Abstract
Background
Huntington’s disease (HD) is a neurodegenerative syndrome that leads to marked decline in cognitive functioning along with uncharacteristic body movements called chorea. There exists no therapeutic agent to address the disease.3-Nitropropionic acid (3-NP) which is a suicide inhibitor of succinate dehydrogenase and a well-known experimental model to study Huntington’s disease, causes substantial impairment in gait and memory through oxidative and neuronal damage.
Purpose
In the present study protective effect of escitalopram against 3-NP induced neurotoxicity was explored.
Methods
Adult female Wistar ratswere subjected to per oral administration of 2 different doses of escitalopram (10 and 20 mg/kg) for 12 days followed by intraperitoneal injection of 3-NP (20 mg/kg) on the last four days.
Results
Intraperitoneal injection of 3-NP lead to significant induction of HD like symptoms in rats such as impaired memory, reduced locomotor activity, hind limb impairment, decreased body weight, oxidative damage and mitochondrial dysfunction. Treatment with 2 different dose of escitalopram helped reverse the mitochondrial enzyme dysfunction along with reversal of behavioural and biochemical anomaly induced by 3-NP. Further, histopathological examination confirmed the neuroprotective potential of escitalopram against 3-NP induced pathological lesions.
Conclusion
The results obtained thus substantiate the claim that escitalopram might play an antioxidant and neuroprotective role against 3-NP induced alterations in rats and can prove to be a promising candidate for the management of HD.
Keywords: Huntington’s disease, Oxidative stress, Neurotoxicity, Antioxidant

Introduction
Huntington’s disease (HD) is a genetic neurodegenerative disorder that is pathologically characterised by the loss of striatal neurons, leading to behavioural symptoms like psychiatric instabilities, dementia, abnormal body movements (chorea) and unintended weight loss.1 The exact pathogenesis leading to Huntington’s is ambiguous. Abnormalities in IT15 gene gives rise to a mutant huntingtin protein with an abnormal expansion of CAG repeat. This mutated protein aggregates in striatal neurons as well as neurons in the cortex, thalamus, hypothalamus, and substantia nigra thereby leading to a sequence of events that includes increased oxidative injury, transcriptional dysregulation, glutamate excitotoxicity, apoptosis and mitochondrial dysfunction. Also studies carried out on HD post-mortem tissue have revealed selective dysfunction of components of the mitochondrial electron transport chain2 which involves alteration in the functions of the complex II (Succinate dehydrogenase) and complex IV (Cytochrome C) of the electron transport chain.3
3-Nitropropionic acid (3-NP) induces neurotoxicity by irreversibly inhibiting succinate dehydrogenase enzyme that forms the complex-II of the electron transport chain, thereby producing chorea and dementia that mirrors the symptoms seen in patients with HD.4 Manipulating the time course of 3-NP injections leads to sustained hyperactivity and hypoactivity seen in early and late stages of HD can also be produced by means of manipulation of time course of 3-NP injections. Since intoxication with 3-NP produces selective striatal lesions, 3-NP is exploited to produce an experimental model of Huntington’s disease.5
Escitalopram, the S-enantiomer of citalopram, is a selective serotonin reuptake inhibitor (SSRI) that has demonstrated antidepressant and anxiolytic activities in preclinical and clinical studies. Selective Serotonin reuptake inhibitors (SSRI’s) selectively block serotonin (5-hydroxytryptamine, 5-HT) reuptake in the pre synaptic cell thereby increasing extracellular serotonin levels. The antioxidant and neuroprotective effects of anti-depressants have been well documented in various studies that have demonstrated the potential of anti-depressants to attenuate the oxidative stress and restore the antioxidant enzyme levels.6 SSRI’s are also known to decrease the excitotoxicity in the cell through suppression of the voltage gated sodium and calcium channels.7 They increase Brain derived neurotropic factor (BDNF) levels.8 Hypothalamic-pituitary-adrenal axis (HPA-axis) hyperactivity is seen in HD patients which is attributed to the dysregulation of the 5-HT neurotransmission can be reversed by escitalopram.9 Thus, in the present study, the possible neuroprotective effect of Escitalopram against 3-nitropropionic acid induced behavioural, biochemical, and mitochondrial dysfunctions in the rat brain was explored
Methods
Chemicals
The drugs used in the study were 3-Nitro Propionic acid (3-NP) and Escitalopram. 3-NP was purchased from sigma chemicals whereas escitalopram was obtained as a gift sample from lupin lab. All analytical grade chemicals were used respectively.
Animal and Treatment Schedule
Adult female Wistar rats (200–230 g) were used in the present study. Prior to experimentation the animals were allowed to acclimatize to the laboratory conditions. The animals were housed under standard conditions of 12 h light/dark cycles and were provided with a standard rat feed and water ad libitum with experiments being strictly conducted between 09:00 and 17:00 h. The study was carried out in accordance with the guidelines of committee for the Purpose of Control and Supervision of Experiments on Animals for use and care of laboratory animals and the protocol was approved by the Institutional Animal Ethics Committee. Animals were randomly divided into 5 groups each containing 6 animals. 3-NP was diluted with saline (pH 7.4) and injected intraperitoneally, whereas escitalopram was dissolved in water and administered per orally. Animals from group I and II received vehicle (oral) and saline (i.p) respectively for 12 days. Group III & IV received two different doses of Escitalopram (p.o) (10 mg/kg & 20 mg/kg) for 12 days. Animals from group II, III & IV were challenged using 3-NP (20 mg/kg) (i.p) on last four consecutive days of treatment (9th to 12th day from the administration of the drug) wherein 3-NP was administered 1 hr. after saline (for group II) and escitalopram (groups II and IV) treatment. Group V animals were administered with a higher dose of Escitalopram (20 mg/kg) for 12 days and saline (i.p) for last four days (9th–12th).
Body Weight Measurement
Animal body weights were recorded on day zero and day 12th respectively. The percent change in body weight was recorded.
Behavioural Assessments
All animals were subjected to a training period of 4 days prior to the start of drug treatment. All behavioural parameters were assessed 1 hr after 3-NP administration and an interval of 30 mins was given between each test.
Elevated Plus Maze Test
Assessment of spatial long term memory was carried out using elevated plus maze apparatus.10 The maze comprised of two open arms crossed with two closed arms and elevated at a height of 50 cm from the ground with the arms connected with central square (10 × 10 cm). Animal was placed at one end of the open arm facing away from the central square and the initial transfer latency was recorded by measuring the time taken by the animal to move into one of the enclosed arms. The animal was allowed to explore the maze for another 30 secs before returning to the home cage. A gentle push was given in case the animal failed to enter into one of the enclosed arms within 90 secs, with the transfer latency (TL) being assigned as 90. TL recordings were carried out on day 0, 8 and 12.
Morris Water Maze test
Cognitive performance was evaluated using Morris water maze apparatus.11 The maze comprised of a circular tank (diameter 160 cm & height 35 cm). The tank was filled with water to a depth of 20 cm and was made opaque by adding milk. The tank was divided into 4 fixed points on its perimeter assigned as 4 quadrants. The tank also comprised of a square platform (escape platform) attached at one end along the circumference of the tank placed at about 2 cm below the water surface and which was of the same colour as that of the rest of the basin to avoid any false positive results. Animals were trained to swim to the platform by placing them gently at a start point in the middle of the rim of a quadrant not containing the escape area with their face to the wall. In case the rat failed to find the platform within 90 s it was guided to the platform and allowed to stay on it for 20 s. Training sessions comprising of four trials in a day for 4 days was provided. In all four trials, starting positions were different (acquisition trial). After the acquisition trials, the time taken by the animals to reach the platform (escape latency) was recorded on day 0, 8 and 12 respectively.
Narrow Beam test
It was carried out to measure the hind limb impairment.12 The narrow beam comprising of a wooden beam with dimensions of 105 cm length and 4 cm width and suspended at a height of 80 cm from the ground with adequate wooden supports (Fig 1). A 20 cm line was drawn at the one end of the beam. Recording was done by placing the animal within the 20 cm starting zone. facing its home cage. The stopwatch was started immediately on releasing the animal and the time taken by the animal to traverse the beam was determined. The maximum time allotted for the task was 2 mins. Falls, if any, were also recorded. The test was performed on day 0, 8 and 12 respectively.
Fig. 1:
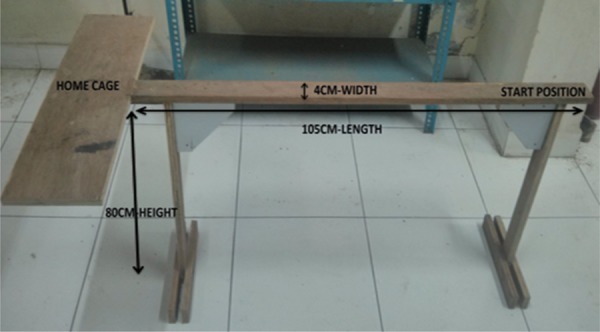
Narrow beam apparatus for rats.
Assessment of gross behavioural activity
It involves assessment of locomotor and rearing activity using a photoactometer on the last day of study.13 An actophotometer consists of infrared sensors and a digital counter. The animal’s movement interrupts the infrared beam, which gets recorded and displayed digitally. This principle is used to count total locomotor activity of an animal. A habituation period of 3 min was given by placing the animal in the cage followed by activity recordings for next 5 mins. The motor activity was detected by infrared photocells. Locomotor activity (horizontal and vertical) was estimated as counts per 5 min.
After behavioural estimations on day 12, the animals were sacrificed using carbon dioxide chamber. A modified method of Sandhir et al14 was used to isolate the rat brain mitochondrial pellets. The whole rat brain was isolated and the cerebellum was separated. The brain was rinsed in ice cold saline and was homogenised with 10 volumes (w/v) ice cold homogenising buffer (10 mM Tris-HCl, 0.44 M sucrose, 10 mM ethylenediaminetetraacetic acid). The homogenate was subsequently centrifuged at 5000 rpm for 30 mins at 4°C to obtain the brain supernatant by discarding the pellets. Estimation of lipid peroxidation (LP), glutathione reductase (GSH), total tissue proteins (TTP), Acetylcholinesterase (AchE) and Nitrite was carried out using the supernatant. The residual supernatant was recentrifuged at 5000 rpm for 45 mins at 4°C to obtain the mitochondrial pellets. The crude pellets were washed with equal volumes of extraction buffer and centrifuged at 5000 rpm for 45 mins at 4°C. The final pellets obtained were suspended in equal volume of ice cold suspending buffer (0.44 M sucrose in 10 mM Tris-HCl, pH7.4). The pellets containing pure mitochondrial fraction were used for the estimations of mitochondrial parameters.
Mitochondrial Parameters
Complex-I (Nicotinamide adenine dinucleotide dehydrogenase (NADH) activity)
NADH activity was measured through spectrophotometry as per the method described by King and Howard.15
Complex-II (succinate dehydrogenase (SDH) activity)
SDH activity was measured through spectrophotometry as per the method described by King.16
Complex IV (Cytochrome oxidase Assay)
Cytochrome oxidase activity was determined according to protocol of Sottocasa et al 17
MTT Assay18
Mitochondrial respiratory chain activity was determined by measuring the rate of MTT reduction as per the method described by Kamboj et al.18
Anti-oxidant Parameters
Lipid peroxidation Measurement
The amount of malondialdehyde (MDA) formed by reaction with TBA was used to estimate lipid peroxidation as per the method described by Ohkawa et al.19
Reduced glutathione
Reduced glutathione (GSH) was assessed according to the method of Ellman.20
Catalase activity
Catalase (CAT) activity was determined according to the method described by Luck21
Estimation of other parameters
Nitrite
Presence of nitrite in the supernatant was determined using Greiss reagent (0.1% N-(1-naphthyl) ethylenediame dihydrochloride, 1% sulphanilamide and 5% phosphoric acid) through colorimetric analysis. 500 microliters of both supernatant and Greiss reagent were mixed and the22 mixture was incubated in dark at room temperature for 10 mins. The absorbance was measured at 540 nm and concentration of nitrite in the supernatant was determined from sodium nitrite standard curve and expressed as µmol/mg protein.
Acetylcholinesterase (AchE) Estimation
The AchE activity was determined by Ellman method.23
Total Tissue Protein Estimation
Total tissue protein in the supernatant was determined by Lowry method.24
Histopathological Examination
On day 12, after behavioural estimations, the animals were sacrificed using carbon dioxide chamber and representative brain samples from each group were preserved in 10% formalin. Staining of the sections was done using hematoxylin and eosin (H&E) and were subsequently examined under the light microscope at different magnifications and photographs were taken. 25
Statistical Analysis
All values were expressed as mean ± standard deviation. Statistical method employed was one way analysis of variance followed by Tukey’s test. Values with P<0.05 were considered as statistically significant.
Results
Effect of Escitalopram on Body Weight in 3-NP treated rats
A significant decline in body weight was observed on day 12 in 3-NP treated animals as compared to the vehicle treated group (p<0.05) that showed no substantial change in body weight. On the other hand, treatment with Escitalopram (10 and 20 mg/kg) attenuated the loss in body weight of 3-NP treated (p<0.05). No significant change in body weights was observed for animals receiving drug Per se (20 mg/kg) (Fig 2).
Fig. 2:
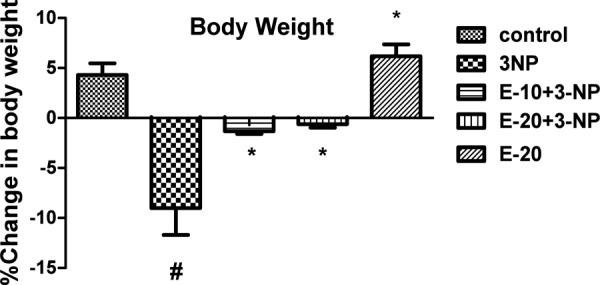
Effect of Escitalopram on Body Weight.
Values are expressed as mean ± standard deviation (SD); n = 6. Significance determined using one way ANOVA followed by Tukey’s test. #Statistically significant from control group (p<0.05).*Statistically significant from 3-NP treated group (p<0.05).
Effect of Escitalopram on Behavioural Parameters
Elevated Plus Maze Test
Significant increase in transfer latency on day 12 was observed for 3-NP treated rats (p<0.05). Escitalopram pre- treatment (10 and 20 mg/kg) significantly decreased the transfer latency (p<0.05). Treatment with only escitalopram (20 mg/kg) did not show any significant change in transfer latency (Fig 3).
Fig. 3:
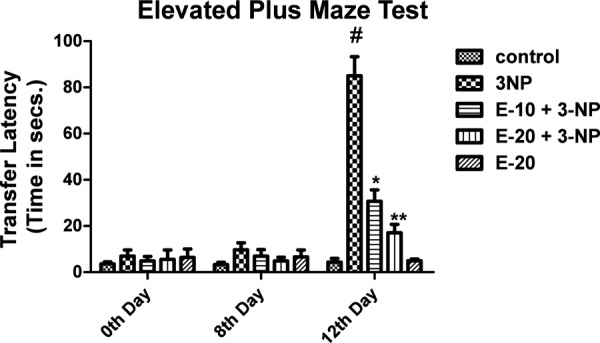
Effect of Escitalopram on Transfer Latency.
Values are expressed as mean ± standard deviation (SD); n = 6. Significance was determined using one way ANOVA followed by Tukey’s test. #Statistically significant from control group (p<0.05).*Statistically significant from 3-NP treated group (p<0.05).
Morris Water Maze Test
Treatment with 3-NP resulted in an increase in escape latency (time taken by the animals to locate the platform) on day 12 (p<0.05). Escitalopram pre-treatment (20 mg/kg) showed improvement in the escape latency (p<0.05). Treatment with vehicle and Escitalopram only (20 mg/kg) did not affect the escape latency time (Fig 4).
Fig. 4:
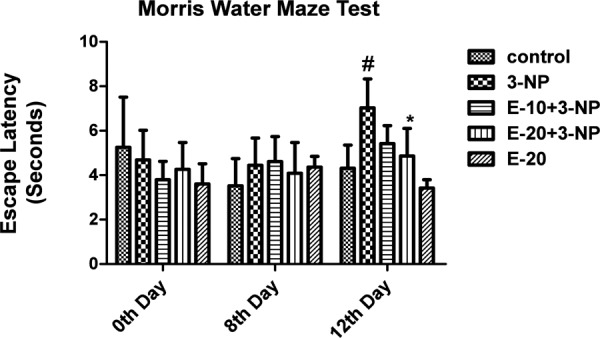
Effect of Escitalopram on Cognitive Impairment.
Values are expressed as mean ± standard deviation (SD); n = 6. Significance was determined using one way ANOVA followed by Tukey’s test. #Statistically significant from control group (p<0.05).*Statistically significant from 3-NP treated group (p<0.05).
Narrow Beam Test
3-NP treatment caused prominent hind limb impairment on day 12 thereby increasing the time taken by the animals to traverse the beam (p<0.05). Pre-treatment with Escitalopram (10 and 20 mg/kg) attenuated the hind limb impairment thereby showing a reduction in time taken by the animals to cross the beam as compared to the animals receiving only 3-NP (p<0.05). Animals subjected to vehicle and per se treatment of Escitalopram (20 mg/kg) showed no significant change (Fig 5).
Fig. 5:
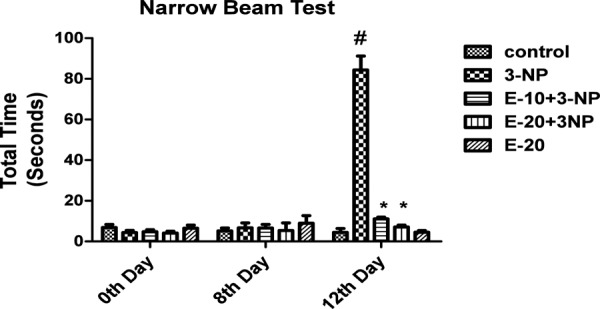
Effect of Escitalopram on Narrow Beam.
Values are expressed as mean ± standard deviation (SD); n = 6. Significance was determined using one way ANOVA followed by Tukey’s test. #Statistically significant from control group (p<0.05).*Statistically significant from 3-NP treated group (p<0.05).
Assessment of gross behavioural activity (Locomotor activity)
A substantial decrease in locomotor as well as rearing activity was observed for 3-NP treated rats (p<0.05). Escitalopram pre-treatment (10 and 20 mg/kg) significantly increased locomotor activity (p<0.05). Per se treatment with Escitalopram (20 mg/kg) and vehicle treatment showed no significant change (Fig 6).
Fig. 6:
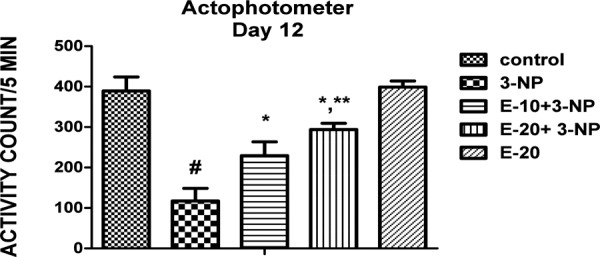
Effect of Escitalopram on Locomotor.
Values are expressed as mean ± standard deviation (SD); n = 6. Significance was determined using one way ANOVA followed by Tukey’s test. #Statistically significant from control group (p<0.05).*Statistically significant from 3-NP treated group (p<0.05).
Effect of Escitalopram on mitochondrial respiratory chain enzymes
3-NP administration significantly reduced all the mitochondrial enzyme complexes (I, II, IV and MTT activities) (p<0.05). Pre-treatment with escitalopram (10 and 20 mg/kg) for 12 days significantly attenuated mitochondrial enzyme dysfunction in a dose dependent manner (p<0.05). Treatment with Escitalopram only (20 mg/kg) did not alter the mitochondrial complexes significantly (Table 1)
Table 1: Effect of Escitalopram on mitochondrial respiratory chain enzymes.
| Groups | Complex-I (μmole NADH oxidized/min/mg protein) | Complex-II (μmole SDH oxidized/min/mg protein) | Complex-IV (μmole cytochrome oxidized/min/mg protein) | MTT reduction (% of control) |
|---|---|---|---|---|
| Values are expressed as mean ± standard deviation (SD); n = 6. | ||||
| Significance was determined using one way ANOVA followed by Tukey’s test. | ||||
| #Significantly different from control group (p<0.05). | ||||
| *Significantly different from 3-NP treated group (p<0.05). | ||||
| **Significantly different from E-10 + 3-NP-treated group (p<0.05). | ||||
| SD- standard deviation; ANOVA - analysis of variance. | ||||
| Control | 9.33 ± 0.34 | 93.06 ± 8.00 | 7.70 ± 0.95 | 100 ± 0.029 |
| 3-NP | 4.40 ± 0.34# | 26.57 ± 2.03# | 3.06 ± 0.25# | 35.77 ± 0.073# |
| E-10 + 3NP | 6.37 ± 0.23* | 50.70 ± 3.24* | 4.35 ± 0.21* | 58.33 ± 0.937* |
| E-20 + 3NP | 7.63 ± 0.40*,** | 73.51 ± 2.45*,** | 6.05 ± 0.37*,** | 73.24 ± 1.327*,** |
| E-20 | 9.74 ± 0.66 | 88.48 ± 5.35 | 8.44 ± 0.82 | 89.01 ± 2.91 |
Effect of Escitalopram on antioxidant parameters (Lipid peroxidation, Reduced glutathione, Catalase)
A significant increase in lipid peroxidation and decrease in catalase enzyme activity and reduced glutathione was seen with 3-NP administration (Table 2). Pre-treatment with Escitalopram (10 and 20 mg/kg) counteracted this effect by decreasing lipid peroxidation and restoring the levels of reduced glutathione and catalase enzyme activity (p<0.05). Per se treatment with escitalopram (20 mg/kg) did not affect the levels of these antioxidant parameters.
Table 2: Effect of Escitalopram on anti-oxidant parameters.
| Groups | Lipid peroxidation (nmole/mg protein) | Reduced glutathione (μmole/mg protein) | Catalase (μmole of H2O2 decomposed/min/mg pro-tein) |
|---|---|---|---|
| Values are expressed as mean ± standard deviation (SD); n = 6. | |||
| Significance was determined using one way ANOVA followed by Tukey’s test. | |||
| #Significantly different from control group (p<0.05). | |||
| *Significantly different from 3-NP treated group (p<0.05). | |||
| **Significantly different from E-10 + 3-NP-treated group (p<0.05). | |||
| Control | 4.92 ± 0.55 | 27.65 ± 3.63 | 8.66 ± 0.91 |
| 3-NP | 9.99 ± 1.09# | 18.11 ± 2.68# | 2.33 ± 0.37# |
| E-10 + 3NP | 7.27 ± 0.13* | 23.06 ± 2.55* | 4.01 ± 0.84* |
| E-20 + 3NP | 5.69 ± 0.68** | 24.83 ± 1.01* | 7.42 ± 0.66** |
| E-20 | 4.24 ± 0.51 | 28.59 ± 0.84 | 8.71 ± 0.49 |
Effect of Escitalopram on Nitrite and AchE enzyme levels
3-NP administration produced significant increase in AchE and nitrite levels (p<0.05) (Table 3). Pre-treatment with Escitalopram (10 and 20 mg/kg) in 3-NP treated animals resulted in significant decrease in AchE levels (p<0.05). Per se treatment with escitalopram (20 mg/kg) did not affect AchE and nitrite levels.
Table 3: Effect of Escitalopram on nitrite and AchE enzyme levels.
| Groups | Nitrite levels (μmole/mg protein) | Acetylcholinesterase levels (μmole of sub-strate hydrolysed/mg protein) |
|---|---|---|
| Values are expressed as mean ± standard deviation (SD); n = 6. | ||
| Significance was determined using one way ANOVA followed by Tukey’s test. | ||
| #Significantly different from control group (p<0.05). | ||
| *Significantly different from 3-NP treated group (p<0.05). | ||
| **Significantly different from E-10 + 3-NP-treated group (p<0.05). | ||
| Control | 0.16 ± 0.018 | 30.91 ± 1.54 |
| 3-NP | 0.27 ± 0.0095# | 69.25 ± 9.08# |
| E-10 + 3NP | 0.21 ± 0.0041* | 37.85 ± 1.47* |
| E-20 + 3NP | 0.18 ± 0.00922*,** | 34.68 ± 0.73*,** |
| E-20 | 0.15 ± 0.0076 | 28.67 ± 0.73 |
Effect of Escitalopram on Histopathological changes
Effect of Escitalopram on the histopathological changes in the brain striatum in 3-NP treated rats is shown in Table 4 and Fig 7.
Table 4: Effect of Escitalopram on the histopathological changes in the brain striatum in 3-NP treated rats.
| Groups | Remarks |
|---|---|
| NAD: No abnormality detected; +: Mild; ++: Moderate; +++: Severe. | |
| Control | NAD. No evidence of degeneration |
| 3-NP | Moderate to severe neurofibrillary degeneration in striatum and hippocampus region. (+++) |
| E-10 + 3-NP | Mild-Moderate foci of degeneration. (++) |
| E-20 + 3-NP | Patchy areas of degeneration are evident in the striatum and hippocampus region. (+) |
| E-20 | NAD. No foci of degeneration. |
Fig. 7:
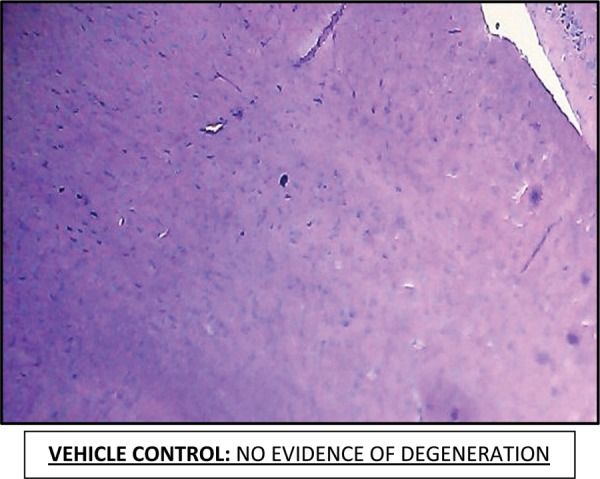
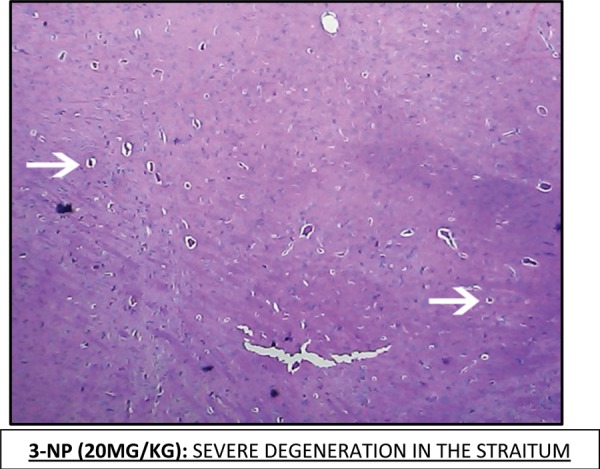
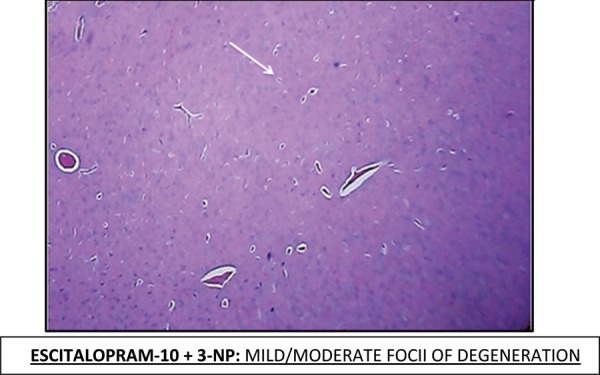
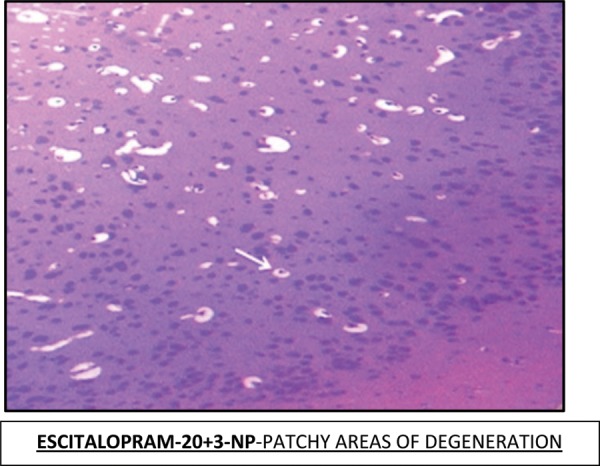
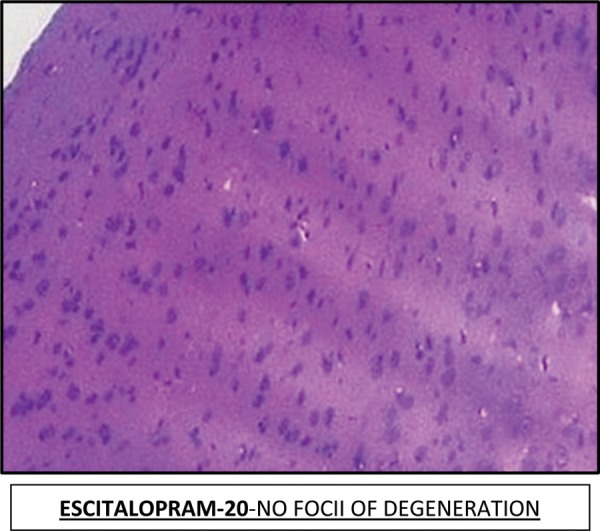
Histopathological pictures from the sections through striatum of brain showing effect of Escitalopram on 3-NP induced pathological lesion.
Note: Areas with degeneration are marked with arrows.
Discussion
In the present study Escitalopram was found to be successful in overcoming the oxidative stress and mitochondrial dysfunction induced by 3-NP which was evaluated using various biochemical, behavioural and mitochondrial parameters.
A hallmark symptom associated with Huntington’s disease is weight loss. It occurs early in HD patients and is independent of calorie intake. It is reported mitochondrial energy production is inversely proportion to the length of the CAG repeats26 The above claim is bolstered in our study wherein administration of 3-NP resulted in substantial decrease in body weight in rats as opposed to those receiving only saline. Escitalopram pre-treatment for 12 days helped to subdue this decrease in body weight indicating its therapeutic usefulness.
3-NP produces locomotion defects that resemble the motor symptoms seen in HD. 3-NP causes death of striatal and leads to conspicuous decline in motor movements.6 Behavioural parameters assessed in the present study to examine the motor incoordination caused by 3-NP comprised of actophotometer and Narrow beam test.3-NP administration resulted in significant hind limb impairment (narrow beam test) and decrease in locomotor and rearing activity (actophotometer). Escitalopram pre-treatment helped to attenuate this motor defects which supports the findings that SSRI’s improve locomotor performance irrespective of serotonin metabolism.27
Another major symptom of Huntington’s disease is memory decline that occurs due to the disruption of striatal–frontal circuits in Huntington’s disease patients. 3-NP produces memory impairment by causing alterations in neural processing and significantly inhibiting Succinate Dehydrogenase SDH.28 Cognitive performance in the present study was evaluated using Elevated Plus maze test and Morris water maze test. 3-NP resulted in significant decrease in spatial long termmemory and cognitive impairment. Escitalopram pre-treatment helped to subdue this cognitive decline, implying its potential to overcome memory dysfunction. The ability of SSRI’s to selectively block serotonin reuptake and increase BDNF levelswhich in turn promotes neurogenesis might help to attenuate these behavioral anomalies induced by 3-NP in rats.29
It has been reported that 3-NP administration tends to alter the oxidants/antioxidant defence system by some anonymous mechanisms.8 Inactivation of Succinate dehydrogenase (SDH) brought about by the irreversibly binding of 3-NPleads to the impairment of electron transport chain leading to decreased energy production and depolarization of membrane potential. This is followed by release of precursors for radical species production and consequently oxidative stress. Generation of superoxide radicals can be brought about by the activation of NMDA receptors by 3-NP allowing calcium influx into the cell, which can further exacerbate oxidative damage.30
GSH is a non-enzymatic antioxidant that plays a vital role in reduction of ROS in brain by interacting directly to detoxify certain ROS. Lipid peroxidation leads to the loss of cell function in conditions like oxidative stress in brain and in neurodegenerative disorders.32 Reaction of O2 with Nitric oxide results in the production of ONOO- which highly cytotoxic and induces hydroxyl radical formationwhich makes it critical in the pathogenesis of neurodegenerative disorders.33 Catalase is an antioxidant enzyme that is essential for protection against oxidative damage to the cell.34
3-NP treatment resulted in significant oxidative stress by decreasing GSH and catalase levels and increasing LPO and nitrite levels. These alterations were overcome by escitalopram pre- treatment. Previous studies have reported that SSRI’s indeed have an antioxidant effect7 in addition to its action on serotonin receptor. Increased metabolism of dopamine and nor epinephrine increases the radical burden and oxidative stress. Escitalopram most likely exerts its antioxidant effect by inhibiting the reuptake of serotonin by means of selective inhibition of presynaptic 5- hydroxytryptamine (5-HT) uptake site. Suppression of immune T cells and P450 enzymes is associated with antidepressant treatment. This effect might help to overcome the oxidative stress.35 However, similar studies carried out in different animal model also suggest the therapeutic role of interventions like bacopa monierra in alzhiemer's model of mice.36
Reduced levels of acetylcholine are one of primary biochemical alterations seen in HD leading to cognitive defects. Acetylcholinesterase (AchE) is an enzyme that catalyses hydrolysis of acetylcholine thereby serving to terminate synaptic transmission.37 3-NP increased AchE levels, whereas Escitalopram administration helped to restore the levels of AchE to normal.
One of the major pathways leading to huntington’s disease is dysfunction in mitochondrial energy metabolism. Experimental and clinical evidences indicate specific impairment of complex II in Huntington’s disease patients. 3-NP acts by selectively causing the impairment of complex-II of mitochondrial respiratory chain thereby leading to decreased ATP levels.38 In this study, 3-NP significantly decreased mitochondrial complex activity (Complexes-I, II, and IV). Complex-II enzyme activity was significantly reduced as compared to other complexes because of the specificity of 3-NP towards succinate dehydrogenase. 3-NP also declined number of viable cells in the mitochondria as estimated by MTT assay.
Escitalopram treatment significantly attenuated the dysfunctional mitochondrial enzyme complex. Inhibition of mitochondrial permeability transition and extracellular catabolism of ATP can be br. ought about by antidepressants. Therefore, it is possible to suggest that changes induced by antidepressants on mitochondrial complexes in this study may be due to modulating ATP levels in the mitochondria of brain.39 Neuroprotective effect of escitalopram was further determined by histopathological examinations.
Conclusion
Above data in the study suggests that escitalopram has antioxidant activity and also the ability to overcome the oxidative damage induced in the brain and presents itself as a potential neuroprotective agent to ameliorate the oxidative stress and neuronal damage induced in HD. Thus, these findings may provide a guide for the clinical trials in humans with HD. Escitalopram is a well-tolerated drug and devoid of potential lethal side effects. The present study thus warrants further investigation.
Authorship Contribution
Shruthi Shetty, Ashwini Hariharan: Experimental, Shruthi Shetty: Writing of manuscript, Aarti G Jagtap: Writing of manuscript & supervision on experiment, Trupti Shirole: Biochemical studies
Acknowledgemnt
The authors gratefully acknowledge the financial support of AICTE, New Delhi, India.
Abbreviations
- BDNF:
Brain derived neurotropic factor Control-Vehicle treated rats
- E-10 + 3-NP:
Escitalopram (10 mg/kg) +3-Nitropropionic acid (20 mg/kg) treated rats
- E-20 + 3-NP:
Escitalopram (20 mg/kg) +3-Nitropropionic acid (20 mg/kg) treated rats
- E-20:
Escitalopram (20 mg/kg) treated rats
- Huntington’s disease:
HD
- 5-hydroxytryptamine:
5-HT
- 3-Nitropropionic acid:
3-NP
- Selective Serotonin reuptake inhibitors:
SSRI’s
Footnotes
This article complies with International Committee of Medical Journal editor’s uniform requirements for manuscript.
Conflict of Interests: None: Source of funding: AICTE, New Delhi, India.
References
- 1.Raymund AC Roos.Huntington’s disease: A clinical review. Orphanet Journal of Rare Diseases. 2010;5:40. doi: 10.1186/1750-1172-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar P, Kalonia H, Kumar A. Huntington’s disease: Pathogenesis to animal models. Pharmacol Rep. 2010;62(1):1–14. doi: 10.1016/s1734-1140(10)70238-3. [DOI] [PubMed] [Google Scholar]
- 3.Beal MF. Energetics in the pathogenesis of neurodegenerative disease. Trends Neurosci. 2000;23(7):298–304. doi: 10.1016/s0166-2236(00)01584-8. [DOI] [PubMed] [Google Scholar]
- 4.Brouillet E, Jacquard C, Bizat N et al. 3-Nitropropionic acid: A mitochondrial toxin to uncover physiopathological mechanisms underlying striatal degeneration in Huntington’s disease. J. Neurochem. 2005;95:1521–40. doi: 10.1111/j.1471-4159.2005.03515.x. [DOI] [PubMed] [Google Scholar]
- 5.Borlongan CV, Koutouzis TK, Sanberg PR. 3-Nitropropionic Acid Animal Model and Huntington’s disease. Neurosci Biobehav Rev. 1997;21(3):289–93. doi: 10.1016/s0149-7634(96)00027-9. [DOI] [PubMed] [Google Scholar]
- 6.Eren I, Naziroğlu M, Demirdaş A. Protective Effects of Lamotrigine, Aripiprazole and Escitalopram on Depression-induced Oxidative Stress in Rat Brain. Neurochem Res. 2007;32(7):1188–95. doi: 10.1007/s11064-007-9289-x. [DOI] [PubMed] [Google Scholar]
- 7.Kumar P, Kumar A. Possible role of sertraline against 3-nitropropionic acid induced behavioral, oxidative stress and mitochondrial dysfunctions in rat brain. Prog in Neuropsychopharmacol&Biol Psychiatry. 2009;33:100–8. doi: 10.1016/j.pnpbp.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Schulte-Herbru¨ggen O, Fuchs E, Abumaria N et al. Effects of Escitalopram on the Regulation of Brain-Derived Neurotrophic Factor and Nerve Growth Factor Protein Levels in a Rat Model of Chronic Stress. J Neurosci Res. 2009;87:2551–60. doi: 10.1002/jnr.22080. [DOI] [PubMed] [Google Scholar]
- 9.Hery M, Semont A, Fache M. P et al. The effects of serotonin on glucocorticoid receptor binding in rat raphe nuclei and hippocampal cells in culture. J. Neurochem. 2000;74:406–13. doi: 10.1046/j.1471-4159.2000.0740406.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee DH, Kim CS, Lee YJ. Astaxanthin protects against MPTP/MPP+- induced mitochondrial dysfunction and ROS production in vivo and in vitro. Food ChemToxicol. 2001;49:271–80. doi: 10.1016/j.fct.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar A, Dogra S, Prakash A. Protective effect of curcumin against aluminium toxicity: possible behavioural and biochemical alterations in rats. Behav Brain Res. 2005;205:384–90. doi: 10.1016/j.bbr.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Allbut HN, Henderson JM. Use of narrow beam test in the rat, 6-hydroxydopamine model of Parkinson’s disease. J Neurosci Methods. 2007;159:195–202. doi: 10.1016/j.jneumeth.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Reddy DS, Kulkarni SK. Possible role of nitric oxide in the nootropic and anti-amnesic effects of neurosteroids on aging and dizocline induced learning impairment. Brain Res. 1998;799:215–19. doi: 10.1016/s0006-8993(98)00419-3. [DOI] [PubMed] [Google Scholar]
- 14.Sandhir R. Mehrortra A, Kamboj SS. Lycopene prevents 3-nitropropionic acid induced mitochondrial oxidative stress and dysfunctions in the nervous system. Neurochem Int. 2010;57:579–87. doi: 10.1016/j.neuint.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 15.King TE, Howard RL. Preparations and properties of soluble NADH dehydrogenases from cardiac muscle. Methods Enzymol. 1967;10:275–94. [Google Scholar]
- 16.King TE. Preparation of succinate dehydrogenase and reconstitution of succinate oxidase. Methods Enzymol. 1967;10:322–31. [Google Scholar]
- 17.Sottocasa GL, Kuylenstierna B, Ernster L et al. An electron-transport system associated with the outer membrane of liver mitochondria: A biochemical and morphological study. J Cell Biol. 1967;32:415–38. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamboj SS, Kumar V, Kamboj A et al. Mitochondrial oxidative stress and dysfunction in rat brain induced by carbofuran exposure. Cell Mol Neurobiol. 2008;28:961–9. doi: 10.1007/s10571-008-9270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohkawa H, Ohini N, Yogi K. Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1967;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 20.Ellman GL. Tissue sulfhydryl groups. Archbiochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 21.Luck H. In: Catalase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. New York: Academic Press; 1971. pp. 885–93. [Google Scholar]
- 22.Green LC, Wagner DA, Glogowski J et al. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 23.Ellman GL, Courtney KD, Andres V, Jr et al. New and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharamcol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL et al. Protein measurement with the Folin phenol reagent. J BiolChem. 1951;193(1):265–75. [PubMed] [Google Scholar]
- 25.Hartz PH. Simultaneous histologic fixation and gross determination of calcification. Am J ClinPathol. 1947;17(9):750–3. doi: 10.1093/ajcp/17.9_ts.750. [DOI] [PubMed] [Google Scholar]
- 26.Aziz NA, van der Burg JM, Landwehrmeyer GB, Brundin P, Stijnen T. EHDI Study Group, Roos RA “Weight loss in Huntington disease increases with higher CAG repeat number.”. Neurology. 2008;71(19):1506–13. doi: 10.1212/01.wnl.0000334276.09729.0e. [DOI] [PubMed] [Google Scholar]
- 27.Sills TL, Greenshaw AJ, Baker GB et al. The potentiating effect of sertraline and fluoxetine on amphetamine-induced locomotor activity is not mediated by serotonin. Psychopharmacology. 1999;143(4):426–32. doi: 10.1007/s002130050968. [DOI] [PubMed] [Google Scholar]
- 28.Keene CD, Rodrigues CM, Eich T et al. A bile acid protects against motor and cognitive deficits and reduces striatal degeneration in the 3-nitropropionic acid model of Huntington’s disease. Exp. Neurol. 2001;171(2):351–60. doi: 10.1006/exnr.2001.7755. [DOI] [PubMed] [Google Scholar]
- 29.Duan W, Peng Q, Masuda N et al. Sertraline slows disease progression and increases neurogenesis in N171–82Q mouse model of Huntington’s disease. Neurobiol Dis. 2008;30(3):312–22. doi: 10.1016/j.nbd.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wullner U, Young A. B, Penney J. B et al. 3-Nitropropionic acid toxicity in the striatum. J. Neurochem. 1994;63(5):1772–81. doi: 10.1046/j.1471-4159.1994.63051772.x. [DOI] [PubMed] [Google Scholar]
- 31.Cruz R, Almaguer MW, Bergado RJA. Glutathione in cognitive function and neurodegeneration. Rev. Neurol. 2003;36(9):877–86. [PubMed] [Google Scholar]
- 32.Bueno NA, Gonzalez PR, Alfaro RA et al. Recovery of motor deficit, cerebellar serotonin and lipid peroxidation levels in the cortex of injured rats. Neurochem. Res. 2010;35(10):1538–45. doi: 10.1007/s11064-010-0213-4. [DOI] [PubMed] [Google Scholar]
- 33.Kleinbongard P, Dejam A, Lauer T et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic. Biol. Med. 2003;35(7):790–6. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 34.Aebi H. In: Catalase. In: Bergmer HU, editor. Methods of enzymatic analysis. Academic press; New York: 1974. pp. 673–684. [Google Scholar]
- 35.Nieuwstraten C, Labiris NR, Holbrook A. Systematic overview of drug interactions with antidepressant medications. Can J Psychiatry. 2006;51(5):300–16. doi: 10.1177/070674370605100506. [DOI] [PubMed] [Google Scholar]
- 36.Saraf MK, Prabhakar S, Anand A. Neuroprotective effect of Bacopa monniera on ischemia induced brain injury. Pharmacol Biochem Behav. 2010;97(2):192–7. doi: 10.1016/j.pbb.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 37.McGeer PL, McGeer EG, Fibiger HC. Choline acetylase and glutamic acid decarboxylase in Huntington’s chorea. A preliminary study. Neurology. 1973;(9):912–7. doi: 10.1212/wnl.23.9.912. [DOI] [PubMed] [Google Scholar]
- 38.Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol. Rev. 2000;80(1):315–60. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- 39.Zhang WH, Wang H, Wang X et al. Nortriptyline protects mitochondria and reduces cerebral ischemia/hypoxia injury. Stroke. 2008;39(2):455–62. doi: 10.1161/STROKEAHA.107.496810. [DOI] [PMC free article] [PubMed] [Google Scholar]


