Abstract
Obesity is characterized by an increase in the infiltration of monocytes into the adipose tissue, causing an inflammatory condition associated with, for example, the development of insulin resistance. Thus, anti-inflammatory-based treatments could emerge as a novel and interesting approach. It has been reported that Chilean native fruits maqui (Aristotelia chilensis) and calafate (Berberis microphylla) present high contents of polyphenols, which are known for their antioxidant and anti-inflammatory properties. The aim of this study was to evaluate the ability of extracts of these fruits to block the pathogenic interaction between adipocytes and macrophages in vitro and to compare its effect with blueberry (Vaccinium corymbosum) extract treatment, which has been already described to possess several biomedical benefits. RAW264.7 macrophages were treated with 5 μg/mL lipopolysaccharides (LPS), with conditioned media (CM) from fully differentiated 3T3-L1 adipocytes, or in a coculture (CC) with 3T3-L1 adipocytes, in the presence or absence of 100 μM [total polyphenolic content] of each extract for 24 h. The gene expression and secretion profile of several inflammatory markers were evaluated. Nitric oxide secretion induced by LPS, CM, and CC was reduced by the presence of maqui (−12.2%, −45.6%, and −14.7%, respectively) and calafate (−27.6%, −43.9%, and −11.8%, respectively) extracts. Gene expression of inducible nitric oxide synthase and TNF-α was inhibited and of IL-10 was induced by maqui and calafate extract incubation. In conclusion, the extracts of these fruits present important inhibitory-like features over the inflammatory response of the interaction between adipocytes and macrophages, comprising a potential therapeutic tool against comorbidities associated with obesity development.
Key Words: : adiposity, antioxidant activity, anti-inflammatory, 3T3-L1 cells, RAW264.7 cells
Introduction
Obesity is often associated with a low-grade chronic inflammation of the white adipose tissue (WAT), being a potential mechanism by which insulin resistance occurs.1 Adipose tissue inflammation is due to macrophage infiltration, and the cross talk between these inflammatory macrophages and resident adipocytes appears as a key factor to the development of associated comorbidities.2,3 Thus, several inflammatory products produced by this interaction, such as TNF-α, MCP-1, and nitric oxide (NO), correlate with increased body adiposity4 and appear to participate in the induction and maintenance of the chronic inflammatory state associated with obesity.5 Also, WAT overgrowth leads to downregulation of anti-inflammatory products, for example, adiponectin.6 Therefore, a reduction in the inflammatory status based on anti-inflammatory agents could constitute a potential treatment to avoid adverse obesity-associated consequences, such as insulin resistance.
In this sense, the use of native South American fruits is currently been claimed internationally,7,8 specially driven by their important content of polyphenols. Polyphenols are metabolites with well-known positive health effects.9 Anthocyanins, the main polyphenolic compounds, have been reported to possess antioxidant and anti-inflammatory features. It has been identified that the Chilean native fruits maqui and calafate present a high content of anthocyanins.10 Therefore, in the present study, the ability of these fruit extracts to modulate the inflammatory response of an in vitro adipocyte–macrophage interaction was evaluated and further compared to the effect of blueberry (Vaccinium corymbosum) extracts as control. The former has been shown to possess important anti-inflammatory and antioxidant features.11–14
Materials and Methods
Fruit extracts
Ripe fruits of maqui (Aristotelia chilensis), calafate (Berberis microphylla), and blueberry (V. corymbosum; as control) were obtained from SAAUTCHILE (Valdivia, Chile). Fruits were dried for 72 h at 40°C in a heating oven, pulverized in a grinder, and extracted with methanol:water (1:1) for 24 h with stirring. Methanol was evaporated with a rotavapor, and the resulting aqueous extracts were maintained at −20°C until further assays were performed.
Characterization of extracts
Total polyphenolic content
Total polyphenol content (TP) was determined by the Folin-Ciocalteu colorimetric method15 and expressed as gallic acid equivalents (GAE).
Total anthocyanins
Total anthocyanins (TA) were determined by the differential pH method.16 Absorbance was measured at 515 and 700 nm using pH 1.0 and 4.5 buffers, respectively, with a molar extinction coefficient of 26,900. Results were expressed as mg cyanidin-3-glucoside equivalents/100 g dry weight (DW).
Antioxidant activity
The antioxidant activity (AA) was carried out by the ferric reducing/antioxidant power (FRAP) colorimetric assay17 at 593 nm. Results were expressed as mmol Fe+2/100 g DW.
Liquid chromatography coupled to mass spectrometry procedure
Samples were filtered (0.45 mm) and analyzed using a liquid chromatography coupled to mass spectrometry (LC-MS) system. The chromatographic systems LC-MS consisted of a HPLC Agilent 1100 (Agilent Technologies, Inc., Santa Clara, CA, USA) connected through a split to an Esquire 4000 ion trap LC/MS system (Bruker Daltoniks, Bremen, Germany). A C18 column (Luna 150×4.6 mm, 5 μm, 100 Å; Phenomenex, Inc., Torrance, CA, USA) was used; at the exit of the column, a splitter system divided the eluant in two fractions, one of them to an UV detector and the second to the mass spectrometer. A volume of 20 μL was injected. The mobile phases were water:acetonitrile:formic acid (87:3:10% v/v/v, solvent A) and water:acetonitrile:formic acid (40:50:10% v/v/v, solvent B) at a flow rate of 0.8 mL/min according to the following elution gradient: 0–15 min, 6% B; 15–30 min, 30% B; 30–35 min, 50% B; 35–41 min, 60% B; and 41–50 min, 6% B. Phenolic compounds were detected at 520 nm. The mass spectral data were acquired in a positive mode. Ionization (nebulization) was performed with nitrogen as drying gas at 55 psi, 365°C, flow rate of 10 L/min, and capillary voltage of 3000 V. The trap parameters were set in ion charge control using manufacturer default parameters. Collision-induced dissociation was performed by collisions with the helium background gas present in the trap. Fragmentation was set with Smart Frag.
Cell culture
The 3T3-L1 mouse preadipocytes and RAW264.7 mouse macrophages were obtained from the Laboratory of Cellular and Molecular Biology (INTA, University of Chile) and from the Laboratory of Biochemistry, Metabolism and Drug Resistance (ICBM, University of Chile), respectively. Both cell lines were maintained at 37°C in a humidified atmosphere containing 5% CO2. The 3T3-L1 cells were cultured in DMEM containing 4.5 g/L glucose and 10% calf serum. Two days after full confluence, cells were differentiated by incubation with 0.5 mM isobutylmethylxanthine, 1 mM dexamethasone, and 10 mg/mL insulin (all reagents from Sigma-Aldrich, St. Louis, MO, USA) in 4.5 g/L glucose DMEM supplemented with 10% fetal bovine serum for 2 days, and for the next 2 days with 10 mg/mL insulin in 4.5 g/L glucose DMEM supplemented with 10% fetal bovine serum. Thereafter, cells were maintained and refed every 2 or 3 days with media, without any hormones, until the cells reached a fully differentiated phenotype (14–16 days). These adipocytes were then utilized for coculture (CC) with macrophages and for conditioned media (CM) production. Regarding the preparation of CM, fully differentiated adipocytes were cultured in DMEM containing 4.5 g/L glucose and 10% fetal bovine serum for 24 h. Then, media were saved at 20°C for further assays. Macrophages were cultured in DMEM containing 4.5 g/L glucose and 10% fetal bovine serum. Cells were activated either with 5 μg/mL lipopolysaccharides (LPS; Sigma-Aldrich) or CM from adipocytes for 24 h in the presence or absence of 100 μM [TP] of each extract for 24 h. Cells and media were stored at −20°C for further determinations. Finally, a CC between adipocytes and macrophages was performed according to a previously described protocol.18
Secretion assays
The amount of nitrite in cell-free culture supernatants was measured using the Griess reagent according to the manufacturer's protocol (Sigma-Aldrich). IL-10 secretion to culture media was measured using the Mouse IL-10 ELISA Kit (Merck Millipore, Billerica, MA, USA). Cell viability was assessed using the LDH Cytotoxicity Assay Kit from Cayman Chemical Company (Ann Arbor, MI, USA) according to the manufacturer's indications.
Gene expression assays
Total RNA was isolated from samples using Trizol (Invitrogen, Paisley, United Kingdom), according to the supplier's protocol. Purified RNA (2 mg) was then treated with DNase (DNAfree kit; Ambion, Austin, TX, USA) and used to generate first-strand cDNA with M-MLV reverse transcriptase (Invitrogen), utilizing random hexamers (Invitrogen) and dNTP mix (Bioline, London, United Kingdom), according to the manufacturer's protocol. The resultant cDNA was amplified with specific primers for mouse inducible nitric oxide synthase (iNOS), TNF-α, IL-10, MCP-1, and ADIPOQ in a total volume of 10 μL. Real-time PCR was performed in a Stratagene Mx3000P System (Agilent Technologies) following the manufacturer's recommendations (Applied Biosystems, Foster City, CA, USA). All the expression levels of the target genes studied were normalized by the expression of cyclophilin as the selected internal control (also supplied by Applied Biosystems). Fold change between groups was calculated by the 2(−ΔΔCt) method.
Statistical analyses
Data are expressed as mean±SD. Differences were assessed using one-way ANOVA followed by the Tukey post hoc test. All statistical analyses were performed with the GraphPad Prism 6.0 statistical package (GraphPad Software, Inc., San Diego, CA, USA).
Results
Characterization of extracts
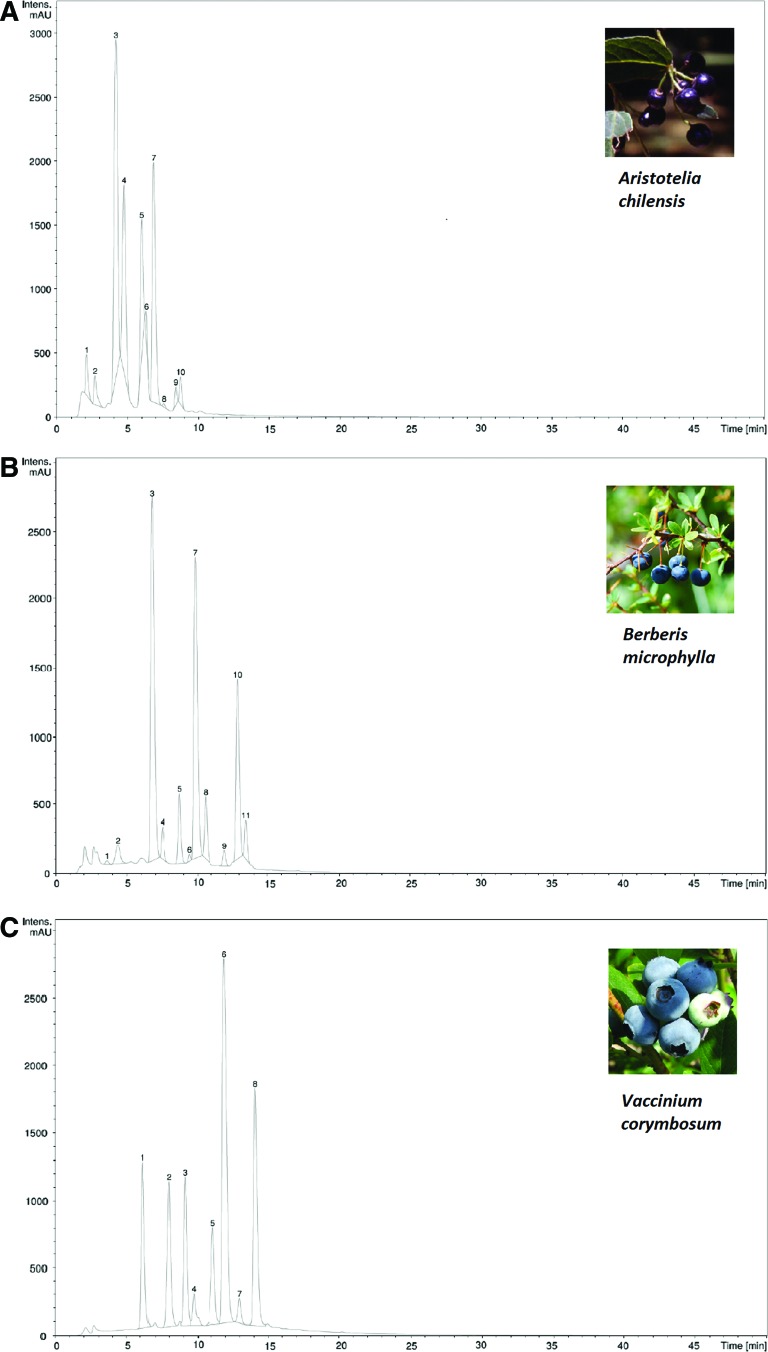
Table 1 shows TP, TA, and AA of the studied extracts. All three determinations presented higher levels in maqui, followed by calafate and blueberry extracts. Moreover, an LC-MS identification was performed comparing m/z signals and fragment ions of the anthocyanin pattern of all extracts (Fig. 1 and Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/jmf). Regarding maqui extracts, the main compound identified was delphinidin-3-sambubioside-5-glucoside (m/z: 759.5, peak 3, 36.5%), followed by delphinidin-3-galactoside (m/z: 465.3, peak 7, 23.8%) and delphinidin-3,5-O-diglucoside (m/z: 627.4, peak 4, 18.9%). Other compounds identified were glucosides of cyanidin. In the case of calafate fruit extract, the most abundant anthocyanin was delphinidin-3-glucoside (m/z: 465.3; peak 3, 36.1%), followed by petunidin and malvidin, both conjugated to 3-glucoside or 3-galactoside (m/z: 479.1, peak 7, 29.9% and m/z: 493.2, peak 10, 15.2%, respectively). Other anthocyanins detected in calafate were glycosylated derivatives of cyanidin, malvidin, peonidin, and petunidin. Finally, regarding blueberry fruit extracts (which was used as control), the major anthocyanin identified was malvidin-3-glucoside or conjugated to galactose (m/z: 493.4, peak 6, 35.4%) followed by malvidin-3-arabinoside (m/z: 463.3, peak 8, 19.9%). Glucosides of cyanidin, delphinidin, petunidin, and peonidin were also identified.
Table 1.
Total Polyphenolic and Anthocyanin Content and Antioxidant Capacity of Maqui, Calafate, and Blueberry Extracts
| Maqui | Calafate | Blueberry | |
|---|---|---|---|
| Total polyphenols (mg GAE/100 g DW) | 1906.5±73.2a | 1344.2±10.5b | 1229.6±20.9b |
| Total anthocyanins (mg C-3-G E/100 g DW) | 72.7±0.1a | 31.5±0.8b | 20.1±1.2c |
| Antioxidant activity (mmol Fe+2/100 g DW) | 38.9±1.7a | 11.7±1.8b | 5.9±0.1c |
One-way ANOVA followed by Tukey's post hoc tests were performed to identify statistical differences among groups.
Different letters mean statistical difference of at least P<.05.
GAE, gallic acid equivalents; DW, dry weight; C-3-G E, cyanidin-3-glucoside equivalents.
FIG. 1.
HPLC-UV chromatogram detected at 520 nm of all studied extracts. In maqui extracts (A), peak 2: cyanidin-3,5-diglucoside; 3: delphinidin-3-sambubioside-5-glucoside; 4: delphinidin-3,5-O-diglucoside; 5: cyanidin-3-sambubioside-5-glucoside; 6: delphinidin-3-sambubioside; 7: delphinidin-3-galactoside or delphinidin-3-galactoside; 9: cyanidin-3-sambubioside; 10: cyanidin-3-glucoside or cyanidin-3-galactoside were identified. In calafate extracts (B), peak 1: cyanidin-3,5-diglucoside or cyanidin-3-soforoside; 2: petunidin-3-O-galactoside-5-O-glucoside or petunidin-3-O-glucoside-5-O-glucoside; 3: delphinidin-3- glucoside or delphinidin-3- galactoside; 5: cyanidin-3-glucoside or cyanidin-3-galactoside; 6: cyanidin-3-rutinoside; 7: petunidin-3-galactoside or petunidin-3-glucoside; 8: petunidin-3-rutinoside; 9: peonidin-3-galactoside or peonidin-3-glucoside; 10: malvidin-3-galactoside or malvidin-3-glucoside; 11: malvidin-3-rutinoside were identified. Finally, in blueberry extracts (C), peak 1: delphinidin-3-glucoside or delphinidin-3-galactoside; 2: delphinidin-3-arabinoside; 3: petunidin-3-galactoside or petunidin-3-glucoside; 4: cyanidin-3-arabinoside or cyanidin-3-xiloside; 5: petunidin-3-arabinoside; 6: malvidin-3-glucoside or malvidin-3-galactoside; 7: peonidin-3-arabinoside; 8: malvidin-3-arabinoside were identified. Intens., intensity; mAU, milli-absorbance units; min, minutes. Color images available online at www.liebertpub.com/jmf
Maqui and calafate extracts inhibit RAW264.7 macrophage activation by LPS and 3T3-L1 CM
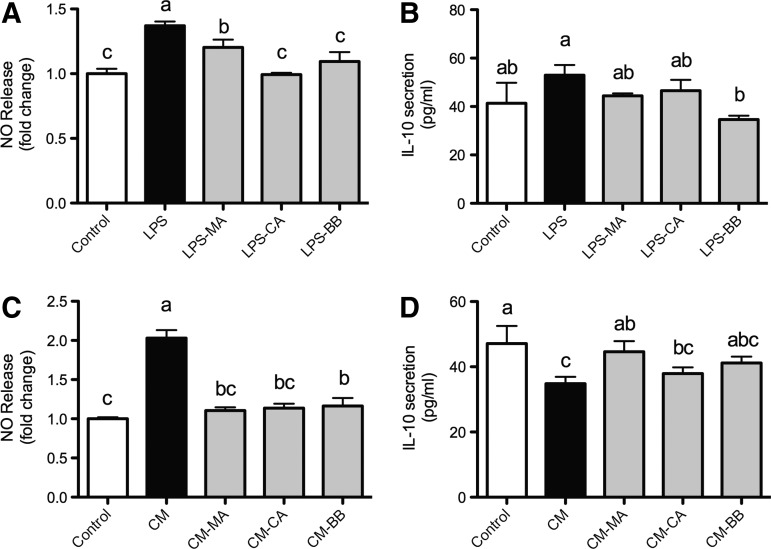
All extracts significantly prevented LPS-induced NO secretion by macrophages; however, the calafate extract induced the more drastic effect (−27.6%, Fig. 2A). Furthermore, neither LPS nor the extracts exerted a significant modulation of IL-10 secretion (Fig. 2B). When the effect of adipocyte CM on RAW264.7 cells was analyzed, the macrophage inflammatory activity was observed, resulting in an increase (103%) and decrease (−26.1%) of NO and IL-10 secretion, respectively (Fig. 2C, D). Regarding pretreatment, a prevention of the CM-induced NO release by maqui (−45.6%), calafate (−44%), and blueberry (−42.7%) extracts was observed, and a restoration of IL-10 secretion to control levels only by maqui extracts (−5.4% with respect to control).
FIG. 2.
Nitric oxide and IL-10 release by RAW264.7 macrophages. Nitric oxide (A) and IL-10 (B) release were detected in mouse macrophages that were pretreated for 1 h with 100 μM [total polyphenol] extracts of maqui, calafate, and blueberry, and then treated for 24 h with 5 μg/mL LPS. Likewise, both nitric oxide (C) and IL-10 (D) were detected when macrophages were treated in similar conditions, but activated with conditioned media from mature 3T3-L1 adipocytes instead of LPS. Data (n=3) are expressed as mean±SD. One-way ANOVA followed by Tukey post hoc test were performed to identify statistical differences among groups. NO, nitric oxide; LPS, lipopolysaccharide; CM, conditioned media; MA, maqui; CA, calafate; BB, blueberry. Different letters mean statistical difference of at least P<.05.
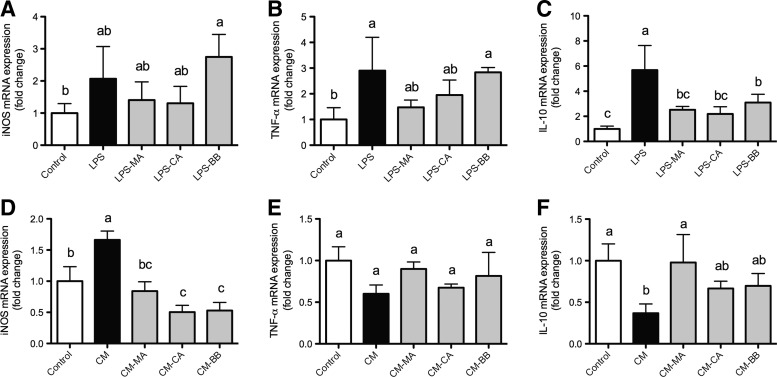
To evaluate whether the extracts would modulate the gene expression of cytokines associated with certain inflammatory pathways, the relative change of iNOS and TNF-α and IL-10 mRNA expression was determined. It was observed that LPS increased iNOS (106.9%) and TNF-α (190.4%) expression (Fig. 3A, B), indicating successful RAW264.7 macrophage activation. However, IL-10 an anti-inflammatory cytokine was also increased with LPS treatment (325.7%) (Fig. 3C). When the effects of the extracts were assessed, a protective effect (tendency) was observed only with maqui (−32.2%) and calafate (−36.9%) extracts over iNOS expression (Fig. 3A). A same pattern was observed on TNF-α gene expression (−49.3% reduction by maqui and −32.8% by calafate extracts) (Fig. 3B). Finally, all extracts significantly prevented LPS-induced IL-10 secretion (−30.6% to −36.4%) (Fig. 3C).
FIG. 3.
Gene expression analysis in RAW264.7 macrophages. iNOS (A), TNF-α (B), and IL-10 (C) transcript contents were detected in mouse macrophages that were pretreated for 1 h with 100 μM [total polyphenol] extracts of maqui, calafate, and blueberry, and then treated for 24 h with 5 μg/mL LPS. Likewise, iNOS (D), TNF-α (E), and IL-10 (F) transcript contents were detected when macrophages were treated in similar conditions, but activated with conditioned media from mature 3T3-L1 adipocytes instead of LPS. Data (n=3) are expressed as mean±SD. One-way ANOVA followed by Tukey post hoc test were performed to identify statistical differences among groups. Different letters mean statistical difference of at least P<.05.
On the other hand, it was observed that the CM treatment was able to significantly activate macrophages, inducing an increase (66.2%) and decrease (−63.1%) in iNOS and IL-10 gene expression, respectively (Fig. 3D, F). Regarding pretreatments, it was observed that calafate extracts induced the highest inhibition (−69.7%) followed by blueberry (−68.2%) and maqui (−49.4%) extracts, when compared with CM treatment (Fig. 3D). In the case of IL-10 gene expression, only maqui extract was able to completely revert the effect of CM treatment (to −2.2% reduction with respect to control) (Fig. 3F). No effects were observed in the TNF-α mRNA expression (Fig. 3E).
Maqui and calafate extracts inhibit the inflammatory response induced in a 3T3-L1 and RAW267.4 CC
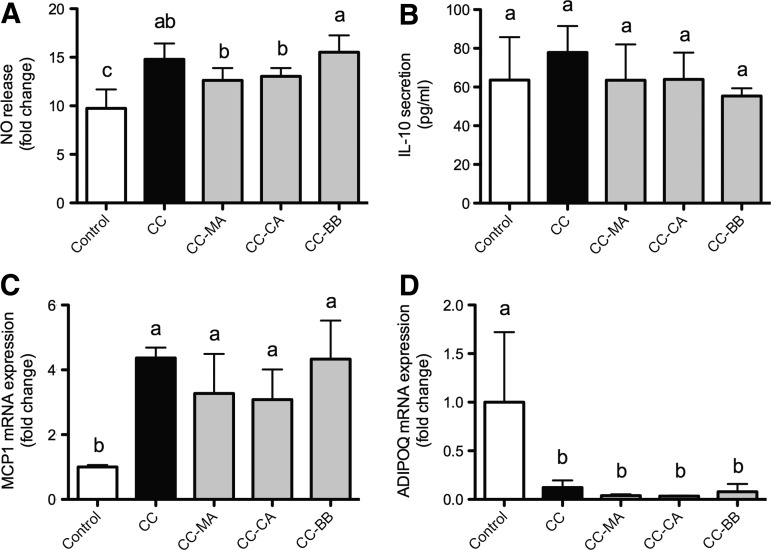
To further evaluate the anti-inflammatory effects of the native fruit extracts, 3T3-L1 and RAW267.4 were cultured in direct contact with each other, aiming to obtain a more realistic model of obesity-related inflammation. In this CC, a significant increase of NO levels with respect to the control culture was observed (51.8%). Only maqui (−14.7%) and calafate (−11.8%) extracts induced a slight prevention of this inflammatory secretion profile (Fig. 4A). On the other hand, no effects were observed in IL-10 secretion (Fig. 4B).
FIG. 4.
Nitric oxide and IL-10 release, and gene expression analyses in coculture of 3T3-L1 adipocytes and RAW264.7 macrophages. Nitric oxide (A) and IL-10 (B) release, and MCP-1 (C) and ADIPOQ (D) transcript contents were detected in a coculture between mouse adipocytes and macrophages, in the presence of 100 μM [total polyphenol] extracts of maqui, calafate, and blueberry, after 24 h. As control, we used coculture without extracts. Data (n=3) are expressed as mean±SD. One-way ANOVA followed by Tukey post hoc test were performed to identify statistical differences among groups. Different letters mean statistical difference of at least P<.05.
Finally, the expression levels of inflammatory (MCP-1) and anti-inflammatory (ADIPOQ) transcripts were assessed. Cell-to-cell interaction caused a significant increase (337%) and decrease (−87.7%) of MCP-1 and ADIPOQ mRNA expression, respectively, indicating a successful establishment of the inflammatory model (Fig. 4C, D). Although extract pretreatments were not able to significantly modify both adipokine expressions, maqui (−25.1%) and calafate (−29.5%) extracts induced a slight reduction in MCP-1 mRNA content when comparing to the CC group (Fig. 4C).
Discussion
It has been recognized that increased body adiposity is habitually accompanied by an increased systemic oxidative stress and a low-grade inflammation condition in the adipose tissue. In this sense, tools aiming to counteract these inflammatory processes are welcome to be investigated to set up novel protocols for prevention and/or treatment of obesity-related illnesses. The present article describes how polyphenolic-rich extracts from two Chilean native fruits inhibited inflammatory features in vitro.
As it has been working with crude extracts, a proper exhaustive characterization of each material used was performed. The maqui extract presented the higher antioxidant potential among all extracts analyzed. These results could be attributed to the structural features of the polyphenols and/or other compounds present in each fruit extract. Compared to previously published works, TP reported in the present study was lower than those reported in maqui (4570 mg GAE/100g DW19), calafate (3490 mg GAE/100 g DW20), and blueberry extracts (5660 mg catechin equivalents/100 g fresh weight [FW]21). This could be explained by the use of a nonacidified hydroalcoholic extract, which probably resulted in a decrease extraction efficiency of anthocyanins and other compounds. With respect to AA and TA in methanolic extracts, in previous studies a lower AA has been reported (12.32 mmol Fe+2/100 g FW), measured by the FRAP method. Although in this study a shorter extraction time was performed, that could affect the results.22 On the other hand, a higher content of TA has been found (211.9 mg delphinidin-3-glucoside equivalents/100 g DW), although this measurement was performed by HPLC-PAD-MS.23 Meanwhile, values of 2.53, 17.81, and 17.88 μmol/g FW of TA in blueberry, calafate, and maqui extracts have been described, respectively.10 Regarding LC-MS compound identification, delphinidin-3-sambubioside-5-glucoside was also identified previously as a main phenolic compound in maqui.23–25 In the case of calafate extract, the reported anthocyanin pattern agrees with the one previously found.10,26 Finally, in blueberry extracts, the compounds observed are in accordance with those reported before,21,27 with malvidin-3-glucoside, malvidin-3-galactoside, malvidin-3-arabinoside, and delphinidin-3-galactoside as major components. Thus, the identification of anthocyanins by LC-MS in the three extracts studied was consistent with previous reports by other authors. In maqui and calafate fruit extracts, delphinidin and petunidin (both glycosylated) predominated. On the other hand, malvidin was the major compound detected in blueberry. These differences in composition may explain, in part, the higher TP content and AA of maqui and calafate.
We found that the anthocyanin/polyphenol ratio in maqui, calafate, and blueberry extracts was 0.038, 0.023, and 0.016, respectively. Indeed, at the same concentration of TPs (100 μM) when compared with maqui extracts, calafate presented −38.5%, while blueberry presented −57.1% of TA content. These data were not related to the anti-inflammatory effect observed in the present study, suggesting that biomedical features found do not depend on concentration, but on the combination of anthocyanins. Therefore, it is necessary that future studies focus on assessing the effect of each pure compound and the synergistic interactions between them.
In the present work, we observed that LPS and CM from mature adipocytes exerted differential impact on macrophages, TNF-α and iNOS mRNA expression. One possible explication could be related to the fact that adipocytes can also secrete TNF-α,28 and this molecule acts mediating iNOS gene expression on macrophages.29 Therefore, CM could not cause a significant effect on TNF-α mRNA levels, but on iNOS expression.
Another controversial result observed was the differential IL-10 gene expression between LPS and CM induced in macrophages. Since IL-10 is recognized as an anti-inflammatory cytokine, a downregulation against the stimulus applied should be expected; however, the opposite outcome was observed when cells were incubated with LPS. In this sense, it has been reported that IL-10 could present a proinflammatory feature when endotoxemia is induced.30 Furthermore, IL-10 is recognized as an anti-inflammatory cytokine secreted by the M2 macrophage subset. Thus, the upregulation observed when cells were incubated with LPS could be due to an M2b phenotype polarization. This macrophage subset is characterized with an IL-10/TNF-α secretory profile and is active with LPS and other immune complex treatments.31,32 In this regard, the results observed in the CM induction model were of great importance, since these are closer to a real adipocyte–macrophage interaction.
Nevertheless, overall in the present article, it was reported that the pre- and co-treatment with the three fruit extracts reduced the expression/secretion of inflammatory markers on 3T3-L1 adipocytes, macrophages, and a mixed coculture. This outcome was especially evident for maqui and calafate. Thus, the fact that these former extracts present better results suggests that a combination or specific compounds could exist, ascribed only to these native fruits that are related to higher healthy potential. Several previous reports support the present findings. Regarding the antioxidant and anti-inflammatory activities of the maqui fruit, it has been observed that this fruit extract induced a reduction in lipid peroxidation and presented important antioxidant effects on the rat heart.33 Moreover, subfractions of maqui have been described to prevent LDL oxidation and protect against oxidative stress in human endothelial cells.34 On the other hand, it has been described25 that an anthocyanin-enriched fraction of maqui improves fasting glucose and glucose intolerance in diet-induced hyperglycemic obese mice, diminishes glucose production, improves insulin-stimulated glucose-6-phosphatase inhibition on hepatic cells, and increases basal and insulin-stimulated glucose uptake in myotubes. It was also observed that crude extracts of maqui inhibited the α-glucosidase and α-amylase activity, improving postprandial glucose tolerance in diabetic patients.19 Likewise, it was described that subfractions of maqui showed anti-inflammatory effects in tetradecanoylphorbol acetate-induced formation of mouse ear edema.35 Finally, it has been reported that maqui extracts inhibited lipid accumulation in differentiating 3T3-L1 cells, and the production of NO and prostaglandin E2 and the expression of iNOS and cyclooxygenase-2 in RAW264.7 macrophages.36 The present work adds novel information related to a novel obesity-related anti-inflammatory feature. Finally, regarding calafate, it has been observed that chloramphenicol-induced reactive oxygen species production was inhibited by water Berberis extracts in human isolated blood cells.37 Therefore, the anti-inflammatory features observed in the present work by the calafate extract could be ascribed to its antioxidant properties.
Efforts must be made to reduce diabetes incidence worldwide. Possibly, major improvements can be accomplished counteracting the establishment of the inflammatory state in WAT. Novel strategies or specific compounds that are able to diminish this inflammation are highly valuated. In the present work, maqui and calafate extracts were able to modulate the proinflammatory state generated by the interaction between adipocytes and macrophages. These interesting outcomes must be further studied to generate new therapeutic tools against inflammation-related insulin resistance.
Supplementary Material
Acknowledgments
The authors thank Dr. Cecilia Rojas (Laboratory of Cellular and Molecular Biology, INTA, University of Chile) and Dr. Juan Diego Maya (Laboratory of Biochemistry, Metabolism and Drug Resistance, ICBM, University of Chile) for kindly providing 3T3-L1 and RAW264.7 vials, respectively. This work was supported by the FONDECYT grant 11110219 (CONICYT, Chile).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Dandona P, Aljada A, Bandyopadhyay A: Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 2004;25:4–7 [DOI] [PubMed] [Google Scholar]
- 2.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr.: Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu H, Barnes GT, Yang Q, et al. : Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrante AW, Jr.: Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med 2007;262:408–414 [DOI] [PubMed] [Google Scholar]
- 5.Shoelson SE, Lee J, Goldfine AB: Inflammation and insulin resistance. J Clin Invest 2006;116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeda N, Shimomura I, Kishida K, et al. : Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 2002;8:731–737 [DOI] [PubMed] [Google Scholar]
- 7.Schreckinger ME, Lotton J, Lila MA, de Mejia EG: Berries from South America: a comprehensive review on chemistry, health potential, and commercialization. J Med Food 2010;13:233–246 [DOI] [PubMed] [Google Scholar]
- 8.Costa AGV, Garcia-Diaz DF, Jimenez P, Silva PI: Bioactive compounds and health benefits of exotic tropical red-black berries. J Funct Foods 2013;5:539–549 [Google Scholar]
- 9.Pandey KB, Rizvi SI: Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med Cell Longevity 2009;2:270–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiz A, Hermosin-Gutierrez I, Mardones C, et al. : Polyphenols and antioxidant activity of calafate (Berberis microphylla) fruits and other native berries from Southern Chile. J Agric Food Chem 2010;58:6081–6089 [DOI] [PubMed] [Google Scholar]
- 11.Graf BA, Milbury PE, Blumberg JB: Flavonols, flavones, flavanones, and human health: epidemiological evidence. J Med Food 2005;8:281–290 [DOI] [PubMed] [Google Scholar]
- 12.Schmidt BM, Erdman JW, Jr., Lila MA: Differential effects of blueberry proanthocyanidins on androgen sensitive and insensitive human prostate cancer cell lines. Cancer Lett 2006;231:240–246 [DOI] [PubMed] [Google Scholar]
- 13.McDougall GJ, Ross HA, Ikeji M, Stewart D: Berry extracts exert different antiproliferative effects against cervical and colon cancer cells grown in vitro. J Agric Food Chem 2008;56:3016–3023 [DOI] [PubMed] [Google Scholar]
- 14.DeFuria J, Bennett G, Strissel KJ, et al. : Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J Nutr 2009;139:1510–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singleton V, Rossi J: Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 1965;16:144–158 [Google Scholar]
- 16.Wrolstad R: Color and pigment analyses in fruit products. Oregon Agr Expt Sta Bul 1976;624:1–17 [Google Scholar]
- 17.Benzie IF, Strain JJ: The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1996;239:70–76 [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Diaz DF, Campion J, Quintero P, Milagro FI, Moreno-Aliaga MJ, Martinez JA: Vitamin C modulates the interaction between adipocytes and macrophages. Mol Nutr Food Res 2011;55Suppl 2:S257–S263 [DOI] [PubMed] [Google Scholar]
- 19.Rubilar M, Jara C, Poo Y, et al. : Extracts of maqui (Aristotelia chilensis) and murta (Ugni molinae Turcz.): sources of antioxidant compounds and alpha-glucosidase/alpha-amylase inhibitors. J Agric Food Chem 2011;59:1630–1637 [DOI] [PubMed] [Google Scholar]
- 20.Mariangel E, Reyes-Diaz M, Lobos W, Bensch E, Schalchli H, Ibarra P: The antioxidant properties of calafate (Berberis microphylla) fruits from four different locations in southern Chile. Cienc Investig Agrar 2013;40:161–170 [Google Scholar]
- 21.Torri E, Lemos M, Caliari V, Kassuya CA, Bastos JK, Andrade SF: Anti-inflammatory and antinociceptive properties of blueberry extract (Vaccinium corymbosum). J Pharm Pharmacol 2007;59:591–596 [DOI] [PubMed] [Google Scholar]
- 22.Araya H, Clavijo C, Herrera C: Capacidad antioxidante de frutas y verduras cultivados en Chile. Arch Latinoam Nutr 2006;56:361–365 [PubMed] [Google Scholar]
- 23.Escribano-Bailon MT, Alcalde-Eon C, Munoz O, Rivas-Gonzalo JC, Santos-Buelga C: Anthocyanins in berries of Maqui (Aristotelia chilensis (Mol.) Stuntz). Phytochem Anal 2006;17:8–14 [DOI] [PubMed] [Google Scholar]
- 24.Cespedes CL, Valdez-Morales M, Avila JG, El-Hafidi M, Alarcon J, Paredes-Lopez O: Phytochemical profile and the antioxidant activity of Chilean wild black-berry fruits, Aristotelia chilensis (Mol) Stuntz (Elaeocarpaceae). Food Chem 2010;119:886–895 [Google Scholar]
- 25.Rojo LE, Ribnicky D, Logendra S, et al. : In vitro and in vivo anti-diabetic effects of anthocyanins from Maqui Berry (Aristotelia chilensis). Food Chem 2012;131:387–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruiz A, Hermosin-Gutierrez I, Vergara C, et al. : Anthocyanin profiles in south Patagonian wild berries by HPLC-DAD-ESI-MS/MS. Food Res Int 2013;51:706–713 [Google Scholar]
- 27.Kader F, Rovel B, Girardin M, Metche M: Fractionation and identification of the phenolic compounds of Highbush blueberries (Vaccinium corymbosum, L). Food Chem 1996;5535–40 [Google Scholar]
- 28.Sewter CP, Digby JE, Blows F, Prins J, O'Rahilly S: Regulation of tumour necrosis factor-alpha release from human adipose tissue in vitro. J Endocrinol 1999;163:33–38 [DOI] [PubMed] [Google Scholar]
- 29.Fonseca SG, Romao PR, Figueiredo F, et al. : TNF-alpha mediates the induction of nitric oxide synthase in macrophages but not in neutrophils in experimental cutaneous leishmaniasis. Eur J Immunol 2003;33:2297–2306 [DOI] [PubMed] [Google Scholar]
- 30.Lauw FN, Pajkrt D, Hack CE, Kurimoto M, van Deventer SJ, van der Poll T: Proinflammatory effects of IL-10 during human endotoxemia. J Immunol 2000;165:2783–2789 [DOI] [PubMed] [Google Scholar]
- 31.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M: The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004;25:677–686 [DOI] [PubMed] [Google Scholar]
- 32.Rees AJ: Monocyte and macrophage biology: an overview. Semin Nephrol 2010;30:216–233 [DOI] [PubMed] [Google Scholar]
- 33.Cespedes CL, El-Hafidi M, Pavon N, Alarcon J: Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), Maqui. Food Chem 2008;107:820–829 [Google Scholar]
- 34.Miranda-Rottmann S, Aspillaga AA, Perez DD, Vasquez L, Martinez AL, Leighton F: Juice and phenolic fractions of the berry Aristotelia chilensis inhibit LDL oxidation in vitro and protect human endothelial cells against oxidative stress. J Agric Food Chem 2002;50:7542–7547 [DOI] [PubMed] [Google Scholar]
- 35.Céspedes CL, Alarcon J, Avila JG, Nieto A: Anti-inflammatory Activity of Aristotelia chilensis Mol. (Stuntz) (Elaeocarpaceae). B Latinoam Caribe Pl 2010;9:127–135 [Google Scholar]
- 36.Schreckinger ME, Wang JZ, Yousef G, Lila MA, de Mejia EG: Antioxidant Capacity and in Vitro Inhibition of Adipogenesis and Inflammation by Phenolic Extracts of Vaccinium floribundum and Aristotelia chilensis. J Agric Food Chem 2010;58:8966–8976 [DOI] [PubMed] [Google Scholar]
- 37.Albrecht C, Pellarin G, Rojas MJ, Albesa I, Eraso AF: Beneficial effect of Berberis buxifolia Lam, Ziziphus mistol Griseb and Prosopis alba extracts on oxidative stress induced by chloramphenicol. Medicina (B Aires) 2010;70:65–70 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.