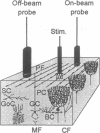
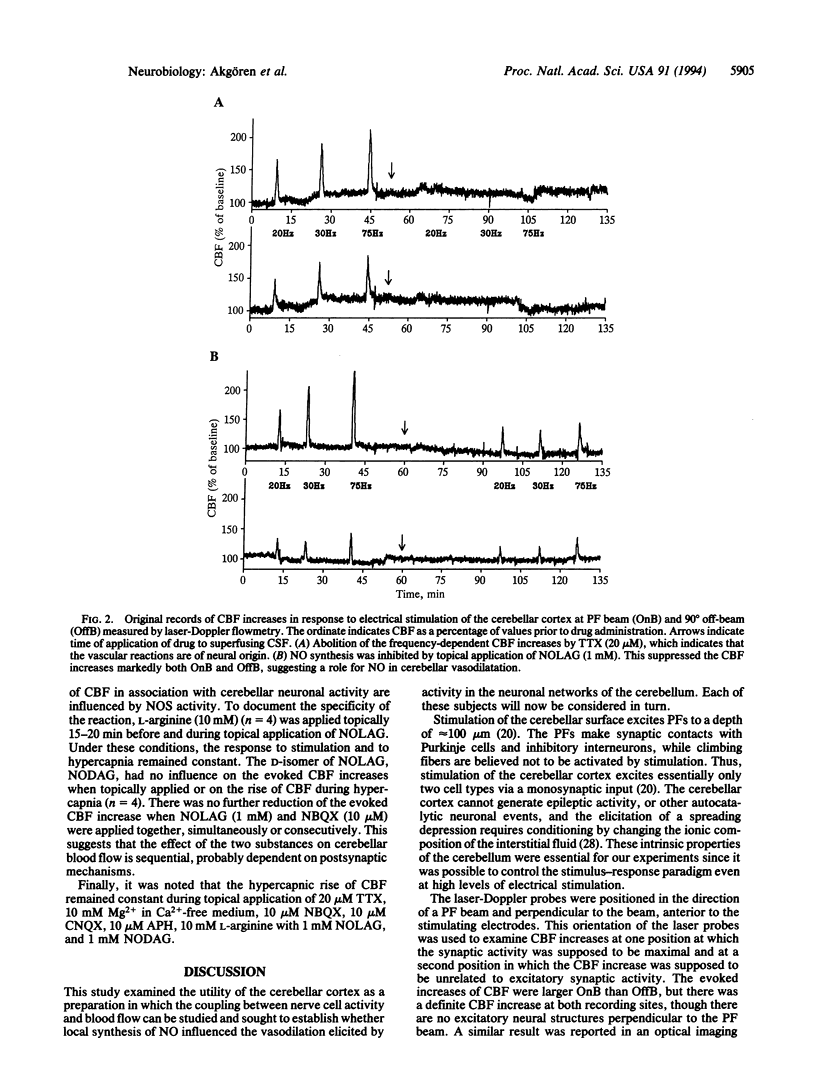
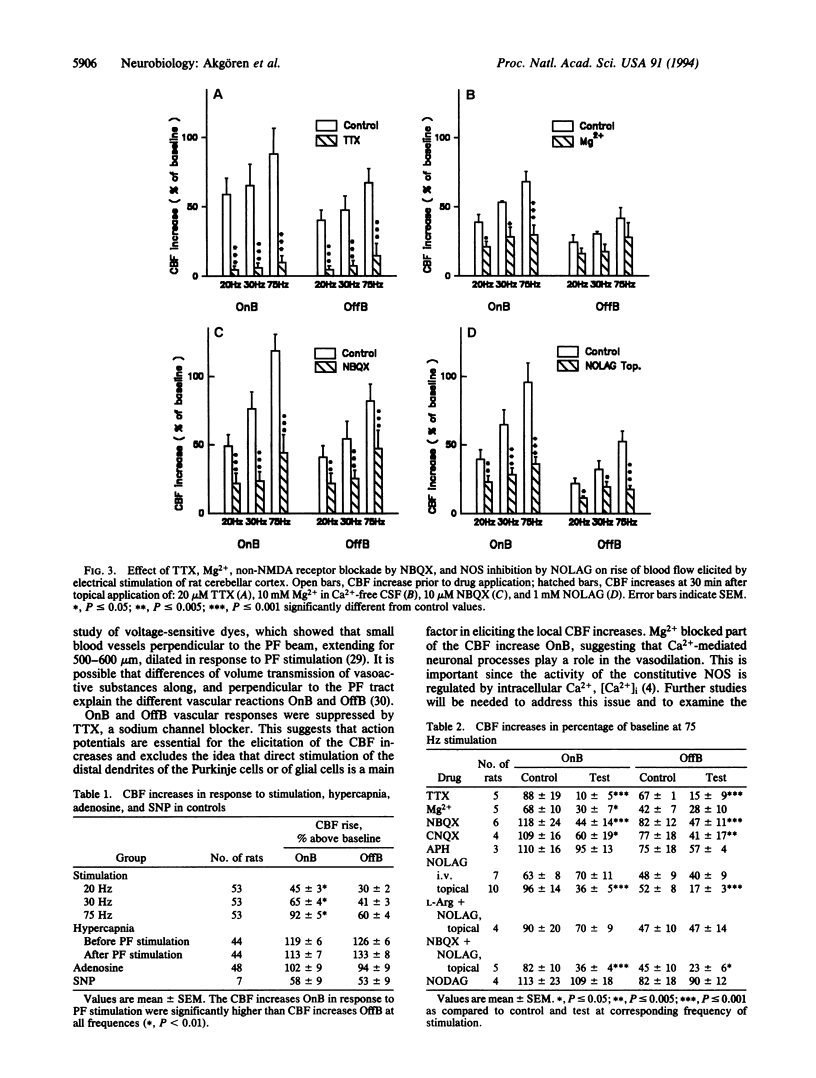
Abstract
The endothelium-derived relaxing factor, probably nitric oxide (NO), is a potent vasodilator that regulates the vascular tone in several vascular beds, including the brain. We explored the possibility that NO might be of importance for the increase of cerebral blood flow (CBF) associated with activity of the well-defined neuronal circuits of the rat cerebellar cortex. Laser-Doppler flowmetry was used to measure increases of cerebellar blood flow evoked by trains of electrical stimulations of the dorsal surface. The evoked increases of CBF were frequency-dependent, being larger on than off the parallel fiber tracts, suggesting that conduction along parallel fibers and synaptic activation of target cells were important for the increase of CBF. This was verified experimentally since the evoked CBF increases were abolished by tetrodotoxin and reduced by 10 mM Mg2+ and selective antagonists for non-N-methyl-D-aspartate receptors. The cerebellar cortex contains high levels of NO synthase. This raised the possibility that NO was involved in the increase of CBF associated with neuronal activation. NO synthase inhibition by topical application of NG-nitro-L-arginine attenuated the evoked CBF increase by about 50%. This effect was partially reversed by pretreatment with L-arginine, the natural substrate for the enzyme, while NG-nitro-D-arginine, the inactive enantiomer, had no effect on the evoked CBF increases. Simultaneous blockade of non-N-methyl-D-aspartate receptors and NO synthase had no further suppressing effect on the blood flow increase than either substance alone, suggesting that the NO-dependent flow rise was dependent on postsynaptic mechanisms. These findings are consistent with the idea that local synthesis of NO is involved in the transduction mechanism between neuronal activity and increased CBF.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Inanami O., Sato A. Nitric oxide (NO) is involved in increased cerebral cortical blood flow following stimulation of the nucleus basalis of Meynert in anesthetized rats. Neurosci Lett. 1992 May 25;139(2):201–204. doi: 10.1016/0304-3940(92)90552-i. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Dirnagl U., Kaplan B., Jacewicz M., Pulsinelli W. Continuous measurement of cerebral cortical blood flow by laser-Doppler flowmetry in a rat stroke model. J Cereb Blood Flow Metab. 1989 Oct;9(5):589–596. doi: 10.1038/jcbfm.1989.84. [DOI] [PubMed] [Google Scholar]
- Dirnagl U., Lindauer U., Villringer A. Role of nitric oxide in the coupling of cerebral blood flow to neuronal activation in rats. Neurosci Lett. 1993 Jan 4;149(1):43–46. doi: 10.1016/0304-3940(93)90343-j. [DOI] [PubMed] [Google Scholar]
- Dwyer M. A., Bredt D. S., Snyder S. H. Nitric oxide synthase: irreversible inhibition by L-NG-nitroarginine in brain in vitro and in vivo. Biochem Biophys Res Commun. 1991 May 15;176(3):1136–1141. doi: 10.1016/0006-291x(91)90403-t. [DOI] [PubMed] [Google Scholar]
- Elias S. A., Yae H., Ebner T. J. Optical imaging of parallel fiber activation in the rat cerebellar cortex: spatial effects of excitatory amino acids. Neuroscience. 1993 Feb;52(4):771–786. doi: 10.1016/0306-4522(93)90528-n. [DOI] [PubMed] [Google Scholar]
- Faraci F. M., Breese K. R. Nitric oxide mediates vasodilatation in response to activation of N-methyl-D-aspartate receptors in brain. Circ Res. 1993 Feb;72(2):476–480. doi: 10.1161/01.res.72.2.476. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Gorsky L. D., Pollock J. S., Schmidt H. H., Heller M., Murad F. Regional distribution of EDRF/NO-synthesizing enzyme(s) in rat brain. Biochem Biophys Res Commun. 1990 Apr 30;168(2):727–732. doi: 10.1016/0006-291x(90)92382-a. [DOI] [PubMed] [Google Scholar]
- Gally J. A., Montague P. R., Reeke G. N., Jr, Edelman G. M. The NO hypothesis: possible effects of a short-lived, rapidly diffusible signal in the development and function of the nervous system. Proc Natl Acad Sci U S A. 1990 May;87(9):3547–3551. doi: 10.1073/pnas.87.9.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J., Beaumont P. S. Excitatory amino acid receptors in the parallel fibre pathway in rat cerebellar slices. Neurosci Lett. 1989 Dec 15;107(1-3):151–156. doi: 10.1016/0304-3940(89)90808-2. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988 Nov 24;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Glaum S. R., Slater N. T., Rossi D. J., Miller R. J. Role of metabotropic glutamate (ACPD) receptors at the parallel fiber-Purkinje cell synapse. J Neurophysiol. 1992 Oct;68(4):1453–1462. doi: 10.1152/jn.1992.68.4.1453. [DOI] [PubMed] [Google Scholar]
- Haberl R. L., Heizer M. L., Ellis E. F. Laser-Doppler assessment of brain microcirculation: effect of local alterations. Am J Physiol. 1989 Apr;256(4 Pt 2):H1255–H1260. doi: 10.1152/ajpheart.1989.256.4.H1255. [DOI] [PubMed] [Google Scholar]
- Hirano T., Hagiwara S. Synaptic transmission between rat cerebellar granule and Purkinje cells in dissociated cell culture: effects of excitatory-amino acid transmitter antagonists. Proc Natl Acad Sci U S A. 1988 Feb;85(3):934–938. doi: 10.1073/pnas.85.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. Does nitric oxide mediate the increases in cerebral blood flow elicited by hypercapnia? Proc Natl Acad Sci U S A. 1992 May 1;89(9):3913–3916. doi: 10.1073/pnas.89.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C., Zhang F., Xu X. Role of nitric oxide synthase-containing vascular nerves in cerebrovasodilation elicited from cerebellum. Am J Physiol. 1993 Apr;264(4 Pt 2):R738–R746. doi: 10.1152/ajpregu.1993.264.4.R738. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovách A. G., Szabó C., Benyó Z., Csáki C., Greenberg J. H., Reivich M. Effects of NG-nitro-L-arginine and L-arginine on regional cerebral blood flow in the cat. J Physiol. 1992 Apr;449:183–196. doi: 10.1113/jphysiol.1992.sp019081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koźniewska E., Oseka M., Styś T. Effects of endothelium-derived nitric oxide on cerebral circulation during normoxia and hypoxia in the rat. J Cereb Blood Flow Metab. 1992 Mar;12(2):311–317. doi: 10.1038/jcbfm.1992.43. [DOI] [PubMed] [Google Scholar]
- Lauritzen M., Rice M. E., Okada Y., Nicholson C. Quisqualate, kainate and NMDA can initiate spreading depression in the turtle cerebellum. Brain Res. 1988 Dec 20;475(2):317–327. doi: 10.1016/0006-8993(88)90620-8. [DOI] [PubMed] [Google Scholar]
- Levi G., Gallo V. Release studies related to the neurotransmitter role of glutamate in the cerebellum: an overview. Neurochem Res. 1986 Dec;11(12):1627–1642. doi: 10.1007/BF00967741. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Northington F. J., Matherne G. P., Berne R. M. Competitive inhibition of nitric oxide synthase prevents the cortical hyperemia associated with peripheral nerve stimulation. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6649–6652. doi: 10.1073/pnas.89.14.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Sekiguchi M. Synaptic receptors and intracellular signal transduction in the cerebellum. Neurosci Res. 1991 Jan;9(4):213–237. doi: 10.1016/0168-0102(91)90023-r. [DOI] [PubMed] [Google Scholar]
- Pelligrino D. A., Koenig H. M., Albrecht R. F. Nitric oxide synthesis and regional cerebral blood flow responses to hypercapnia and hypoxia in the rat. J Cereb Blood Flow Metab. 1993 Jan;13(1):80–87. doi: 10.1038/jcbfm.1993.10. [DOI] [PubMed] [Google Scholar]
- Rice M. E., Okada Y. C., Nicholson C. Anisotropic and heterogeneous diffusion in the turtle cerebellum: implications for volume transmission. J Neurophysiol. 1993 Nov;70(5):2035–2044. doi: 10.1152/jn.1993.70.5.2035. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Gagne G. D., Nakane M., Pollock J. S., Miller M. F., Murad F. Mapping of neural nitric oxide synthase in the rat suggests frequent co-localization with NADPH diaphorase but not with soluble guanylyl cyclase, and novel paraneural functions for nitrinergic signal transduction. J Histochem Cytochem. 1992 Oct;40(10):1439–1456. doi: 10.1177/40.10.1382087. [DOI] [PubMed] [Google Scholar]
- Sheardown M. J., Nielsen E. O., Hansen A. J., Jacobsen P., Honoré T. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science. 1990 Feb 2;247(4942):571–574. doi: 10.1126/science.2154034. [DOI] [PubMed] [Google Scholar]
- Southam E., Morris R., Garthwaite J. Sources and targets of nitric oxide in rat cerebellum. Neurosci Lett. 1992 Mar 30;137(2):241–244. doi: 10.1016/0304-3940(92)90413-2. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46(4):755–784. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]
- Wang Q., Kjaer T., Jørgensen M. B., Paulson O. B., Lassen N. A., Diemer N. H., Lou H. C. Nitric oxide does not act as a mediator coupling cerebral blood flow to neural activity following somatosensory stimuli in rats. Neurol Res. 1993 Feb;15(1):33–36. doi: 10.1080/01616412.1993.11740103. [DOI] [PubMed] [Google Scholar]
- Wang Q., Paulson O. B., Lassen N. A. Effect of nitric oxide blockade by NG-nitro-L-arginine on cerebral blood flow response to changes in carbon dioxide tension. J Cereb Blood Flow Metab. 1992 Nov;12(6):947–953. doi: 10.1038/jcbfm.1992.131. [DOI] [PubMed] [Google Scholar]