Abstract
Construct, face and predictive validities are necessary for any disease model. Although rodent models are used to investigate the neurobiology of autism, however, till date there is no such ideal animal model which can fulfill all the above said validities. Available drug therapy to treat autism is very limited and less effective. In this review, we summarize the work done with rodent models of autism and highlight different validities. We found that, very few studies have studied all the validities in a single study and none of the study fulfilled all the validities. We also reviewed the drugs used in the treatment of autism. Here we propose the limitations of available animal models. We also propose the urgent need of additional models to fulfill all the validities and to understand autism in a better way.
Keywords: Autism, Drug therapy, Animal model, Construct validity, Face validity
 Introduction
Introduction
According to Diagnostic and Statistical Manual (DSM) IV, autism has been defined as a pervasive developmental disorder (PDD) involving three main domains as a symptomatic phenotype: (1) altered social communication, (2) inflexible language and, (3) repetitive sensory-motor behavior1 But DSM V has redefined Autism Spectrum Disorder (ASD) symptoms into dyads as (i) Social interaction and social communication deficits; (ii) Restricted, repetitive patterns of behavior, interests, or activities.2 It has been reported that autism is caused by the environmental factors only, but recent reports are in the favor of involvement of neurodevelopmental and genetic factors too.3,4 Although, the children who are born with autism, can be diagnosed by 2-4 years,5,6 yet the child suffers with autism throughout the whole life because there is no effective treatment for this disorder till now. In the pathogenesis of autism, genetic, environmental and neurobiological factors are very crucial in which imbalance of the inhibitory-excitatory system plays an important role beside other factors like immune dysregulation and oxidative stress.7 Therefore, animal models should mimic the parameters and factors, that are considered as developmental precursors of anatomical, functional and behavioral manifestations. Future studies on these manifestations may help elucidate the factors and processes that bring about the unfolding of autistic behavior.
Animal model and diseases
Some neurodevelopmental disorders like autism are strictly limited to humans. The interview is the best method to assess autism and in human where language plays an important role, but in animals we can assess the symptoms of autism by their specific behavioral parameters. Mice, rats, guinea pigs and voles (Microtus) have been already reviewed as animal model of and for autism.8 Experiments on these models are easy and can be helpful to find out the mechanism of action of particular pathway which is involved in the pathogenesis of ASD. Now a days people are working on zebrafish (Danio rerio) and fly (Drosophila sp.) model system that help to study key molecules and pathways of dysfunctional neurodevelopmental processing in autism.9,10 Due to low maintenance cost, small size, embryonic transparency and rapid development life cycle in these two species, it will have the advantage and make these species an attractive genetic and molecular experimental model yet they don’t mimic all of the construct validity. In the future research on the cause and cure for autism, animal model will play a very important role.
In most of the studies (Table 1) investigator has used only one causative agent, either chemical or genetic to produce the disease in the animal. As recent reports are in the favor of involvement of neurodevelopmental and genetic factors in autism,3,4 besides the environmental factors so that the used causative agent should also use the combination of the above said factors. Interaction of molecular and environmental factors cannot be studied in humans, so we need an animal model to study autism. Currently, no specified accepted animal model of and for autism has been developed, although research on primates and rodents are on peak. The main challenge lies in the fact that an animal model relevant to a psychiatric condition in human like ASD should fit several criteria (Figure 1) usually described as a construct, face and predictive validity.11 Construct validity mainly relies upon the identity of causation between the animal phenotype and the human disease. Face validity implies that the phenomenological aspect observed in the animal is similar to the one observed in patients and predictive validity means that treatments that suppress or reduce some of the symptoms of the disease in humans also reduce the symptoms found in the animal model.
Table 1: Comparative analysis of the parameters studied in different animal models of Autism Spectrum Disorder.
| S no | Causative agent and Mod-el organism | Disease | Measured part of brain | Molecular Measure-ments | Behavioral Measure-ments | Findings |
|---|---|---|---|---|---|---|
| 1. | Chromo-some-engineered mouse model. | Autism | Not specified | Calcium Meas-urement in Neu-ronal Cell Culture | Morris Water Task Three-Chambered Social Interaction Ultrasonic Vocaliza-tion Barnes Maze Task | Seems to replicate various aspects of human autistic phenotypes and validates the relevance of the human chromosome abnormality.12 |
| 2. | Wistar Han Rat Rat | Autism | Prefrontal cortex | Microcircuit alterations | LTP | Layer 5 pyramidal neu-rons connected to neighboring neurons.13 |
| 3. | Loss-of-function mutation in NL4-KO and NL4-cDNA mice | Autism | Overall brain | Reduced Brain Volume | Selective Deficits of Social Interaction and Social Memory Vocalization | Selective perturbation of social behavior and vocalization.14 |
| 4. | BALB/c Mice. | Autism | Not specified | Not evaluated | Water Maze Hanging Wire Grip Strength Mid-air Righting | Seems to replicate various aspects of human autistic phenotypes.15 |
| 5. | Sprague Dawley female rats. | Autism | Overall brain | NeuN GABA | Acoustic startle response and prepulse Inhibi-tion Mechanical and thermal nociception | Disrupts circuitry involved in sensory processing.16 |
| 6. | Sprague Dawley Rats. | Autism | Cerebral cortex and pons | Morphological abnormalities | Not evaluated | Abnormal development of the cortical plate abnormal migration and/or distribution of TH-positive and 5-HT neurons Abnormal running nerve tract at the pons.17 |
| 7. | BALB/c Mice. | Autism | Not specified | Microarray analy-sis | Not evaluated | 21 genes were up regulat-ed. 18 genes were down regulated.18 |
| 8. | Pilocarpine induced epilepsy in wistar rats. | Epilepsy | Not specified | Not evaluated | Social discrimination test | Study describes changes in the patterns of social behavior of rats with temporal lobe epilepsy.32 |
| 9. | Knockin mice. | Autsim | Hippocampus neurons | Measurement of GABAR and NMDAR mediated evoked potential. | Not evaluated | R704C substitution in NLG3 in Murine embryonic fibroblast cell, iN was studied. Decreases in AMPA-R medited synaptic transmission and no change in GABA-R mrdiated synaptic transmission were observed which causes autism associated phenotypes.48 |
| 10. | C57BL/6N fe-male mice. | ASD | Not specified | Immune system dysregulation analysis | PPI, Social preference, Repetitive marble bury-ing, and Open field exploration, | Hyperresponsive CD4+, CD25+ and CD3+ GITR+ T cell observed which also causes dysregulation in cytokines in CSF. Repetitive and anxiety like behavior abnormalities also observed.49 |
| 11. | Zebrafish | ASD | Brain stem | Chemical-genetics approach | Not evaluated | Repression of ascl1b causes 5HT neuronal differentiation failure in VPA treated embryo which implicated in neuropsychiatric disorders.50 |
| 12. | Point muta-tion In SHANK gene in mice. | ASD | Not specified | Measurement of synaptic protein and receptor. | Not evaluated | Reduction of spine volume, spine elongation supported delayed maturation and dysregulation of glutamatergic synaptic transmission were observed in ASD animal model.51 |
| 13. | Wistar rats. | ASD | Hippocampus | Measurements of Neuroligin and Neurexin signal-ing. | Not evaluated | Mutation in ProSAP2/SHANK3 alters synaptic transmission causes disturbances in AMPA and NMDA mediated synaptic transmission in ASD.52 |
| 14. | Wistar rats. | Autism | Not specified | Not evaluated | Open field test social interaction test eight-arm radial maze learning assay | Influence on learning per-formance and behavior after birth.53 |
| 15. | Evans rats. | Autism | Not specified | Not evaluated | Sunflower seed eating task, Vermicelli Handling task, Sensory reporting and gating test. | Auditory unresponsivity and skilled motor performance were found in VPA treated rats.54 |
| 16. | Sprague-Dawley Rats. | Autism | Ventrolateral orbital cortex | Not evaluated | Forced swimming test Locomotion activity | VLO produced an antide-pressant-like effect.55 |
| 17. | C57BL6/J mice. | ASD | Medial Anterior Olfactory Nucleus, the Central and Medial Amygdaloid nuclei, and the Nucleus Accumbens | µ-opioid receptors (MOR) expression gene. | Locomotion, exploration (sniffing, rearing, digging, and climbing), social investigation (following, sniffing nose, body, and ano-genital region of the partner), and self-grooming | Functional loss of MOR gene (Oprm1−/− mice) showed social impair-ments and core autistic symptoms.56 |
| 18. | Female C57BL6/J mice. | ASD | Not specified | qRT-PCR for Pten and Peg3gene expression study | Social interaction, Habitua-tion/dishabituation olfactory test, Elevated plus maze. | Reduced gene expression after Glufosinate ammonium exposure causes memory impairments, brain structural modifications, astrogliosis, and disturbances of the glutamate homeostasis in adult mice.57 |
Fig. 1:
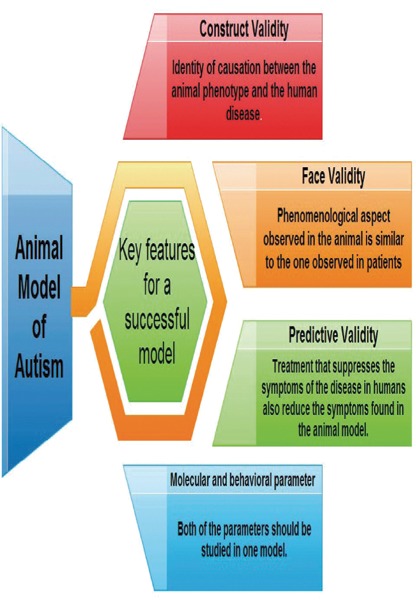
Key features required for any disease model.
Present animal models and their limitations in autism
Most experimental observations of autism explain the correlation with the different parameters and these correlations often exist without any base. In experimental studies of autism the definite causal relationship between the parameters is required and that must be established in one model organism. Structural, functional and cognitive studies demands bridging all the parameters from genetics to biochemistry. Here we have analyzed few animal models of autism and found that in none of the model both molecular and behavioral parameters were assessed which are the key diagnostic criteria for autism.
Loss of function mutation and chromosome engineering are currently used methods to establish animal model in rat and mouse,12–14 beside VPA as chemical or infectious agent15–17 and Influenza virus.18 Although autistic like behavior is expressed by the model organisms, but final diagnosis of autism is still far way away in current scenario. However, the prefrontal cortex is highly implicated in autism and responsible for the alteration in functions related to cognition, language, sociability and emotion,13 but the author didn’t study these parameters. In one of the research author study the effect of sodium valproate (VPA) in children exposed in utero and demonstrated similar behavioral and neuroanatomical abnormalities as reported in autistic animals.15 The neurodevelopmental effects of this antiepileptic agent were examined in mice following its pre- or postnatal administration, but the connection with molecular parameters was lacking in the study.
Multiple case studies have explained an association of autism and epilepsy.19–21 Comorbidity study of autism and epilepsy might pull doors towards treatment of Autism Spectrum Disorder.
Co morbidity of autism and epilepsy
Autism shows some followup disease like epilepsy or seizures.22 Clinical and pathophysiological studies between autism and epilepsy gives evidence of comorbidity. Approximately 30% autsitic children show epilepsy associated symptoms that is continued upto adolescence, and a substantial, but smaller, the proportion of children with epilepsy is on the autism spectrum.23–25 There are various reports showing variable incidence rate, but the rate is sufficiently high to understand the seriousness of this comorbidity. Some studies show the incidence of epilepsy in the autistic individual to be between 5–40%. ASD frequently occurs along with other mental impairments, such as learning disability, seizure disorders, and attention deficit hyperactivity disorder (ADHD). Autism-epilepsy phenotype helps to identify the complex relationship between ASD and epilepsy. This complexity is one of the factors that explain the reason of poorer cognitive behavior in co-expressed ASD-epilepsy patient than those in autism or epilepsy alone.26,27 Comorbidity can account via shared neuronal network between autism and epilepsy that leads to imbalance in excitatory/inhibitory pathways, abnormalities in connectivity and disrupted synaptic plasticity. ASD and epilepsy associated fragile X syndrome (FXS), rett syndrome (RTT) and tuberous sclerosis (TSC) can be the result of genetic alteration. The severity of impairment and symptoms associated with autism or with particular epilepsy syndromes reflects focal or global, structurally abnormal or dysfunctional neuronal networks. Pathophysiology of autism can be understood not only by studying epilepsy phenotypes, common molecular and genetic mechanism also help as well. The autism-epilepsy phenotype provides novel model to study new interventions in studying positive modulating effects on social cognitive outcome.28 Autism is strongly correlated with reduced neuronal cell size and increased cell packing density confined to the limbic system, including the hippocampus, the amygdala, entorhinal cortex, anterior cingulate cortex, mammilary bodies, and the septum.29,30 Along with these reports some report shows decreased dendritic complexities in Golgi staining of hippocampal pyramidal cells in autistic condition.31 Further research have been verified the seizures associated malformations by studying altered GABAergic interneurons packing in CA1 and CA3 hippocampal subfields.30 Further research defined only behavioral parameter in the support of ASD model induced by epilepsy.32 Abnormal EEG reports of focal and localized epilepsy also demonstrated developmental regression, intellectual disability and some autistic like features.33
Now a days research in ASD is on a very advanced stage and enough number of reports suggest that for a perfect model for ASD both the behavioral and molecular parameters should be evaluated in a same model organism and these parameters should show the connection with early suggested studies. Few of the studies have evaluated the molecular parameters,17 but their behavioral phenotypes remain to be controversial or not evaluated. Therefore, to obtain optimal diagnostic approach, validated data collection and appropriate longitudinal follow-up may help in treatment services in both epilepsy and autism.
Autism and translational research
In case of autism, translational research is very much needed. In translational research, we can replicate some specific brain behavior relationship in children with autism to develop an animal model of the same. A study has shown the differential exploration behavior in the autistic and normal child due to smaller cerebellar vermis.34
Such type of study can be designed to develop the animal model for autism to investigate the genetic or nongenetic cause of autism. Study on mutant GS guinea pig with malformation of cerebellar vermal lobules,35 Purkinje cell degeneration mutant mice36,37 and rat with cerebellar lesions38 show differential exploration behavior.
Drug therapy in autism
Currently, there is no specific drug in the market that is approved to treat symptoms of autism. Different pathobiological targets have been reported to be linked to autism in previous clinical and preclinical studies that may help to find a pathway to develop a new drug for the treatment of autistic children. Fig 2 summarizes the percentage of various drugs under different developmental stages.39 These drugs act on different targets and help to cure the behavioral as well as neurological symptoms.
Fig. 2:
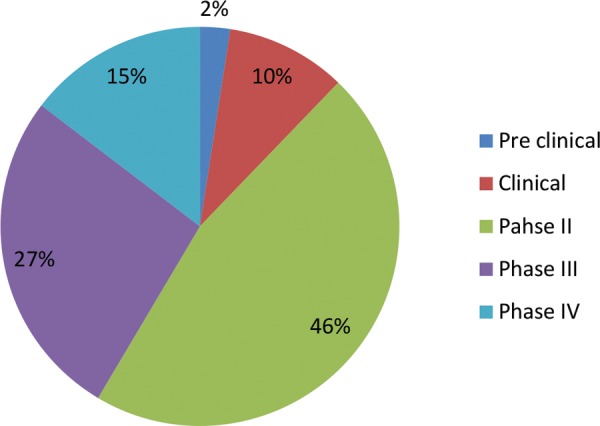
Percentage of drugs at different level of clinical trials.
Disrupt neuroregulation of opioid systems in the body, considered to be involved in impaired social communication in autistic patients.40 Fluctuated doses of morphine and the endogenous opioid β-endorphin act in opposite ways to each other such as low doses stimulate the frequency of social interaction in animals whether higher doses disrupt it. In maternal-infant attachment studies also reported the opiods influence on feelings of social comfort and blocking separation distress reactions.41 These studies supports the involvement of opioid systems in formation of social bonding in human that is generally altered in autism.42,43 Neuropeptide Org 2766 and naltrexone are the examples of endogenous opioid systems that are the new therapeutic intervention for autism.
Brain imaging, neurochemical and genetic studies have been suggested the association of monoaminergic neurotransmitter systems (dopamine, noradrenaline, serotonin etc) in pathophysilogy of the psychiatric disorders such as hyperactivity disorder,44 mood disorder,45 Obsessive Compulsive Disorder (OCD),46 anxiety disorder,47 and schizophrenia and these neurotransmitters are affected by the medications. In autism the involvement of these neurotransmitters are not necessary and that is why the drug treatment in autism is not that much effective as in other psychiatric disorders.
Conclusion
Autism can be diagnosed only by the behavioral parameters in the clinical setup. It is very hard to replicate autism in animal models with all the clinical hallmarks. Based on Figure 1, an animal model of autism would show impairment in social interaction, communication with repetitive behavior. Beside these behavioral parameters all the molecular marker of autism should be also defined in an animal model of autism. Proper evaluation of both behavioral and molecular parameters in the same animal model can give us the better animal model of autism.
Future perspective
However, to produce an animal model of autism by using only one chemical or one mutation in particular gene do not fulfill the construct validity of the model. The face validity of animal model of autism solely based on behavioral assays would be incomplete. The construct, face and predictive validity must be fulfilled by any successful animal model of autism to replicate a combination of the behavioral, neuropathological, immunological, biochemical and genetic basis for autistic disorder. The use of greater number of causative agents in the development of the model can give us more realistic animal model of autism. As the drug therapy is very limited for autism, so that we need a drug urgently for this disease. For the drug development process, a successful animal model of disease is very much required. More than one pathway is involved in the pathogenesis of autism, so we require a drug or any herbal compound that can act on all the affected pathways.
Authorship Contribution
Rakesh Ruhela: Concept preparation, literature search, review writing and diagram preparation, Ajay Prakash: Helped with pharmacotherapy portion of review, Bikash Medhi: Provided technical guidance and did proof reading.
Acknowledgment
This research was supported by a grant from the Department of Biotechnology, Govt. Of India.
Footnotes
The article complies with International Committee of Medical Journal editor’s uniform requirements for manuscript.
Competing Interests: : None; Source of funding: DBT
References
- 1.Georgiades S, Szatmari P, Zwaigenbaum L et al. Structure of the autism symptom phenotype: a proposed multidimensional model. Am Acad Child Adolesc Psychiatry. 2007;46:188–96. doi: 10.1097/01.chi.0000242236.90763.7f. [DOI] [PubMed] [Google Scholar]
- 2.Crawley JN. Translational animal models of autism and neurodevelopmental disorders. Dialogues in Clinical Neuroscience. 2012;14(3):293–305. doi: 10.31887/DCNS.2012.14.3/jcrawley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lacroix A, Guidetti M, Roge B et al. Recognition of emotional and nonemotional facial expressions: A comparison between Williams syndrome and autism. Research in Developmental Disabilities. 2009;30:976–985. doi: 10.1016/j.ridd.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Niklasson L, Rasmussen P, O´ skarsdo´ ttir S et al. Autism, ADHD, mental retardation and behavior problems in 100 individuals with 22q11 deletion syndrome. Research in Developmental Disabilities. 2009;30:763–773. doi: 10.1016/j.ridd.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Kuban KCK, O’Shea TM, Allred EN et al. Positive screening on the Modified Checklist for Autism in Toddlers (M-CHAT) in extremely low gestational age newborns. The Journal of Pediatrics. 2009;154:535–540. doi: 10.1016/j.jpeds.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matson JL, Wilkins J, Sevin JA et al. Reliability and item content of the Baby and Infant Screen for Children with autism Traits (BISCUIT): Parts 1–3. Research in Autism Spectrum Disorders. 2009;3:336–344. [Google Scholar]
- 7.Feng T, Ann MM, Robert HL. Effects of histone deacetylase inhibitor Trichostatin A on epigenetic changes and transcriptional activation of Bdnf promoter 1 by rat hippocampal neurons. Annals of the New York Academy of Sciences. 2010;1199:186–193. doi: 10.1111/j.1749-6632.2009.05175.x. [DOI] [PubMed] [Google Scholar]
- 8.Sabine MK, Poustka A. Animal models of autism. Drug Discovery Today: Disease Models. 2006;3(4):313–318. [Google Scholar]
- 9.Tropepe V, Sive HL. Can zebrafish be used as a model to study the neurodevelopmental causes of autism? Genes Brain Behav. 2003;2:268–281. doi: 10.1034/j.1601-183x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 10.McBride SM, Choi CH, Wang Y et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45:753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 11.McKinney WT, Bunney WE., Jr Animal model of depression. Review of evidence: implications for research. Arch. Gen. Psychiatry. 1969;21:240–248. doi: 10.1001/archpsyc.1969.01740200112015. [DOI] [PubMed] [Google Scholar]
- 12.Nakatani J, Tamada K, Hatanaka F et al. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell. 2009;137:1235–1246. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rinaldi T, Perrodin C, Markram H. Hyper-Connectivity and Hyper-Plasticity in the Medial Prefrontal Cortex in the Valproic Acid Animal Model of Autism. Front Neural Circuits. 2008;2:4. doi: 10.3389/neuro.04.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephane J, Konstantin R, Kurt H et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. PNAS. 2008;105(5):1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner GC, Reuhl KR, Cheh M et al. A New Neurobehavioral Model of Autism in Mice: Pre- and Postnatal Exposure to Sodium Valproate J Autism Dev Disord. 2006;36:779–793. doi: 10.1007/s10803-006-0117-y. [DOI] [PubMed] [Google Scholar]
- 16.Dendrinos G, Hemelt M, Keller A. Prenatal VPA exposure and changes in sensory processing by the superior colliculus. Front Integr Neurosci. 2011 Oct 20;5:68. doi: 10.3389/fnint.2011.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makiko K, Tetsuo O, Seiji S et al. Observation of fetal brain in a rat valproate-induced autism model:a developmental neurotoxicity study. J. Devl Neuroscience. 2009;27:399–405. doi: 10.1016/j.ijdevneu.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Fatemi SH, Pearce DA, Brooks AI et al. Prenatal Viral Infection in Mouse Causes Differential Expression of Genes in Brains of Mouse Progeny: A Potential Animal Model for Schizophrenia and Autism. Synapse. 2005;57:91–99. doi: 10.1002/syn.20162. [DOI] [PubMed] [Google Scholar]
- 19.Gubbay SS, Lobascher M, Kingerlee P. A neurologic appraisal of autistic children: results of a western Australian survey. Dev Med Child Neurol. 1970;12:422–9. doi: 10.1111/j.1469-8749.1970.tb01935.x. [DOI] [PubMed] [Google Scholar]
- 20.Tuchman RF, Rapin I, Shinnar S. Autistic and dysphasic children: II. Epilepsy. Pediatrics. 1991;88:1219–1225. [PubMed] [Google Scholar]
- 21.Olsson I, Steffenburg S, Gillberg C. Epilepsy in autism and autistic like conditions. Arch Neurol. 1998;45:666–668. doi: 10.1001/archneur.1988.00520300086024. [DOI] [PubMed] [Google Scholar]
- 22.Kanner L. Autistic disturbances of affective contact. Acta Paedopsychiatr. 1968;35(4):100–36. [PubMed] [Google Scholar]
- 23.Tuchman R, Rapin I. Epilepsy in autism. Lancet Neurol. 2002;1:352–358. doi: 10.1016/s1474-4422(02)00160-6. [DOI] [PubMed] [Google Scholar]
- 24.Pavone P, Incorpora G, Fiumara A et al. Epilepsy is not a prominent feature of primary autism. Neuropediatrics. 2004;35:207–210. doi: 10.1055/s-2004-821079. [DOI] [PubMed] [Google Scholar]
- 25.Gabis L, Pomeroy J, Andriola MR. Autism and epilepsy: cause, consequence, comorbidity, or coincidence? Epilepsy Behav. 2005;7:652–656. doi: 10.1016/j.yebeh.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Danielsson S, Gillberg IC, Billstedt E et al. Epilepsy in young adults with autism: a prospective population-based follow-up study of 120 individuals diagnosed in childhood. Epilepsia. 2005;46:918–923. doi: 10.1111/j.1528-1167.2005.57504.x. [DOI] [PubMed] [Google Scholar]
- 27.Hara H. Autism and epilepsy: a retrospective follow-up study. Brain & development. 2007;29:486–490. doi: 10.1016/j.braindev.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Tuchman R, Solomon LM. Convulsing toward the pathophysiology of autism. Brain Developmental. 2009;31:95–103. doi: 10.1016/j.braindev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bauman ML, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35:866–874. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- 30.Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci. 2005;23(2-3):183–7. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Raymond G, Bauman ML, Kemper TL. Hippocampus in autism: A Golgi study. Acta Neuropathology. 1996;91:117–119. doi: 10.1007/s004010050401. [DOI] [PubMed] [Google Scholar]
- 32.Joao CMM, Paula JM, Roberta MC et al. Temporal lobe epilepsy and social behavior: An animal model for autism? Epilepsy & Behavior. 2008;13:43–46. doi: 10.1016/j.yebeh.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 33.El Achkar CM, Spence SJ. Clinical characteristics of children and young adults with co-occurring autism spectrum disorder and epilepsy. Eplilepsy Behav. 2015;S15255050(14):00689–1. doi: 10.1016/j.yebeh.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 34.Pierce K, Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol Psychiatry. 2001;49:655–664. doi: 10.1016/s0006-3223(00)01008-8. [DOI] [PubMed] [Google Scholar]
- 35.Caston J, Yon E, Mellier D et al. An animal model of autism: behavioural studies in the GS guinea pig. Eur J Neurosci. 1998;10:2677–2684. doi: 10.1046/j.1460-9568.1998.00272.x. [DOI] [PubMed] [Google Scholar]
- 36.Lalonde R, Manseau M, Boetz MI. Exploration and habituation in Purkinje cell degeneration mutant mice. Brain Research. 1989;479:201–203. doi: 10.1016/0006-8993(89)91354-1. [DOI] [PubMed] [Google Scholar]
- 37.Kamiguchi H, Hlavin ML, Lemmon V. Role of L1 in neural development: what the knockouts tell us. Mol Cell Neurosci. 1998;12:48–55. doi: 10.1006/mcne.1998.0702. [DOI] [PubMed] [Google Scholar]
- 38.Leaton RN, Supple WF., Jr Medial cerebellum and long-term habituation of acoustic startle in rats. Behav Neurosci. 1991;105:804–816. doi: 10.1037//0735-7044.105.6.804. [DOI] [PubMed] [Google Scholar]
- 39.Kumar B, Prakash A, Kumar R et al. Drug therapy in autism: a present and future perspective. Pharmacological Reports. 2012;64:1291–1304. doi: 10.1016/s1734-1140(12)70927-1. [DOI] [PubMed] [Google Scholar]
- 40.Benton D. in. P.F. Brain 1988 The role of opioid mechanisms in social interaction and attachment. In: Rodgers R.J, Cooper S.J, editors. Endorphins, Opiates and Behavioural Processes. Chichester, England: John Wiley & Sons; pp. 217–235. [Google Scholar]
- 41.Panksepp J, Herman B, Connor R et al. The biology of social attachments: opiates alleviate separation distress. Biol Psychiatry. 1978;13:607–618. [PubMed] [Google Scholar]
- 42.Panksepp J. A neurochemical theory of autism. Trends Neurosci. 1979;2:174–177. [Google Scholar]
- 43.Deutsch SI. Rationale for the administration of opiate antagonists in treating infantile autism. Am J Ment Defic. 1986;90(6):631–5. [PubMed] [Google Scholar]
- 44.David JG, Tatyana DS, Raul RG et al. Hyperactivity, elevated dopaminergic transmission, and response to amphetamine in M1 muscarinic acetylcholine receptor-deficient mice. PNAS. 2001;98(26):15312–15317. doi: 10.1073/pnas.261583798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.David JD, Samuel G. The role of dopamine in mood disorders. Comprehensive Psychiatry. 1992;33(2):115–120. doi: 10.1016/0010-440x(92)90007-d. [DOI] [PubMed] [Google Scholar]
- 46.Denys D, Zohar J, Westenberg HG. The role of dopamine in obsessive-compulsive disorder: preclinical and clinical evidence. J Clin Psychiatry. 2004;65(Suppl 14):11–7. [PubMed] [Google Scholar]
- 47.Franklin RS, Anissa AD, Diana M et al. Dopamine Transporters, D2 Receptors, and Dopamine Release in Generalized Social Anxiety Disorder. Depress Anxiety. 2009;26(5):411–418. doi: 10.1002/da.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chanda S, Samuele M, Marius W et al. Neurons generated by direct conversion of fibroblasts reproduce synaptic phenotype caused by autism-associated neuroligin-3 mutation. PNAS. 2013;110(41):16622–16627. doi: 10.1073/pnas.1316240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsiao EY, Sara W, McBride Janet Chow et al. Modeling an autism risk factor in mice leads to permanent immune dysregulation. PNAS. 2012;109(31):12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacob J, Vanessa RSM, Sean CC et al. Valproic acid silencing of ascl1b/Ascl1 results in the failure of serotonergic differentiation in a zebrafish model of fetal valproate syndrome. Disease Models & Mechanisms. 2014;7:107–117. doi: 10.1242/dmm.013219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang YH, Michael D. Ehlers. Modeling Autism by SHANK Gene Mutations in Mice. Neuron. 2013;78(1):8–27. doi: 10.1016/j.neuron.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Magali HA, Charlotte JT, Andreas MG et al. Garner. Autism-Associated Mutations in ProSAP2/Shank3 Impair Synaptic Transmission and Neurexin–Neuroligin Mediated Transsynaptic Signaling. J. Neurosci. 2012;32(43):14966–14978. doi: 10.1523/JNEUROSCI.2215-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Narita M, Oyabu A, Imura Y et al. Nonexploratory movement and behavioral alterations in a thalidomide or valproic acid-induced autism model rat. Neuroscience Research. 2010;66:2–6. doi: 10.1016/j.neures.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Reynolds S, Alexandre M, Darragh PD. Sensory and Motor Characterization in the Postnatal Valproate Rat Model of Autism. Dev Neurosci. 2012;34:258–267. doi: 10.1159/000336646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xinga B, Zhaoa Y, Zhangc H et al. Microinjection of valproic acid into the ventrolateral orbital cortex exerts an antidepressant-like effect in the rat forced swim test. Brain Research Bulletin. 2011;85:153–157. doi: 10.1016/j.brainresbull.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Gigliucci V, Leonzino M, Busnelli M et al. (2014) Region specific up-regulation of oxytocin receptors in the opioid Oprm1−/−mouse model of autism. Frontiers in Pediatrics. 2014;2:91. doi: 10.3389/fped.2014.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laugeray A, Herzine A, Perche O et al. Pre- and postnatal exposure to low dose glufosinate ammonium induces autism-like phenotypes in mice. Front Behav Neurosci. 2014;8:390. doi: 10.3389/fnbeh.2014.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]


