Abstract
In this study, red ginseng extract (RGE) was converted into high-content minor ginsenosides by fermenting with Bgp1 enzymes at 37°C for 5 days. Compared to the RGE, the minor ginsenoside contents were increased in fermented red ginseng extract (FRGE). Moreover, the amount of minor ginsenosides such as Rh1 (11%) and Rg2 (16%) was slightly augmented, while the level of Rg3 (33%) was significantly increased after bioconversion. Furthermore, we also examined and compared the effect of RGE and FRGE on the differentiation and mineralization of preosteoblastic MC3T3-E1 cells. Similarly, the level of mRNA expression of intracellular alkaline phosphatase (ALP) activity, type-1 collagen (Col-I) was also increased. Based on the comparison, it is clear that the FRGE has improved effects on bone formation and differentiation of preosteoblastic MC3T3-E1 cells.
Key Words: : β-glycosidase, bone formation, differentiation, mineralization, osteoblast, Panax ginseng
Introduction
Ginseng, the root of Panax ginseng C.A. Meyer, has been widely used in Asian and Western countries for millions of years.1 For the last decades, particularly, red ginseng has been used as a common tonic with numerous biological and pharmacological activities. The most valuable major active ingredients of P. ginseng are the saponins, commonly known as ginsenosides, which are triterpene glucosides.2,3 These ginsenosides in their natural state are difficult to absorb in the human gastrointestinal tract due to their high molecular weight, low solubility, and poor permeability through the cell membrane.4,5 Bea and his colleagues studied the conversion of ginsenosides in the human gastrointestinal tract by the gut microorganism,6 but recently, enzymatic biotransformation methods have been introduced for the conversion of major ginsenosides into minor ginsenosides.7,8 The recombinant β-glucosidase 1 and 3 (Bgp1, Bgp3) from Microbacterium esteraromaticum were used for the bioconversion of the major ginsenosides Rb1, Re, and Rg1 into compound K (CK), Rg2, and Rh1, respectively.7,8 β-Glucosidase (Bgp) hydrolyzes two molecules of glucose or glucose-substituted molecules by acting on β-1,4 bond of a compound.9 As previously reported, β-galactosidase from Aspergillus oryzae and β-D-glucosidase from Bacteroides sp. have been used for the bioconversion of major ginsenosides Re and Rg1 into minor ginsenosides Rg2 and Rh1, respectively.10,11 The beneficial effect of enzymatic biotransformation is to convert major ginsenosides of red ginseng extract (RGE) into minor ginsenosides to improve their pharmacological efficacies.
Minor ginsenosides exhibit potent pharmacological effects such as antiallergy, anti-inflammatory, antitumor, antidiabetic, and antiosteoporosis agents.12–14 Till now, very few studies have used fermented red ginseng extract (FRGE) for studying bone formation and osteoporosis. Osteoporosis is a common bone disease, especially in women, and it affects more than 12% of the population throughout the world.15 During osteoporosis, there is more bone resorption compared to bone formation due to high activity of osteoclasts. Hence, significant reductions in bone mass result in increased bone fracture risk. Currently, different drugs such as hormone receptor therapy (HRT), alendronate, and risedronate have been used for treating osteoporosis. However, prolonged usage of these drugs causes some side effects such as nausea and vomiting, chest pain and sore throat, and difficulty in swallowing.16–19 Osteoblasts are bone-forming cells, their differentiation and mineralization are controlled by various bone-forming proteins. Among the osteogenic markers, bone morphogenetic proteins (BMPs) are considered important osteogenic proteins that play a vital role in the stimulation and differentiation of osteoblastic cells. After differentiation and mineralization, these cells induce the expression of bone formation with specific markers such ALP, Col-I, osteopontin (OPN), and osteocalcin (OCN).20
Cultured MC3T3-E1 cells act similar to in vivo osteoblasts by secreting OCN, ALP, Col-I, and mineralization of extracellular matrix.21,22 Thus, the effects of FRGE on the function of osteoblastic MC3T3-E1 cells along with the differentiation and mineralization markers were investigated.
Materials and Methods
Materials
Six-year-old red ginseng was purchased and soaked with 10 volume of 50% alcohol. It was extracted at 85°C±1°C for 12 h, filtered with 5-μm cartridge, and then vacuum evaporated. This manufactured RGE was kindly provided by the Ginseng Resources Bank of Kyung Hee University in South Korea. Five grams of crude extract of red ginseng was dissolved in 100 mL distilled water and autoclaved. Bgp1 (GenBank accession number 603820) has high β-glucosidase with a very specific hydrolase activity. Previously, in our laboratory, Bgp1 was isolated from Microbacterium esteraromaticum GS514 found in the ginseng field and cloned in Escherichia coli BL21. The encoded gene (Bgp1) was amplified from the genomic DNA by polymerase chain reaction (PCR) using a DNA polymerase kit. It was amplified with these primers: Bgp1/R (5-GGA ATT CCT ATC GGG CCG C-3) and Bgp1/F (5-GGC CCA TAT GTG CGG ATG CCC TAC C-3). To generate a maltose-binding protein (MBP)-Bgp1 gene fusion, the amplified fragments were digested with Nde1 and EcoR1, inserted into pMAL-c5X, and then the amplified gene (Bgp1) was sequenced and confirmed.8
Enzymatic characterization of Bgp1
The Bgp1 was grown in the LB containing ampicillin (50 μg/mL) at 37°C until the OD600 reached to 0.4. At this point, we added 0.5 mM IPTG (Isopropyl-β-D-thiogalactopyranoside). After 9-h incubation at 28°C with 47 g, the cells were harvested at 5000 g for 30 min at 4°C. Twenty millimolars sodium phosphate buffer (pH 7.0) was used twice for washing the cells and then resuspended in the same buffer. Cells were lysed by sonication and the debris was removed by centrifugation at 9000 g for 20 min at 4°C.
The activity of crude enzymes was checked by using pNP-β-D-glucopyranoside as a substrate in 20 mM sodium phosphate buffer (pH 7.0) at 37°C. After 5 min, the reaction was stopped by adding Na2CO3 with a final concentration of 0.1 M, and pNP release was measured at OD465.
Enzymatic bioconversion of RGE
Five grams of RGE was dissolved in 100 mL distal water and autoclaved. One milliliter of the solution was mixed with 1 mL Bgp1 crude enzyme (1:1 v/v). The sample was then incubated for 3, 5, and 7 days at 37°C with 44 g. Two hundred fifty microliters of sample was taken out and mixed with the same volume of 80% n-butanol and then centrifuged at 12225 g for 2 min. This process was repeated thrice to obtain the crude saponin fraction, and then the upper layer was analyzed by thin layer chromatography (TLC) and high-performance liquid chromatography (HPLC).
TLC analysis
TLC analysis was performed by using the silica gel plates (Merck KGaA, Darmstadt, Germany) with the developing solvent CHCl3:CH3OH:H2O (65:35:10 v/v/v, lower phases). The spots on the TLC were detected by spraying with 10% (v/v) H2SO4 followed by heating at 110°C for 3 to 5 min.
HPLC analysis
The evaporated sample was dissolved in 250 μL methanol. This experiment employed a C18 (250×4.6 mm, 5 μm) column using acetonitrile (solvent A) and water (solvent B) as mobile phase at 85% B for 5 min, 79% B for 20 min, 42% B for 55 min, 10% B for 12 min, and 85% B for 18 min, all at 1.6 mL min−1, and the sample was detected at 230 nm by HPLC.
Cell cultures and differentiation
The mouse cell line MC3T3-E1 (RCB1126) was obtained from the RIKEN Cell Bank (Tsukuba, Japan). The cells were cultured in alpha modification of Eagle's Medium (α-MEM; GIBCO, Grand Island, NY, USA) with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, 100 mg/mL streptomycin and then incubated at 37°C in 5% CO2 atmosphere. To induce differentiation, cells were plated onto a 6- or 12-well plate and allowed to grow to 80% to 90% confluence. At confluence (day 0), cells were transferred to the α-MEM that contains 10% FBS, 1% penicillin–streptomycin, 10 mM β-glycerophosphate, and 100 μg/mL ascorbic acid as a differentiation medium (DM) for an additional 6 to 24 days.
Cell viability assay
The MC3T3-E1 cells were seeded at a density of 5×103 cells per well in a 96-well microplate, suspended in the α-MEM with 10% (v/v) FBS and 1% (v/v) penicillin streptomycin (P/S), and then incubated for 24 h at 37°C in a 5% CO2 atmosphere. After 24 h, the cells were washed with a phosphate-buffered saline (PBS) buffer, and the medium was changed with serum-free media, including 0 to 100 μg/mL concentration of FRGE. After 72 h, cell viability was measured by MTT assay solutions. Ten microliters of MTT solution (5 mg/mL) was added to each well, and then the plates were incubated for an additional 3–4 h. After a 4-h incubation, MTT solutions were removed and 100 μL dimethyl sulfoxide (DMSO) was added to dissolve formazan products,20 and the plates were shaken for 5 min. Finally, OD was measured at 570 nm using ELISA reader.
ALP activity assay
The MC3T3-E1 cells were cultured at a density of 5×103 cells per well in a 24-well microplate, suspended in the α-MEM with 10% (v/v) FBS and 1% (v/v) P/S, and then incubated for 24 h at 37°C in a 5% CO2 atmosphere. After 24 h, the medium was replaced with the DM with or without FRGE, for additional time of 14 days. Media were changed every 2 days. The ALP was measured after 14 days of treatment. The cells were washed thrice with PBS buffer, suspended in 10 mM Tris/HCl (pH 7.5), including 0.1% Triton X-100, 2 mM MgSO4, and incubated at 37°C for 2 h. Then, the reactions were stopped by adding 50 μL 1.0 N NaOH,23 and the absorbance was measured at 405 nm. The protein concentration was assayed by using the Smart BCA protein assay kit, and p-nitrophenol phosphate was used as a substrate for the determination of ALP activity.
Collagen content
To measure the collagen content, cells were treated with the same reagents as mentioned in the ALP assay. After 14 days of treatment, Sirius Red-based colorimetric assay was used for measurement of Col-I content.24 After washing the cells with PBS, 1 mL Bouin's fluid was added to each well and then incubated for 1 h. After fascination, the fixation Bouin's fluid was removed and rinsed by tap water for 10–15 min. The culture wells were air-dried and stained by the Sirius Red dye reagent for 1 h using a mild shaker; then, the Sirius Red dye solution was removed and washed with 0.1 N HCl to remove the nonbound dye. After washing, 300 μL of 0.01N HCl and 300 μL of 0.1 N NaOH solution were added to dissolve the stained material, and the absorbance was measured at 550 nm.
Alizarin Red staining
The MC3T3-E1 cells were seeded as mentioned above. After a 24-h incubation, the medium was changed with the DM, supplemented with or without FRGE for an additional time of 24 days. After every 2 days, the media were changed. On the 24th day of incubation, the cultured cells were washed twice with the PBS buffer, fixed with 70% ethanol, and incubated for 1 h at 37°C in 5% CO2 atmosphere. Then, the cells were stained with 40 mM Alizarin Red S for 30 min using a mild shaker. For the enumeration of bound dye, we added 10% cetylpyridinium chloride and shaken for 15 min. The absorbance of the solubilized Alizarin Red S stain was measured at 570 nm.
RNA isolation and reverse-transcriptase PCR
For reverse-transcriptase PCR (RT-PCR), the cells were cultured and treated in the same manner as mentioned in the ALP assay. After 14 days of treatment, total RNA was isolated from cultured MC3T3-E1 cells treated with FRGE, using TRIzol LS reagents (Invitrogen, Carlsbad, CA, USA). For making first-strand cDNA, the Thermo Scientific cDNA synthesis kit (Onebio, Lithuania) was used. The samples were incubated at 42°C for 60 min, followed by 70°C for 5 min. The cDNA products were subjected to PCR amplification with gene-specific primers. RT-PCR amplification was performed using a Light Cycler System (Roche, Mortlake, Australia) with a SYBR Green qPCR Super Mix UDG kit (Invitrogen). Amplifications were performed for 30 cycles with the following: first 30 cycles (at 95°C for 4 min, at 95°C for 30 sec, at 60°C for 30 sec) and second 30 cycles (at 72°C for 1 min 30 sec, at 72°C for 8 min). The PCR products were subjected to 0.8% agarose gel electrophoresis 100 V for 25 min. The relative expression levels of target genes against the endogenous reference glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were calculated using the delta cycle threshold (Ct).
Statistical analysis
The results are representative of at least three independent experiments and expressed as the mean±SEM. Comparison of the control and treatment groups was performed using analysis of variance, and the statistical significance was analyzed by using Duncan's test. Differences of *P<.05 were considered statistically significant.
Results
The crude extract of ginsenoside hydrolyzing gene consisting of 2496 bp was cloned and expressed in E. coli., encoding 831 amino acids, which have homology to the glycosyl hydrolase family 3.25 To get the maximum yield of soluble active enzymes, it was incubated with 0.5 mM IPTG at 28°C for 9 h.7,8 Based on the TLC analysis, the incubated sample of the 5th day was good for bioconversion of crude extract of major ginsenosides to minor ginsenosides (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/jmf).
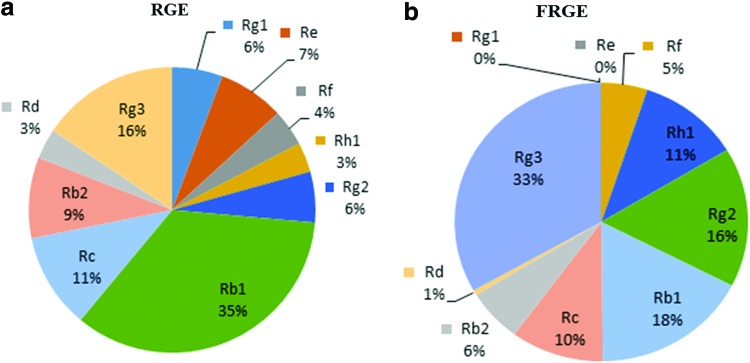
After TLC analysis, the conversion yield of crude extract of ginsenoside by Bgp1 was further confirmed by HPLC quantitatively. The control and the conversion of crude extract of major ginsenosides (Rg1, Re, Rb1, Rb2, Rc, and Rd) to minor ginsenosides (Rh1, Rg2, and Rg3) are represented by Figure 1a and b, respectively. Our results showed that Bgp1 hydrolyzes specifically a glucose molecule at C-20. Hence, the amount of Rg3 (33%) increased double than those of Rh1 (11%) and Rg2 (16%) (Fig. 1b).
FIG. 1.
High-performance liquid chromatography (HPLC) analysis of the biotransformation of crude extract of ginsenoside by Bgp1. (a) Red ginseng extract (RGE), (b) fermented red ginseng extract (FRGE). From the HPLC analysis, it is concluded that Rg1 and Re are fully converted into other minor ginsenosides, whereas a high amount of Rg3 (33%) was identified compared to other minor ginsenosides (Rf, Rh1, and Rg2). Color images available online at www.liebertpub.com/jmf
Effect of FRGE on preosteoblastic MC3T3-E1 cell viability
To study cellular toxicity effects of FRGE on the differentiation and mineralization of preosteoblastic MC3T3-E1 cells, cells were treated at various concentrations of FRGE (1–100 μg/mL) for 72 h. Compared to control and RGE, FRGE did not show any cellular toxicity up to 100 μg/mL; therefore, concentrations from 1 to 100 μg/mL of FRGE were employed in further experimental studies (Fig. 2a).
FIG. 2.
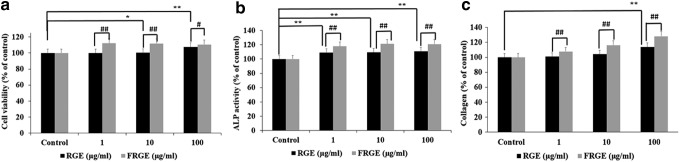
Effect of FRGE on the growth of MC3T3-E1 cells (a), ALP (b), and (c) Col-I of osteoblastic MC3T3-E1 cells. Data were expressed as a percentage of control. *P<.05, **P<.01, control versus RGE, #P<.05, ##P<.01 RGE versus FRGE.
FRGE stimulates ALP activity in mouse preosteoblastic MC3T3-E1 cells
To examine the effect of FRGE on the growth and differentiation of osteoblastic MC3T3-E1cells, the ALP activity was observed. Preosteoblastic MC3T3-E1 cells were cultured in the DM in the presence/absence of FRGE, and the cellular ALP activity was tested with pNPP as the substrate. Compared to the control, a significant increase was observed in ALP activity, as indicated in Figure 2b.
Effect of FRGE on the intracellular Col-I content in mouse preosteoblastic MC3T3-E1 cells
The effects of RGE and FRGE on collagen production in osteoblastic MC3T3-E1 cells are shown in Figure 2c. The collagen content of MC3T3-E1 cells was significantly increased with treatments of 1 to 100 μg/mL FRGE compared to RGE.
Effect of FRGE on in vitro mineralization
To assess the osteoblast growth and differentiation, extracellular matrix mineralization is also considered a major portion of bone formation. Consequently, we evaluated whether FRGE treatment of preosteoblastic MC3T3-E1 cells stimulated calcium deposition and matrix mineralization, a marker of augmented anabolic action in bone formation. To assess mineralized nodule formation and extracellular matrix calcium deposition, the preosteoblastic MC3T3-E1 cells were stained with Alizarin Red S dye, which combines with calcium ions. Our results showed that the mineralized nodules increased in MC3T3-E1 cells compared to untreated cells (Fig. 3a). The results also indicated that mineralized nodules were more bright red in color by Alizarin Red S staining (Fig. 3b). It can be seen from Figure 3b, that the FRGE might improve mineralization and bone formation in MC3T3-E1 preosteoblastic cells compared to RGE.
FIG. 3.
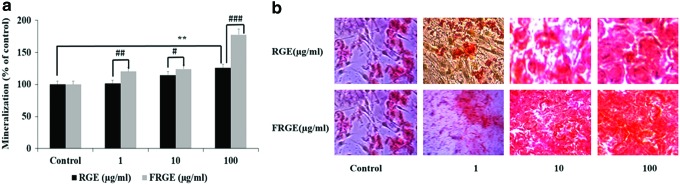
Effect of FRGE on the growth of MC3T3-E1 cells. For mineralization, Alizarin Red S staining (a, b), MC3T3-E1 cells were exposed to a culture medium containing β-glycerophosphate and ascorbic acid for 24 days. **P<.01, control versus RGE, #P<.05, ##P<.01, ###P<.001 RGE versus FRGE. Color images available online at www.liebertpub.com/jmf
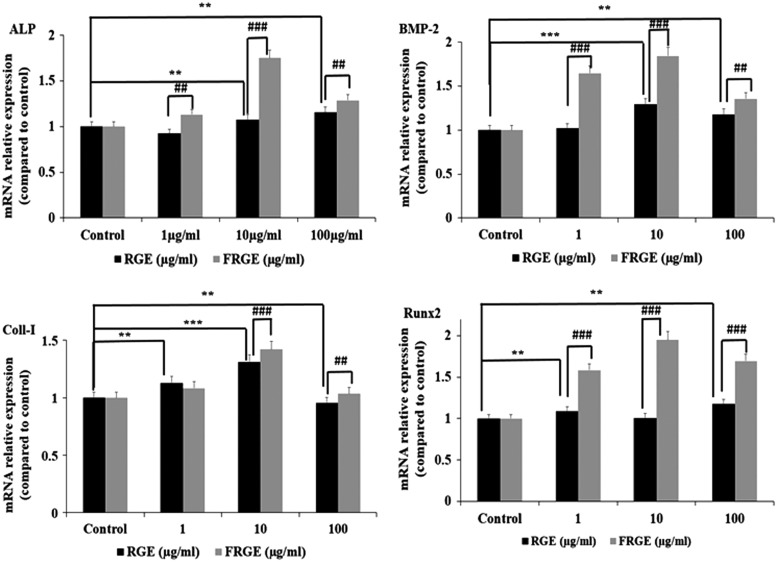
Expression of BMP-2, Runx2, and OCN mRNA in osteoblastic cells after FRGE treatments
To investigate the FRGE stimulation of growth and differentiation in MC3T3-E1 cells, we further investigated the simulative effects of FRGE on the mRNA expression of BMP-2, Runx2, ALP, and OCN for the differentiation and mineralization of MC3T3-E1 cells. Compared to control (RGE), a significant upregulation of BMP-2, ALP, OCN, and Runx-2 mRNA expressions were observed (Fig. 4a–d). These results suggested that FRGE enhances the BMP-2/Runx2 signaling pathway and hence more effectively induces osteoblast growth and differentiation compared to RGE.
FIG. 4.
Effects of FRGE treated with β-glycosidase 1 (Bgp1) on ALP, BMP-2, Col-I, and on Runx-2 mRNA expression level in MC3T3-E1 cells were examined by reverse-transcriptase polymerase chain reaction. MC3T3-E1 cells were treated with (1, 10, and 100 μg/mL) or without FRGE fermented by β-glycosidase 1 (Bgp1) for 12 days. The GAPDH mRNA level was analyzed in the same samples as a reference gene. Each value is the mean±SEM of three to five independent experiments. **P<.01, ***P<.001 control versus RGE, ##P<.01, ###P<.001 RGE versus FRGE. Hence, 10 μg/mL provides a significant effect on ALP, BMP-2, Col-I, and on Runx-2 expression in MC3T3-E1 cells. BMP, bone morphogenetic protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Discussion
Ginsenosides are the major triterpenoids in ginseng and are differentiated by different sugar molecules at different positions. As previously reported that major ginsenosides show low solubility in the human gastrointestinal tract4,5; therefore, it can be transformed into minor ginsenosides to increase their pharmacological effects. Recently, the minor ginsenosides Rg5 and Rk1 have been shown to stimulate murine osteoblastic MC3T3-E1 cells.14
The Bgp1 enzyme hydrolyzes the glucose molecule attached to C-20 position to attain the ginsenoside pathway: Rb1→Rd→20(S)-Rg3; ginsenoside Re→Rg2 and Rg1→Rh1. Among the natural products, P. ginseng is a potential candidate for stimulating bone formation, osteoblast differentiation, and mineralization. However, few studies have reported the effect of FRGE on the proliferation, differentiation, and mineralization of preosteoblastic MC3T3-E1 cells. Thus, in the current study, the efficiency and efficacy of FRGE on growth and differentiation in mouse calvaria-origin preosteoblastic MC3T3-E1 were assessed.
Compared to RGE, the FRGE shows a greater protective and an anabolic effect on the bone matrix development by stimulating osteoblast cell growth and also by increasing collagenous protein production, which is an important agent for bone matrix maturation. After treatment with FRGE at various concentrations for 14 days, Col-I production was significantly augmented compared to the control group (Fig. 4c). It stimulates the synthesis of collagen and upregulates osteoblastic MC3T3-E1 cell growth and differentiation. BMPs, especially BMP-2, play a central role in bone remodeling. During bone formation, BMP-2 attached to type-I and type-II serine/threonine kinase receptors (BMPR-I and BMPR-II) (Fig. 4b), and downstream signaling pathways are activated along with the transcription factors/SMAD complexes such as SMAD1/5/8. After the phosphorylation of SMAD1/5/8 on serine residues, they make a complex with SMAD4 that is considered a common mediator. Thus, this complex is transported into the nucleus, where it stimulates osteoblastic differentiation and mineralization of specific genetic factors.26,27 Until now, quite a few natural compounds such as osthole, fraxetin, and daidzein have been suggested to prompt osteoblast growth and differentiation by initiation of the BMP/Runx2 signaling pathway,28–30 and mRNA expression of ALP is also increased (Fig. 4a). Our results show that the mRNA expression of BMP-2 significantly increases in MC3T3-E1 cells with the treatment of FRGE (Fig. 4b). These results support our hypothesis that the BMP-2 signaling system may play an important role in the FRGE-mediated cell maturation and differentiation in osteoblasts. Furthermore, we studied the beneficial effect of FRGE on another vital osteoblast-specific gene such as Col-I (Fig. 4c). Moreover, Runx2, an important transcription factor for the growth and differentiation of osteoblasts, was significantly increased (Fig. 4d). Previous studies have demonstrated that ginsenosides and flavonoids stimulate osteoblast growth and differentiation by initiation of BMP-2 expression.31,32 Thus, our results also demonstrate that FRGE may induce osteoblast differentiation and mineralization by the induction of BMP-2/SMAD and Runx2 signaling pathways compared to RGE. These observations support the previous report of ginsenoside Rd on osteoblastic markers such as ALP, Col-I, and OCN through potentiation of the AMP protein kinase using BMP-2 signaling pathway.32
To sum up, a number of osteogenic signaling pathways are positively implicated in the regulation of bone formation, including the bone morphogenetic protein 2 (BMP2), Wnt, and Runx2 pathways. Among the BMP family proteins, BMP-2 is an effective osteo influencing signal protein, stimulating osteoblast differentiation and bone formation as well.33,34 Similarly, Runx2, the vital downstream target of BMP-2, and an important transcriptional regulator of osteoblast differentiation and mineralization.35 In our results, this hypothesis is supported by the effect of FRGE on bone mineralization and differentiation. In this work, we found that FRGE promotes osteoblast differentiation, mineralization, ALP activity, and Col-I.
In conclusion, Bgp1 is an effective hydrolyzing enzyme, which cuts glucose molecule at C-20 position, but it cannot hydrolyze the glucose at C-3 position. The HPLC analysis shows that after biotransformation, Re and Rg1 are fully converted into minor ginsenosides Rg2 (16%) and Rh1 (11%), respectively, and the amount of Rg3 doubles (33%). Compared to RGE, the present study recommended that the FRGE can increase bone formation by stimulating osteoblast cell growth, differentiation, and cellular level of ALP and Col-I synthesis. After Bgp1 biotransformation, the precise documentation of the active compound such as Rg3, Rg2, and Rh1 would be expedient for simplifying the stimulatory effect of FRGE on the differentiation and mineralization of preosteoblastic MC3T3-E1 cells and for facilitating proper bone formation.
Supplementary Material
Acknowledgments
This research was supported by the Korea Institute of Planning & Evaluation for Technology in Food, Agriculture, Forestry, & Fisheries (KIPET NO: 313038-03-1-SB010) and also supported by a grant from the Next-Generation BioGreen 21 Program (SSAC, grant#: PJ00952903).
Author Disclosure Statement
The authors declare that there is no conflict of interest.
References
- 1.Sengupta S, Toh SA, Sellers LA, et al. : Modulating angiogenesis: The yin and the yang in ginseng. Circulation 2004;110:1219–1225 [DOI] [PubMed] [Google Scholar]
- 2.Attele AS, Wu JA, Yuan CS: Ginseng pharmacology: Multiple constituents and multiple actions. Biol Pharm Bull 1999;58:1685–1693 [DOI] [PubMed] [Google Scholar]
- 3.Yuan CS, Wu JA, Osinski J: Ginsenoside variability in American ginseng samples. Am J Clin Nutr 2002;75:600–601 [DOI] [PubMed] [Google Scholar]
- 4.Tawab MA, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M: Degradation of ginsenosides in humans after oral administration. Drug Metabol Dispos 2003;31:1065–1071 [DOI] [PubMed] [Google Scholar]
- 5.Xu QF, Fang XL, Chen DF: Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J Ethnopharmacol 2003;84:187–192 [DOI] [PubMed] [Google Scholar]
- 6.Bae EA, Han MJ, Kim EJ, Kim DH: Transformation of ginseng saponins to ginsenoside Rh2 by acids and human intestinal bacteria and biological activities of their transformants. Arch Pharm Res 2004;27:61–67 [DOI] [PubMed] [Google Scholar]
- 7.Quan LH, Min JW, Jin Y, Wang C, Kim YJ, Yang DC: Enzymatic biotransformation of ginsenoside Rb1 to compound K by recombinant beta-glucosidase from Microbacterium esteraromaticum. J Agric Food Chem 2012;60:3776–3781 [DOI] [PubMed] [Google Scholar]
- 8.Quan LH, Min JW, Sathiyamoorthy S, Yang DU, Kim YJ, Yang DC: Biotransformation of ginsenosides Re and Rg1 into ginsenosides Rg2 and Rh1 by recombinant β-glucosidase. Biotechnol Lett 2012;34:913–917 [DOI] [PubMed] [Google Scholar]
- 9.Pei J, Pang Q, Zhao L, Fan S, Shi H: Thermoanaerobacterium thermosaccharolyticum β-glucosidase: A glucose-tolerant enzyme with high specific activity for cellobiose. Biotechnol Biofuels 2012;5:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko SR, Choi KJ, Suzuki K, Suzuki Y: Enzymatic preparation of ginsenosides Rg2, Rh1, and F1. Chem Pharm Bull 2003;51:404–408 [DOI] [PubMed] [Google Scholar]
- 11.Bae EA, Shin JE, Kim DH: Metabolism of ginsenoside Re by human intestinal microflora and its estrogenic effect. Biol Pharm Bull 2005;28:1903–1908 [DOI] [PubMed] [Google Scholar]
- 12.Choi JR, Hong SW, Kim Y, et al. : Jang: Metabolic activities of ginseng and its constituents, ginsenoside Rb1 and Rg1, by human intestinal microflora. J Ginseng Res 2011;35:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia L, Zhao Y: Current evaluation of the millennium phytomedicine-ginseng (I): Etymology, pharmacognosy, phytochemistry, market and regulations. Curr Med Chem 2009;16:2475–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddiqi MH, Siddiqi MZ, Ahn S, Kang S, Kim YJ, Veerappan K, Yang DC: Stimulative effect of ginsenosides Rg5:Rk1 on murine osteoblastic MC3T3-E1 cells. Phytother Res 2014;28:1447–1455 [DOI] [PubMed] [Google Scholar]
- 15.Rodan GA, Martin TJ: Therapeutic approaches to bone diseases. Science 2000;289:1508–1514 [DOI] [PubMed] [Google Scholar]
- 16.Lloyd M: Treatment of postmenopausal osteoporosis. N Engl J Med 1998;338:736–746 [DOI] [PubMed] [Google Scholar]
- 17.Body JJ: Calcitonin for the long-term prevention and treatment of postmenopausal steoporosis. Bone 2002;30:75S–79S [DOI] [PubMed] [Google Scholar]
- 18.Tremollieres F, Lopes P: Specific estrogen receptor modulators (SERMs). Presse Med 2002;31:1323–1328 [PubMed] [Google Scholar]
- 19.Watts NB: Bisphosphonate treatment of osteoporosis. Clin Geriatr Med 2003;19:395–414 [DOI] [PubMed] [Google Scholar]
- 20.Quarles LD, Yohay DA, Lever LW, Caton R, Wenstrup RJ: Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: An in vitro model of osteoblast development. J Bone Miner Res 1992;7:683–690 [DOI] [PubMed] [Google Scholar]
- 21.Lian JB, Stein GS, Canalis E, Robey PG, Boskey AL: Bone formation: Osteoblast lineage cells, growth factors, matrix proteins and the mineralization process. In: Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism (Favus MJ, ed.). Lippincott Williams & Wilkins, Philadelphia, 1999, pp. 14–29 [Google Scholar]
- 22.Green LM, Reade JL, Ware CF: Rapid colorimetric assay for cell viability: Application to the quantitation of cytotoxic and growth inhibitory lymphokines. J Immunol Methods 1984;70:257–268 [DOI] [PubMed] [Google Scholar]
- 23.Tietz NW, Burtis CA, Duncan P, Ervin K, Petitclerc CJ, Rinker AD, Shuey D, Zygowicz ER: A reference method for measurement of alkaline phosphatase activity in human serum. Clin Chem 1983;29:751–761 [PubMed] [Google Scholar]
- 24.Tullberg-Reinert H, Jundt G: In situ measurement of collagen synthesis by human bone cells with a sirius red-based colorimetric microassay: Effects of transforming growth factor beta2 and ascorbic acid 2-phosphate. Histochem Cell Biol 1999;112:271–276 [DOI] [PubMed] [Google Scholar]
- 25.Varghese JN, Hrmova M, Fincher GB: Three-dimensional structure of a barley beta-D-glucan exohydrolase, a family 3 glycosyl hydrolase. Structure 1999;15:179–190 [DOI] [PubMed] [Google Scholar]
- 26.Sykaras N, Opperman LA: Bone morphogenetic proteins (BMPs): How do they function and what can they offer the clinician? J Oral Sci 2003;45:57–73 [DOI] [PubMed] [Google Scholar]
- 27.Nohe A, Keating E, Knaus P, Petersen NO: Signal transduction of bone morphogenetic protein receptors. Cell Signal 2004;16:291–299 [DOI] [PubMed] [Google Scholar]
- 28.Jia TL, Wang HZ, Xie LP, Wang XY, Zhang RQ: Daidzein enhances osteoblast growth that may be mediated by increased bone morphogenetic protein (BMP) production. Biochem Pharmacol 2003;65:709–715 [DOI] [PubMed] [Google Scholar]
- 29.Kuo PL, Hsu YL, Chang CH, Chang JK: Osthole-mediated cell differentiation through bone morphogenetic protein-2/p38 and extracellular signal-regulated kinase 1/2 pathway in human osteoblast cells. J Pharmacol Exp Ther 2005;314:1290–1299 [DOI] [PubMed] [Google Scholar]
- 30.Kuo PL, Huang YT, Chang CH, Chang JK: Bone morphogenetic protein-2 and −4 (BMP-2 and −4) mediates fraxetin-induced maturation and differentiation in human osteoblast-like cell lines. Biol Pharm Bull 2006;29:119–124 [DOI] [PubMed] [Google Scholar]
- 31.Garrett IR: Anabolic agents and the bone morphogenetic protein pathway. Curr Top Dev Biol 2007;78:127–171 [DOI] [PubMed] [Google Scholar]
- 32.Kim DY, Park YG, Quan HY, Kim SJ, Jung MS, Chung SH: Ginsenoside Rd stimulates the differentiation and mineralization of osteoblastic MC3T3-E1 cells by activating AMP-activated protein kinase via the BMP-2 signaling pathway. Fitoterapia 2012;83:215–222 [DOI] [PubMed] [Google Scholar]
- 33.Lee MH, Kim YJ, Kim HJ, Park HD, Kang AR, Kyung HM, Sung JH, Wozney JM, Ryoo HM: BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-beta 1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J Biol Chem 2003;278:34387–34394 [DOI] [PubMed] [Google Scholar]
- 34.Gori F, Thomas T, Hicok KC, Spelsberg TC, Riggs BL: Differentiation of human marrow stromal precursor cells: Bone morphogenetic protein-2 increases OSF2/CBFA1, enhances osteoblast commitment, and inhibits late adipocyte maturation. J Bone Miner Res 1999;14:1522–1535 [DOI] [PubMed] [Google Scholar]
- 35.Phimphilai M, Zhoa ZR, Boules H, Roca H, Franceschi RT: BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J Bone Miner Res 2006;21:637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.