Abstract
Many nutrient biomarkers are altered by inflammation. We calculated adjustment factors for retinol and ferritin by using meta-analyses of studies containing the respective biomarker and 2 acute phase proteins in serum, C-reactive protein (CRP), and α1-acid glycoprotein (AGP). With the use of CRP and AGP we identified 4 groups in each study: reference (CRP ≤5 mg/L, AGP ≤1 g/L), incubation (CRP >5 mg/L, AGP ≤1 g/L), early convalescence (CRP >5 mg/L, AGP >1 g/L), and late convalescence (CRP ≤5 mg/L, AGP >1 g/L). For each biomarker, ratios of the geometric means of the reference to each inflammation group concentration were used to calculate adjustment factors for retinol (1.13, 1.24, and 1.11) and ferritin (0.77, 0.53, and 0.75) for the incubation, early, and late convalescent groups, respectively. The application of the meta-analysis factors in more recent studies compares well with study-specific factors. The same method was used to calculate adjustment factors for soluble transferrin receptor (sTfR) and body iron stores (BISs) in Lao children. We found no advantage in adjusting sTfR for inflammation; in fact, adjustment decreased iron deficiency. Neither adjusted (10% <0 mg/kg) nor nonadjusted (12% <0 mg/kg) BISs detected as much iron deficiency as did ferritin (18% <12μg/L) and adjusted ferritin (21% <12 μg/L) unless the cutoff for BISs was increased from 0 to <3 mg/kg. However, we could find no evidence that the larger number of children identified as having BISs <3 mg/kg had risks of anemia comparable to those identified by using ferritin <12 μg/L. In conclusion, both corrected and uncorrected ferritin concentrations <12 μg/L are associated with more iron deficiency and anemia than either sTfR >8.3 mg/L or BISs <0 mg/kg in Lao children.
Keywords: acute phase proteins, ferritin, iron, retinol, soluble transferrin receptor
Introduction
Biochemical nutritional status is usually assessed by measuring the quantity of a nutrient or biomarker in blood or other body fluid. Disease impacts directly and indirectly on these measurements. Direct effects of illness on nutrient concentrations can operate through a decrease in appetite, whereas indirect effects are usually through alterations in immune activity. Researchers are generally aware that, during illness, measurements of nutrient concentrations may be misleading indicators of nutritional status and that the effects of disease on nutritional status of patients will be influenced by both the duration and severity of an illness. When the illness subsides, a period of subclinical inflammation usually follows during which nutritional biomarkers may continue to be misleading. Thus, in an apparently healthy population, if there is subclinical inflammation, nutritional biomarkers may not accurately reflect status. Verbal reports from a subject or family member can provide information on recent morbidity, and such reports have sometimes been shown to associate significantly with abnormal biochemical profiles of child health (1); however, to accurately interpret nutritional indices, specific biomarkers of subclinical inflammation must be obtained to quantify residual effects of morbidity on nutritional status (2, 3).
The acute phase protein (APP)7 C-reactive protein (CRP) has been used widely to monitor inflammatory processes (4). Serum concentrations of CRP and other APPs [e.g., serum amyloid A (SAA) and α1-antichymotrypsin (ACT)] increase rapidly within the first 6 h of the onset of an infection or trauma and reach maximum concentrations between 24 and 48 h (5, 6). The concentrations of many serum nutrients or nutritional biomarkers also change rapidly in the same time interval [e.g., retinol (7), iron and zinc (8), 25-hydroxycholecalciferol (vitamin D) (9), and ferritin (10)], and the concentrations of several others are known to be low in the presence of elevated inflammatory proteins [e.g., carotenoids (11), pyridoxal phosphate (12), selenium, and vitamin C (4)]. The rapid increase in CRP, SAA, and ACT concentrations illustrates the acute response to trauma and, with the disappearance of the inflammatory stimulus, concentrations decrease rapidly early in convalescence (13). Nutritional biomarkers may not return to normal as quickly as CRP as was shown by ferritin after in vivo administration of inflammatory cytokines in humans (10). Another protein, α1-acid glycoprotein (AGP), is more suitable to monitor the inflammatory response during convalescence (13). AGP increases more slowly than CRP and may take 2–5 d to achieve maximum concentrations (5, 14) and can then remain elevated for some time during convalescence or chronic infection (13, 15).
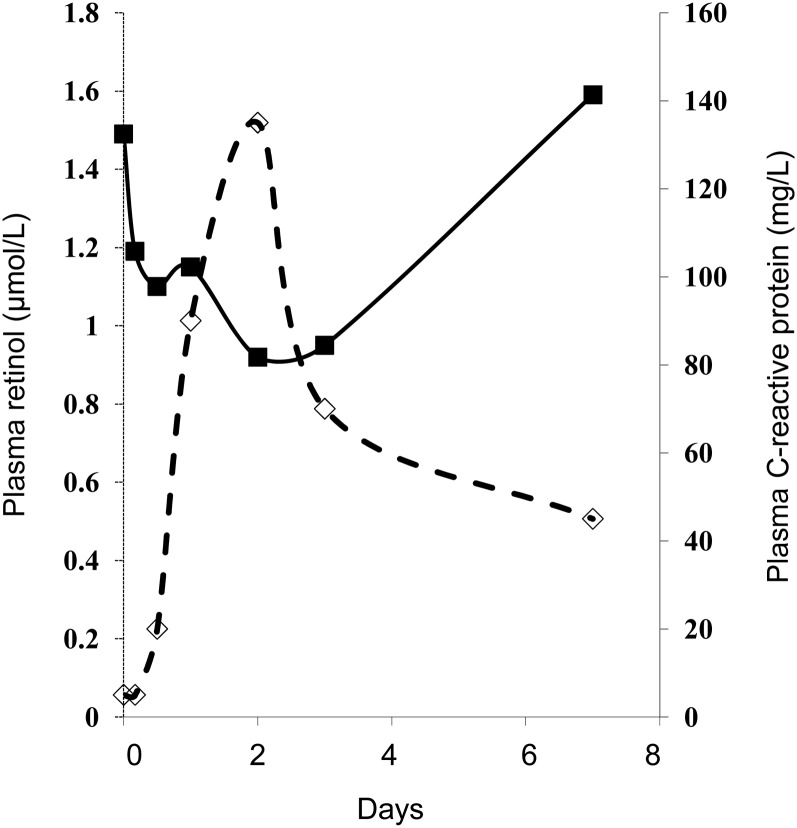
Surgery provides the opportunity to examine the influence of an altered inflammatory state on plasma nutrient concentrations without the complications of disease (9). Community studies showed that concentrations of both plasma retinol and retinol binding protein are depressed in cases in which there is exposure to inflammation (16–18), and sequential monitoring of plasma retinol concentration before and after surgical trauma showed a rapid decrease followed by a gradual return to normal values during recovery (Figure 1) (7). The apparent return to normal by plasma retinol concentrations as CRP concentrations normalized suggests that there were no long-term effects of the surgery on vitamin A status.
FIGURE 1.
Changes in plasma retinol and C-reactive protein concentrations in a group of nutritionally adequate patients in response to uncomplicated orthopedic surgery. A rapid decrease in plasma retinol concentration (solid line) coincides with an increase in C-reactive protein (dashed line) in the first 48 h after surgery. Data from reference (7).
Plasma ferritin concentrations are also influenced by inflammation, and the rapid increase in response to surgery increased in parallel with CRP but then remained elevated in association with AGP when CRP declined (10). The behavior of ferritin indicates that no single APP will fully reflect the behavior of nutrient biomarker concentrations during an inflammatory response. In the following section, we show how we used the 2 plasma APP concentrations, CRP and AGP, to provide a method of correcting plasma retinol and ferritin concentrations for the influence of subclinical inflammation. We also used the same method to adjust concentrations of soluble transferrin receptor (sTfR) and body iron stores (BISs) and discuss the results.
Methods
The data used in this article were obtained from published work (Table 1). To calculate adjustment factors from studies in apparently healthy subjects, articles were identified in which the 2 serum APPs were measured together with the relevant nutrient. The 2 APPs were used to define the reference group and 3 inflammation groups and ratios of the respective nutrient concentrations of the reference group and each inflammation group provided the adjustment values. The ratios obtained from the meta-analyses on the retinol and ferritin data are shown as means (95% CIs) (Table 2) (2, 3).
TABLE 1.
Studies used for 2 meta-analyses to calculate adjustment factors for retinol and ferritin1
| Retinol |
|||
| Analysis type and study characteristics | Apparently healthy subjects | All possible groups for 4-group analyses | Ferritin |
| Two-group analyses | |||
| Total number of studies | 152 | 30 | |
| Total number of subjects | 9914 | 8796 | |
| Four-group analyses | |||
| Total number of studies | 43 | 73 | 223 |
| Total number of subjects | 4486 | 4975 | 7848 |
| Infants (studies) | 0 | 1 | 5 |
| Preschool-aged children (studies) | 44 | 54 | 35 |
| Nonpregnant women (studies) | 0 | 0 | 5 |
| Pregnant and lactating women (studies) | 0 | 1 | 6 |
| Men (studies) | 0 | 0 | 3 |
All studies were used for the 2-group analysis. Some studies only included 1 acute phase protein, and some studies were described as asymptomatic (e.g., where subjects were known to be HIV positive, known to live in malaria-endemic areas, or known to have been sick with various symptoms in the previous 2 wk).
Only included studies where there were subjects in the reference and the 3 inflammation groups. The 4 studies analyzed separately excluded Nigerian neonates (acute phase response possibly immature), pregnant women (possible differences in C-reactive protein response from nonpregnant women), and preschool-aged children from Papua New Guinea (possible exposure to malaria).
One study from Guatemala included children aged from 2 mo to 5 y.
Included both preschool-aged and older children.
TABLE 2.
Summary results from 4-group meta-analyses of serum retinol and ferritin in apparently healthy subjects1
| Retinol (n = 4) |
Ferritin (n = 22) |
|||
| Group comparison | Mean (95% CI) | P | Mean (95% CI) | P |
| Reference vs. incubation | 1.15 (0.98, 1.36) | 0.09 | 1.30 (1.15, 1.47) | <0.001 |
| Reference vs. early convalescence | 1.32 (1.12, 1.55) | 0.001 | 1.90 (1.51, 2.37) | <0.001 |
| Reference vs. late convalescence | 1.12 (0.94, 1.34) | 0.18 | 1.36 (1.19, 1.55) | <0.001 |
Values are mean (95% CI) ratios of the geometric mean nutrient concentrations for the respective pairs from the 4-group analyses. For retinol, the 4 studies were all conducted in preschool-aged children, but similar mean ratios (1.14, 1.31, and 1.12) were obtained when 7 studies were included in the analysis by adding Nepalese pregnant women, neonates, and another study in preschool-aged children (2, 3) (see Table 1 for more details).
The retinol adjustment factors from the meta-analysis were compared with those from a specific study in Kenyan men and women. The effects of the 2 methods of adjustment on their retinol values were similar and are shown as medians and quartiles (Table 3). In the case of ferritin, we compared the use of adjustment factors to remove the effects of inflammation with those obtained by other workers in 2 separate studies (19) (Table 4). In addition, in a study in Lao children (20), we examined the effects of inflammation on ferritin, sTfR, and BISs to determine the effects on iron deficiency and anemia (Table 5). In all of the work, significance was assessed on the basis of P < 0.05.
TABLE 3.
Comparison of meta-analysis factors and study-generated factors to adjust plasma retinol concentrations for the influence of subclinical inflammation in apparently healthy HIV-1–seropositive Kenyan adults
| Inflammation group |
|||||
| Reference | Incubation | Early convalescence | Late convalescence | All subjects | |
| n | 66 | 18 | 65 | 14 | 163 |
| Plasma retinol,1 μmol/L | 1.33 (1.00–1.56) | 1.25 (0.84–1.54) | 1.01 (0.61–1.33) | 1.10 (0.84–1.41) | 1.16 (0.87–1.47) |
| Plasma retinol <0.7 μmol/L, n (%) | 6 (9) | 4 (22) | 19 (29) | 3 (21) | 32 (20) |
| Adjustment factors | — | 1.13 | 1.24 | 1.11 | — |
| Plasma retinol after adjustment,2 μmol/L | 1.33 (1.00–1.56) | 1.41 (0.94–1.74) | 1.25 (0.75–1.65) | 1.22 (0.93–1.56) | 1.31 (0.94–1.62) |
| Adjusted plasma retinol <0.7 μmol/L, n (%) | 6 (9) | 2 (11) | 15 (23) | 1 (7) | 24 (15) |
TABLE 4.
Adjustment factors for serum ferritin from the meta-analysis and 2 other studies1
| Study | Incubation | Early convalescence | Late convalescence |
| Meta-analysis (3) | 0.77 | 0.53 | 0.75 |
| Kenyan preschool-aged children (19) | 0.54 | 0.35 | 0.62 |
| Liberian preschool-aged children2 | 0.68 | 0.38 | 0.72 |
The studies by Grant et al. (19) and Kupka et al. were both conducted in malaria-endemic areas. There were 680 Kenyan children of whom 42 were malaria positive. There were 1090 Liberian preschool-aged children who were malaria-antigen negative.
R Kupka, P Mathema, and R Johnson, UNICEF, Liberia, personal communication, 2011.
TABLE 5.
Impact of inflammation on risk of anemia in children with serum ferritin <12 μg/L compared with those with body iron stores <3 and <0 mg/kg1
| Variable | Reference group, n (%) | Combined inflammation groups, n (%) | P2 | |
| Inflammation-corrected ferritin concentrations <12 μg/L (n = 102) | Hemoglobin <110 g/L | 29 (50) | 34 (77.3) | 0.004 |
| Hemoglobin ≥110 g/L | 29 (50) | 10 (22.7) | ||
| Inflammation-corrected body iron stores <3 mg/kg (n = 143) | Hemoglobin <110 g/L | 42 (52.5) | 41 (65.1) | 0.089 |
| Hemoglobin ≥110 g/L | 38 (47.5) | 22 (34.9) | ||
| Inflammation-corrected body iron stores <0 mg/kg (n = 73) | Hemoglobin <110 g/L | 23 (57.5) | 29 (87.9) | 0.004 |
| Hemoglobin ≥110 g/L | 17 (42.5) | 4 (12.1) |
Data from reference 20.
P values indicate differences in proportions of anemia between reference and the combined inflammation groups of data from subjects with ferritin <12 μg/L or body iron stores <3 and <0 mg/kg (chi-square tests).
Results and Discussion
Categorizing subclinical inflammation in apparently healthy people.
Nutrient concentrations are influenced by disease, and in any community there will be people who are sick and those who are apparently healthy. The apparently healthy group includes 4 groups; the really healthy (reference group, i.e., those with no evidence of subclinical illness), those who have recently been infected but are not yet showing signs of illness (incubation group), and those who are in early and late convalescence after an infection. Our methods are intended for subjects who are apparently healthy; that is, those subjects who in a survey or an intervention study are not excluded because they are sick. Therefore, a person with a cold or a child with “candles” running from his/her nose would be regarded as apparently healthy and included unless he or she had a fever or some other evidence of sickness. Persons who are apparently healthy but found subsequently to have a positive malarial parasitemia should be included, but the results should be noted and possibly adjusted separately.
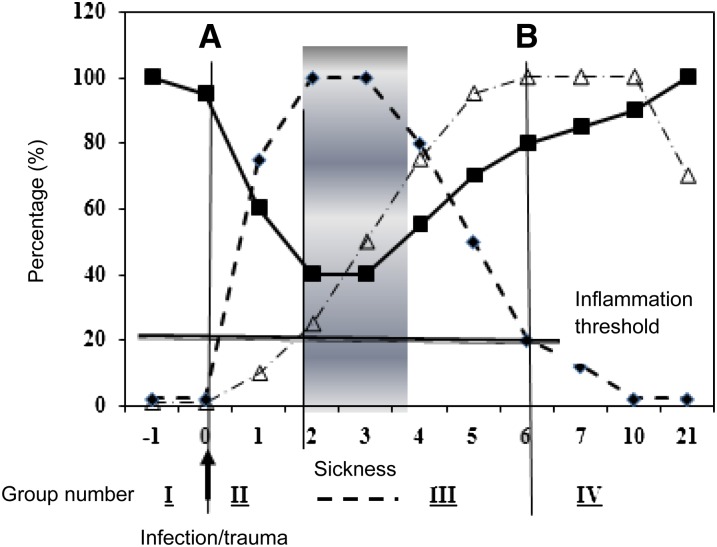
To determine the impact of subclinical infection on plasma nutrient or biomarker concentrations, we carried out 2 meta-analyses that included studies containing the relevant serum biomarker plus CRP and AGP (2, 3). Figure 2 illustrates the hypothetical relation between the inflammatory proteins CRP and AGP and the biomarker of vitamin A status, plasma retinol concentration, in an apparently healthy population of children or adults (21). By using elevated concentrations of the APPs CRP (>5 mg/L) and AGP (>1 g/L), the 4 groups described above were identified. Because the reference group displays no evidence of inflammation, its nutritional status should be representative of the true nutritional status of the community.
FIGURE 2.
Model of the behavior of the plasma acute phase proteins CRP and AGP and retinol concentrations after an infection or trauma. The ordinate axis indicates arbitrary % values where zero represents normal and 20% the inflammation threshold of CRP (>5 mg/L) and AGP (>1 g/L); 100% represents a normal serum retinol concentration or the maximum increase in CRP or AGP concentrations. The figure shows the rapid increase in CRP (line with short dashes) and decrease in retinol concentrations (solid line) after an infection stimulus at time zero (A). The CRP concentration reaches a plateau when clinical evidence of sickness appears and retinol reaches its nadir. AGP (line with combination of short and long dashes) concentrations increase more slowly from time zero and only pass the inflammation threshold 2–5 d later. As sickness wanes, concentrations of CRP decrease, retinol increases, and AGP reaches a plateau. In the final stage of convalescence, CRP concentrations return to normal (B), leaving only AGP elevated. The horizontal solid line at 20% is an arbitrary inflammation threshold. See text for details. The model is used to categorize subjects in the following groups: I, reference (no elevated acute phase proteins); II, incubation (only CRP increased); III, early convalescence (CRP and AGP both above the inflammation threshold); and IV, late convalescence (only AGP is raised). AGP, α1-acid glycoprotein; CRP, C-reactive protein. Modified from reference 21.
The 3 inflammation groups are as follows: “incubation,” “early convalescence,” and “late convalescence.” The incubation group includes those subjects who were recently exposed to disease or trauma but have not yet developed any signs or symptoms of disease. In these subjects CRP will be elevated but AGP will be normal. Early convalescence follows the disappearance of clinical symptoms; therefore, CRP concentrations will be declining but still >5 mg/L and AGP will also be elevated. In the late-convalescent group only AGP will be elevated. Between the incubation and the convalescence phases there will be a period of sickness that may be of varying duration and intensity but during which nutrient concentrations will be influenced both by the acute phase response and potentially by many other factors including dehydration; anorexia; permeability changes in vasculature, gut, and kidney; increased tissue requirement to combat the infection; and other factors. However, in convalescence, only residual acute phase effects should be present because we assume that homeostasis will have been restored because the subjects appear healthy.
Also included in Figure 2 is the hypothetical behavior of plasma retinol concentrations. During the incubation phase, plasma retinol concentrations decrease as CRP increases. During sickness the concentration continues to decrease because we found that concentrations of retinol in early convalescence are lower than those in the incubation phase (2, 11, 21). During convalescence, however, as inflammation decreases and a person progresses into the late-convalescent phase, plasma retinol concentrations increase and normalize.
Meta-analyses to calculate adjustment factors for serum retinol and ferritin concentrations.
The method used to calculate the adjustment factors was the same for retinol and ferritin. The only difference between the 2 nutrients is that retinol is depressed by inflammation whereas ferritin is increased. The studies used for the 2 meta-analyses are shown in Table 1. To be eligible for inclusion in the 4-group meta-analyses, studies had to include measurements of both an acute and a chronic APP. All of the studies except for 1 included CRP and AGP. The exception was a study in 3074 Pakistani preschool-aged children in whom ACT was measured in place of CRP (22). Performing the analyses with and without the Pakistani children made no significant difference to the adjustment factors for either retinol or ferritin, so the study was included in the final results.
We carried out 2- and 4-group analyses (2, 3, 23). Only the 4-group analysis is described because we believe that the adjustment factors obtained provide more accurate estimates of the effects of inflammation than the 2-group analyses. We used the procedure described in Figure 2 to allocate subjects to their appropriate group: reference (no elevated APPs), incubation (CRP >5 mg/L), early convalescence (CRP elevated and AGP >1 g/L), and late convalescence (only AGP elevated). We then calculated mean log nutrient concentrations for each group and compared groups with one another for all studies. There were 7 studies that provided data for the 4-group analysis, as follows: 4 in apparently healthy preschool-aged children (22, 24, 25, and Guatemalan data; and H Delgado, Institute of Nutrition for Central America and Panama, personal communication, 2002). 1 in Nepalese pregnant women (26), 1 in Nigerian neonates (27), and 1 in preschool-aged children from Papua New Guinea (28) in whom there was possible exposure to malaria. Because the acute phase response may be altered in neonates (immature), in pregnancy, and on exposure to malaria, we conducted the 4 and 7 studies separately. Four-group analysis resulted in 6 pairs of comparisons of mean log nutrient concentrations for each study (2). Of these, only 3 had practical use for calculation of adjustment factors—i.e., the ratios between the reference group and each of the 3 inflammation groups. Each of the 3 pairs was summarized by study and then analyzed as below.
The summary statistic (effect size) was the difference between 2 log means, and the variability associated with each summary statistic was related to sample sizes. In general, studies with a larger number of samples will have smaller variability than those with smaller numbers. To combine the summary statistics of all of the studies, traditional weights were calculated on the basis of the inverse of the within-study variance. Thus, studies with a large variance, and therefore a relatively imprecise estimate of the study summary, received less weight than a study with a smaller variance. In the 4-group analysis, weights were computed from the sum of the variances for the 4 groups and on the total sample size for the 4 groups.
To estimate the variability of the overall summary statistic and to provide study-to-study variation, the random-effects model was used for all of the analyses reported because it allowed for small differences between studies and enabled the generation of valid SDs. The data obtained for group comparisons are summarized in Table 2. As described earlier, we conducted the 4-group meta-analysis for retinol with both 4 and 7 studies. The mean ratios for the 7 studies were 1.14, 1.31, and 1.12 and were indistinguishable from the results for the 4-study analysis shown in Table 2.
Adjustment factors for retinol.
The mean ratios for retinol shown in Table 2 represent the differences between retinol in the 3 inflammation groups from those in the reference group. The ratios provide estimates of the mean depression in plasma retinol concentrations defined by the respective types of inflammation and converted to multiplication factors of 1.13, 1.24, and 1.11. Thus, in a community study, plasma retinol concentrations of subjects found to have subclinical inflammation with the characteristics of the incubation group (CRP >5 mg/L and AGP ≤1 g/L) should be multiplied by 1.13 to remove the effects of inflammation. Those in the early- and late-convalescent groups should be multiplied by 1.24 and 1.11, respectively. If the adjustment is appropriate and correct, the final mean values of the inflammation groups should be very similar to those of the reference group.
In a separate study to test the meta-analysis, we used the meta-analysis adjustment factors on plasma retinol concentrations obtained from a group of male and female apparently healthy Kenyan adults who had previously tested positive for HIV but were currently without symptoms (Table 3) (11). The median plasma retinol concentration for the whole group was 1.16 μmol/L compared with 1.33 μmol/L for the reference group. After adjustment of the retinol values in each of the 3 inflammation groups, the combined median for the 3 groups increased from 1.04 to 1.29 μmol/L, and the overall median for the 4 groups was 1.31 μmol/L. The total number of subjects with retinol concentrations <0.7 μmol/L decreased from 20% to 15% after adjustment. It should be noted that the Kenyan subjects were adults with very different characteristics from the apparently healthy preschool-aged children from whom the adjustment factors were derived. Despite the difference, removal of the effects of inflammation from plasma retinol increased the concentration of the whole group to that of the reference group.
Adjustment factors for ferritin.
Blood ferritin concentrations are recommended as the best marker of iron deficiency by the WHO (29), and cutoffs of <12 μg/L (children <5 y) and <15 μg/L (≥5 y) indicate deficient iron stores. Unfortunately, ferritin concentrations increase rapidly on exposure to infection or trauma (10) and remain high during chronic inflammation (15). By using the techniques described above for retinol, we obtained adjustment factors of 0.77, 0.53, and 0.75 for the incubation and early- and late convalescent groups, respectively (Table 2) (3). As can be seen in Table 1, data were obtained from a wide selection of studies including infants, children, women, and men. There was evidence of variations in summary ratios among the subgroups but no significant differences. Furthermore, the meta-analysis adjustment factors for ferritin are not too dissimilar from those produced with other data from preschool-aged children in Kenya and Liberia (Table 4) (19; and F Sandalinas, A Wise, K Baawo, K Faigao, DI Thurnham, SR Lynch, and R Kupka, unpublished results, 2013). Grant et al. (19) also showed that the increases in estimates of iron deficiency generated from their own data (26.9% to 40.7%) were very similar to those obtained by using the meta-analysis adjustment factors (39.1%). Furthermore, the reductions in ferritin concentrations brought about by the respective adjustment factors were 30% (meta-analysis) (3) and 32% in both Kenya (19) and Liberia (F Sandalinas, A Wise, K Baawo, K Faigao, DI Thurnham, SR Lynch, and R Kupka, unpublished results, 2013). Therefore, adjustment factors produced by the meta-analysis may be generally applicable in other studies in which the numbers of subjects are insufficient to be used to calculate their own adjustment factors.
Serum sTfRs and BISs.
Serum sTfRs are derived mostly from developing RBCs so they reflect the intensity of erythropoiesis and demand for iron, and the concentration increases in iron deficiency anemia (30). Values of sTfR >8.3 mg/L are frequently used to indicate iron deficiency (31). We investigated the possibility of producing adjustment factors for sTfR by using the same method described above on data from preschool-aged children and nonpregnant or lactating women in Laos (20). Our comments are restricted to the 482 preschool-aged children in whom there was more inflammation (43%) than in the women (14%), although we found similar effects in both groups. However, whereas correcting ferritin for inflammation in the Lao children increased iron deficiency from 18% to 21%, correcting sTfR by the same method actually reduced the prevalence from 5% to 4%. The reason for the reduction in iron deficiency may be because both iron deficiency and inflammation increase sTfR values. However, the 2 inflammation groups in which there were higher proportions of sTfR >8.3 mg/L were the 2 convalescent groups. Convalescence could well be associated with a greater intensity of erythropoiesis than that in the reference or incubation groups, because it follows the period of sickness when erythropoiesis may have been inhibited. Therefore, we decided not to adjust sTfR concentrations because this might reduce genuine iron deficiency (20).
BISs.
Serum sTfR concentrations (mg/L) were used together with ferritin (μg/L) in the calculation of BISs for the preschool-aged Lao children (20). We used the method of Cook et al. (32) to calculate the stores of iron, but because of the uncertainties of the value of adjusting sTfR for inflammation we only adjusted ferritin in the calculation, as follows:
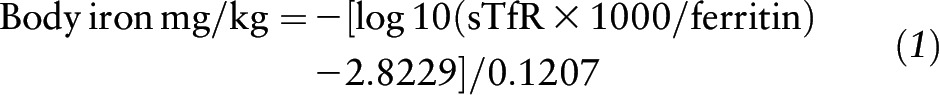 |
With the use of uncorrected ferritin concentrations to calculate BISs, there were 12% of children with BISs <0, indicating iron deficiency, and this value increased to 14% with the use of inflammation-adjusted ferritin values. That is, we were able to detect only two-thirds of the iron deficiency in the children by using low BISs (14%) compared with our ability to detect iron deficiency by using low ferritin concentrations (21%). The situation was similar in the Lao women (20).
Comparison of ferritin concentrations and BISs to assess iron deficiency and the risk of anemia.
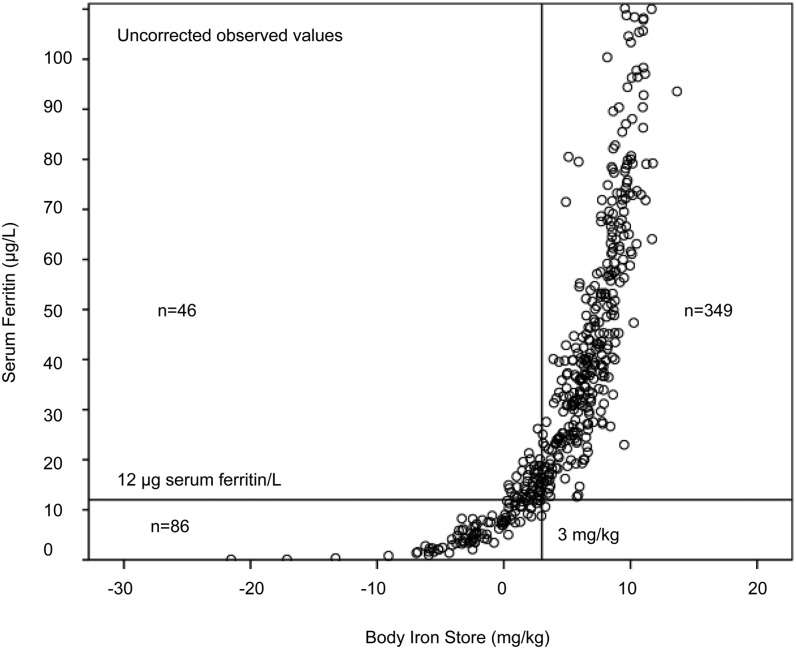
We reasoned that because the cutoff proposed to detect low body iron when using ferritin concentrations was not 0 but <12 μg/L in children <5 y, that we should use a higher cutoff for BISs to give a comparable amount of iron deficiency to that obtained from low ferritin concentrations. We plotted uncorrected data for ferritin and BISs values from the Lao children to illustrate the relation between ferritin and body iron concentrations (Figure 3). Those children with low ferritin concentrations had the lowest body iron, but only 62 of the 86 children with low ferritin were in the <0-mg/kg iron store category. To include all children with a ferritin concentration <12 μg/L the cutoff for low BISs has to be increased to 3 mg/kg. This categorizes 132 (27%) of children with uncorrected low body iron and includes all ferritin values <12 μg/L after correcting for inflammation. A low BISs will increase the risk of anemia when dietary iron is insufficient to maintain or support erythropoiesis. Therefore, if <3 mg/kg is adopted as the cutoff for low body iron, is there any evidence that the larger group of children with low body iron (<3 mg/kg) has the same or a greater risk of anemia as those with specifically low ferritin concentrations?
FIGURE 3.
Relation between ferritin and body iron stores in preschool-aged Lao children. Plasma ferritin and soluble transferrin receptor concentrations were used to calculate body iron stores with the use of the Cook et al. equation (32). The plot shows that a cutoff of 3 mg/kg body iron stores is required in order to include all ferritin concentrations <12 μg/L (20).
One way of answering this question is to compare the proportions of children with anemia in those with and without inflammation. The rationale for this approach is that because the absorption of iron is blocked by hepcidin during infection (33), such children will be more dependent on endogenous iron stores for erythropoiesis than those without infection, who are able to make use of any dietary iron. Thus, children recovering from infection and in convalescence should show more anemia than those who have not been ill recently (i.e., reference group).
The data in Table 5 show inflammation-corrected ferritin and BISs. Most of the children in the combined inflammation groups are in early or late convalescence, and there is more anemia in those groups than in the corresponding reference group. However, only children categorized by ferritin <12 μg/L or BISs <0 mg/kg (a smaller group of children with ferritin <12 μg/L) showed significantly greater proportions of anemia in those with inflammation. If the revised cutoff of <3 mg/kg for iron stores is used, the proportion of children with anemia in the inflammation group is not different from that in the reference group. That is, those children within the group with BISs <3 mg/kg but whose ferritin is ≥12 μg/L do not have the same risk of anemia as those in the group with ferritin values <12 μg/L. Therefore, ferritin concentrations <12 μg/L appear to be more useful than BISs <3 mg/kg to identify the larger number of children with iron deficiency and at risk of anemia.
Conclusions
Inflammation alters serum nutrient biomarker concentrations in apparently healthy people. Retinol is depressed, whereas ferritin and sTfR are increased. By categorizing subjects by stage of inflammation, adjustment factors can be calculated to increase retinol and reduce ferritin concentrations. Concentrations of sTfR and BISs detected smaller numbers of people with iron deficiency than did ferritin in the studied population, and the effects of adjustment for inflammation on both biomarkers were smaller than the effects on ferritin and of questionable benefit. We investigated changing the cutoff for BISs from 0 to <3 mg/kg but found no evidence that the larger number of children identified had comparable risks of anemia to those identified with the use of ferritin <12 μg/L. Both corrected and uncorrected ferritin concentrations <12 μg/L indicate more iron deficiency and anemia than either sTfR >8.3 mg/L or BISs <0 mg/kg in Lao children.
Acknowledgments
DIT wrote the manuscript; DIT and CAN-C collaborated on the vitamin A and ferritin meta-analyses; and DIT and JK conducted the iron biomarker studies in the Lao data. All authors read and approved the final manuscript. We thank Dr. H Delgado from the Institute of Nutrition for Central America and Panama for the Guatemalan data on preschool children. We also thank Drs. R Kupka, P Mathema, and R Johnson (UNICEF, Liberia) for data on preschool aged Liberian children.
Footnotes
Abbreviations used: ACT, α1-antichymotrypsin; AGP, α1-acid glycoprotein; APP, acute phase protein; BIS, body iron store; CRP, C-reactive protein; SAA, serum amyloid A; sTfR, soluble transferrin receptor.
References
- 1.Rousham EK, Northrop-Clewes CA, Lunn PG. Maternal reports of child illness and the biochemical status of the child: the use of morbidity interviews in rural Bangladesh. Br J Nutr 1998;80:451–6. [PubMed] [Google Scholar]
- 2.Thurnham DI, McCabe GP, Northrop-Clewes CA, Nestel P. Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: meta-analysis. Lancet 2003;362:2052–8. [DOI] [PubMed] [Google Scholar]
- 3.Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr 2010;92:546–55. [DOI] [PubMed] [Google Scholar]
- 4.Galloway P, McMillan DC, Sattar N. Effect of the inflammatory response on trace element and vitamin status. Ann Clin Biochem 2000;37:289–97. [DOI] [PubMed] [Google Scholar]
- 5.Fleck A, Myers MA. Diagnostic and prognostic significance of acute phase proteins. In: Gordon AH, Koj A, editors. The acute phase response to injury and infection. 1st ed. Amsterdam: Elsevier Scientific Publishers; 1985. p. 249–71.
- 6.Calvin J, Neale G, Fotherby KJ, Price CP. The relative merits of acute phase proteins in the recognition of inflammatory conditions. Ann Clin Biochem 1988;25:60–6. [DOI] [PubMed] [Google Scholar]
- 7.Louw JA, Werbeck A, Louw ME, Kotze TJ, Cooper R, Labadarios D. Blood vitamin concentrations during the acute-phase response. Crit Care Med 1992;20:934–41. [DOI] [PubMed] [Google Scholar]
- 8.Beisel WR. Trace element in infectious processes. Med Clin North Am 1976;60:831–49. [DOI] [PubMed] [Google Scholar]
- 9.Reid D, Toole BJ, Knox S, Talwar D, Harten J, O'Reilly DS, Blackwell S, Kinsella J, McMillan DC, Wallace AM. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am J Clin Nutr 2011;93:1006–11. [DOI] [PubMed] [Google Scholar]
- 10.Feelders RA, Vreugdenhil G, Eggermont AM, Kuiper-Kramer PA, van Eijk HG, Swaak AJ. Regulation of iron metabolism in the acute-phase response: interferon gamma and tumour necrosis factor alpha induce hypoferraemia, ferritin production and a decrease in circulating transferrin receptors in cancer patients. Eur J Clin Invest 1998;28:520–7. [DOI] [PubMed] [Google Scholar]
- 11.Thurnham DI, Mburu AS, Mwaniki DL, Muniu EM, Alumasa F, de Wagt A. Using plasma acute-phase protein concentrations to interpret nutritional biomarkers in apparently healthy HIV-1-seropositive Kenyan adults. Br J Nutr 2008;100:174–82. [DOI] [PubMed] [Google Scholar]
- 12.Bates CJ, Pentieva KD, Prentice A, Mansoor MA, Finch S. Plasma pyridoxal phosphate and pyridoxic acid and their relationship to plasma homocysteine in a representative sample of British men and women aged 65 years and over. Br J Nutr 1999;81:191–201. [PubMed] [Google Scholar]
- 13.Thompson D, Milford-Ward A, Whicher JT. The value of acute phase protein measurements in clinical practice. Ann Clin Biochem 1992;29:123–31. [DOI] [PubMed] [Google Scholar]
- 14.Stuart J, Whicher JT. Tests for detecting and monitoring the acute phase response. Arch Dis Child 1988;63:115–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Northrop-Clewes CA. Interpreting indicators of iron status during an acute phase response—lessons from malaria and human immunodeficiency virus. Ann Clin Biochem 2008;45:18–32. [DOI] [PubMed] [Google Scholar]
- 16.Thurnham DI, Singkamani R. The acute phase response and vitamin A status in malaria. Trans R Soc Trop Med Hyg 1991;85:194–9. [DOI] [PubMed] [Google Scholar]
- 17.Das BS, Thurnham DI, Das DB. Plasma alpha-tocopherol, retinol, and carotenoids in children with falciparum malaria. Am J Clin Nutr 1996;64:94–100. [DOI] [PubMed] [Google Scholar]
- 18.Rosales FJ, Topping JD, Smith JE, Shankar AH, Ross AC. Relation of serum retinol to acute phase proteins and malarial morbidity in Papua New Guinea children. Am J Clin Nutr 2000;71:1582–8. [DOI] [PubMed] [Google Scholar]
- 19.Grant FK, Suchdev PS, Flores-Ayala R, Cole CR, Ramakrishnan U, Ruth LJ, Martorell R. Correcting for inflammation changes estimates of iron deficiency among rural Kenyan preschool children. J Nutr 2012;142:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knowles J, Thurnham DI, Phengdy B, Houamboun K, Philavong K, Keomoungkhone I, Keovilay K. Impact of inflammation on the biomarkers of iron status in a cross-sectional survey of Lao women and children. Br J Nutr 2013;110:2285–97. [DOI] [PubMed] [Google Scholar]
- 21.Thurnham DI, Mburu AS, Mwaniki DL, De Wagt A. Micronutrients in childhood and the influence of subclinical inflammation. Proc Nutr Soc 2005;64:502–9. [DOI] [PubMed] [Google Scholar]
- 22.Paracha PI, Jamil A, Northrop-Clewes CA, Thurnham DI. Interpretation of vitamin A status in apparently healthy Pakistani children by using markers of subclinical infection. Am J Clin Nutr 2000;72:1164–9. [DOI] [PubMed] [Google Scholar]
- 23.Thurnham DI, McCabe GP. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. In: Rogers LM, editor. Priorities in the assessment of vitamin A and iron status in populations; 2010 Sep 15–17; Panama City, Panama. Geneva: World Health Organization; 2012. p. 63–80.
- 24.Freire W, Dirren H, Mora JO. Diagnostico de la situacion alimentaria, nutricional y de salud de las poblacion Ecuatoriana menor de cinco anos. Quito (Equador): CONADE & Ministerio de Salud Publico; 1988.
- 25.Filteau SM, Morris SS, Raynes JG, Arthur P, Ross DA, Kirkwood B, Tomkins AM, Gyapong JO. Vitamin A supplementation, morbidity and serum acute phase proteins in young Ghanaian children. Am J Clin Nutr 1995;62:434–8. [DOI] [PubMed]
- 26.Christian P, Schultz K, Stoltzfus MC. Hyporetinolemia, illness symptoms and APP response in pregnant women with and without night blindness. Am J Clin Nutr 1988;67:1237–43. [DOI] [PubMed]
- 27.Adelekan DA, Northrop-Clewes CA, Owa JA, Oyedeji AO, Owoeye AA, Thurnham DI. Use of biomarkers of sub-clinical infection, nutrition and neonatal maturity to interpret plasma retinol in Nigerian neonates. Br J Nutr 2003;90:353–61. [DOI] [PubMed]
- 28.Shankar AH, Genton B, Semba RA, Baisor M, Paino J, Tamja S, Adiguma T, Wu L, Rare L, Tielsch JM, et al. . Effect of vitamin A supplementation on morbidity due to Plasmodium falciparum in young children in Papua New Guinea: a randomised trial. Lancet 1999;354:203–9. [DOI] [PubMed]
- 29.World Health Organization; Centers for Disease Control and Prevention. Assessing the iron status of populations. Geneva: WHO Press; 2007.
- 30.Northrop-Clewes CA, Thurnham DI. Biomarkers for the differentiation of anemia and their clinical usefulness. J Blood Med. 2013;4:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr 2004;134:3127–32. [DOI] [PubMed] [Google Scholar]
- 32.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood 2003;101:3359–64. [DOI] [PubMed] [Google Scholar]
- 33.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003;102:783–8. [DOI] [PubMed] [Google Scholar]