Abstract
We sought to review the effectiveness of interventions designed to improve adherence to antiretroviral therapy (ART) from studies included in a recent Cochrane review that reported a clinical and an adherence outcome, with at least 80% follow-up for 6 months or more. Data were extracted independently and in duplicate, with an adjudicator for disagreements. Risk of bias was assessed using the Cochrane Risk of Bias tool. Of 182 relevant studies in the Cochrane review, 49 were related to ART. Statistical pooling was not warranted due to heterogeneity in interventions, participants, treatments, adherence measures and outcomes. Many studies had high risk of bias in elements of design and outcome ascertainment. Only 10 studies improved both adherence and clinical outcomes. These used the following interventions: adherence counselling (two studies); a once-daily regimen (compared to twice daily); text messaging; web-based cognitive behavioral intervention; face-to-face multi-session intensive behavioral interventions (two studies); contingency management; modified directly observed therapy; and nurse-delivered home visits combined with telephone calls. Patient-related adherence interventions were the most frequently tested. Uniform adherence measures and higher quality studies of younger populations are encouraged.
Introduction
Adherence is defined as the “extent to which patients take medications as prescribed by their health care providers.”1 A broader definition, “the extent to which a person's behaviour—taking medication, following a diet, or executing lifestyle changes—corresponds with agreed recommendations from a health care provider,”2 incorporates and recognizes the other factors that can influence adherence, including the fact that patients may disagree with a prescribed regimen. For example, lifestyles of substance abuse may have negative effects on adherence to medication.3–6 Likewise, the factors affecting adherence may differ in specific subgroups, such as pregnant women for whom concerns for fetal safety may be a reason for not taking medication as prescribed.7 Irrespective of the underlying factors for poor adherence, it has nefarious consequences on clinical outcomes, reduces quality of life, and wastes medication.2
This is especially true for human immunodeficiency virus (HIV) infection. Currently, close to 35 million people are living with HIV worldwide.8 The advent of antiretroviral therapy (ART) has led to important reductions in the morbidity and mortality due to HIV. ART reduces HIV viral load to undetectable levels in the serum, yet even when the virus is undetectable, replication is still taking place in lymphatic reservoirs.9 This implies that high levels of adherence to uninterrupted ART are required to maintain prolonged viral suppression.10
Recent evidence suggests that viral suppression may still occur at thresholds of adherence as low as 80%,11,12 yet the highest levels of adherence are expected since they correlate with better clinical and immunological outcomes.13,14 The benefits of ART are limited by poor adherence, which often leads to treatment failure, more resistant viral strains, progression to acquired immune deficiency syndrome (AIDS), higher mortality rates, higher hospitalization rates, and longer stays in hospital.10,15–18 All these consequences increase health care costs—an unfortunate situation given that two-thirds of the people living with HIV are in the most economically disenfranchised region of the world, sub-Saharan Africa.8 Poor adherence also has public health implications, as the transmission of resistant strains leave the newly infected with limited therapeutic options.19, 20 The literature reports that adherence rates of 90% or more can be found in only about 62% of observational studies, with higher rates among men who have sex with men (MSM), in individuals at an early stage of infection, and in developing countries.21
Given the above, it is critical from a public and individual perspective to investigate and describe the interventions that are effective in improving adherence to ART. Systematic reviews report that there is limited evidence on interventions to improve adherence to ART in the pediatric population;22 yet older adults are less likely to be non-adherent;23 there may be benefit in addressing mental health issues that affect adherence;5,24,25 motivational interviewing may offer some benefit,26 and reduce viral load in youth.27 Weekly reminder text messages28–30 and treatment supporters29 also improve adherence and reduce viral load in low resource settings. Food security, community health workers, and social networks may improve adherence in displaced persons living with HIV. 31 One review of adherence interventions in World Health Organisation (WHO) stratum A (low mortality rates) reported that most interventions employed in this setting had no effect,32 while a review of studies based in the United States identified 10 effective evidence-based interventions for improving adherence to ART. These interventions included interactive discussion sessions, pager messages, and home visits.33
A recent update of a comprehensive review of trials of adherence interventions showed substantial increase in the number of trials over the past 5 years, especially including a large increase in the number of HIV trials.34 In view of this new evidence and the unique features of adherence to HIV regimens, we undertook to review in detail, the trials of interventions to improve adherence with HIV regimens.
Methods
Types of studies
This review builds upon a previous review covering high quality studies [randomized controlled trials (RCTs) with at least 80% complete follow up for 6 months or more] on all adherence interventions to prescribed medication for any condition except addiction.34 The search was updated to include all such studies published up to December 2013, on adherence to ART.
Participants
We included all participants, irrespective of age, living with HIV and receiving ART.
Interventions
All adherence enhancing interventions were included, and categorized according to the dimensions proposed by the WHO:2
• social- and economic-related interventions;
• health system/health care team-related interventions;
• therapy-related interventions;
• condition (co-morbidity)-related interventions;
• patient-related interventions.
Interventions were categorized as “complex” if they belonged to more than one of these categories.
Outcomes
We included only studies that reported both adherence measures (as reported by the authors) and clinical outcomes such as viral load (change or proportion with undetectable levels), T-lymphocyte cell count (change), progression to AIDS, mortality and other co-morbidities.
Search methods for identification of studies
The search strategy for the main review is reported in detail elsewhere.34 In brief, The Cochrane Library, MEDLINE, CINAHL, EMBASE, International Pharmaceutical Abstracts (IPA), PsycINFO (all via OVID), and Sociological Abstracts (via CSA) were searched to December 2013 using combinations of search terms such as: patient compliance, adherence, non-compliance, clinical trials, randomized, controlled, regimen, treatment, drug therapy, medication etc., adapted for each database.
Data collection and analyses
Randomized trials on interventions to improve adherence to ART identified in the previous review were selected. The results of the updated search were screened for duplicates, then screened for relevance. The full texts of the selected articles were used for further screening and data extraction. Full text articles were read independently by two authors. A third author adjudicated when there were disagreements on the decision to include or on the data extracted. Study authors were contacted to verify extracted data and given 2 weeks to respond, after which we proceeded with the review. The following data were extracted: full referencing, study setting and design, characteristics of the participants, interventions, measurements, comparisons and outcomes; whether the interventions improved adherence or clinical outcomes; and cost.
Risk of bias
For each of the studies, two authors working independently evaluated random sequence generation, allocation concealment, and selective outcome reporting. For each of the outcomes reported, we evaluated blinding of study personnel, clinic staff, and participants. We also checked for other sources of bias, including power to detect a statistically significant difference in the primary outcome as reported by the authors or based on a sample size of at least 50 per arm. We noted whether there was high, low, or unclear risk of bias (insufficient information reported to make a judgment). Disagreements in these judgments and their justifications were adjudicated by a third author who made a final decision.
Analyses and reporting
We planned to pool sufficiently similar studies using random effects meta-analysis. Odds ratios (95% confidence intervals) were reported for binary data; mean differences (and standard deviation) were reported for continuous data and statistical heterogeneity was assessed using the Q test and the I2. Studies that could not be pooled were synthesized narratively. Our findings are reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.35 We paid specific attention to the following subgroups of interest: category of intervention, low versus high income settings, interventions with a theoretical framework, and technology based interventions.
Results
Results of search and update
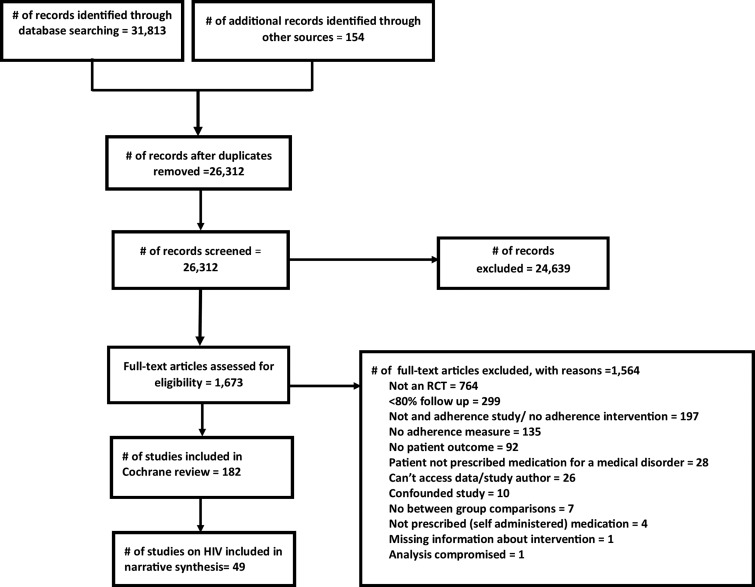
The latest Cochrane review identified 182 studies, of which 49 were about HIV.34 The flow of studies during the screening process is displayed in Fig. 1.
FIG. 1.
Flow diagram of study selection.
Characteristics of included studies
A summary of all the included HIV studies is reported in Table 1. The heterogeneity of the studies, in terms of interventions characteristics, participants, treatment regimens, adherence measures, and clinical outcome measures precluded any form of quantitative synthesis.
Table 1.
Characteristics of Included Studies
| Study ID | Country | Sample size | Theory-based? | Category of intervention | Participants<18 | Intervention | Intervention target | Comparison | Clinical outcomes | Adherence outcomes | Improvement in adherence?* | Improvement in clinical outcomes?* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abrahams, 2010 | South Africa | 274 | Yes | Patient-related | Yes | Telephone counselling | Patients, caregivers, patients' family or friends | Standard care | Depressive symptomology | Pill count/syrup measure, attendance at follow-up | No | No |
| Andrade, 2005 | USA | 64 | No | Patient-related | No | Disease Management Assistance System (DMAS) device, combined with monthly adherence counselling | Patients | Adherence counselling | CD4 counts, viral load, neuropsychological status, mood disorders and substance use | aACTG Baseline Adherence Questionnaire and electronic monitoring | No | Yes |
| Basso, 2013 | Brazil | 121 | Yes | Patient-related | No | CUIDADO (CARE) psychosocial intervention | Patient | Usual care | Viral load | bMEMS | No | No |
| Berrien, 2004 | USA | 37 | Yes | Complex | Yes | Structured home visits for education on adherence and HIV | Patient and caregiver | Usual care | Viral load, CD4 count | Self-report, pharmacy refill | Yes | No |
| Chung, 2011 | Kenya | 400 | Yes | Patient-related | No | Counselling, counselling plus alarm, alarm device | Patient | Usual care | Viral load, CD4 count, mortality | Pharmacy refill records | Yes | Yes |
| Collier, 2005 | USA, Puerto Rico, and Italy | 282 | Not Reported | Patient-related | No | Serial, supportive telephone calls | Patient | The usual adherence support measures | Virologic failure | Self-report | No | No |
| Cooper, 2010 | UK | 87 | Not Reported | Therapy- related | No | Once nightly regimen | Patient | Twice daily regimen | Viral load, cHAART beliefs & intrusiveness | bMEMS | Yes | Yes |
| Dejesus, 2009 | USA and Puerto Rico | 300 | No | Therapy-related | No | Single tablet regimen | Patient | ART | Virologic suppression, health related quality of life, HIV symptom index | Visual analog scale (VAS), pill count | No | Yes |
| DiIorio, 2008 | USA | 247 | Not Reported | Patient-related | No | Motivational interviewing | Patient | Usual care | CD4 cell count, viral load | bMEMS | Yes | No |
| Duncan, 2012 | Not reported | 76 | Yes | Patient-related | No | Mindfulness-based stress reduction | Patient | Standard care | CD4 counts, side effects, depression, perceived stress, positive and negative affect | aACTG self-report adherence measure, visual analogue scale | No | Yes |
| Fisher, 2011 | USA | 564 | Yes | Patient-related | No | Life-windows intervention (interactive computer-based ARV adherence promotion intervention) | Patient | Standard of care | Viral load | Self-report | Yes | No |
| Goggin, 2013 | USA | 204 | Yes | Patient-related | No | dMI-CBT/MDOT, MI-CBT | Patient | Standard care | Viral load | Electronic drug monitor (EDM) | No | No |
| Hersch, 2013 | Not reported | 168 | Yes | Patient-related | No | Life-steps program (computer delivered stress, mood and adherence management intervention) | Patient | Wait-list control | Viral load, self-reported outcomes | aSelf-report (ACTG) | Yes | Yes |
| Holstad, 2011 | USA | 207 | Yes | Patient-related | No | eKHARMA intervention | Patient | Health promotion program | Viral load, CD4 count | cMEMS | No | Yes |
| Johnson, 2011 | USA | 249 | Yes | Condition-related | No | BALANCE project experimental intervention based on management of side-effects | Patient | Usual care | fSECope questionnaire | Self-report | Yes | No |
| Kalichman, 2011 | USA | 436 | Yes | Patient-related | No | Integrated intervention enhancing patients' decision making skills | Patient | Attention control condition | STI infection, adherence and prevention strategies and risk compensation beliefs | Unannounced pill counts | Yes | Yes |
| Kalichman, 2013 | USA | 446 | Yes | Patient-related | No | Standard adherence counseling, pictograph-guided adherence counseling | Patient | General health improvement counseling | Viral load (blood test and medical records) | Pill count, pharmacy records | No | No |
| Knobel, 1999 | Spain | 170 | Not reported | Patient-related | No | Individual advise | Patient | Conventional care | Viral load, CD4 counts | Self-report, pill counts | Yes | Yes |
| Kunutsor, 2011 | Uganda | 174 | No | Social and economic factors | No | Treatment supporter initiative (trained confidante supports patient with antiretroviral therapy) | Patients, patients' family or friends | Standard adherence intervention | Mortality, clinic attendance | Pill count, self-report | Yes | No |
| Lester, 2010 | Kenya | 538 | Not reported | Patient-related | No | Text messages | Patient | Usual care | Viral load | Self-report | Yes | Yes |
| Letourneau, 2013 | Not reported | 34 | Yes | Complex | Yes | gMultisystemic therapy | Patients, caregivers, patients' family or friends | Usual care with motivational interviewing | Viral load, CD4 count | Self-reported adherence | No | No |
| Maitland, 2008 | Not reported | 96 | Not reported | Therapy-related | No | Once daily dosing | Patient | Usual dosing (twice daily) | Hospital Anxiety and Depression (HAD) score, CD4 cell count, viral load | bMEMS | Yes | No |
| Naar-King, 2013 | USA | 76 | Yes | Patient-related | Yes | Motivational enhancement system for adherence (MESA), a computer delivered motivational intervention | Patient | Motivational enhancement system for health (MESH) | Viral load | Pharmacy fill records | Yes | Uncertain |
| Parienti, 2007 | France | 62 | No | Therapy-related | No | Once a day dosing | Patient | Twice a day dosage | Viral control | bMEMS | No | No |
| Parsons, 2007 | USA | 143 | Yes | Patient-related | No | Project PLUS intervention (providing information and motivation to change behavior) | Patient | Education condition | Viral load, CD4 cell count | Self-report interview | Yes | Yes |
| Pearson, 2007 | Mozambique | 350 | Yes | Complex | No | Peer delivered modified directly observed therapy (MDOT) | Patients, caregivers | Standard care | CD4 cell count, mortality | Self-report - interview | Yes | No |
| Portsmouth, 2005 | UK | 43 | Not reported | Therapy-related | No | Once daily regimen | Patients | Twice daily regimen | Medical Outcomes Study HIV Health Survey (MOS-HIV) questionnaire, viral load, CD4 count, cognitive outcomes | bMEMS | Yes | No |
| Pradier, 2003 | France | 244 | Yes | Patient-related | No | Education and counselling | Patient | Standard care | Viral load, cHAART symptom scale, adverse events, toxicity | Self-report | Yes | No |
| Purcell, 2007 | USA | 966 | Yes | Complex | No | Peer mentoring intervention | Patient | Video discussion intervention | Sexual behavior, injection behavior, utilization of HIV care | Self-report | No | No |
| Pyne, 2011 | USA | 276 | Yes | Patient-related | No | hHIV translating initiatives for depression into effective solutions (HIVTIDES) | Patient | Usual care | Depression symptom severity, health status, severity of HIV symptoms | aACTG assessment - | No | Yes |
| Rawlings, 2003 | USA | 195 | Not reported | Complex | No | The Tools for Health and Empowerment (THE) course 11-module educational program for HIV-infected patients and their informal caregivers | Patient and caregiver | Routine counselling | Viral load | MEMS | No | No |
| Remien, 2005 | USA | 215 | Yes | Complex | No | A four-session couple-focused adherence program | Patient and partner | Usual care | Viral load, CD4 count | bMEMS | No | No |
| Robbins, 2013 | USA | 333 | Yes | Patient-related | No | Phone adherence intervention | Patient | Standard care | Time to virological failure, quality of life, symptom distress scores | Self-report | No | No |
| Romero Jimenez, 2013 | Not reported | 188 | Not Reported | Patient-related | No | Pharmacotherapy follow up | Patient | Usual care | Viral load | Simplified Medication Adherence Questionnaire (SMAQ) | No | Yes |
| Rosen, 2007 | USA | 56 | Yes | Patient-related | Uncertain | Contingency management to promote adherence | Patient | Supportive counselling condition | Viral load | bMEMS | Yes | Yes |
| Sabin, 2010 | China | 68 | Yes | Patient-related | No | Counselling based on electronic drug monitoring data | Patient | Standard care | Viral load, CD4 count | EDM, self-report survey | Yes | No |
| Samet, 2005 | USA | 151 | Yes | Patient-related | No | iAdherence to Drugs for HIV, an Experimental Randomized Enhancement (ADHERE) | Patient | Standard care | Viral load, CD4 count, alcohol severity and consumption | aACTG & bMEMS | No | No |
| Sarna, 2008 | Kenya | 234 | Not Reported | Patient-related | No | Modified directly observed therapy (M-DOT) | Patient | Standard care | Viral load, CD4 count, Beck depression inventory, body weight | Self-report, pill count | Yes | Yes |
| Simoni, 2007 | USA | 136 | Yes | Complex | No | Peer support | Patient | Standard care | Viral load, depressive symptomatology | jEDM, self-report | No | No |
| Simoni, 2009 | USA | 226 | Not reported | Complex | No | Peer support, pager messaging | Patient | Usual care | Viral load | Self-report, jEDM | Yes | No |
| Simoni, 2011 | China | 70 | Yes | Patient-related | No | Enhanced intervention arm (choice of electronic reminder device, three counselling session and minimal intervention) | Patients, patients' family or friends | Minimal intervention arm (education, pillbox and referral to peer support group) | Viral load, CD4 count | Self-report, MEMS | Yes | No |
| Simoni, 2013 | USA | 40 | Yes | Patient-related | No | Cognitive Behavior Theory Applied to Adherence (CBT-AD) | Patient | Usual care | Viral load, CD4 count | VAS, EDM | No | No |
| Sorensen, 2007 | USA | 66 | Not reported | Complex | Uncertain | Voucher intervention (vouchers exchangeable for goods and services & medication coaching) | Patient | Medication coaching | Weight, self-reported health (SF 36), viral load, CD4 | MEMS, pill count, self-report - questionnaire | Yes | No |
| Taiwo, 2010 | Nigeria | 499 | Not reported | Complex | Yes | Treatment partner intervention | Patients, caregivers, patients' family or friends | Standard care | CD4 counts, side effects, depression, perceived stress, positive and negative affect (PANAS) | Pharmacy fill record | Yes | No |
| Tsai, 2013 | USA | 135 | Not reported | Patient-related | No | Directly observed Fluoxetine treatment for depression | Patient | Referral for psychiatric treatment | Viral load | Pill count | No | No |
| Tuldra, 2000 | Spain | 116 | Yes | Patient-related | No | Psychoeducative intervention | Patient | Standard care | Viral load | Self-report- questionnaire | No | No |
| Wagner, 2013 | USA | 60 | Yes | Patient-related | No | Adherence readiness program (ARP) | Patient | Usual care | Viral load | Mean dose taking, mean dose timing | Yes | No |
| Wang, 2010 | China | 116 | Not reported | Complex | No | Nurse-delivered home visits | Patients, caregivers, patients' family or friends | Usual care | Quality of life (WHOQOL-BREF), symptoms of depression (SDS) | Self-report - questionnaire | Yes | Yes |
| Weber, 2004 | Switzerland | 60 | Yes | Patient-related | No | CBT | Patient | Standard care | Viral load, CD4 count, psychosocial measures | MEMS, self-report questionnaire | Yes | No |
Did intervention improve at least one adherence or clinical outcome.
ACTG, AIDS Clinical Trials Group.
MEMS, Medication Event Monitoring System.
HAART, Highly Active Anti-Retroviral Therapy.
MI-CBT/MDOT, Motivational Interviewing-Cognitive Behavioral Therapy/ Modified Directly Observed Therapy.
KHARMA, Keeping Healthy and Active with Risk Reduction and Medication Adherence (behavior change intervention).
The SECope questionnaire covers positive emotion focused coping, social support seeking, non-adherence, information seeking, and taking side effect medications.
A home and community-based therapy with treatment delivered at home or settings and times convenient for the families and empowering patients to manage issues that may arise.
HIVTIDES is a computer-based intervention that draws on the social cognitive theory and technology acceptance model.
Multicomponent motivational interviewing based intervention.
Electronic drug monitoring.
Studies
We extracted data from 49 RCTs published between 1999 and 2013. Two of them recruited from more than one country.36,37 Twenty-four were conducted in the USA,38–61 two in the UK,62,63 two in Spain,64,65 three in Kenya,66–68 two in France,69,70 three in China,71–73 one each in Brazil,74 Mozambique,75 Switzerland,76 Uganda,77 South Africa,78 and Nigeria.79 The country in which the study was conducted was not reported in five studies.80–84 The median sample size was 170 (min=34; max 966).
Participants
The participants in these studies were all people receiving antiretroviral therapy either curatively or as post-exposure prophylaxis. Some of them had co-morbidities, such as depressive symptoms, or had been identified as having challenges with adherence. Only five included participants aged less than 18 years.39,47,78,79,82
Interventions
Based on the factors responsible for poor adherence, the interventions were categorized as patient related (n=31),36,38,40–43,45–48,50,53–55,58,60,61,64–68,70–72,74,76,78,80,81,84 complex (n=11),39,49,51,52,56,57,59,73,75,79,82 therapy related (n=5),37,62,63,69,83 social and economic factors (n=1),77 and condition related (n=1).44 The interventions in thirty-nine of these studies targeted only the patient;36–38,40–50,53–71,74,76,80,81,83,84 the rest included, in addition to the patient, the patients' family care-givers and friends (they may have received counselling, education, home visits, or participated in observing the patient take medication).39,51,52,72,73,75,77–79,82 Thirty of these interventions were theory based (i.e., they explicitly applied a theoretical framework in the development of the intervention).39,41–50,52–55,57,58,61,65,66,70–72,74–76,78,80,81 Our attempts to group these interventions into meaningful categories did not fully address the detail and complexity of each intervention. More information on the interventions can be found in Table 1.
Outcomes
The most commonly reported clinical outcomes were viral load or CD4 count.36–43,45,47,48,51–72,74–76,79–84 Eleven studies addressed psychosocial outcomes, including depressive symptoms.38,50,53,57,68,73,76,78–80,83 A variety of measures were used for adherence (singly or in combination), including self-reported measures (the visual analogue scale, ACTG, SMAQ, questionnaires),36–39,41,44,48–50,53,55–59,64,65,67,68,70–73,75–77,80–82,84 pill counts,37,45–47,59,60,64,68,77,78 electronic devices (MEMS or EDM), 40,42,43,51,52,54–59,62,69,71,72,74,76,83 and pharmacy refill records.45–47,66,79 Only 22 of the included studies measured adverse events that may arise due to the intervention.36,37,40,45,48,51–54,59,60,62,63,65–67,69,70,72,78,80,83 Only five reported the cost of the intervention. 42,67,68,75,76
Risk of bias in included studies
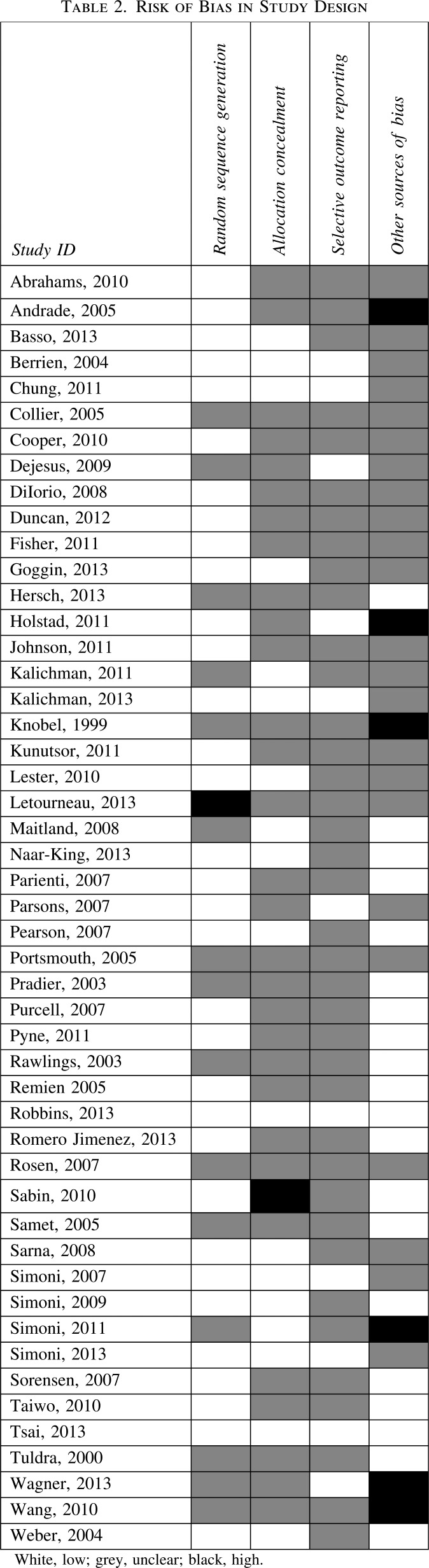
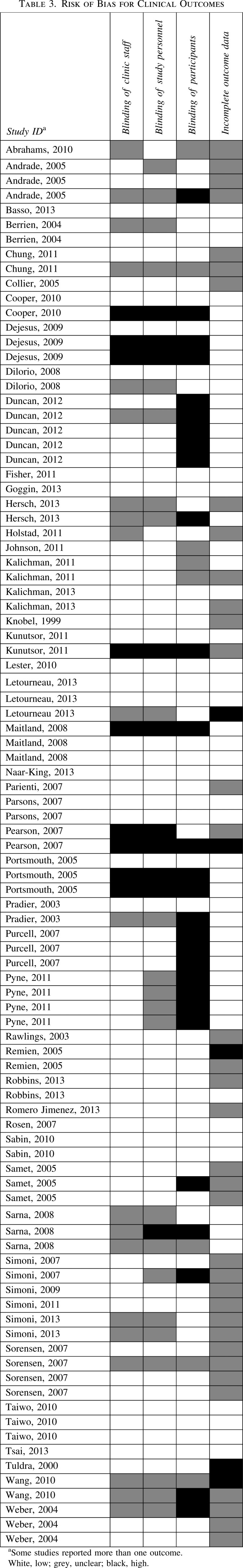
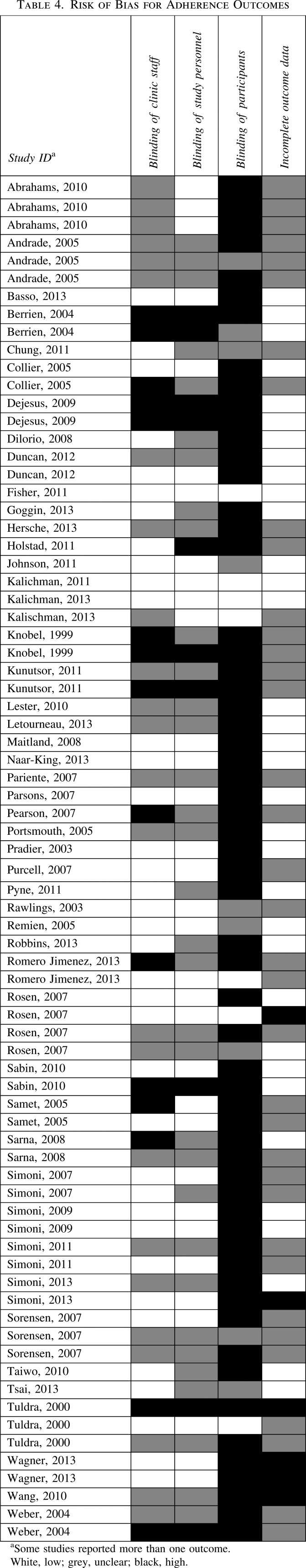
Risk of bias assessments are summarized in Tables 2, 3, and 4.
Random sequence generation
Most studies (33/49) reported an appropriate method of random sequence generation, except for 15, in which it was impossible to make a judgement,36,37,46,51,54,55,61,63–65,70,72,73,81,83 and one in which we judged the risk of bias to be high because some participants were allocated to the intervention arm without randomization.82
Allocation concealment
In most studies (30/49) it was impossible to determine how allocation was concealed. Seventeen of them reported appropriate measures of allocation concealment. One study was judged to be at high risk of bias because the randomization envelopes used were not sealed.71
Selective outcome reporting
Most of the time it was impossible to judge whether there was selective outcome reporting (38/49). The rest of the studies were judged to be at low risk of bias.
Other sources of bias
Six studies were exposed to other sources of bias. In one of these, some of the participants in both arms received an additional adherence intervention and the data collected on lab results did not coincide with follow-up time.43 In the second study, the intervention was very complex with multiple components seemingly biased towards finding an effect.73 In the third, the same nurses were involved in care for both groups, with a potential for contamination.72 In the fourth, the intervention was related to drug abuse despite a low prevalence among the study participants; as such the findings might not be generalizable to other populations.38 The fifth was an open study with self-reported measures of adherence;64 and in the last, the same counsellor provided services to participants in the intervention and control groups.61 We judged that at least 11 of the included studies were underpowered to show statistically significant differences in the primary outcome.38,39,47,58,60,61,63,65,74,76,82
Risk of bias for adherence outcomes
The 49 included studies reported on 76 adherence outcomes. Risk of bias for measuring adherence outcomes was generally high. Clinic staff were not blinded for 12 outcomes (12 studies);36,37,39,55,64,65,68,71,75–77,84 study personnel were not blinded for 8 outcomes (8 studies);36,37,39,64,65,71,76,77 participants were not blinded for 60 outcomes (39 studies);36–40,42,43,47–50,53–59,61,63–65,67–80,82–84 and there was incomplete data for five outcomes (5 studies).54,58,61,65,73
Risk of bias for clinical outcomes
The 49 included studies reported on 95 clinical outcomes. Clinic staff were not blinded for 9 outcomes (6 studies);37,62,63,75,77,83 study personnel were not blinded for 10 outcomes (7 studies).37,62,63,68,75,77,83 participants were not blinded for 28 outcomes (17 studies);37,38,49,50,55,57,62,63,68,70,73,75–77,80,81,83 there was evidence of incomplete outcome data for 2 outcomes (2 studies).65,73
Characteristics of excluded studies
In the parent Cochrane review, 1564 studies were excluded. Reasons for exclusion appear in Fig. 1, with additional information in that review.34
Effects of interventions
The effects of interventions are summarized in Table 1. Twenty-seven studies improved at least one adherence outcome,39–41,44,46–48,54,56,59,61–64,66–68,70–73,75–77,79,81,83 but only 16 improved at least one clinical outcome.37,38,43,46,48,50,54,62,64,66–68,73,80,81,84 Six studies reported improvements in clinical outcomes with no improvements in adherence.37,38,43,50,80,84 Only 10 studies improved both adherence and clinical outcomes.46,48,54,62,64,66–68,73,81
Details of the latter studies serve to illuminate the most potent interventions among the studies in this review. Chung and colleagues66 conducted a randomized controlled factorial design trial in 361 treatment naïve HIV-infected adults in Nairobi, Kenya initiating ART. The participants were randomized to one of four arms: counselling (three counselling sessions around ART initiation), alarm (pocket electronic pill reminder carried for 6 months), counselling plus alarm, and a control arm (neither counselling nor alarm). Adherence was measured using pharmacy refill records and the cut-off point for virological failure was greater than or equal to 5,000 copies/mL at least 4 months after initiating ART and participants were followed for 18 months. In this study, adherence counselling at initiation reduced the odds of virological failure [Hazard ratio (HR) 0.41; 95% CI 0.21–0.81; p=0.01] and poor adherence (HR 0.71; 95% CI 0.49–1.01; p=0.055) compared to no counselling. The use of an alarm device had no effect.
Knobel and colleagues64 in an open label randomized trial, provided either individual advice or conventional care to 170 HIV-infected adults receiving ART. The intervention group received individualized counselling and assessments which consisted of adaptation of treatment to the patient's lifestyle, detailed information about ART, phone support (for questions or medication-related problems), and monthly visits to the HIV day clinic. Adherence was measured using self-report and pill counts and follow up was for 24 weeks. Participants in the intervention group were more adherent [risk ratio (RR) 1.45; 95% CI 1.16–1.82; p=0.002] and had higher viral load suppression (1.98±0.7log 10/mL in the intervention group compared to 1.02±0.5log 10/mL in the control group; p=0.04).
In a multicenter open label randomized trial conducted in 9 UK sites,62 87 participants were randomized to either a once daily (didanosine, lamivudine, and efavirenz at night) versus a twice daily dosing regimen (zidovudine, lamivudine, and efavirenz) for 48 weeks. Adherence was estimated as a combination of persistence (duration on treatment) and execution (taking medication based on MEMS caps). The threshold for undetectable viral load in this study was less than 50 copies/mL. The once-daily group had significantly better adherence (p=0.0327) and were more likely to have undetectable viral load (p=0.001) at 48 weeks. There was no improvement in participants' beliefs about ART.
Lester and colleagues67 conducted a parallel group randomized controlled trial in three clinics in Kenya. In this study, 538 participants initiating ART (regimens determined based on national guidelines) were randomized to receive a short weekly text message or usual care. The message was “How are you?” and participants were required to respond “fine” or “bad”. They would be called by a study nurse for additional support if they didn't respond within 48 h or responded “bad”. The threshold for undetectable viral load was less than 400 copies/mL. Follow-up was for 48 weeks. Self-reported non-adherence (RR 0.81, 95% CI 0.69–0.94; p=0.006) and virological failure (RR 0.84, 95% CI 0.71–0.99; p=0.04) were lower in the intervention group.
Hersch and colleagues81 investigated the efficacy of a web-based programme to improve adherence to medication in people receiving ART. One hundred and sixty-eight adults were randomized to receive the web-based programme or a waitlist. The web-based program was an electronic adaptation of the Life-Steps intervention (a single session cognitive behavioral medication adherence program) with additional modules for stress reduction and mood management. The intervention group showed a slower decline in adherence rates (MEMS caps) than the control group (t=2.03, p<0.05) and a faster decline in viral load (t = −2.263, p=0.024) at 9 months.
Kalichman and colleagues46 conducted a randomized trial among 436 HIV-infected individuals in Georgia, USA to compare an integrated intensive behavioral intervention to an attention control group. This intervention (based on the conflict theory of decision making) involved one 45-min individual goal-setting session and five 120-min group sessions (which covered education on HIV, its treatment, decisional balance in various scenarios, safe sex practices, and adherence), then one final 60-min individual session to establish a personalized plan for treatment decisions, adherence, and safe sex. They used unannounced telephone-based pill counts to measure adherence to ART. At 9 months more participants in the control group reported new sexually transmitted infections [adjusted odds ratio (aOR) 3.0; p<0.05; 95% CI 1.01– 9.04] and greater adherence (Wald χ2=4.1; p<0.05). There was no effect on viral load.
Parsons and colleagues48 evaluated the effectiveness of Project PLUS (Positive Living through Understanding and Support). One hundred and forty three HIV-infected adults were randomized to Project PLUS or an educational intervention. Project PLUS entailed 8-sessions of motivational interviewing and cognitive-behavioral skills building. At 3 months, participants in the intervention group had significant decreases in viral load (a 1.0 log reduction in viral load OR 2.7; p=0.03), increases in CD4 cell count (10% or greater increase in CD4 count; OR=3.4; p=0.013) and adherence, measured using a timeline follow-back interview to estimate number of days adherence (F [1, 111]=4.1; p<0.05 and percentage of doses consumed (F[1, 107]=4.0; p<0.05). These effects were absent at 6 months.
Rosen and colleagues54 randomized 56 poorly adherent HIV-infected adults with a history of illicit substance use to receive 16 weeks of contingency management-based counselling or supportive counselling. Contingency management (CM) involves reinforcing medication taking with rewards. Participants in both arms were encouraged participate in weekly one-on-one counselling sessions. In the intervention group, adherence issues were discussed alongside printouts of MEMS-generated pill taking behavior. They also received monthly letters summarizing their MEMS data. The CM intervention was boosted with raffles for prizes to reward good adherence (potential total earnings were 800 dollars on average). The average MEMS-measured adherence increased significantly more in the intervention group (t=2.5, p=0.01). Viral load was lower in the intervention group at 16 weeks (F=6.0; p=0.02). These differences did not persist beyond the 16 week intervention period.
Sarna and colleagues68 conducted a randomized controlled trial to investigate the efficacy of modified directly observed therapy (mDOT) compared to standard care among 234 HIV-infected adults in Mombasa, Kenya. The mDOT group did twice weekly visits to the health center for a nurse to observe pill ingestion, receive adherence support, and collect medication, over a 24-week period. They were followed for another 24 weeks. For weeks 1–24, non-adherence (reported missed doses) was lower in the intervention group (9.1% vs. 19.1%; p=0.04). This difference did not persist in weeks 25–48. Viral suppression was more likely in the intervention group at week 72 (90% vs. 65.2%; p=0.027) for patients with depression. There were no differences in CD4 count, body mass index, or survival.
Wang and colleagues73 conducted a randomized parallel group trial of nurse-delivered home visits combined with telephone calls, compared to usual care in 116 HIV-infected past or active heroin users in Hunan, China. During these home visits, two qualified nurses provided information on HIV medication and adherence, introduced adherence management skills, reinforced motivation, mobilized family support, and lowered discrimination among family members. Electronic pill boxes and alarms were also provided. Four visits were given over 8 months. Phone calls were made by the same nurses who provided the visits every 2 weeks to assess adherence and well-being, and to provide more support. Participants in the intervention group were more likely to report 100% of pills taken (Fisher's exact=14.3, p<0.001) and to report taking pills on time (Fisher's exact=18.64, p<0.001). The intervention group also had better depression scores (F=5.58; p=0.02).
Effects by category
We attempted to narratively summarize sufficiently similar studies based on the category of intervention, low versus high income settings, interventions with a theoretical framework, and technology based interventions. Thirty-one interventions focused on patient-related adherence difficulties.36,38,40–43,45–48,50,53–55,58,60,61,64–68,70–72,74,76,78,80,81,84 Only 8 (26%) of them improved both adherence and clinical outcomes.46,48,54,64,66–68,81 One of 5 (20%) of the interventions targeting therapy-related adherence difficulties improved both adherence and clinical outcomes.62 One of 11 (9.1%) complex interventions improved adherence and clinical outcomes.73 None of the interventions for condition-related44 and socioeconomic difficulties77 was successful for both adherence and clinical outcomes.
Effects by setting
Five of the 33 interventions (15%) tested in high income settings were successful,46 48,54,62,64 compared to four of eleven (36%) in low income settings.66–68,73
Effects by theoretical framework
Five of the 30 (17%) studies with an explicit theoretical framework were successful in improving both adherence and clinical outcomes.46,48,54,66,81
Effects by use of technology
Five studies used technology-based interventions (interactive computers assisted sessions, phone calls, text messaging, and pagers).36,50,56,67,78 Only one (20%) of these improved both adherence and clinical outcomes.67
Discussion
Summary of main findings
In this systematic review of “high end” randomized trials of interventions to improve adherence to ART, we found that few interventions successfully improved both adherence and clinical outcomes. No clear factors could be identified that would explain why some interventions were more successful than others. Despite our purposeful selection of studies with at least 6 months follow-up and no more than 20% attrition, we still found high risks of bias across outcomes.
This report highlights a number of issues on the current state of the evidence. First, there is a paucity of high quality studies in low resource settings despite the higher disease burden for HIV.8 Second, the role of theoretical underpinnings in adherence research for HIV is unclear. One would assume that interventions based on some theory of behavior would stand a better chance of improving adherence. We found no evidence to support this. We postulate that the complexity of adherence behavior may be beyond the scope of any one single theory and that novel theories are warranted.
Most of the studies identified either targeted the patient dimension of adherence, therapy-related issues, or were complex. Not much research has been conducted on addressing socioeconomic (eliminating competing socioeconomic priorities that interfere with adherence) or condition-related limitations (symptom severity and level of disability) to adherence. However, many studies addressed depression as a co-morbidity that could affect adherence behaviors. We noted that the complexity of the intervention did not seem to correlate with outcomes. There were very few studies addressing adherence enhancement in adolescents and children, even though close to two million children aged 15 or less are currently receiving ART.8
A multitude of techniques were used to measure adherence, ranging from self-reported measures to electronic drug monitoring. This highlights the lack of a gold standard for measuring adherence,85 and the need to associate adherence measures with clinical outcomes. Many of the strategies used to ascertain levels of adherence and clinical outcome were at high risk of bias.
Agreements or disagreements with other reviews
Other reviews on interventions to improve adherence to ART have identified similar limitations, including the paucity of research on adherence enhancing interventions in younger populations,22 the need for behavioral theories that are relevant to Africa, the lack of data on cost-effectiveness,86 and methodological limitations.87 More complex interventions are not necessarily better than simple ones.87 One also reported finding studies that improved clinical outcomes without improvements in adherence, suggesting an alternate mechanism for health outcomes in children.22 Two context specific systematic reviews report that reminders may have beneficial effects on adherence in sub-Saharan Africa, though these effects are small.88,89 A third noted poor methodological quality and few effective interventions in developed countries.32 However, given the more stringent criteria used in this review, many trials included in other reviews might have been excluded here.
Limitations and strengths
This review has limitations. We sought to summarize data on studies with sufficient follow-up (at least 6 months) and limited attrition (at least 80% follow-up), but this strategy might have led to the exclusion of some studies that would shed more light on our findings. However, even in this subset of higher quality studies, we still found high risks of bias in study design and outcome assessment in many studies. The diversity in participants, interventions, comparisons, and outcomes measured prevented us from conducting any statistical pooling.
On the other hand, our choice of eligibility criteria purposefully helps us identify studies with low attrition bias, reporting on longer term adherence and clinical outcomes. Long-term outcomes are of importance with ART because it is a lifelong treatment. Clinical outcomes are useful in corroborating findings from imperfect adherence measures, and are the ultimate endpoint of treatment.
Conclusions
Our findings support testing more interventions to address adherence challenges, the need to develop a gold standard (or uniform measures) for adherence outcome ascertainment, and the investigation of adherence enhancing interventions using robust designs in younger populations and high disease burden settings.
Contributor Information
Collaborators: the Patient Adherence Review (PAR) Team
Acknowledgments
This review was partly supported by a Knowledge Synthesis Grant, KRS 262115, from the Canadian Institutes of Health Research (RB Haynes, Principal Investigator). Our thanks to Norma Brown for assisting with articles screening, and Sarah Quayyum for assisting with data extraction. Additionally, our thanks to Juan Manuel Reyes for assisting with the translation of the study published in Spanish. The PAR team members are: Norma Brown, Robby Nieuwlaat, Rebecca Jeffery, Thomas Agoritsas, Alfonso Iorio, Emma Iserman, Reem A Mustafa, Dawn Jedraszewski, Chris Cotoi (Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, Canada), Niraj Mistry (Department of Pediatrics, St. Michael's Hospital, Toronto, Canada), and Susan Jack (School of Nursing, Faculty of Health Sciences, McMaster University, Hamilton, Canada).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–497 [DOI] [PubMed] [Google Scholar]
- 2.WHO. Adherence to long-term therapies: Evidence for action. http://www.who.int/chp/knowledge/publications/adherence_report/en/ (Last accessed December4, 2014)
- 3.Chesney MA. Factors affecting adherence to antiretroviral therapy. Clin Infect Dis 2000;30:S171–S176 [DOI] [PubMed] [Google Scholar]
- 4.Rajasingham R, Mimiaga MJ, White JM, Pinkston MM, Baden RP, Mitty JA. A systematic review of behavioral and treatment outcome studies among HIV-infected men who have sex with men who abuse crystal methamphetamine. AIDS Patient Care STDS 2012;26:36–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez A, Barinas J, O'Cleirigh C. Substance use: Impact on adherence and HIV medical treatment. Curr HIV/AIDS Rep 2011;8:223–234 [DOI] [PubMed] [Google Scholar]
- 6.Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend 2010;112:178–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitalis D. Factors affecting antiretroviral therapy adherence among HIV-positive pregnant and postpartum women: An adapted systematic review. Int J STD AIDS 2013;24:427–432 [DOI] [PubMed] [Google Scholar]
- 8.UNAIDS. Global Report: UNAIDS report on the global AIDS epidemic 2013. http://www.unaids.org/en/resources/publications/2012/name,76121,en.asp (Last accessed December4, 2014)
- 9.Montaner JS, Reiss P, Cooper D, et al. A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: The INCAS Trial. Italy, The Netherlands, Canada and Australia Study. JAMA 1998;279:930–937 [DOI] [PubMed] [Google Scholar]
- 10.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000;133:21–30 [DOI] [PubMed] [Google Scholar]
- 11.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis 2006;43:939–941 [DOI] [PubMed] [Google Scholar]
- 12.Apisarnthanarak A, Mundy LM. Long-term outcomes of HIV-infected patients with <95% rates of adherence to nonnucleoside reverse-transcriptase inhibitors. Clin Infect Dis 2010;51:115–117 [DOI] [PubMed] [Google Scholar]
- 13.Mannheimer S, Friedland G, Matts J, Child C, Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis 2002;34:1115–1121 [DOI] [PubMed] [Google Scholar]
- 14.Haubrich RH, Little SJ, Currier JS, et al. The value of patient-reported adherence to antiretroviral therapy in predicting virologic and immunologic response. California Collaborative Treatment Group. AIDS 1999;13:1099–1107 [DOI] [PubMed] [Google Scholar]
- 15.Gill CJ, Hamer DH, Simon JL, Thea DM, Sabin LL. No room for complacency about adherence to antiretroviral therapy in sub-Saharan Africa. AIDS 2005;19:1243–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia de Olalla P, Knobel H, Carmona A, Guelar A, Lopez-Colomes JL, Cayla JA. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. J Acquir Immune Defic Syndr 2002;30:105–110 [DOI] [PubMed] [Google Scholar]
- 17.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS 2001;15:1181–1183 [DOI] [PubMed] [Google Scholar]
- 18.Knobel H, Codina C, Miro JM, et al. [The recommendations of GESIDA/SEFH/PNS for improving adherence to antiretroviral treatment. AIDS Study Group of the Spanish Society of Hospital Pharmacy and the National Plan on AIDS of the Minister of Health and Consumers]. Enferm Infecc Microbiol Clin 2000;18:27–39 [PubMed] [Google Scholar]
- 19.Boden D, Hurley A, Zhang L, et al. HIV-1 drug resistance in newly infected individuals. JAMA 1999;282:1135–1141 [DOI] [PubMed] [Google Scholar]
- 20.Hecht FM, Grant RM, Petropoulos CJ, et al. Sexual transmission of an HIV-1 variant resistant to multiple reverse-transcriptase and protease inhibitors. N Engl J Med 1998;339:307–311 [DOI] [PubMed] [Google Scholar]
- 21.Ortego C, Huedo-Medina TB, Llorca J, et al. Adherence to highly active antiretroviral therapy (HAART): A meta-analysis. AIDS Behav 2011;15:1381–1396 [DOI] [PubMed] [Google Scholar]
- 22.Bain-Brickley D, Butler LM, Kennedy GE, Rutherford GW. Interventions to improve adherence to antiretroviral therapy in children with HIV infection. Cochrane Database Syst Rev 2011:CD009513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghidei L, Simone MJ, Salow MJ, et al. Aging, antiretrovirals, and adherence: A meta analysis of adherence among older HIV-infected individuals. Drugs Aging 2013;30:809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nel A, Kagee A. Common mental health problems and antiretroviral therapy adherence. AIDS Care 2011;23:1360–1365 [DOI] [PubMed] [Google Scholar]
- 25.Sin NL, Dimatteo MR. Depression treatment enhances adherence to antiretroviral therapy: A meta-analysis. Ann Behav Med 2014;47:259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill S, Kavookjian J. Motivational interviewing as a behavioral intervention to increase HAART adherence in patients who are HIV-positive: A systematic review of the literature. AIDS Care 2012;24:583–592 [DOI] [PubMed] [Google Scholar]
- 27.Mbuagbaw L, Ye C, Thabane L. Motivational interviewing for improving outcomes in youth living with HIV. Cochrane Library 2012;9:CD009748. [DOI] [PubMed] [Google Scholar]
- 28.Horvath T, Azman H, Kennedy GE, Rutherford GW. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev 2012;3:CD009756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills EJ, Nachega J, Lester RT, et al. Adherence interventions to improve adherence to antiretroviral therapy in low income settings: An individual patient data network meta-analysis. Value Health 2013;16:A361 [Google Scholar]
- 30.Mbuagbaw L, van der Kop ML, Lester RT, et al. Mobile phone text messages for improving adherence to antiretroviral therapy (ART): An individual patient data meta-analysis of randomised trials. BMJ Open 2013;3:e003950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendelsohn JB, Schilperoord M, Spiegel P, Ross DA. Adherence to antiretroviral therapy and treatment outcomes among conflict-affected and forcibly displaced populations: A systematic review. Confl Health 2012;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathes T, Pieper D, Antoine SL, Eikermann M. Adherence-enhancing interventions for highly active antiretroviral therapy in HIV-infected patients. A systematic review. HIV Med 2013;14:583–595 [DOI] [PubMed] [Google Scholar]
- 33.Charania MR, Marshall KJ, Lyles CM, et al. Identification of evidence-based interventions for promoting HIV medication adherence: Findings from a systematic review of U.S.-based studies, 1996–2011. AIDS Behav 2014;9:646–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database System Rev 2014;2:CD000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol 2009;62:1006–1012 [DOI] [PubMed] [Google Scholar]
- 36.Collier AC, Ribaudo H, Mukherjee AL, Feinberg J, Fischl MA, Chesney M. A randomized study of serial telephone call support to increase adherence and thereby improve virologic outcome in persons initiating antiretroviral therapy. J Infect Dis 2005;192:1398–1406 [DOI] [PubMed] [Google Scholar]
- 37.Dejesus E, Young B, Morales-Ramirez JO, et al. Simplification of antiretroviral therapy to a single-tablet regimen consisting of efavirenz, emtricitabine, and tenofovir disoproxil fumarate versus unmodified antiretroviral therapy in virologically suppressed HIV-1-infected patients. J Acquir Immune Defic Syndr 2009;51:163–174 [DOI] [PubMed] [Google Scholar]
- 38.Andrade AS, McGruder HF, Wu AW, et al. A programmable prompting device improves adherence to highly active antiretroviral therapy in HIV-infected subjects with memory impairment. Clin Infect Dis 2005;41:875–882 [DOI] [PubMed] [Google Scholar]
- 39.Berrien VM, Salazar JC, Reynolds E, McKay K. Adherence to antiretroviral therapy in HIV-infected pediatric patients improves with home-based intensive nursing intervention. AIDS Patient Care STDS 2004;18:355–363 [DOI] [PubMed] [Google Scholar]
- 40.DiIorio C, McCarty F, Resnicow K, et al. Using motivational interviewing to promote adherence to antiretroviral medications: A randomized controlled study. AIDS Care 2008;20:273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher JD, Amico KR, Fisher WA, et al. Computer-based intervention in HIV clinical care setting improves antiretroviral adherence: The LifeWindows Project. AIDS Behav 2011;15:1635–1646 [DOI] [PubMed] [Google Scholar]
- 42.Goggin K, Gerkovich MM, Williams KB, et al. A randomized controlled trial examining the efficacy of motivational counseling with observed therapy for antiretroviral therapy adherence. AIDS Behav 2013;17:1992–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holstad MM, DiIorio C, Kelley ME, Resnicow K, Sharma S. Group motivational interviewing to promote adherence to antiretroviral medications and risk reduction behaviors in HIV infected women. AIDS Behav 2011;15:885–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson MO, Dilworth SE, Taylor JM, Neilands TB. Improving coping skills for self-management of treatment side effects can reduce antiretroviral medication nonadherence among people living with HIV. Ann Behav Med 2011;41:83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalichman SC, Cherry C, Kalichman MO, et al. Randomized clinical trial of HIV treatment adherence counseling interventions for people living with HIV and limited health literacy. J Acquir Immune Defic Syndr 2013;63:42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalichman SC, Cherry C, Kalichman MO, et al. Integrated behavioral intervention to improve HIV/AIDS treatment adherence and reduce HIV transmission. Am J Public Health 2011;101:531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naar-King S, Outlaw AY, Sarr M, et al. Motivational Enhancement System for Adherence (MESA): Pilot randomized trial of a brief computer-delivered prevention intervention for youth initiating antiretroviral treatment. J Pediatr Psychol 2013;38:638–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: A randomized controlled trial. J Acquir Immune Defic Syndr 2007;46:443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Purcell DW, Latka MH, Metsch LR, et al. Results from a randomized controlled trial of a peer-mentoring intervention to reduce HIV transmission and increase access to care and adherence to HIV medications among HIV-seropositive injection drug users. J Acquir Immune Defic Syndr 2007;46:S35–S47 [DOI] [PubMed] [Google Scholar]
- 50.Pyne JM, Fortney JC, Curran GM, et al. Effectiveness of collaborative care for depression in human immunodeficiency virus clinics. Arch Intern Med 2011;171:23–31 [DOI] [PubMed] [Google Scholar]
- 51.Rawlings MK, Thompson MA, Farthing CF, et al. Impact of an educational program on efficacy and adherence with a twice-daily lamivudine/zidovudine/abacavir regimen in underrepresented HIV-infected patients. J Acquir Immune Defic Syndr 2003;34:174–183 [DOI] [PubMed] [Google Scholar]
- 52.Remien RH, Stirratt MJ, Dolezal C, et al. Couple-focused support to improve HIV medication adherence: A randomized controlled trial. AIDS 2005;19:807–814 [DOI] [PubMed] [Google Scholar]
- 53.Robbins GK, Testa MA, Su M, et al. Site nurse-initiated adherence and symptom support telephone calls for HIV-positive individuals starting antiretroviral therapy, ACTG 5031: Substudy of ACTG 384. HIV Clin Trials 2013;14:235–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosen MI, Dieckhaus K, McMahon TJ, et al. Improved adherence with contingency management. AIDS Patient Care STDS 2007;21:30–40 [DOI] [PubMed] [Google Scholar]
- 55.Samet JH, Horton NJ, Meli S, et al. A randomized controlled trial to enhance antiretroviral therapy adherence in patients with a history of alcohol problems. Antivir Ther 2005;10:83–93 [DOI] [PubMed] [Google Scholar]
- 56.Simoni JM, Huh D, Frick PA, et al. Peer support and pager messaging to promote antiretroviral modifying therapy in Seattle: A randomized controlled trial. J Acquir Immune Defic Syndr 2009;52:465–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simoni JM, Pantalone DW, Plummer MD, Huang B. A randomized controlled trial of a peer support intervention targeting antiretroviral medication adherence and depressive symptomatology in HIV-positive men and women. Health Psychol 2007;26:488–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simoni JM, Wiebe JS, Sauceda JA, et al. A preliminary RCT of CBT-AD for adherence and depression among HIV-positive Latinos on the U.S.-Mexico border: The Nuevo Dia study. AIDS Behav 2013;17:2816–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sorensen JL, Haug NA, Delucchi KL, et al. Voucher reinforcement improves medication adherence in HIV-positive methadone patients: A randomized trial. Drug Alcohol Depend 2007;88:54–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsai AC, Karasic DH, Hammer GP, et al. Directly observed antidepressant medication treatment and HIV outcomes among homeless and marginally housed HIV-positive adults: A randomized controlled trial. Am J Public Health 2013;103:308–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wagner GJ, Lovely P, Schneider S. Pilot controlled trial of the adherence readiness program: An intervention to assess and sustain HIV antiretroviral adherence readiness. AIDS Behav 2013;17:3059–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cooper V, Horne R, Gellaitry G, et al. The impact of once-nightly versus twice-daily dosing and baseline beliefs about HAART on adherence to efavirenz-based HAART over 48 weeks: The NOCTE study. J Acquir Immune Defic Syndr 2010;53:369–377 [DOI] [PubMed] [Google Scholar]
- 63.Portsmouth SD, Osorio J, McCormick K, Gazzard BG, Moyle GJ. Better maintained adherence on switching from twice-daily to once-daily therapy for HIV: A 24-week randomized trial of treatment simplification using stavudine prolonged-release capsules. HIV Med 2005;6:185–190 [DOI] [PubMed] [Google Scholar]
- 64.Knobel H, Carmona A, Lopez JL, et al. [Adherence to very active antiretroviral treatment: impact of individualized assessment]. Enferm Infecc Microbiol Clin. 1999;17:78–81 [PubMed] [Google Scholar]
- 65.Tuldra A, Fumaz CR, Ferrer MJ, et al. Prospective randomized two-arm controlled study to determine the efficacy of a specific intervention to improve long-term adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2000;25:221–228 [DOI] [PubMed] [Google Scholar]
- 66.Chung MH, Richardson BA, Tapia K, et al. A randomized controlled trial comparing the effects of counseling and alarm device on HAART adherence and virologic outcomes. PLoS Med 2011;8:e1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): A randomised trial. Lancet 2010;376:1838–1845 [DOI] [PubMed] [Google Scholar]
- 68.Sarna A, Luchters S, Geibel S, et al. Short- and long-term efficacy of modified directly observed antiretroviral treatment in Mombasa, Kenya: A randomized trial. J Acquir Immune Defic Syndr 2008;48:611–619 [DOI] [PubMed] [Google Scholar]
- 69.Parienti JJ, Massari V, Reliquet V, et al. Effect of twice-daily nevirapine on adherence in HIV-1-infected patients: A randomized controlled study. AIDS 2007;21:2217–2222 [DOI] [PubMed] [Google Scholar]
- 70.Pradier C, Bentz L, Spire B, et al. Efficacy of an educational and counseling intervention on adherence to highly active antiretroviral therapy: French prospective controlled study. HIV Clin Trials 2003;4:121–131 [DOI] [PubMed] [Google Scholar]
- 71.Sabin LL, DeSilva MB, Hamer DH, et al. Using electronic drug monitor feedback to improve adherence to antiretroviral therapy among HIV-positive patients in China. AIDS Behav 2010;14:580–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simoni JM, Chen WT, Huh D, et al. A preliminary randomized controlled trial of a nurse-delivered medication adherence intervention among HIV-positive outpatients initiating antiretroviral therapy in Beijing, China. AIDS Behav 2011;15:919–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang H, Zhou J, Huang L, Li X, Fennie KP, Williams AB. Effects of nurse-delivered home visits combined with telephone calls on medication adherence and quality of life in HIV-infected heroin users in Hunan of China. J Clin Nurs 2010;19:380–388 [DOI] [PubMed] [Google Scholar]
- 74.Basso CR, Helena ET, Caraciolo JM, Paiva V, Nemes MI. Exploring ART intake scenes in a human rights-based intervention to improve adherence: A randomized controlled trial. AIDS Behav 2013;17:181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pearson CR, Micek MA, Simoni JM, et al. Randomized control trial of peer-delivered, modified directly observed therapy for HAART in Mozambique. J Acquir Immune Defic Syndr 2007;46:238–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weber R, Christen L, Christen S, et al. Effect of individual cognitive behaviour intervention on adherence to antiretroviral therapy: Prospective randomized trial. Antivir Ther 2004;9:85–95 [PubMed] [Google Scholar]
- 77.Kunutsor S, Walley J, Katabira E, et al. Improving clinic attendance and adherence to antiretroviral therapy through a treatment supporter intervention in Uganda: A randomized controlled trial. AIDS Behav 2011;15:1795–1802 [DOI] [PubMed] [Google Scholar]
- 78.Abrahams N, Jewkes R, Lombard C, Mathews S, Campbell J, Meel B. Impact of telephonic psycho-social support on adherence to post-exposure prophylaxis (PEP) after rape. AIDS Care 2010;22:1173–1181 [DOI] [PubMed] [Google Scholar]
- 79.Taiwo BO, Idoko JA, Welty LJ, et al. Assessing the viorologic and adherence benefits of patient-selected HIV treatment partners in a resource-limited setting. J Acquir Immune Defic Syndr 2010;54:85–92 [DOI] [PubMed] [Google Scholar]
- 80.Duncan LG, Moskowitz JT, Neilands TB, Dilworth SE, Hecht FM, Johnson MO. Mindfulness-based stress reduction for HIV treatment side effects: A randomized, wait-list controlled trial. J Pain Symptom Manage 2012;43:161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hersch RK, Cook RF, Billings DW, et al. Test of a web-based program to improve adherence to HIV medications. AIDS Behav 2013;17:2963–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Letourneau EJ, Ellis DA, Naar-King S, Chapman JE, Cunningham PB, Fowler S. Multisystemic therapy for poorly adherent youth with HIV: Results from a pilot randomized controlled trial. AIDS Care 2013;25:507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maitland D, Jackson A, Osorio J, Mandalia S, Gazzard BG, Moyle GJ. Switching from twice-daily abacavir and lamivudine to the once-daily fixed-dose combination tablet of abacavir and lamivudine improves patient adherence and satisfaction with therapy. HIV Med 2008;9:667–672 [DOI] [PubMed] [Google Scholar]
- 84.Romero Jimenez RM, Calleja Hernandez MA, Chaparro Recio M, Martinez Martinez F, Sanjurjo Saez M. Effect of pharmacotherapy follow-up on treatment adherence and virologic and immune response in patients with human immunodeficiency virus. Latin Am J Pharma 2013;32:441–447 [Google Scholar]
- 85.Mayer KH, Stone VE. Strategies for optimizing adherence to highly active antiretroviral therapy: Lessons from research and clinical practice. Clin Infect Dis 2001;33:865–872 [DOI] [PubMed] [Google Scholar]
- 86.Bärnighausen T, Chaiyachati K, Chimbindi N, Peoples A, Haberer J, Newell M-L. Interventions to increase antiretroviral adherence in sub-Saharan Africa: A systematic review of evaluation studies. Lancet Infect Dis 2011;11:942–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chaiyachati KH, Ogbuoji O, Price M, Suthar AB, Negussie EK, Barnighausen T. Interventions to improve adherence to antiretroviral therapy: A rapid systematic review. AIDS 2014;28:S187–S204 [DOI] [PubMed] [Google Scholar]
- 88.Mathes T, Antoine S-L, Pieper D. Adherence-enhancing interventions for active antiretroviral therapy in sub-Saharan Africa: A systematic review and meta-analysis. Sexual Health 2014;11:230–239 [DOI] [PubMed] [Google Scholar]
- 89.Barnighausen T, Chaiyachati K, Chimbindi N, Peoples A, Haberer J, Newell ML. Interventions to increase antiretroviral adherence in sub-Saharan Africa: A systematic review of evaluation studies. Lancet Infect Dis 2011;11:942–951 [DOI] [PMC free article] [PubMed] [Google Scholar]