Dear Editor:
In Asia, an estimated 4.8 million people were living with HIV in 2012.1 Women accounted for a growing proportion of HIV infections, increasing from 21% in 1990 to 35% in 2009. Gender-based analysis in clinical and operational research is still considered understudied. Better understanding of the differences in clinical management, care delivery, and outcomes between males and females in this region may enhance individualization of treatment and improve efficacy and safety outcomes of highly active antiretroviral therapy (HAART). The study objective was to evaluate the effect of sex on viro-immunological responses to HAART, treatment outcomes, and toxicities in a regional observational cohort in the Asia-Pacific.
Established in 2003, the TREAT Asia HIV Observational Database (TAHOD) is a collaborative observational cohort study involving 21 sites in 12 countries in the Asia-Pacific region. Detailed methods have previously been published.2 Briefly, observational data on a range of demographic variables, clinical outcomes, and laboratory testing results are semi-annually transferred to a common data management center. Ethical approvals are obtained from participating sites, the coordinating center, and the data management and biostatistics center.
Endpoints for this analysis included changes in CD4 count, proportions of patients with undetectable HIV viral load at 96 weeks after HAART initiation, rates of new AIDS or death, loss to follow-up (defined as not seen in clinic for >12 months), treatment change due to toxicity, and frequency of CD4 and HIV viral load monitoring. Stepwise multivariate linear regression and logistic regression models were used to evaluate the independent associations of baseline variables with change in CD4 counts and proportion of patients reaching undetectable HIV viral load (<500 copies/mL) at 96 weeks. Multivariate Cox proportional hazards models were used to determine predictors of new AIDS or death, loss to follow-up, and treatment change due to toxicity. P<0.05 for a two-sided test was considered to be statistically significant. Data management and statistical analyses were performed using SAS for Windows (SAS Institute Inc., Cary, NC), and Stata (StataCorp, STATA 10.1 for Windows, College Station, TX).
A total of 3899 patients were evaluated with a median follow-up of 3.6 years. At HAART initiation, 71% were male and the median age was 35 years. The median CD4 cell count was 113 cells/μL and 47% of patients had a baseline CD4 cell count below 200 cells/μL. The median CD4 cell count at HAART initiation was 108 cells/μL for males and 128 cells/μL for females (p=0.094). Significant gender-related differences were found in the following baseline parameters: median age (35 years in males vs. 34 years in females, p<0.001), reported injecting drug use (8% vs. 2%, p<0.001), CDC category C (45% vs. 34%, p<0.001), HIV viral load (median 4.9 log vs. 4.8 log, p=0.03), positive HBsAg (12% vs. 6%, p<0.001), positive anti-HCV (15% vs. 9%, p<0.001), and presence of anemia (hemoglobin <13 vs. <11 g/dL, or 52% vs. 44%, p<0.001). More females were taking non-nucleoside reverse transcriptase inhibitor-based combination ART at baseline (85% vs. 71%, p<0.001). Frequency of HIV viral load monitoring differed by sex (median days between tests 180 days for males vs. 238 days for females, p<0.001). There was no statistical difference in the frequency of CD4 monitoring (median days between tests 162 days for males vs. 169.5 days for females, p=0.055).
There was no significant difference by gender in CD4 change at 96 weeks (225 vs. 223 cells/μL, p=0.60). However, females were more likely to reach undetectable HIV viral load at 96 weeks after adjustment for other variables (OR 1.7, 95% CI 1.1–2.6, p=0.024). On the other hand, younger age (<25), IDU, and prior use of mono/double ART were less likely to reach undetectable HIV viral load. Table 1 summarizes the factors associated with undetectable HIV viral load.
Table 1.
Factors Associated with Undetectable HIV Viral Load at 96 Weeks After HAART Initiation (n=1359)
| Variable | OR | 95% CI | p Value |
|---|---|---|---|
| Gender | |||
| Male | 1 | ||
| Female | 1.7 | (1.1, 2.6) | 0.024 |
| Age at HAART initiation (year) | |||
| ≤25 | 1 | ||
| 26–35 | 2.0 | (1.2, 3.5) | 0.011 |
| 36–45 | 2.4 | (1.4, 4.3) | 0.002 |
| >45 | 3.1 | (1.6, 6.1) | 0.001 |
| Mode of infection | |||
| Heterosexual contact | 1 | ||
| Homosexual contact | 1.3 | (0.9, 2.1) | 0.165 |
| Injecting drug use | 0.2 | (0.1, 0.5) | <0.001 |
| Blood products | 1.1 | (0.2, 5.0) | 0.926 |
| Other/ unknown | 1.6 | (0.7, 3.6) | 0.283 |
| Prior treatment (mono or double) before HAART initiation | |||
| No | 1 | ||
| Yes | 0.4 | (0.3, 0.6) | <0.001 |
CI, confidence interval; HAART, highly active antiretroviral therapy; OR, odds ratio.
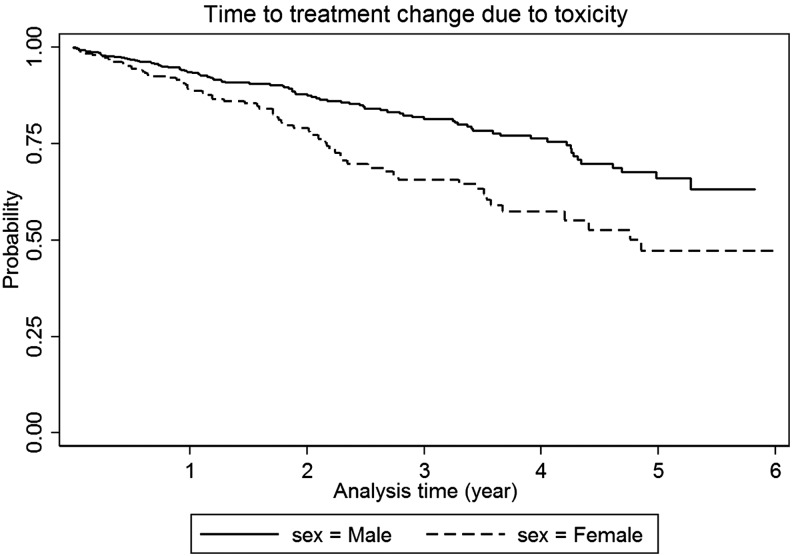
Gender was not a predictor of new AIDS events or death (adjusted HR 0.83, 95% CI 0.62–1.10, p=0.186) or loss to follow-up (adjusted HR 1.04, 95% CI 0.8–1.2, p=0.796). Females were more likely to change treatment regimens due to toxicity (adjusted HR 1.9, 95% CI 1.4–2.7, p<0.001; Fig. 1). Common adverse events leading to treatment change in males were lipodystrophy (30%), skin rash (22%), anemia (13%), peripheral neuropathy (10%), abnormal liver function (2%), and diarrhea (2%); and in females were lipodystrophy (43%), skin rash (24%), anemia (10%), peripheral neuropathy (7%), and lactic acidosis (3%).
FIG. 1.
Time to treatment change due to toxicity by gender.
This multi-center observational cohort study showed that females were more likely to reach undetectable HIV viral load at 96 weeks after HAART initiation. However, there were no gender differences in immunological response, and risk of developing AIDS or death. The frequency of CD4 cell count monitoring was similar, but females received less HIV viral load testing. The difference could be explained by the fact that more females (80%), compared with 57% of males, were from sites in lower middle/low income countries in which viral load monitoring is less frequent or absent.
In our study, Asian males had a higher proportion of CDC category C disease (45% vs. 34%, p<0.001) and lower median CD4 cell counts at baseline (108 vs. 128 cells/μL, p=0.094) when compared with females, although there was no significant difference in the risk of developing AIDS or death. Males in sub-Saharan Africa have been reported to have higher mortality than females, largely due to late presentation at more advanced HIV disease.3,4 Studies in Asian countries also reported difference in health-seeking behavior. Being male has been associated with late access to ART in northern Thailand and China's free ART program.5,6
Consistent with previous studies,7 females were more likely to switch treatment regimens due to toxicity (adjusted HR 1.9, 95% CI 1.4–2.7, p<0.001). A meta-analysis reported that females are more prone to develop treatment-related toxicities.8 Susceptibility to adverse events may differ based on pharmacokinetics.9 The contributing factors and mechanisms for higher drug exposures in females include differences in body weight and composition, renal clearance, and P-glycoprotein activity.10,11
Our study has several limitations. Non-adherence is a primary cause of virological failure, but ARV drug adherence data were not included in the analysis. Information on past ARV exposure for prevention of mother-to-child transmission is not available in the database, which may have affected treatment responses. In addition, socioeconomic factors are important determinants of gender difference in treatment outcomes but they are not routinely collected.
In conclusion, we observed no significant difference by sex in the risk of developing new AIDS events or death within our regional cohort. A higher proportion of females achieved undetectable HIV viral load at 96 weeks after HAART initiation. More males had late disease presentation, while females were more susceptible to treatment-related adverse events. Continued research on gender differences in health-seeking behavior, treatment access and outcomes is warranted.
Acknowledgments
Study funders included the US National Institutes of Health as part of the International Epidemiologic Databases to Evaluate AIDS (NIAID/NICHD/NCI; U01AI069907), the Dutch Ministry of Foreign Affairs through Stichting Aids Fonds, and the Australian Government Department of Health and Ageing. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.United Nation AIDS Programme (UNAIDS): UNAIDS Report on the Global AIDS Epidemic 2013. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf (Last accessed November15, 2014).
- 2.Zhou J, Kumarasamy N, Ditangco R, et al. . The TREAT Asia HIV Observational Database: Baseline and retrospective data. J Acquir Immune Defic Syndr 2005;38:174–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornell M, Myer L, Kaplan R, et al. . The impact of gender and income on survival and retention in a South African antiretroviral therapy programme. Trop Med Intl Health 2009;14:722–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alibhai A, Kipp W, Saunders LD, et al. . Gender-related mortality for HIV-infected patients on highly active antiretroviral therapy (HAART) in rural Uganda. Intl J Women's Health 2010;2:45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Coeur S, Collins IJ, Pannetier J, et al. . Gender and access to HIV testing and antiretroviral treatments in Thailand: Why do women have more and earlier access? Soc Sci Med 2009;69:846–853 [DOI] [PubMed] [Google Scholar]
- 6.Wen Y, Zhao D, Dou Z, et al. . Some patient-related factors associated with late access to ART in China's free ART program. AIDS Care 2011;23:1226–1235 [DOI] [PubMed] [Google Scholar]
- 7.Currier JS, Spino C, Grimes J, et al. . Differences between women and men in adverse events and CD4+ responses to nucleoside analogue therapy for HIV infection. The Aids Clinical Trials Group 175 Team. J Acquir Immune Defic Syndr 2000;24:316–324 [DOI] [PubMed] [Google Scholar]
- 8.Umeh OC, Currier JC. Sex differences in pharmacokinetics and toxicity of antiretroviral therapy. Expert Opin Drug Metab Toxicol 2006;2:273–283 [DOI] [PubMed] [Google Scholar]
- 9.Clark R. Sex differences in antiretroviral therapy-associated intolerance and adverse events. Drug Saf 2005;28:1075–1083 [DOI] [PubMed] [Google Scholar]
- 10.Floridia M, Giuliano M, Palmisano L, et al. . Gender differences in the treatment of HIV infection. Pharmacol Res 2008;58:173–182 [DOI] [PubMed] [Google Scholar]
- 11.Gandhi M, Aweeka F, Greenblatt RM, et al. . Sex differences in pharmacokinetics and pharmacodynamics. Ann Rev Pharmacol Toxicol 2004;44:499–523 [DOI] [PubMed] [Google Scholar]