coding genes. Little is currently known about the role of lncRNAs in PAD. The 9p21 locus, which is associated with several vascular disease phenotypes, harbors polymorphisms within a lncRNA known as ANRIL (antisense non-coding RNA in the INK4 locus). ANRIL has been shown to recruit polycomb repressive complexes to the promoter of CDKN2B,101 and directly silence the expression of this atheroprotective and anti-aneurysmal gene.55, 102 The association of 9p21 locus with atherosclerotic vascular disease could be mediated by this indirect epigenetic pathway involving ANRIL, through trans-regulation of CDKN2B.
Rare variant association studies
Common genetic susceptibility variants do not fully explain the heritability of complex diseases and the extent to which rare variants contribute to disease susceptibility is not known.103 The common disease-rare variant concept has been illustrated by several reports, including the association of uncommon PCSK9 variants with CHD susceptibility104 and of rare CFH variants with macular degeneration.105 Association studies of rare variants in gene coding regions are a logical next step to complement genome-wide analysis of common variants. New genotyping arrays allow testing the association of rare (defined as minor allele frequency <1%) functional variants with traits of interest. Such an approach has been successful in identifying rare genetic variants associated with complex traits such as insulin resistance.106
Network and pathway analyses
Identifying disease susceptibility genes/variants by itself may not provide insights into the relevant pathophysiologic pathways. Genes often act in networks to influence susceptibility to complex diseases and such effects are unlikely to be identified by SNP-level analyses. Knowledge-based approaches such as enrichment analysis, and network and pathway analysis may provide insights into how genes and proteins interact to influence disease susceptibility.107 The Gene Ontology108 resource provides annotation of the biology and function of gene and protein sequences based on their homology across multiple organisms, using a common vocabulary. KEGG (Kyoto Encyclopedia of Genes and Genomes),109 a database of biological systems that integrates genomic, chemical and systemic functional information, provides a link between genes and higher order processes, such as pathways. Reactome110 is a curated, peer-reviewed database of reactions and pathways that allows representation of intermediary metabolism, regulatory pathways, and signal transduction, and high-level processes, such as the cell cycle.
Microbiome
Periodontal disease has been associated with atherosclerosis, suggesting that bacteria from the oral cavity may contribute to the development of atherosclerosis and cardiovascular disease. Using quantitative PCR, Koren et al111 found bacterial DNA in atherosclerotic plaque and then used 454 pyrosequencing of 16S rRNA genes to demonstrate that several bacterial phylotypes were common to the atherosclerotic plaque and oral or gut samples of the same individual. Several recent studies suggest an association between gut-derived circulating metabolites and angiographic coronary artery disease.112, 113 Wang et al112 have shown that gut microbiome derived metabolites choline, betaine, and trimethylamine-N-oxide (TMAO) are associated with the presence of coronary artery disease. Furthermore, higher levels of TMAO were associated with increased risk of adverse cardiovascular events over in patients referred for coronary angiography.113 These reports motivate additional studies in carefully phenotyped PAD cohorts to identify new pathophysiologic pathways associated with PAD, novel metabolomic markers of disease initiation and progression, and new targets for therapy, such as altering gut/dental flora by dietary intervention and probiotics.
SUMMARY
In spite of relatively large sample sizes, GWAS for PAD have not been as successful as those for CHD. This is likely because susceptibility variants have modest effects, PAD is phenotypically heterogenous and genetic susceptibility variants differ across the subtypes of PAD. Genetic variants may also interact with risk factors such as smoking and diabetes in predisposing to PAD. Thus much larger sample sizes will necessary to identify susceptibility variants with small effects sizes and genomic association analyses will need to be stratified based on the presence versus absence of risk factors such as diabetes and smoking. Results of the genomic association studies so far confirm the presence of pleiotropy across the various forms of atherosclerotic vascular disease and variants found to be associated with CHD, AAA and carotid artery disease should be tested for association with PAD. New directions include the use of whole genome/exome sequencing to uncover the genetic basis of rare forms of PAD, deploying new technologies such as RNAseq to identify genes that are differentially expressed in the initiation and progression of PAD, investigating the role of rare variants and structural variation as susceptibility factors and the study of oral and gut microbiomes in the pathogenesis of PAD.
Supplementary Material
Table 1.
Ascertaining PAD in the clinical setting
| Symptomatic PAD. The classical symptom of PAD is intermittent claudication but less than one third of patients with abnormal ankle brachial index (ABI) (< 0.9) have claudication. Additional clinical presentations include atypical leg discomfort, critical limb ischemia (including rest pain, gangrene) and history of amputation or revascularization for limb ischemia. |
| Abnormal ankle brachial index (ABI).8 ABI – the ratio of systolic BP at the ankle to the systolic BP in the arm – is an established noninvasive measure of PAD that is inversely related to disease severity. When segmental blood pressures are combined with Doppler, disease location can also be ascertained. An ABI of < 0.90 is 95% sensitive in detecting stenosis of 50% or greater (determined angiographically) involving the lower extremities.9, 10 There are two main limitations of the ABI as a phenotype of PAD. Since the ABI becomes abnormal only with hemodynamically significant lesions, disease of lesser severity may be missed. Use of post-exercise ABIs increases the sensitivity and partially addresses this problem. ABI may be falsely elevated due to medial calcification that typically occurs in the elderly and diabetic subjects and leads to poorly compressible arteries. 11 |
| Atherosclerotic plaque on imaging. Several imaging modalities can detect atherosclerotic plaque in lower extremity arteries: Ultrasound- Arterial ultrasound is a noninvasive imaging modality that is more sensitive than the ABI in identifying subclinical PAD. In addition to visualizing plaque burden, hemodynamic assessment with Doppler is also possible. Methods for quantifying plaque burden are not yet available. Intravascular ultrasound provides more accurate estimates of plaque burden but is invasive in nature. Angiography- Conventional angiography is the gold standard for assessment of luminal narrowing of the peripheral arteries but is invasive and expensive. Furthermore it may not provide an accurate measure of the true burden of atherosclerosis much of which could be accommodated in the arterial wall due to remodeling. Angiography can also be performed by computed tomography or by magnetic resonance imaging. |
Table 2.
Genetic loci associated with inflammatory arterial diseases in genome-wide associated studies
| Disease | Involvement of peripheral arteries | Genes implicated |
|---|---|---|
| ANCA-associated vasculitis62 | Inflammation of small-sized blood vessels | SERPINA1, HLA-DP, COL11A2 |
| Kawasaki's disease 63-66 | Inflammation of medium- and small-sized blood vessels | FAM167A – ELK, CD40, HLA-DQB2–HLA-DOB FCGR2A, ZFHX3, LNX1, CAMK2D, CSMD1, TCP1 |
| Behcet's disease67-70 | Inflammation of large, medium-and small-sized blood vessels | GIMAP, STAT4, ERAP1, IL23R - IL12RB2, IL10, CCR1-CCR3, KLRC4 |
ANCA=Anti neutrophil cytoplasmic antibody
Table 3.
SNPs associated with PAD in genome-wide associated studies
| SNP | Locus | Cases (n) | Controls (n) | OR (95% CI) | P-value | RAF* | Nearest gene |
|---|---|---|---|---|---|---|---|
| rs10757278-G | 9p21 | 2599 | 15012 | 1.14 (1.07, 1.22 | 6.1×10−5 | 0.42-0.51 | CDKN2A/CDKN2B |
| rs1051730-C | 15q24 | 2738 | 29,964 | 1.19 (1.12, 1.27) | 1.4×10−7 | 0.27-0.37 | CHRNA |
| rs7025486-A | 9q33 | 3690 | 12,271 | 1.14 (1.07, 1.21) | 3.9×10−5 | 0.23 | DAB12 |
| rs1902341-G | 3p23 | 195 699 |
1358 1540 |
1.31 (1.18, 1.46) | 5×10−7 | 0.397 | OSBPL |
| rs6584389-C | 10 | 3409 | 68,002 | 1.17(1.10, 1.25) | 2.3×10−6 | 0.50 | PAX2 |
| rs653178-C | 12q24 | 1641 740 |
1604 1051 |
1.22 (1.13, 1.32) | 6.46×10−7 | 0.49 | SH2B3-ATXN |
in controls. OR= odds ratio; RAF= risk allele frequency in controls
Table 4.
Challenges in identifying genetic determinants of peripheral arterial disease
| Challenge | Comment |
|---|---|
| Phenotypic heterogeneity | Genomic association analyses stratifying by subtypes of PAD could address phenotypic heterogeneity. |
| Genetic heterogeneity | Increasing sample size in case-control association studies may help in addressing genetic heterogeneity. |
| Modest effect sizes of genetic variants | Uncovering variants of small effects requires large sample sizes, and recognition of this fact has motivated assembly of genetic consortia for common diseases. |
| Gene-gene and gene-environment interactions | Identifying such interactions will require large sample sizes and precise measures of environmental factors e.g. pack years in the case of smoking. |
| Rare variants | Both common and rare variants likely influence PAD susceptibility. To identify rare variants that influence susceptibility to PAD, very large sample sizes will be required. |
| Structural variants | Additional studies are needed to investigate the association of structural genetic variants with complex diseases such as PAD. |
Abbreviations: PAD, peripheral arterial disease; ABI, ankle brachial index.
Acknowledgments
Sources of Funding
IJK is supported by Grant U01HG006379 from the National Human Genome Research Institute. NJL is supported by K08HL10360503 from the National Heart Lung and Blood Institute.
Nonstandard Abbreviations and Acronyms
- AAA
abdominal aortic aneurysm
- ABI
ankle brachial index
- CHD
coronary heart disease
- eQTL
expression quantitative trait loci
- GWAS
genome-wide association studies
- lncRNA
long noncoding RNA
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- miR
microRNA
- OR
odds ratio
- PAD
peripheral arterial disease
- RNAseq
RNA sequencing
- SNP
single-nucleotide polymorphism
Footnotes
Financial disclosures: None of the authors have involvement with any organization or entity with a financial interest in the subject matter discussed in the manuscript
References
- 1.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 2.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738–-743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 4.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–699. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 5.Mahoney EM, Wang K, Cohen DJ, Hirsch AT, Alberts MJ, Eagle K, Mosse F, Jackson JD, Steg PG, Bhatt DL. One-year costs in patients with a history of or at risk for atherothrombosis in the United States. Circ Cardiovasc Qual Outcomes. 2008;1:38–45. doi: 10.1161/CIRCOUTCOMES.108.775247. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch AT, Hartman L, Town RJ, Virnig BA. National health care costs of peripheral arterial disease in the Medicare population. Vasc Med. 2008;13:209–215. doi: 10.1177/1358863X08089277. [DOI] [PubMed] [Google Scholar]
- 7.Iglehart JK. Prioritizing comparative-effectiveness research--IOM recommendations. N Engl J Med. 2009;361:325–328. doi: 10.1056/NEJMp0904133. [DOI] [PubMed] [Google Scholar]
- 8.Grenon SM, Gagnon J, Hsiang Y. Ankle–Brachial Index for Assessment of Peripheral Arterial Disease. N Engl J Med. 2009;361:e40. doi: 10.1056/NEJMvcm0807012. [DOI] [PubMed] [Google Scholar]
- 9.Carter SA. Indirect systolic pressures and pulse waves in arterial occlusive diseases of the lower extremities. Circulation. 1968;37:624–637. doi: 10.1161/01.cir.37.4.624. [DOI] [PubMed] [Google Scholar]
- 10.Kiekara O, Riekkinen H, Soimakallio S, Lansimies E. Correlation of angiographically determined reduction of vascular lumen with lower-limb systolic pressures. Acta Chir Scand. 1985;151:437–440. [PubMed] [Google Scholar]
- 11.Arain FA, Ye Z, Bailey KR, Chen Q, Liu G, Leibson CL, Kullo IJ. Survival in patients with poorly compressible leg arteries. J Am Coll Cardiol. 2012;59:400–407. doi: 10.1016/j.jacc.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leeper NJ, Kullo IJ, Cooke JP. Genetics of peripheral artery disease. Circulation. 2012;125:3220–3228. doi: 10.1161/CIRCULATIONAHA.111.033878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Downing KP, Nead KT, Kojima Y, Assimes T, Maegdefessel L, Quertermous T, Cooke JP, Leeper NJ. The combination of 9p21.3 genotype and biomarker profile improves a peripheral artery disease risk prediction model. Vasc Med. 2014;19:3–8. doi: 10.1177/1358863X13514791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, Stirrups K, Konig IR, Cazier JB, Johansson A, Hall AS, Lee JY, Willer CJ, Chambers JC, Esko T, Folkersen L, Goel A, Grundberg E, Havulinna AS, Ho WK, Hopewell JC, Eriksson N, Kleber ME, Kristiansson K, Lundmark P, Lyytikainen LP, Rafelt S, Shungin D, Strawbridge RJ, Thorleifsson G, Tikkanen E, Van Zuydam N, Voight BF, Waite LL, Zhang W, Ziegler A, Absher D, Altshuler D, Balmforth AJ, Barroso I, Braund PS, Burgdorf C, Claudi-Boehm S, Cox D, Dimitriou M, Do R, Doney AS, El Mokhtari N, Eriksson P, Fischer K, Fontanillas P, Franco-Cereceda A, Gigante B, Groop L, Gustafsson S, Hager J, Hallmans G, Han BG, Hunt SE, Kang HM, Illig T, Kessler T, Knowles JW, Kolovou G, Kuusisto J, Langenberg C, Langford C, Leander K, Lokki ML, Lundmark A, McCarthy MI, Meisinger C, Melander O, Mihailov E, Maouche S, Morris AD, Muller-Nurasyid M, Nikus K, Peden JF, Rayner NW, Rasheed A, Rosinger S, Rubin D, Rumpf MP, Schafer A, Sivananthan M, Song C, Stewart AF, Tan ST, Thorgeirsson G, van der Schoot CE, Wagner PJ, Wells GA, Wild PS, Yang TP, Amouyel P, Arveiler D, Basart H, Boehnke M, Boerwinkle E, Brambilla P, Cambien F, Cupples AL, de Faire U, Dehghan A, Diemert P, Epstein SE, Evans A, Ferrario MM, Ferrieres J, Gauguier D, Go AS, Goodall AH, Gudnason V, Hazen SL, Holm H, Iribarren C, Jang Y, Kahonen M, Kee F, Kim HS, Klopp N, Koenig W, Kratzer W, Kuulasmaa K, Laakso M, Laaksonen R, Lind L, Ouwehand WH, Parish S, Park JE, Pedersen NL, Peters A, Quertermous T, Rader DJ, Salomaa V, Schadt E, Shah SH, Sinisalo J, Stark K, Stefansson K, Tregouet DA, Virtamo J, Wallentin L, Wareham N, Zimmermann ME, Nieminen MS, Hengstenberg C, Sandhu MS, Pastinen T, Syvanen AC, Hovingh GK, Dedoussis G, Franks PW, Lehtimaki T, Metspalu A, Zalloua PA, Siegbahn A, Schreiber S, Ripatti S, Blankenberg SS, Perola M, Clarke R, Boehm BO, O'Donnell C, Reilly MP, Marz W, Collins R, Kathiresan S, Hamsten A, Kooner JS, Thorsteinsdottir U, Danesh J, Palmer CN, Roberts R, Watkins H, Schunkert H, Samani NJ. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang P NK, Olin JW, Cooke JP, Leeper NJ. Clinical and socioeconomic factors associated with unrecognized peripheral artery disease. Vasc Med. 2014;19(4):289–296. doi: 10.1177/1358863X14535475. [DOI] [PubMed] [Google Scholar]
- 16.Kullo IJ, Bailey KR, Kardia SL, Mosley TH, Jr., Boerwinkle E, Turner ST. Ethnic differences in peripheral arterial disease in the NHLBI Genetic Epidemiology Network of Arteriopathy (GENOA) study. Vasc Med. 2003;8:237–242. doi: 10.1191/1358863x03vm511oa. [DOI] [PubMed] [Google Scholar]
- 17.Newman AB, Sutton-Tyrrell K, Vogt MT, Kuller LH. Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index. JAMA. 1993;270:487–489. [PubMed] [Google Scholar]
- 18.Kuller L, Fisher L, McClelland R, Fried L, Cushman M, Jackson S, Manolio T. Differences in prevalence of and risk factors for subclinical vascular disease among black and white participants in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1998;18:283–293. doi: 10.1161/01.atv.18.2.283. [DOI] [PubMed] [Google Scholar]
- 19.Collins TC, Johnson M, Henderson W, Khuri SF, Daley J. Lower extremity nontraumatic amputation among veterans with peripheral arterial disease: is race an independent factor? Med Care. 2002;40:I106–116. doi: 10.1097/00005650-200201001-00012. [DOI] [PubMed] [Google Scholar]
- 20.Freedman DS, Newman WP, 3rd, Tracy RE, Voors AE, Srinivasan SR, Webber LS, Restrepo C, Strong JP, Berenson GS. Black-white differences in aortic fatty streaks in adolescence and early adulthood: the Bogalusa Heart Study. Circulation. 1988;77:856–864. doi: 10.1161/01.cir.77.4.856. [DOI] [PubMed] [Google Scholar]
- 21.Singh S, Bailey KR, Kullo IJ. Ethnic differences in ankle brachial index are present in middle-aged individuals without peripheral arterial disease. Int J Cardiol. 2013;162:228–233. doi: 10.1016/j.ijcard.2011.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valdez R, Yoon PW, Qureshi N, Green RF, Khoury MJ. Family History in Public Health Practice: A Genomic Tool for Disease Prevention and Health Promotion*. Ann Rev of Publ Health. 2010;31:69–87. doi: 10.1146/annurev.publhealth.012809.103621. [DOI] [PubMed] [Google Scholar]
- 23.Guttmacher AE, Collins FS, Carmona RH. The family history--more important than ever. N Engl J Med. 2004;351:2333–2336. doi: 10.1056/NEJMsb042979. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr., White CJ, White J, White RA. ACC/AHA Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 25.Wahlgren CM, Magnusson PK. Genetic influences on peripheral arterial disease in a twin population. Arterioscler Thromb Vasc Biol. 2011;31:678–682. doi: 10.1161/ATVBAHA.110.210385. [DOI] [PubMed] [Google Scholar]
- 26.Wassel CL, Loomba R, Ix JH, Allison MA, Denenberg JO, Criqui MH. Family history of peripheral artery disease is associated with prevalence and severity of peripheral artery disease: the San Diego population study. J Ameri Coll Cardiol. 2011;58:1386–1392. doi: 10.1016/j.jacc.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khaleghi M, Isseh IN, Bailey KR, Kullo IJ. Family History as a Risk Factor for Peripheral Arterial Disease. Am J Cardiol. 2014 doi: 10.1016/j.amjcard.2014.06.029. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barretto S, Ballman KV, Rooke TW, Kullo IJ. Early-onset peripheral arterial occlusive disease: clinical features and determinants of disease severity and location. Vasc Med. 2003;8:95–100. doi: 10.1191/1358863x03vm475oa. [DOI] [PubMed] [Google Scholar]
- 29.Valentine RJ, MacGillivray DC, DeNobile JW, Snyder DA, Rich NM. Intermittent claudication caused by atherosclerosis in patients aged forty years and younger. Surgery. 1990;107:560–565. [PubMed] [Google Scholar]
- 30.Levy PJ, Gonzalez MF, Hornung CA, Chang WW, Haynes JL, Rush DS. A prospective evaluation of atherosclerotic risk factors and hypercoagulability in young adults with premature lower extremity atherosclerosis. J Vas Surg. 1996;23:36–43. doi: 10.1016/s0741-5214(05)80033-3. discussion 43-35. [DOI] [PubMed] [Google Scholar]
- 31.Nitter-Hauge S, Erikssen J, Thaulow E, Vatne K. Angiographic and risk factor characteristics of subjects with early onset ischaemic heart disease. Br Heart J. 1981;46:325–330. doi: 10.1136/hrt.46.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutter CM, Austin MA, Humphries SE. Familial hypercholesterolemia, peripheral arterial disease, and stroke: a HuGE minireview. AmJ Epidemiol. 2004;160:430–435. doi: 10.1093/aje/kwh238. [DOI] [PubMed] [Google Scholar]
- 33.Johnston JD. A case of heterozygous familial hypercholesterolaemia who developed exclusive peripheral vascular disease. Annal Clini Biochem. 1997;34:321–323. doi: 10.1177/000456329703400318. [DOI] [PubMed] [Google Scholar]
- 34.Vitale E, Zuliani G, Baroni L, Bicego L, Grego F, Valerio G, Fellin R. Lipoprotein abnormalities in patients with extra-coronary arteriosclerosis. Atherosclerosis. 1990;81:95–102. doi: 10.1016/0021-9150(90)90015-b. [DOI] [PubMed] [Google Scholar]
- 35.Boers GH, Smals AG, Trijbels FJ, Fowler B, Bakkeren JA, Schoonderwaldt HC, Kleijer WJ, Kloppenborg PW. Heterozygosity for homocystinuria in premature peripheral and cerebral occlusive arterial disease. N Engl J Me. 1985;313:709–715. doi: 10.1056/NEJM198509193131201. [DOI] [PubMed] [Google Scholar]
- 36.Leftheriotis G, Kauffenstein G, Hamel JF, Abraham P, Le Saux O, Willoteaux S, Henrion D, Martin L. The contribution of arterial calcification to peripheral arterial disease in pseudoxanthoma elasticum. PloS one. 2014;9:e96003. doi: 10.1371/journal.pone.0096003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gudmundsson G, Matthiasson SE, Arason H, Johannsson H, Runarsson F, Bjarnason H, Helgadottir K, Thorisdottir S, Ingadottir G, Lindpaintner K, Sainz J, Gudnason V, Frigge ML, Kong A, Gulcher JR, Stefansson K. Localization of a gene for peripheral arterial occlusive disease to chromosome 1p31. Am J Hum Genet. 2002;70:586–592. doi: 10.1086/339251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kullo IJ, Turner ST, Kardia SL, Mosley TH, Jr., Boerwinkle E, Andrade M. A genome-wide linkage scan for ankle-brachial index in African American and non-Hispanic white subjects participating in the GENOA study. Atherosclerosis. 2006;187:433–438. doi: 10.1016/j.atherosclerosis.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Fowkes FG, Connor JM, Smith FB, Wood J, Donnan PT, Lowe GD. Fibrinogen genotype and risk of peripheral atherosclerosis. Lancet. 1992;339:693–696. doi: 10.1016/0140-6736(92)90596-u. [DOI] [PubMed] [Google Scholar]
- 40.Monsalve MV, Young R, Jobsis J, Wiseman SA, Dhamu S, Powell JT, Greenhalgh RM, Humphries SE. DNA polymorphisms of the gene for apolipoprotein B in patients with peripheral arterial disease. Atherosclerosis. 1988;70:123–129. doi: 10.1016/0021-9150(88)90106-2. [DOI] [PubMed] [Google Scholar]
- 41.Fowkes FG, Lee AJ, Hau CM, Cooke A, Connor JM, Lowe GD. Methylene tetrahydrofolate reductase (MTHFR) and nitric oxide synthase (ecNOS) genes and risks of peripheral arterial disease and coronary heart disease: Edinburgh Artery Study. Atherosclerosis. 2000;150:179–185. doi: 10.1016/s0021-9150(99)00366-4. [DOI] [PubMed] [Google Scholar]
- 42.Kullo IJ, Greene MT, Boerwinkle E, Chu J, Turner ST, Kardia SL. Association of polymorphisms in NOS3 with the ankle-brachial index in hypertensive adults. Atherosclerosis. 2008;196:905–912. doi: 10.1016/j.atherosclerosis.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrison AC, Doris PA, Folsom AR, Nieto FJ, Boerwinkle E. G-protein beta3 subunit and alpha-adducin polymorphisms and risk of subclinical and clinical stroke. Stroke. 2001;32:822–829. doi: 10.1161/01.str.32.4.822. [DOI] [PubMed] [Google Scholar]
- 44.Flex A, Gaetani E, Pola R, Santoliquido A, Aloi F, Papaleo P, Dal Lago A, Pola E, Serricchio M, Tondi P, Pola P. The -174 G/C polymorphism of the interleukin-6 gene promoter is associated with peripheral artery occlusive disease. Eur J Vas and Endovasc Surg. 2002;24:264–268. doi: 10.1053/ejvs.2002.1711. [DOI] [PubMed] [Google Scholar]
- 45.Li R, Folsom AR, Sharrett AR, Couper D, Bray M, Tyroler HA. Interaction of the glutathione S-transferase genes and cigarette smoking on risk of lower extremity arterial disease: the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis. 2001;154:729–738. doi: 10.1016/s0021-9150(00)00582-7. [DOI] [PubMed] [Google Scholar]
- 46.Kardia SL, Greene MT, Boerwinkle E, Turner ST, Kullo IJ. Investigating the complex genetic architecture of ankle-brachial index, a measure of peripheral arterial disease, in non-Hispanic whites. BMC medical genomics. 2008;1:16. doi: 10.1186/1755-8794-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wassel CL, Lamina C, Nambi V, Coassin S, Mukamal KJ, Ganesh SK, Jacobs DR, Jr., Franceschini N, Papanicolaou GJ, Gibson Q, Yanek LR, van der Harst P, Ferguson JF, Crawford DC, Waite LL, Allison MA, Criqui MH, McDermott MM, Mehra R, Cupples LA, Hwang SJ, Redline S, Kaplan RC, Heiss G, Rotter JI, Boerwinkle E, Taylor HA, Eraso LH, Haun M, Li M, Meisinger C, O'Connell JR, Shuldiner AR, Tybjaerg-Hansen A, Frikke-Schmidt R, Kollerits B, Rantner B, Dieplinger B, Stadler M, Mueller T, Haltmayer M, Klein-Weigel P, Summerer M, Wichmann HE, Asselbergs FW, Navis G, Mateo Leach I, Brown-Gentry K, Goodloe R, Assimes TL, Becker DM, Cooke JP, Absher DM, Olin JW, Mitchell BD, Reilly MP, Mohler ER, 3rd, North KE, Reiner AP, Kronenberg F, Murabito JM. Genetic determinants of the ankle-brachial index: a meta-analysis of a cardiovascular candidate gene 50K SNP panel in the candidate gene association resource (CARe) consortium. Atherosclerosis. 2012;222:138–147. doi: 10.1016/j.atherosclerosis.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Helgadottir A, Thorleifsson G, Magnusson KP, Gretarsdottir S, Steinthorsdottir V, Manolescu A, Jones GT, Rinkel GJ, Blankensteijn JD, Ronkainen A, Jaaskelainen JE, Kyo Y, Lenk GM, Sakalihasan N, Kostulas K, Gottsater A, Flex A, Stefansson H, Hansen T, Andersen G, Weinsheimer S, Borch-Johnsen K, Jorgensen T, Shah SH, Quyyumi AA, Granger CB, Reilly MP, Austin H, Levey AI, Vaccarino V, Palsdottir E, Walters GB, Jonsdottir T, Snorradottir S, Magnusdottir D, Gudmundsson G, Ferrell RE, Sveinbjornsdottir S, Hernesniemi J, Niemela M, Limet R, Andersen K, Sigurdsson G, Benediktsson R, Verhoeven EL, Teijink JA, Grobbee DE, Rader DJ, Collier DA, Pedersen O, Pola R, Hillert J, Lindblad B, Valdimarsson EM, Magnadottir HB, Wijmenga C, Tromp G, Baas AF, Ruigrok YM, van Rij AM, Kuivaniemi H, Powell JT, Matthiasson SE, Gulcher JR, Thorgeirsson G, Kong A, Thorsteinsdottir U, Stefansson K. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008;40:217–224. doi: 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]
- 49.Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, Stacey SN, Bergthorsson JT, Thorlacius S, Gudmundsson J, Jonsson T, Jakobsdottir M, Saemundsdottir J, Olafsdottir O, Gudmundsson LJ, Bjornsdottir G, Kristjansson K, Skuladottir H, Isaksson HJ, Gudbjartsson T, Jones GT, Mueller T, Gottsater A, Flex A, Aben KK, de Vegt F, Mulders PF, Isla D, Vidal MJ, Asin L, Saez B, Murillo L, Blondal T, Kolbeinsson H, Stefansson JG, Hansdottir I, Runarsdottir V, Pola R, Lindblad B, van Rij AM, Dieplinger B, Haltmayer M, Mayordomo JI, Kiemeney LA, Matthiasson SE, Oskarsson H, Tyrfingsson T, Gudbjartsson DF, Gulcher JR, Jonsson S, Thorsteinsdottir U, Kong A, Stefansson K. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gretarsdottir S, Baas AF, Thorleifsson G, Holm H, den Heijer M, de Vries JP, Kranendonk SE, Zeebregts CJ, van Sterkenburg SM, Geelkerken RH, van Rij AM, Williams MJ, Boll AP, Kostic JP, Jonasdottir A, Walters GB, Masson G, Sulem P, Saemundsdottir J, Mouy M, Magnusson KP, Tromp G, Elmore JR, Sakalihasan N, Limet R, Defraigne JO, Ferrell RE, Ronkainen A, Ruigrok YM, Wijmenga C, Grobbee DE, Shah SH, Granger CB, Quyyumi AA, Vaccarino V, Patel RS, Zafari AM, Levey AI, Austin H, Girelli D, Pignatti PF, Olivieri O, Martinelli N, Malerba G, Trabetti E, Becker LC, Becker DM, Reilly MP, Rader DJ, Mueller T, Dieplinger B, Haltmayer M, Urbonavicius S, Lindblad B, Gottsater A, Gaetani E, Pola R, Wells P, Rodger M, Forgie M, Langlois N, Corral J, Vicente V, Fontcuberta J, Espana F, Grarup N, Jorgensen T, Witte DR, Hansen T, Pedersen O, Aben KK, de Graaf J, Holewijn S, Folkersen L, Franco-Cereceda A, Eriksson P, Collier DA, Stefansson H, Steinthorsdottir V, Rafnar T, Valdimarsson EM, Magnadottir HB, Sveinbjornsdottir S, Olafsson I, Magnusson MK, Palmason R, Haraldsdottir V, Andersen K, Onundarson PT, Thorgeirsson G, Kiemeney LA, Powell JT, Carey DJ, Kuivaniemi H, Lindholt JS, Jones GT, Kong A, Blankensteijn JD, Matthiasson SE, Thorsteinsdottir U, Stefansson K. Genome-wide association study identifies a sequence variant within the DAB2IP gene conferring susceptibility to abdominal aortic aneurysm. Nat Genet. 2010;42:692–697. doi: 10.1038/ng.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koriyama H, Nakagami H, Katsuya T, Sugimoto K, Yamashita H, Takami Y, Maeda S, Kubo M, Takahashi A, Nakamura Y, Ogihara T, Rakugi H, Kaneda Y, Morishita R. Identification of evidence suggestive of an association with peripheral arterial disease at the OSBPL10 locus by genome-wide investigation in the Japanese population. J Atheroscler Thromb. 2010;17:1054–1062. doi: 10.5551/jat.4291. [DOI] [PubMed] [Google Scholar]
- 52.Murabito JM, White CC, Kavousi M, Sun YV, Feitosa MF, Nambi V, Lamina C, Schillert A, Coassin S, Bis JC, Broer L, Crawford DC, Franceschini N, Frikke-Schmidt R, Haun M, Holewijn S, Huffman JE, Hwang SJ, Kiechl S, Kollerits B, Montasser ME, Nolte IM, Rudock ME, Senft A, Teumer A, van der Harst P, Vitart V, Waite LL, Wood AR, Wassel CL, Absher DM, Allison MA, Amin N, Arnold A, Asselbergs FW, Aulchenko Y, Bandinelli S, Barbalic M, Boban M, Brown-Gentry K, Couper DJ, Criqui MH, Dehghan A, den Heijer M, Dieplinger B, Ding J, Dorr M, Espinola-Klein C, Felix SB, Ferrucci L, Folsom AR, Fraedrich G, Gibson Q, Goodloe R, Gunjaca G, Haltmayer M, Heiss G, Hofman A, Kieback A, Kiemeney LA, Kolcic I, Kullo IJ, Kritchevsky SB, Lackner KJ, Li X, Lieb W, Lohman K, Meisinger C, Melzer D, Mohler ER, 3rd, Mudnic I, Mueller T, Navis G, Oberhollenzer F, Olin JW, O'Connell J, O'Donnell CJ, Palmas W, Penninx BW, Petersmann A, Polasek O, Psaty BM, Rantner B, Rice K, Rivadeneira F, Rotter JI, Seldenrijk A, Stadler M, Summerer M, Tanaka T, Tybjaerg-Hansen A, Uitterlinden AG, van Gilst WH, Vermeulen SH, Wild SH, Wild PS, Willeit J, Zeller T, Zemunik T, Zgaga L, Assimes TL, Blankenberg S, Boerwinkle E, Campbell H, Cooke JP, de Graaf J, Herrington D, Kardia SL, Mitchell BD, Murray A, Munzel T, Newman AB, Oostra BA, Rudan I, Shuldiner AR, Snieder H, van Duijn CM, Volker U, Wright AF, Wichmann HE, Wilson JF, Witteman JC, Liu Y, Hayward C, Borecki IB, Ziegler A, North KE, Cupples LA, Kronenberg F. Association between chromosome 9p21 variants and the ankle-brachial index identified by a meta-analysis of 21 genome-wide association studies. Circ Cardiovasc Genet. 2012;5:100–112. doi: 10.1161/CIRCGENETICS.111.961292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harismendy O ND, Song X, Rahim NG, Tanasa B, Heintzman N, Ren B, Fu X, Topol EJ, Rosenfeld MG, Frazer KA. 9p21 DNA variants associated with coronary artery disease impair interferon-y signalling response. Nature. 2011;470:264–268. doi: 10.1038/nature09753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Visel A, Zhu Y, May D, Afzal V, Gong E, Attanasio C, Blow MJ, Cohen JC, Rubin EM, Pennacchio LA. Targeted deletion of the 9p21 non-coding coronary artery disease risk interval in mice. Nature. 2010;464:409–412. doi: 10.1038/nature08801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kojima Y, Downing K, Kundu R, Miller C, Dewey F, Lancero H, Raaz U, Perisic L, Hedin U, Schadt E, Maegdefessel L, Quertermous T, Leeper NJ. Cyclin-dependent kinase inhibitor 2B regulates efferocytosis and atherosclerosis. J Clin Invest. 2014;124:1083–1097. doi: 10.1172/JCI70391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kullo IJ, Shameer K, Jouni H, Lesnick TG, Pathak J, Chute CG, de Andrade M. The ATXN2-SH2B3 locus is associated with peripheral arterial disease: an electronic medical record-based genome-wide association study. Front Genet. 2014;5:166. doi: 10.3389/fgene.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Devalliere J, Charreau B. The adaptor Lnk (SH2B3): an emerging regulator in vascular cells and a link between immune and inflammatory signaling. Biochem Pharmacol. 2011;82:1391–1402. doi: 10.1016/j.bcp.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 58.Devalliere J, Chatelais M, Fitau J, Gerard N, Hulin P, Velazquez L, Turner CE, Charreau B. LNK (SH2B3) is a key regulator of integrin signaling in endothelial cells and targets alpha-parvin to control cell adhesion and migration. FASEB J. 2012;26:2592–2606. doi: 10.1096/fj.11-193383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhernakova A, Elbers CC, Ferwerda B, Romanos J, Trynka G, Dubois PC, de Kovel CG, Franke L, Oosting M, Barisani D, Bardella MT, Joosten LA, Saavalainen P, van Heel DA, Catassi C, Netea MG, Wijmenga C. Evolutionary and functional analysis of celiac risk loci reveals SH2B3 as a protective factor against bacterial infection. Am J Hum Genet. 2010;86:970–977. doi: 10.1016/j.ajhg.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ding K, Kullo IJ. Geographic differences in allele frequencies of susceptibility SNPs for cardiovascular disease. BMC Med Genet. 2011;12:55. doi: 10.1186/1471-2350-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pickrell JK, Coop G, Novembre J, Kudaravalli S, Li JZ, Absher D, Srinivasan BS, Barsh GS, Myers RM, Feldman MW, Pritchard JK. Signals of recent positive selection in a worldwide sample of human populations. Genome Res. 2009;19:826–837. doi: 10.1101/gr.087577.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lyons RT, PA1, Trivedi S, Holle JU, Watts RA, Jayne DR, Baslund B, Brenchley P, Bruchfeld A, Chaudhry AN, Cohen Tervaert JW, Deloukas P, Feighery C, Gross WL, Guillevin L, Gunnarsson I, Harper L, Hrušková Z, Little MA, Martorana D, Neumann T, Ohlsson S, Padmanabhan S, Pusey CD, Salama AD, Sanders JS, Savage CO, Segelmark M, Stegeman CA, Tesař V, Vaglio A, Wieczorek S, Wilde B, Zwerina J, Rees AJ, Clayton DG, Smith KG. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012 Jul 19;367:214–223. doi: 10.1056/NEJMoa1108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burgner DS, D1, Breunis WB, Ng SB, Li Y, Bonnard C, Ling L, Wright VJ, Thalamuthu A, Odam M, Shimizu C, Burns JC, Levin M, Kuijpers TW, Hibberd ML, International Kawasaki Disease Genetics Consortium A genome-wide association study identifies novel and functionally related susceptibility Loci for Kawasaki disease. PLoS Genet. 2009 Jan;5:e1000319. doi: 10.1371/journal.pgen.1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khor DS, CC1, Breunis WB, Lee YC, Shimizu C, Wright VJ, Yeung RS, Tan DE, Sim KS, Wang JJ, Wong TY, Pang J, Mitchell P, Cimaz R, Dahdah N, Cheung YF, Huang GY, Yang W, Park IS, Lee JK, Wu JY, Levin M, Burns JC, Burgner D, Kuijpers TW, Hibberd ML, Hong Kong–Shanghai Kawasaki Disease Genetics Consortium. Korean Kawasaki Disease Genetics Consortium; Taiwan Kawasaki Disease Genetics Consortium. International Kawasaki Disease Genetics Consortium. US Kawasaki Disease Genetics Consortium; Blue Mountains Eye Study Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nat Genet. 2011 Nov 13;43:1241–1246. doi: 10.1038/ng.981. [DOI] [PubMed] [Google Scholar]
- 65.Lee KH, YC1, Chang JS, Chang LY, Huang LM, Chen MR, Liang CD, Chi H, Huang FY, Lee ML, Huang YC, Hwang B, Chiu NC, Hwang KP, Lee PC, Chang LC, Liu YM, Chen YJ, Chen CH, Taiwan Pediatric ID Alliance. Chen YT, Tsai FJ, Wu JY. Two new susceptibility loci for Kawasaki disease identified through genome-wide association analysis. Nat Genet. 2012 Mar 25;44:522–525. doi: 10.1038/ng.2227. [DOI] [PubMed] [Google Scholar]
- 66.Onouchi OK, Y1, Burns JC, Shimizu C, Terai M, Hamada H, Honda T, Suzuki H, Suenaga T, Takeuchi T, Yoshikawa N, Suzuki Y, Yasukawa K, Ebata R, Higashi K, Saji T, Kemmotsu Y, Takatsuki S, Ouchi K, Kishi F, Yoshikawa T, Nagai T, Hamamoto K, Sato Y, Honda A, Kobayashi H, Sato J, Shibuta S, Miyawaki M, Oishi K, Yamaga H, Aoyagi N, Iwahashi S, Miyashita R, Murata Y, Sasago K, Takahashi A, Kamatani N, Kubo M, Tsunoda T, Hata A, Nakamura Y, Tanaka T, Japan Kawasaki Disease Genome Consortium. US Kawasaki Disease Genetics Consortium A genome-wide association study identifies three new risk loci for Kawasaki disease. Nat Genet. 2012 Mar 25;44:517–521. doi: 10.1038/ng.2220. [DOI] [PubMed] [Google Scholar]
- 67.Mizuki MA, N1, Ota M, Ohno S, Shiota T, Kawagoe T, Ito N, Kera J, Okada E, Yatsu K, Song YW, Lee EB, Kitaichi N, Namba K, Horie Y, Takeno M, Sugita S, Mochizuki M, Bahram S, Ishigatsubo Y, Inoko H. Genome-wide association studies identify IL23R-IL12RB2 and IL10 as Behçet's disease susceptibility loci. Nat Genet. 2010 Aug;42:703–706. doi: 10.1038/ng.624. [DOI] [PubMed] [Google Scholar]
- 68.Hou YZ, S1, Du L, Jiang Z, Shu Q, Chen Y, Li F, Zhou Q, Ohno S, Chen R, Kijlstra A, Rosenbaum JT, Yang P. Identification of a susceptibility locus in STAT4 for Behçet's disease in Han Chinese in a genome-wide association study. Arthritis Rheum. 2012 Dec;64:4104–4113. doi: 10.1002/art.37708. [DOI] [PubMed] [Google Scholar]
- 69.Lee HY, YJ1, Wallace GR, Choi YS, Park JA, Choi JY, Song R, Kang YM, Kang SW, Baek HJ, Kitaichi N, Meguro A, Mizuki N, Namba K, Ishida S, Kim J, Niemczyk E, Lee EY, Song YW, Ohno S, Lee EB. Genome-wide association study identifies GIMAP as a novel susceptibility locus for Behcet's disease. Ann Rheum Dis. 2013 Sep 1;72:1510–1516. doi: 10.1136/annrheumdis-2011-200288. [DOI] [PubMed] [Google Scholar]
- 70.Kirino BG, Y1, Ishigatsubo Y, Mizuki N, Tugal-Tutkun I, Seyahi E, Ozyazgan Y, Sacli FS, Erer B, Inoko H, Emrence Z, Cakar A, Abaci N, Ustek D, Satorius C, Ueda A, Takeno M, Kim Y, Wood GM, Ombrello MJ, Meguro A, Gül A, Remmers EF, Kastner DL. Genome-wide association analysis identifies new susceptibility loci for Behçet's disease and epistasis between HLA-B*51 and ERAP1. Nat Genet. 2013 Feb;45:202–207. doi: 10.1038/ng.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kullo IJ, Fan J, Pathak J, Savova GK, Ali Z, Chute CG. Leveraging informatics for genetic studies: use of the electronic medical record to enable a genome-wide association study of peripheral arterial disease. J Am Med Inform Assoc. 2010;17:568–574. doi: 10.1136/jamia.2010.004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCarty CA, Chisholm RL, Chute CG, Kullo IJ, Jarvik G, Larson EB, Li R, Masys DR, Ritchie MD, Roden DM, Struewing JP, Wolf WA, the eMERGE team The eMERGE Network: A consortium of biorepositories linked to electronic medical records data for conducting genomic studies. BMC Med Genom. 2001;4:13. doi: 10.1186/1755-8794-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kho AN, Pacheco JA, Peissig PL, Rasmussen L, Newton KM, Weston N, Crane PK, Pathak J, Chute CG, Bielinski SJ, Kullo IJ, Li R, Manolio TA, Chisholm RL, Denny JC. Electronic medical records for genetic research: results of the eMERGE consortium. Sci Transl Med. 2011;3:79re71. doi: 10.1126/scitranslmed.3001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Q, Smith CY, Bailey KR, Wennberg PW, Kullo IJ. Disease Location Is Associated With Survival in Patients With Peripheral Arterial Disease. J Am Heart Assoc. 2013:2. doi: 10.1161/JAHA.113.000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lusis AJ, Mar R, Pajukanta P. Genetics of atherosclerosis. Ann Rev Genomics Hum Genet. 2004;5:189–218. doi: 10.1146/annurev.genom.5.061903.175930. [DOI] [PubMed] [Google Scholar]
- 76.Nelson MR, Kardia SL, Ferrell RE, Sing CF. A combinatorial partitioning method to identify multilocus genotypic partitions that predict quantitative trait variation. Genome Res. 2001;11:458–470. doi: 10.1101/gr.172901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.St Hilaire C, Ziegler SG, Markello TC, Brusco A, Groden C, Gill F, Carlson-Donohoe H, Lederman RJ, Chen MY, Yang D, Siegenthaler MP, Arduino C, Mancini C, Freudenthal B, Stanescu HC, Zdebik AA, Chaganti RK, Nussbaum RL, Kleta R, Gahl WA, Boehm M. NT5E mutations and arterial calcifications. N Engl J Med. 2011;364:432–442. doi: 10.1056/NEJMoa0912923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shameer K, Klee EW, Dalenberg AK, Kullo IJ. Whole Exome Sequencing Implicates an INO80D Mutation in a Syndrome of Aortic Hypoplasia, Premature Atherosclerosis, and Arterial Stiffness. Circ Cardiovasc Genet. 2014:xx–xx. doi: 10.1161/CIRCGENETICS.113.000233. [DOI] [PubMed] [Google Scholar]
- 79.Morrison AJ, Shen X. Chromatin remodelling beyond transcription: the INO80 and SWR1 complexes. Nat Rev Mole Cell Biol. 2009;10:373–384. doi: 10.1038/nrm2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whitney AR, Diehn M, Popper SJ, Alizadeh AA, Boldrick JC, Relman DA, Brown PO. Individuality and variation in gene expression patterns in human blood. Proc Natl Acad Sci U S A. 2003;100:1896–1901. doi: 10.1073/pnas.252784499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eady JJ, Wortley GM, Wormstone YM, Hughes JC, Astley SB, Foxall RJ, Doleman JF, Elliott RM. Variation in gene expression profiles of peripheral blood mononuclear cells from healthy volunteers. Physiol Genomics. 2005;22:402–411. doi: 10.1152/physiolgenomics.00080.2005. [DOI] [PubMed] [Google Scholar]
- 82.Masud R, Shameer K, Dhar A, Ding K, Kullo IJ. Gene expression profiling of peripheral blood mononuclear cells in the setting of peripheral arterial disease. J Clin Bioinforma. 2012;2:6. doi: 10.1186/2043-9113-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valentine RJ, Verstraete R, Clagett GP, Cohen JC. Premature cardiovascular disease is common in relatives of patients with premature peripheral atherosclerosis. Archives of Internal Medicine. 2000;160:1343–1348. doi: 10.1001/archinte.160.9.1343. [DOI] [PubMed] [Google Scholar]
- 84.Gschwendtner A, Bevan S, Cole JW, Plourde A, Matarin M, Ross-Adams H, Meitinger T, Wichmann E, Mitchell BD, Furie K, Slowik A, Rich SS, Syme PD, MacLeod MJ, Meschia JF, Rosand J, Kittner SJ, Markus HS, Muller-Myhsok B, Dichgans M. Sequence variants on chromosome 9p21.3 confer risk for atherosclerotic stroke. Ann Neurol. 2009;65:531–539. doi: 10.1002/ana.21590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tragante V, Doevendans PA, Nathoe HM, van der Graaf Y, Spiering W, Algra A, de Borst GJ, de Bakker PI, Asselbergs FW. The impact of susceptibility loci for coronary artery disease on other vascular domains and recurrence risk. Eur Heart J. 2013;34:2896–2904. doi: 10.1093/eurheartj/eht222. [DOI] [PubMed] [Google Scholar]
- 86.Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, Thorleifsson G, Helgadottir H, Steinthorsdottir V, Stefansson H, Williams C, Hui J, Beilby J, Warrington NM, James A, Palmer LJ, Koppelman GH, Heinzmann A, Krueger M, Boezen HM, Wheatley A, Altmuller J, Shin HD, Uh ST, Cheong HS, Jonsdottir B, Gislason D, Park CS, Rasmussen LM, Porsbjerg C, Hansen JW, Backer V, Werge T, Janson C, Jonsson UB, Ng MC, Chan J, So WY, Ma R, Shah SH, Granger CB, Quyyumi AA, Levey AI, Vaccarino V, Reilly MP, Rader DJ, Williams MJ, van Rij AM, Jones GT, Trabetti E, Malerba G, Pignatti PF, Boner A, Pescollderungg L, Girelli D, Olivieri O, Martinelli N, Ludviksson BR, Ludviksdottir D, Eyjolfsson GI, Arnar D, Thorgeirsson G, Deichmann K, Thompson PJ, Wjst M, Hall IP, Postma DS, Gislason T, Gulcher J, Kong A, Jonsdottir I, Thorsteinsdottir U, Stefansson K. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41:342–347. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 87.Gieger C, Radhakrishnan A, Cvejic A, Tang W, Porcu E, Pistis G, Serbanovic-Canic J, Elling U, Goodall AH, Labrune Y, Lopez LM, Magi R, Meacham S, Okada Y, Pirastu N, Sorice R, Teumer A, Voss K, Zhang W, Ramirez-Solis R, Bis JC, Ellinghaus D, Gogele M, Hottenga JJ, Langenberg C, Kovacs P, O'Reilly PF, Shin SY, Esko T, Hartiala J, Kanoni S, Murgia F, Parsa A, Stephens J, van der Harst P, Ellen van der Schoot C, Allayee H, Attwood A, Balkau B, Bastardot F, Basu S, Baumeister SE, Biino G, Bomba L, Bonnefond A, Cambien F, Chambers JC, Cucca F, D'Adamo P, Davies G, de Boer RA, de Geus EJ, Doring A, Elliott P, Erdmann J, Evans DM, Falchi M, Feng W, Folsom AR, Frazer IH, Gibson QD, Glazer NL, Hammond C, Hartikainen AL, Heckbert SR, Hengstenberg C, Hersch M, Illig T, Loos RJ, Jolley J, Khaw KT, Kuhnel B, Kyrtsonis MC, Lagou V, Lloyd-Jones H, Lumley T, Mangino M, Maschio A, Mateo Leach I, McKnight B, Memari Y, Mitchell BD, Montgomery GW, Nakamura Y, Nauck M, Navis G, Nothlings U, Nolte IM, Porteous DJ, Pouta A, Pramstaller PP, Pullat J, Ring SM, Rotter JI, Ruggiero D, Ruokonen A, Sala C, Samani NJ, Sambrook J, Schlessinger D, Schreiber S, Schunkert H, Scott J, Smith NL, Snieder H, Starr JM, Stumvoll M, Takahashi A, Tang WH, Taylor K, Tenesa A, Lay Thein S, Tonjes A, Uda M, Ulivi S, van Veldhuisen DJ, Visscher PM, Volker U, Wichmann HE, Wiggins KL, Willemsen G, Yang TP, Hua Zhao J, Zitting P, Bradley JR, Dedoussis GV, Gasparini P, Hazen SL, Metspalu A, Pirastu M, Shuldiner AR, Joost van Pelt L, Zwaginga JJ, Boomsma DI, Deary IJ, Franke A, Froguel P, Ganesh SK, Jarvelin MR, Martin NG, Meisinger C, Psaty BM, Spector TD, Wareham NJ, Akkerman JW, Ciullo M, Deloukas P, Greinacher A, Jupe S, Kamatani N, Khadake J, Kooner JS, Penninger J, Prokopenko I, Stemple D, Toniolo D, Wernisch L, Sanna S, Hicks AA, Rendon A, Ferreira MA, Ouwehand WH, Soranzo N. New gene functions in megakaryopoiesis and platelet formation. Nature. 2011;480:201–208. doi: 10.1038/nature10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shameer K, Denny JC, Ding K, Jouni H, Crosslin DR, de Andrade M, Chute CG, Peissig P, Pacheco JA, Li R, Bastarache L, Kho AN, Ritchie MD, Masys DR, Chisholm RL, Larson EB, McCarty CA, Roden DM, Jarvik GP, Kullo IJ. A genome- and phenome-wide association study to identify genetic variants influencing platelet count and volume and their pleiotropic effects. Hum Genet. 2013 doi: 10.1007/s00439-013-1355-7. (doi: 10.1007/s00439-013-1355-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, Morahan G, Nerup J, Nierras C, Plagnol V, Pociot F, Schuilenburg H, Smyth DJ, Stevens H, Todd JA, Walker NM, Rich SS. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takizawa H, Nishimura S, Takayama N, Oda A, Nishikii H, Morita Y, Kakinuma S, Yamazaki S, Okamura S, Tamura N, Goto S, Sawaguchi A, Manabe I, Takatsu K, Nakauchi H, Takaki S, Eto K. Lnk regulates integrin alphaIIbbeta3 outside-in signaling in mouse platelets, leading to stabilization of thrombus development in vivo. J Clin Invest. 2010;120:179–190. doi: 10.1172/JCI39503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Handy DE, Castro R, Loscalzo J. Epigenetic modifications: basic mechanisms and role in cardiovascular disease. Circulation. 2011;123:2145–2156. doi: 10.1161/CIRCULATIONAHA.110.956839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Breitling LP. Current genetics and epigenetics of smoking/tobacco-related cardiovascular disease. Arterioscler Thromb Vasc Biol. 2013;33:1468–1472. doi: 10.1161/ATVBAHA.112.300157. [DOI] [PubMed] [Google Scholar]
- 93.Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- 94.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 95.Peschansky VJ, Wahlestedt C. Non-coding RNAs as direct and indirect modulators of epigenetic regulation. Epigenetics. 2014;9:3–12. doi: 10.4161/epi.27473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aryal B, Rotllan N, Fernandez-Hernando C. Noncoding RNAs and atherosclerosis. Curr Atheroscler Rep. 2014;16:407. doi: 10.1007/s11883-014-0407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hamburg NM, Leeper NJ. Therapeutic Potential of Modulating MicroRNA in Peripheral Artery Disease. Curr Vasc Pharmacol. 2013 doi: 10.2174/15701611113119990014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Caporali A, Meloni M, Vollenkle C, Bonci D, Sala-Newby GB, Addis R, Spinetti G, Losa S, Masson R, Baker AH, Agami R, le Sage C, Condorelli G, Madeddu P, Martelli F, Emanueli C. Deregulation of microRNA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Circulation. 2011;123:282–291. doi: 10.1161/CIRCULATIONAHA.110.952325. [DOI] [PubMed] [Google Scholar]
- 99.Leeper NJ, Cooke JP. MicroRNA and mechanisms of impaired angiogenesis in diabetes mellitus. Circulation. 2011;123:236–238. doi: 10.1161/CIRCULATIONAHA.110.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stather PW, Sylvius N, Wild JB, Choke E, Sayers RD, Bown MJ. Differential microRNA expression profiles in peripheral arterial disease. Circ Cardiovasc Genet. 2013;6:490–497. doi: 10.1161/circgenetics.111.000053. [DOI] [PubMed] [Google Scholar]
- 101.Kotake Y, Nakagawa T, Kitagawa K, Suzuki S, Liu N, Kitagawa M, Xiong Y. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene. 2011;30:1956–1962. doi: 10.1038/onc.2010.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leeper NJ, Raiesdana A, Kojima Y, Kundu RK, Cheng H, Maegdefessel L, Toh R, Ahn GO, Ali ZA, Anderson DR, Miller CL, Roberts SC, Spin JM, de Almeida PE, Wu JC, Xu B, Cheng K, Quertermous M, Kundu S, Kortekaas KE, Berzin E, Downing KP, Dalman RL, Tsao PS, Schadt EE, Owens GK, Quertermous T. Loss of CDKN2B promotes p53-dependent smooth muscle cell apoptosis and aneurysm formation. Arterioscler Thromb Vasc Biol. 2013;33:e1–e10. doi: 10.1161/ATVBAHA.112.300399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, McCarroll SA, Visscher PM. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cohen JC, Boerwinkle E, Mosley TH, Jr., Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 105.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huyghe JR, Jackson AU, Fogarty MP, Buchkovich ML, Stancakova A, Stringham HM, Sim X, Yang L, Fuchsberger C, Cederberg H, Chines PS, Teslovich TM, Romm JM, Ling H, McMullen I, Ingersoll R, Pugh EW, Doheny KF, Neale BM, Daly MJ, Kuusisto J, Scott LJ, Kang HM, Collins FS, Abecasis GR, Watanabe RM, Boehnke M, Laakso M, Mohlke KL. Exome array analysis identifies new loci and low-frequency variants influencing insulin processing and secretion. Nat Genet. 2013;45:197–201. doi: 10.1038/ng.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu S, Lusis AJ, Drake TA. A systems-based framework for understanding complex metabolic and cardiovascular disorders. J. Lipid Res. 2009;50(Suppl):S358–363. doi: 10.1194/jlr.R800067-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic acids research. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Croft D, O'Kelly G, Wu G, Haw R, Gillespie M, Matthews L, Caudy M, Garapati P, Gopinath G, Jassal B. Reactome: a database of reactions, pathways and biological processes. Nucleic acids research. 2010 doi: 10.1093/nar/gkq1018. gkq1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koren O, Spor A, Felin J, Fak F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, Backhed F. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Nat Acad Scien of the United States of America. 2011;108(Suppl 1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.