Abstract
Objective:
To examine vertical transmission rates of Cytomegalovirus, Toxoplasma Gondii and Rubella infections according to amniotic fluid PCR analysis.
Methods:
A retrospective analysis of mid-trimester amniocenteses performed in in pregnancies with diagnosed maternal infection by Cytomegavirus (CMV), Rubella or Toxoplasma gondii during 1994-2008 was performed. Vertical transmission rates were observed according to the presence of the infectious agent's DNA in the amniotic fluid. A univariate regression model was also performed to investigate possible correlations between transmission and epidemiological parameters.
Results:
Overall, 7033 amniocenteses were performed during study's period, of which 166 (2.4%) with the indication of maternal infection by CMV, Rubella or Toxoplasma. Mean maternal age was 27.4 ± 2.5 years and the mean gestational age at amniocentesis was 18.7 ± 2.5 weeks. Vertical transmission was observed in 21 cases (12.7%). Transmission rate was 17.3% in cases with infection from CMV, 9.5% from Toxoplasma gondii and 7.8% from Rubella (P = .05). Maternal age was the only parameter being significantly associated with increased risk for vertical transmission (P = .04).
Conclusions:
According to our results, overall vertical transmission rate marginally exceeds 10%. CMV infection is characterized by relatively higher transplacental transmission rate, while increased maternal age appears to be associated with a higher risk for vertical transmission
Keywords: Amniocentesis, cytomegalovirus, infection, rubella, toxoplasma, vertical transmission
INTRODUCTION
Perinatal infection is the cause of 2–3% of overall congenital anomalies. TORCH is an acronym that encompasses the most common perinatal infections including Toxoplasmosis, Other (syphilis, parvovirus B19, varicella-zoster), Rubella, Cytomegalovirus (CMV) and Herpes.[1] Although these infections are considered to have minor clinical impact on mother in the majority of cases, they may cause serious consequences for fetus, increasing perinatal morbidity, and even mortality. Severity of neonatal disease is mostly correlated with gestational age at which maternal infection occurs as well as with former maternal immune status. Prompt treatment has been shown to improve neonatal outcomes even if vertical transmission is confirmed.[2,3,4,5] Therefore, early diagnosis of such cases is critical in an effort to reduce the possibility of a seriously affected child.
Serum screening for specific IgM and IgG antibodies in the maternal blood is the first step in the diagnostic approach. Indeed, the presence of IgM antibodies is associated with acute infection. However, as IgM antibodies may persist for months or even years after the infection, this examination may lead to false-positive results. Nevertheless, anxiety that such a result causes to parents may often lead to unnecessary pregnancy termination.[2,6,7,8] According to reports, 1 out of 5 women proceed in abortion just after the announcement of a positive IgM test for Toxoplasma gondii.[9,10] IgG avidity test may have a higher specificity in the detection of acute infection; however, it still cannot identify a neonatal infection.[6,8,11]
Transplacental transmission of maternal infection to the fetus may be confirmed by amniocentesis. Polymerase chain reaction (PCR) analysis of the amniotic fluid is characterized by high sensitivity and specificity regarding the detection of an affected embryo.[5,8,12] Indeed, Bessières et al. demonstrated a 91% sensitivity and 99.5% specificity of PCR analysis of the amniotic fluid for the diagnosis of congenital toxoplasmosis, therefore demonstrating the diagnostic supremacy of this method in the detection of transplacental transmission.[4]
Main objective of the present study was to record transmission rates of CMV, T. gondii and rubella, as confirmed by second trimester amniocentesis. In addition, their potential correlation with epidemiological parameters as well as outcome of affected cases was also examined.
METHODS
In Greece, all pregnant women are offered screening for CMV, rubella and toxoplasmosis infection in the first trimester of pregnancy. In cases where a recent infection is suspected, a mid-trimester amniocentesis is offered followed by real-time PCR analysis for infectious agent's DNA. This was a retrospective study of all amniocenteses conducted in a single institution, following the diagnosis of a first trimester maternal infection by CMV, rubella and toxoplasmosis. All cases with the former infections were diagnosed by positive maternal serum specific IgM antibodies during the first trimester of pregnancy. Patients were advised not to perform amniocentesis before 20th gestational week regarding CMV and rubella and 18th gestational week regarding T. gondii because of the increased possibility of false-negative results. However, in case of maternal request and given the relative small interval until the limit of 24th gestational week for pregnancy termination according to Greek legislation, amniocentesis could also be performed earlier than suggested. Data were collected from the medical records of all the patients during the period 1994–2008. Institutional Review Board approved the present study.
Epidemiological characteristics available for analysis included maternal age, parity, and ethnicity. Specifically, we estimated mean values of maternal age and parity, as well as rates of women aged ≥35 years old, nulliparous and natives. Placenta location and gestational age at the amniocentesis procedure were also recorded. All the aforementioned parameters were studied for the total of patients as well as for each specific infection independently.
The main objective of our analysis was to define the rate of vertical transplacental transmission in cases where specific IgM antibodies were detected in the maternal serum during the first trimester of the present pregnancy. Transmission was confirmed by real-time PCR analysis of the amniotic fluid aspirated by amniocentesis. Vertical transmission rate was estimated for the total of cases while a sub-analysis was also made for the three different studied maternal infections. In addition, a univariate regression analysis was used in order to correlate vertical transmission confirmed by second trimester amniocentesis with epidemiological characteristics. The amniocentesis procedure was conducted as described thoroughly in a previous report.[13]
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Science version 17.0 (SPSS Inc., Chicago, IL, USA). Independent samples t-test was used for continuous data to assess mean values and standard deviations. Fisher's exact test analysis was used to compare cross-tabulated variables. Univariate logistic regression model (relative risk; 95% confidence interval) was used to correlate the vertical transmission rate of the different infections of the study with epidemiological parameters. Differences between groups were considered statistically significant at P < 0.05. All P values were two-sided.
RESULTS
There were overall 7033 amniocenteses performed during the period of the study, of which 166 (2.4%) were performed with the indication of maternal infection by CMV, rubella, and toxoplasmosis. Mean maternal age of the women was 27.4 ± 4.9 years and 13 (7.8%) were older than 35 years old. Mean parity was 1.5, while 110 (66.3%) women were nulliparous. Mean gestational week at the time of the amniocentesis was 18.7 ± 2.5 weeks. Placenta location was mainly anterior (54.2%). There were 2 of 166 cases that involved twin pregnancies (1.2%). No significant difference was observed in terms of maternal age, parity, placenta location and gestational age at amniocentesis between the different groups. However, a significantly higher percentage of native Greek women were infected by CMV in comparison to the other infections. No abnormal ultrasound markers were detected in any case with vertical transmission while no signs of intrauterine growth restriction were also detected. Comparison of women's epidemiological characteristics according to the different types of maternal infection is presented in Table 1. Overview of amniocenteses performed because of maternal infection is presented in Figure 1.
Table 1.
Epidemiological characteristics of women performing amniocentesis according to the different types of maternal infection
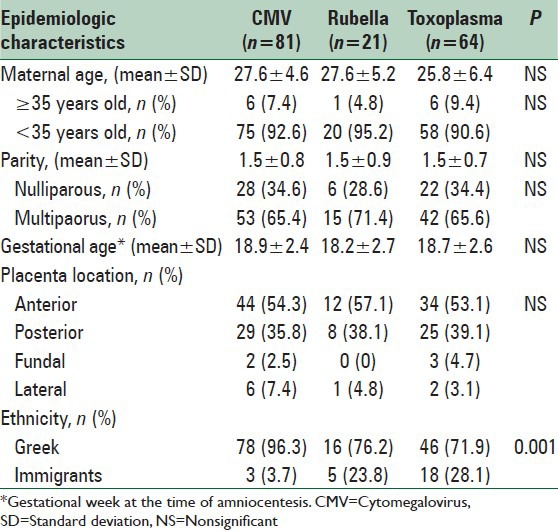
Figure 1.
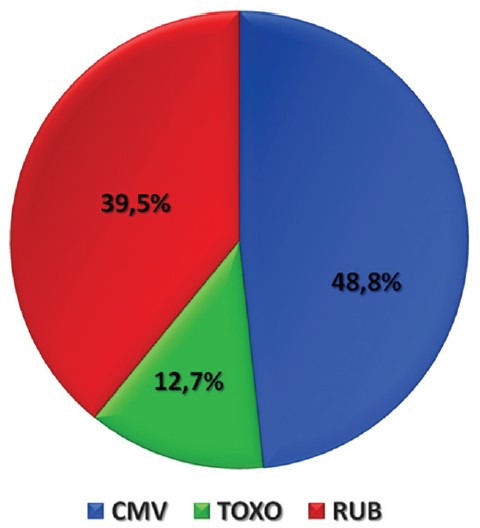
Overview of amniocenteses performed with the indication of maternal infection during 1994–2008
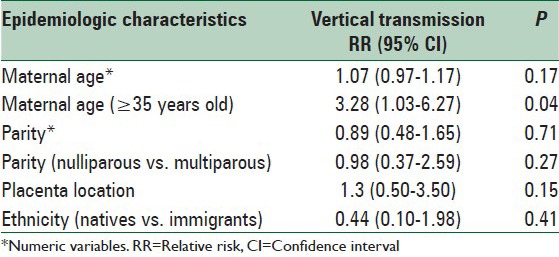
Vertical transmission was confirmed in 21 cases out of 166 cases (12.7%), all of which were singleton pregnancies. According to the type of infection, transmission rate was 17.3% for cases affected by CMV (n = 14/81), 9.5% for T. gondii (n = 2/21) and 7.8% for rubella (n = 5/64), the difference being marginally significantly different (P = 0.05). When correlating the possibility of vertical transmission with epidemiological aspects, maternal age ≥35 years old was the only parameter found to significantly increase risk for transmission. Thus, vertical transmission was 30.4% in women ≥35 years old while 11.9% for women <35 years old (P = 0.04). Univariate regression model between vertical transmission and epidemiological aspects is presented in Table 2.
Table 2.
Univariate logistic regression model correlating vertical transmission with the epidemiological characteristics
DISCUSSION
The present study showed that transplacental transmission of CMV, rubella and toxoplasmosis was confirmed in 12.7% of all cases examined. More specifically, the vertical transmission rate was 17.3% for CMV, 4.7% for T. gondii and 9.5% for rubella.
Cytomegalovirus remains the virus most commonly responsible for congenital infection in the developed world. Transplacental transmission following primary viral infection approaches the rate of 40% while in case of recurrent infection this may not exceed 1%.[2,14] Overall transmission rate in the present study was estimated to be 17.3%. Rahav et al., demonstrated a vertical transmission of CMV in about 30% of mothers infected by CMV during pregnancy.[15]
Cytomegalovirus infection during pregnancy does not necessarily prove infant damage, even when vertical transmission is confirmed. According to literature, only 10–15% of newborns affected by the virus at the intrauterine period will develop mild to serious complications. The rest of embryos will be delivered free of symptoms, and only 10% of them are expected to suffer neurodevelopmental defects later in life, such as deafness and earing problems necessitating early earing survey.[16,17] However, abortion rate is relatively high in pregnancies complicated by CMV infection.[15] Finally, regarding therapy, there is no vaccine or another prophylactic measure available in order to prevent such an infection. Nevertheless, Nigro et al. report that fetal damage may be limited in case anti-CMV monoclonal antibodies are infused into an infected pregnant woman.[18]
Infection with the protozoon T. gondii is a widely-spread disease, affecting 1 out of 3 persons worldwide. The gestational week of maternal infection is proportional to the transplacental transmission but inversely proportional to the severity of fetal disease.[3] In our analysis, vertical transmission of the protozoan was confirmed in 9.5% of the infected mothers after second trimester amniocentesis. Relatively, Yamada et al. detected T. gondii DNA in the amniotic fluid in 17.6% of infected pregnant women.[8,19,20]
There is no agreement in the literature concerning prevention of T. gondii transmission. However, it is suggested that infected pregnant women should receive treatment (1st trimester infection: Spiramycin, 2nd trimester virus-PCR positive on amniotic fluid: Stop spiramycin then treatment by pyrimethamin and sulfadoxin or pyrimethamin and sulfadiazine.[4,5] Although congenital infection may lead to severe lesions, as already mentioned, the vast majority of infected fetuses are born free of symptoms. Characteristically, Villena et al., analyzing a population of live-born infants that were characterized by intrauterine infection, reported that 87% of them were asymptomic. In addition, of the remaining 13% in the same study, only 3% suffered from severe disease.[5] Therefore, also in case of toxoplasmosis, proof of vertical transmission may not necessarily confirm fetal damage.
Risk for congenital rubella syndrome increases when infection occurs in early stages of pregnancy. Specifically, the percentage of infants with congenital malformation exceeds 50% in cases of infection during the first trimester of pregnancy while the relative percentage is significantly reduced after the 20th gestational week.[20,21,22,23,24] However, maternal viremia is not a proof of vertical transmission to the embryo, and fetal infection does not necessarily correspond to fetal damage. Namaei et al. reported that none of the infants of gravidas that received a measles-rubella vaccine at the interval between 3 months before and 3 months after conception appeared to experience viral consequences. This may be the reason why authors support detailed information provided to the mother in order to prevent unnecessary voluntary interruption of pregnancies.[21,25]
Prenatal diagnostic exploration should be recommended in case of positive PCR in order to detect the affected embryos, having more precise information concerning the fetal health status before the decision of pregnancy's termination.[2,6,12,26] Studies have demonstrated an association between viral load in amniotic fluid and the risk of a symptomatic infant, whereas further investigation with a noninvasive diagnostic procedure might postulate a method to evaluate potential fetal affection.[26,27,28,29]
The main limitation of the present study is the nonavailability of follow-up of cases in which vertical transmission was diagnosed. However, our center is a private diagnostic center where pregnancies are referred for prenatal diagnosis and are afterward treated by private physicians or hospitals. In addition, amniocenteses were also in certain cases performed earlier than recommended, potentially increasing the possibility of false-negative results. However, the merit of the study was to elucidate on the vertical transmission rates in cases of maternal infection by CMV, rubella, and T. gondii. To the best of our knowledge, this study is one amongst only a few presenting a relatively large series of amniocenteses performed with the indication of maternal infection. Besides, our observation that maternal age may be associated with vertical transmission possibility is an interesting outcome necessitating further research.
CONCLUSIONS
Cytomegavirus infection is characterized by significantly increased transmission rates than rubella and T. gondii. However, vertical transmission rate was observed to be lower than 20% for cytomegavirus and then 10% for rubella and T. gondii. Pregnancy termination should not be the initial approach before confirming vertical transmission and reporting to parents the evidence-based possibility of severe congenital disease so that they may make a justified decision regarding their affected fetus.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Stegmann BJ, Carey JC. TORCH Infections. Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes infections. Curr Womens Health Rep. 2002;2:253–8. [PubMed] [Google Scholar]
- 2.Goegebuer T, Van Meensel B, Beuselinck K, Cossey V, Van Ranst M, Hanssens M, et al. Clinical predictive value of real-time PCR quantification of human cytomegalovirus DNA in amniotic fluid samples. J Clin Microbiol. 2009;47:660–5. doi: 10.1128/JCM.01576-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bessières MH, Berrebi A, Cassaing S, Fillaux J, Cambus JP, Berry A, et al. Diagnosis of congenital toxoplasmosis: Prenatal and neonatal evaluation of methods used in Toulouse University Hospital and incidence of congenital toxoplasmosis. Mem Inst Oswaldo Cruz. 2009;104:389–92. doi: 10.1590/s0074-02762009000200038. [DOI] [PubMed] [Google Scholar]
- 4.Gras L, Wallon M, Pollak A, Cortina-Borja M, Evengard B, Hayde M, et al. Association between prenatal treatment and clinical manifestations of congenital toxoplasmosis in infancy: A cohort study in 13 European centres. Acta Paediatr. 2005;94:1721–31. doi: 10.1111/j.1651-2227.2005.tb01844.x. [DOI] [PubMed] [Google Scholar]
- 5.Villena I, Ancelle T, Delmas C, Garcia P, Brezin AP, Thulliez P, et al. Congenital toxoplasmosis in France in 2007: First results from a national surveillance system. Euro Surveill. 2010;15 doi: 10.2807/ese.15.25.19600-en. pii: 19600. [DOI] [PubMed] [Google Scholar]
- 6.Schlesinger Y. Routine screening for CMV in pregnancy: Opening the Pandora box? Isr Med Assoc J. 2007;9:395–7. [PubMed] [Google Scholar]
- 7.Bobic B, Sibalic D, Djurkovic-Djakovic O. High levels of IgM antibodies specific for Toxoplasma gondii in pregnancy 12 years after primary toxoplasma infection. Case report. Gynecol Obstet Invest. 1991;31:182–4. doi: 10.1159/000293151. [DOI] [PubMed] [Google Scholar]
- 8.Yamada H, Nishikawa A, Yamamoto T, Mizue Y, Yamada T, Morizane M, et al. Prospective study of congenital toxoplasmosis screening with use of IgG avidity and multiplex nested PCR methods. J Clin Microbiol. 2011;49:2552–6. doi: 10.1128/JCM.02092-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Candolfi E, Ramirez R, Hadju MP, Shubert C, Remington JS. The Vitek immunodiagnostic assay for detection of immunoglobulin M toxoplasma antibodies. Clin Diagn Lab Immunol. 1994;1:401–5. doi: 10.1128/cdli.1.4.401-405.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liesenfeld O, Press C, Montoya JG, Gill R, Isaac-Renton JL, Hedman K, et al. False-positive results in immunoglobulin M (IgM) toxoplasma antibody tests and importance of confirmatory testing: The Platelia Toxo IgM test. J Clin Microbiol. 1997;35:174–8. doi: 10.1128/jcm.35.1.174-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedman K, Lappalainen M, Seppäiä I, Mäkelä O. Recent primary toxoplasma infection indicated by a low avidity of specific IgG. J Infect Dis. 1989;159:736–40. doi: 10.1093/infdis/159.4.736. [DOI] [PubMed] [Google Scholar]
- 12.Revello MG, Gerna G. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin Microbiol Rev. 2002;15:680–715. doi: 10.1128/CMR.15.4.680-715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margioula-Siarkou C, Karkanaki A, Kalogiannidis I, Petousis S, Dagklis T, Mavromatidis G, et al. Operator experience reduces the risk of second trimester amniocentesis-related adverse outcomes. Eur J Obstet Gynecol Reprod Biol. 2013;169:230–3. doi: 10.1016/j.ejogrb.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Lazzarotto T, Lanari M. Why is cytomegalovirus the most frequent cause of congenital infection? Expert Rev Anti Infect Ther. 2011;9:841–3. doi: 10.1586/eri.11.109. [DOI] [PubMed] [Google Scholar]
- 15.Rahav G, Gabbay R, Ornoy A, Shechtman S, Arnon J, Diav-Citrin O. Primary versus nonprimary cytomegalovirus infection during pregnancy, Israel. Emerg Infect Dis. 2007;13:1791–3. doi: 10.3201/eid1311.061289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaytant MA, Steegers EA, Semmekrot BA, Merkus HM, Galama JM. Congenital cytomegalovirus infection: Review of the epidemiology and outcome. Obstet Gynecol Surv. 2002;57:245–56. doi: 10.1097/00006254-200204000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Gaytant MA, Rours GI, Steegers EA, Galama JM, Semmekrot BA. Congenital cytomegalovirus infection after recurrent infection: Case reports and review of the literature. Eur J Pediatr. 2003;162:248–53. doi: 10.1007/s00431-002-1115-5. [DOI] [PubMed] [Google Scholar]
- 18.Nigro G, Adler SP, La Torre R, Best AM Congenital Cytomegalovirus Collaborating Group. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350–62. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- 19.Cook AJ, Gilbert RE, Buffolano W, Zufferey J, Petersen E, Jenum PA, et al. Sources of toxoplasma infection in pregnant women: European multicentre case-control study. European Research Network on Congenital Toxoplasmosis. BMJ. 2000;321:142–7. doi: 10.1136/bmj.321.7254.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varella IS, Canti IC, Santos BR, Coppini AZ, Argondizzo LC, Tonin C, et al. Prevalence of acute toxoplasmosis infection among 41,112 pregnant women and the mother-to-child transmission rate in a public hospital in South Brazil. Mem Inst Oswaldo Cruz. 2009;104:383–8. doi: 10.1590/s0074-02762009000200037. [DOI] [PubMed] [Google Scholar]
- 21.De Santis M, Cavaliere AF, Straface G, Caruso A. Rubella infection in pregnancy. Reprod Toxicol. 2006;21:390–8. doi: 10.1016/j.reprotox.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Peckham C. Congenital rubella in the United Kingdom before 1970: The prevaccine era. Rev Infect Dis. 1985;7(Suppl 1):S11–6. doi: 10.1093/clinids/7.supplement_1.s11. [DOI] [PubMed] [Google Scholar]
- 23.Ghidini A, Lynch L. Prenatal diagnosis and significance of fetal infections. West J Med. 1993;159:366–73. [PMC free article] [PubMed] [Google Scholar]
- 24.Turbadkar D, Mathur M, Rele M. Seroprevalence of torch infection in bad obstetric history. Indian J Med Microbiol. 2003;21:108–10. [PubMed] [Google Scholar]
- 25.Namaei MH, Ziaee M, Naseh N. Congenital rubella syndrome in infants of women vaccinated during or just before pregnancy with measles-rubella vaccine. Indian J Med Res. 2008;127:551–4. [PubMed] [Google Scholar]
- 26.Guerra B, Lazzarotto T, Quarta S, Lanari M, Bovicelli L, Nicolosi A, et al. Prenatal diagnosis of symptomatic congenital cytomegalovirus infection. Am J Obstet Gynecol. 2000;183:476–82. doi: 10.1067/mob.2000.106347. [DOI] [PubMed] [Google Scholar]
- 27.Lazzarotto T, Gabrielli L, Foschini MP, Lanari M, Guerra B, Eusebi V, et al. Congenital cytomegalovirus infection in twin pregnancies: Viral load in the amniotic fluid and pregnancy outcome. Pediatrics. 2003;112:e153–7. doi: 10.1542/peds.112.2.e153. [DOI] [PubMed] [Google Scholar]
- 28.Gouarin S, Gault E, Vabret A, Cointe D, Rozenberg F, Grangeot-Keros L, et al. Real-time PCR quantification of human cytomegalovirus DNA in amniotic fluid samples from mothers with primary infection. J Clin Microbiol. 2002;40:1767–72. doi: 10.1128/JCM.40.5.1767-1772.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guerina NG. Congenital infection with Toxoplasma gondii. Pediatr Ann. 1994;23:138. doi: 10.3928/0090-4481-19940301-07. [DOI] [PubMed] [Google Scholar]


