Abstract
In this study, we evaluated the analgesic and anti-inflammatory activities of a 70% ethanol extract from Rosa taiwanensis Nakai (RTEtOH). The analgesic effect was determined using acetic acid-induced writhing response and formalin test. The anti-inflammatory activity was evaluated by λ-carrageenan-induced paw edema in mice. The anti-inflammatory mechanism of RTEtOH was examined by measuring the levels of cyclooxygenase-2 (COX-2), nitric oxide (NO), tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, and malondialdehyde (MDA) in the paw edema tissue and the activities of superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione reductase (GRd) in the liver tissue. The betulinic acid and oleanolic acid contents of RTEtOH were assayed by HPLC. The results showed that RTEtOH decreased the acetic acid-induced writhing responses (1.0 g/kg) and the late phase of the formalin-induced licking time (0.5 and 1.0 g/kg). In the anti-inflammatory models, RTEtOH (0.5 and 1.0 g/kg) reduced the paw edema at 3, 4, and 5 h after λ-carrageenan administration. Moreover, the anti-inflammatory mechanisms might be due to the decreased levels of COX-2, TNF-α, IL-1β, and IL-6, as well as the inhibition of NO and MDA levels through increasing the activities of SOD, GPx, and GRd. The contents of two active compounds, betulinic acid and oleanolic acid, were quantitatively determined. This study demonstrated the analgesic and anti-inflammatory activities of RTEtOH and provided evidence to support its therapeutic use in inflammatory diseases.
Key Words: : acetic acid writhing response, anti-inflammatory, λ-carrageenan, formalin test, Rosa taiwanensis
Introduction
Previous reports have provided evidence that inflammation plays an important role in the pathogenesis of many diseases, such as cancer, arthritis, neurodegenerative disease, diabetes mellitus, and other serious diseases.1–3 Inflammatory process involves a series of events involving various cytokines and mediators, such as prostaglandins (PGs) and leukotrienes. During the inflammation response, cytokines (e.g., tumor necrosis factor-α [TNF-α] and interleukin [IL]-1β) induce the production of many proinflammatory mediators, such as PGs and nitric oxide (NO).4 Treatment of inflammatory diseases is directed against inflammatory processes. Nonsteroidal anti-inflammatory agents (NSAIDs) have been used to treat pain and inflammatory diseases for a long time. However, NSAIDs are known to be limited clinically because of their side effects, such as gastrointestinal hemorrhage.5 In recent years, anti-inflammatory activities of herbs have been comprehensively investigated and some medicinal plants or plant-derived chemical compounds are good sources of anti-inflammatory agents.6–8
Rosa taiwanensis Nakai belongs to the family Rosaceae and is an endemic species of Taiwan. The phytochemical investigations on R. taiwanensis have revealed that it contains phenols, unsaturated acids, abscisic acid, flavones, sterols, betulinic acid, and oleanolic acid.9 In Taiwan, R. taiwanensis, also a folk medicine, is used to treat swelling, pain, and liver diseases. Although this plant has a long history of medicinal use, there is no scientific research reported on its analgesic and anti-inflammatory effects and mechanisms of action of R. taiwanensis, yet.
The λ-carrageenan-induced paw edema is an animal model commonly used to evaluate the anti-inflammatory effects of traditional medicines, natural products, and compounds. In this study, the analgesic effect of R. taiwanensis was evaluated using the formalin test and acetic acid-induced writhing response. The anti-inflammatory effect was investigated using the λ-carrageenan-induced paw edema test in mice. To investigate the mechanism of the anti-inflammatory effects, we also examined the levels of cyclooxygenase-2 (COX-2), NO, TNF-α, IL-1β, and malondialdehyde (MDA) in the edema of the paw and the activities of superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione reductase (GRd) in the liver.
Materials and Methods
Plant material
The plants of R. taiwanensis were collected from Hualian County, Taiwan as described in Flora of Taiwan and identified by Dr. Sheng-Yu Lu, Taipei Botanical Garden (Taipei, Taiwan). A plant specimen has been deposited in the School of Chinese Pharmaceutical Sciences and Chinese Medicine Resources (Taichung, Taiwan).
Preparation of plant extract
Dried plants of R. taiwanensis were cut into small pieces and ground into a coarse powder. The coarse powder (402 g) was extracted with 1L of 70% ethanol for four times. The extracts were filtered, combined, and concentrated under reduced pressure with a vacuum rotary evaporator. The remaining solution was lyophilized and 40.88 g (10.17% net gain) of crude extract (RTEtOH) was yielded. The RTEtOH extract was stored in a refrigerator before the experiment.
Chemicals
The λ-carrageenan, indomethacin, and Griess reagent, were purchased from Sigma-Aldrich Chemical Co (St. Louis, MO, USA). Formalin was purchased from Nihon Shiyaku Industry Ltd. (Taipei, Taiwan). The SOD, GPx, and GRd activity assay kits were purchased from Randox Laboratory Ltd. (Antrim, United Kingdom). Murine TNF-α, IL-1β, and IL-6 enzyme-linked immunosorbent assay (ELISA) Development Kits were purchased from BioLegend, Inc. (San Diego, CA, USA). The anti-COX-2 antibodies were purchased from Santa Cruz (rabbit polyclonal to COX-2, sc-650; Santa Cruz, CA, USA). All of the other reagents used were of analytical grade.
HPLC analysis of components
The contents of oleanolic acid and betulinic acid were determined by HPLC analysis compared with standard references. A modular Shimadzu LC-10AT system comprising of an LC-10AT vp pump, SCL-10A vp system controller, SPD-M10A vp photodiode array detector, SIL-10A auto injector (Shimadzu, Kyoto, Japan), and Ascentis C18 column (5 μm, 250 cm×4.6 mm, analytical) (Supelco, Bellefonte, PA, USA) was used. Separations were done in the isocratic mode using acetonitrile: 0.2% formic acid (22:78; v/v) at a flow rate of 0.8 mL min−1. The injection volume was 20 μL and the UV detection wavelength was 210 nm. Each determination was carried out in triplicate.
Experimental animals
Male ICR mice (20–25 g) were purchased from BioLasco Charles River Technology (Taipei, Taiwan). They were maintained in the animal center of China Medical University at 22°C±1°C, relative humidity 55%±5%, with a light and dark cycle of 12 h for at least 1 week before the experiment. Animals were provided with a rodent diet and clean water ad libitum. Animal tests and procedures used in this study were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the Committee on Animal Research, China Medical University.
Acute toxicity study
The acute toxicology test in mice was carried out according to the method of Liao et al.10 Male ICR mice were randomly divided into three groups (10 mice per group). Two groups of mice were administered with two different doses of RTEtOH (2.5 g and 5 g/kg, p.o.), respectively. The experimental mice were provided with forage and water ad libitum, and they were kept under regular observation for 14 days for any mortality or behavioral changes. The behavioral changes closely observed were hyperactivity, tremors, ataxia, convulsions, salivation, diarrhea, lethargy, sleep, and coma.
Acetic acid-induced writhing response
The writhing test in mice was carried out using the method of Koster et al.11 The writhes were induced by intraperitoneal injection of 1.0% acetic acid (v/v, 0.1 mL/10 g body weight). Five randomly selected groups of mice were administered with solvent control (0.5% CMC, p.o.), indomethacin (20 mg/kg, p.o.) or three different doses of RTEtOH (0.1, 0.5, and 1.0 g/kg, p.o.), respectively 60 min before the chemical stimulus. The data were recorded 5 min after acetic acid injection. The number of muscular contractions was counted for 5–15 min after acetic acid injection and writhe numbers during the 10 min were recorded. The total writhe numbers during 10 min were recorded and were expressed as writhing numbers.
Formalin test
The formalin test used in this study was according to the method of Tjølsen et al.12 Pain was induced by injecting 20 μL of 5% formalin in saline subcutaneously into the right hind paw of each mouse. Indomethacin (20 mg/kg, p.o.) and RTEtOH (0.1, 0.5, and 1.0 g/kg, p.o.) were administered 60 min before formalin injection. Mice were individually placed in a transparent Plexiglas cage (25×15×15 cm). The time (in seconds) spent in licking and biting response of the injected paw was taken as an indicator of pain response. Pain responses were measured for 0–5 min (early phase or neurogenic pain) and 20–30 min (late phase or inflammatory pain) after formalin injection.
λ-Carrageenan-induced mice paw edema
The anti-inflammatory activity of RTEtOH was determined by the λ-carrageenan-induced edema test in the hind paws of mice.13 Male ICR mice (10 per each group) were fasted for 24 h before the experiment with free access to water. Fifty microliters of a 1% λ-carrageenan suspension in saline was injected into the plantar side of right hind paws of the mice. Paw volume was measured immediately at 1, 2, 3, 4, and 5 h after the administration of the λ-carrageenan using a plethysmometer. Indomethacin (20 mg/kg, p.o.) and RTEtOH (0.1, 0.5, and 1.0 g/kg, p.o.) were administered at the 2nd hour after λ-carrageenan injection. The control group was given an equal volume of saline. The degree of swelling was evaluated by the delta volume (a–b), where a and b were the volume of the right hind paw after and before the λ-carrageenan treatment, respectively.
In another experiment, the whole right hind paw and liver tissues were taken at the 5th hour. The right hind paw tissue was rinsed in ice-cold normal saline, and immediately placed in four volumes of cold normal saline and finally homogenized at 4°C. The homogenate was centrifuged at 8,000 g for 5 min. The supernatant was obtained and stored at −80°C for the COX-2, NO, TNF-α, IL-1β, and MDA assays.
On the other hand, the whole liver tissue was rinsed in ice-cold normal saline, and immediately placed in an equal volume of cold normal saline and finally homogenized at 4°C. Then the homogenate was centrifuged at 8,000 g for 5 min. The supernatant was obtained and stored at −80°C for the antioxidant enzymes (SOD, GPx, and GRd) activity assays.
MDA assay
MDA was evaluated by the thiobarbituric acid reacting substance (TBARS) method.14 Briefly, MDA reacted with thiobarbituric acid in an acidic condition with high temperature (above 90°C) and formed a red-complex TBARS. The absorbance of TBARS was determined at 532 nm. MDA levels were expressed as nanomoles per milligram of protein.
NO assay
NO was measured based on the method of Moshage et al.15 For nitrite determination, NO3− was converted into NO2− utilizing nitrate reductase. NO2− reacted with sulfanilic acid to produce diazonium ion and coupled with N-(1-naphthyl) ethylenediamine to form the chromophoric aeroderivative (purplish red). The absorbance of the final product was determined at 540 nm. Values obtained by this procedure represent the sum of nitrite and nitrate.
Western blotting for COX-2
Freshly isolated paw tissue was homogenized in a lyses buffer. The protein concentration of the tissue homogenate and the cytosolic and microsomal fractions were determined according to the method of Lowry et al.16 One hundred micrograms of protein from paw homogenates or 50 μg of protein from purified microsome were loaded per lane on 8% or 12% polyacrylamide gels and electrophoresis was performed. Proteins were then transferred onto nitrocellulose membranes. The membrane was blocked overnight with buffer and then incubated with primary antibodies for 1 h using 1:1000 dilution of rabbit anti-COX-2 (100-fold dilution, 2 h at 25°C; Santa Cruz). The membranes were then washed three times in TBST solution containing Tris buffer solution (TBS) with 0.1% Tween-20 for 15 min, incubated with 1:1000 dilution of alkaline phosphatase-conjugated anti-rabbit IgG as the second antibody for 1 h. The protein was visualized with an enhanced chemiluminescence western blotting detection kit (Amersham, Arlington Heights, IL, USA), and exposed to Xray film for 3 min.
TNF-α, IL-1β, and IL-6 assays
TNF-α, IL-1β, and IL-5 were measured using ELISA. Assays were performed according to the manufacturer's instructions. The capture antibody of TNF-α, IL-1β, or IL-6 was seeded to each well of a 96-well plate overnight. Next day, a second set of biotinylated antibody was incubated with sample tissues or standard antigens in the plate before streptavidin was finally added. The color of the reaction converted from purple to yellow and was recorded at 450 nm. The concentrations of TNF-α, IL-1β, and IL-6 in each sample were expressed as picogram per milligram (pg/mg) protein for cytokine concentration.
Antioxidant enzymatic activity measurements
The following biochemical parameters were analyzed to evaluate the antioxidant activities of RTEtOH by the methods given below. SOD enzymatic activity was determined in accordance with the method of Misra and Fridovich17 at room temperature. One hundred microliters of liver homogenate supernatant was added to 880 μL (0.05 M, pH 10.2, 0.1 mM EDTA) carbonate buffer. Twenty microliters of 30 mM epinephrine (in 0.05% acetic acid) was added to the mixture at 480 nm for 4 min on a Hitachi U 2000 Spectrophotometer. The enzymatic activity was expressed as the amount of enzyme that inhibits the oxidation of epinephrine by 50%, which is equal to 1 U.
GPx enzyme activity was determined according to the method of Flohe and Gunzler18 at 37°C. A reaction mixture consisting of 500 μL phosphate buffer, 100 μL 0.01 M GSH (reduced form), 100 μL 1.5 mM NADPH and 100 μL GRd (0.24 U). One hundred microliters of the supernatant was added to the reaction mixture and incubated at 37°C for 10 min. Then 50 μL of 12 mM t-butyl hydroperoxide was added to 450 μL of the tissue reaction mixture and measured at 340 nm for 180 sec. The molar extinction coefficient of 6.22×10−3 was used to determine the enzymatic activity. One unit of activity was equal to the mM of NADPH oxidized/min per mg protein.
GRd enzyme activity was determined by the method of Carlberg and Mannervik19 at 37°C. Fifty microliters of NADPH (2 mM) in 10 mM Tris buffer (pH 7.0) was added in a cuvette containing 50 μL of GSSG (20 mM) in phosphate buffer. One hundred microliters of the supernatant was added to the NADPH-GSSG buffered solution and measured at 340 nm for 3 min. The molar extinction coefficient of 6.22×10−3 was used to determine the GRd enzyme activity. One unit of activity was equal to the mM of NADPH oxidized/min per mg protein.
Statistical analysis
All data were represented as mean±SEM. Statistical analyses were performed with the SPSS software and analyzed with one-way analysis of variance followed by Scheffe's multiple range test. P<.05 was considered statistically significant.
Results
Chromatographic analysis of RTEtOH
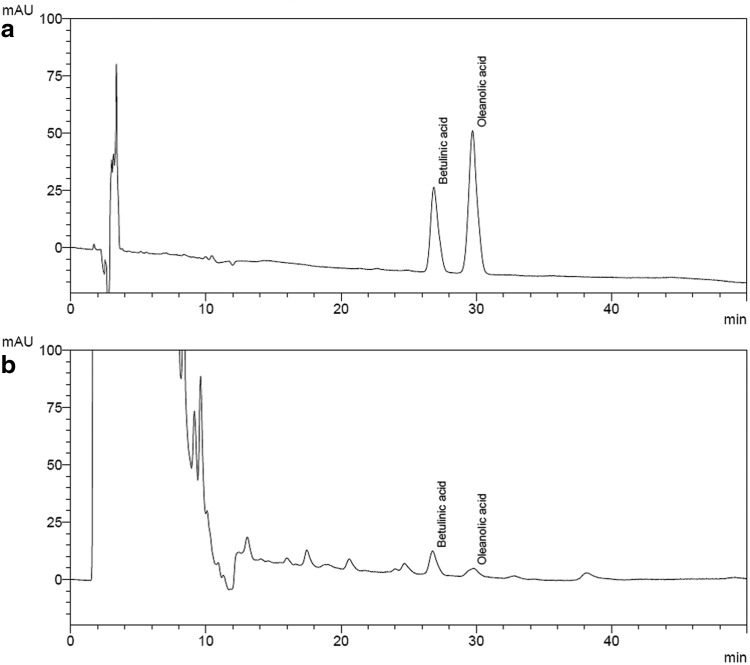
As shown in Figure 1, the results demonstrated that the retention times of betulinic acid and oleanolic acid were 26.86 and 29.71 min, respectively. According to the calibration curve, the content of betulinic acid and oleanolic acid in RTEtOH were about 588.3 μg/g of extract and 64.4 μg/g of extract, respectively.
FIG. 1.
HPLC chromatographs of (a) Standards and (b) RTEtOH. Peaks: (1) betulinic acid; (2) oleanolic acid. Ascentis C18 column (5 μm, 250 cm×4.6 mm, analytical), mobile phase: acetonitrile and 0.2% formic acid (22:78; v/v) in the isocratic mode, low rate: 0.8 mL min−1, UV detection wavelength: 210 nm.
Acute toxicity study
The result showed that the oral administration of RTEtOH for 14 days did not cause any behavioral changes and no mortality was observed. Therefore, the LD50 value of RTEtOH was concluded to be greater than 5 g/kg in mice. This result indicated that RTEtOH practically has no acute toxicity.
Acetic-acid-induced writhing response
Intraperitoneal injection of acetic acid induced 44.16±2.91 writhes in the control group. The writhing responses were significantly reduced by treatment with 1.0 g/kg RTEtOH (23.71±2.20, P<.01) and 20 mg/kg indomethacin (16.80±2.31, P<.001) (Fig. 2.).
FIG. 2.
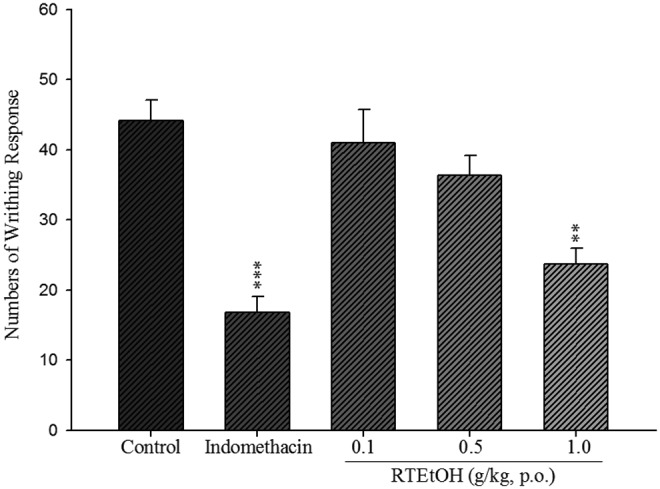
Analgesic effect of RTEtOH and indomethacin on acetic acid-induced writhing response in mice. Each value represents mean±SEM (n=10). Each value was represented as mean±SEM. **P<.01, ***P<.001 when compared with the solvent control group (one-way analysis of variance [ANOVA] followed by Scheffe's multiple range test).
Formalin test
The results showed that in the early phase, both RTEtOH and indomethacin-treated groups did not show any significant inhibitions as compared with the control group (Fig. 3a). In the late phase, however, the licking and biting response duration induced by subcutaneous injection of formalin was 124.33±11.11 sec. Doses of 0.5 and 1.0 g/kg RTEtOH, respectively significantly reduced nociceptive responses (60.60±8.28, P<.01 and 55.66±6.42, P<.001), similar to indomethacin (20 mg/kg) (53.42±4.90, P<.001) (Fig. 3b).
FIG. 3.
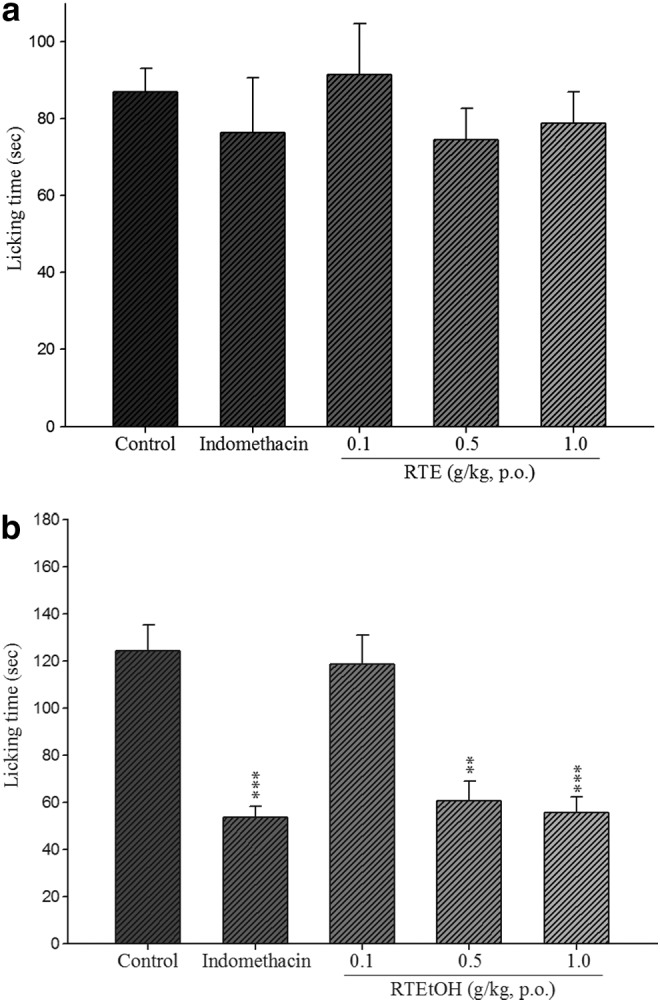
Effect of RTEtOH and indomethacin on (a) early phase and (b) late phase of formalin test in mice. Each value represents mean±SEM (n=10). **P<.01 and ***P<.001 as compared with the solvent control group (one-way ANOVA followed by Scheffe's multiple range test).
Effects of RTEtOH on λ-carrageenan-induced mice paw edema
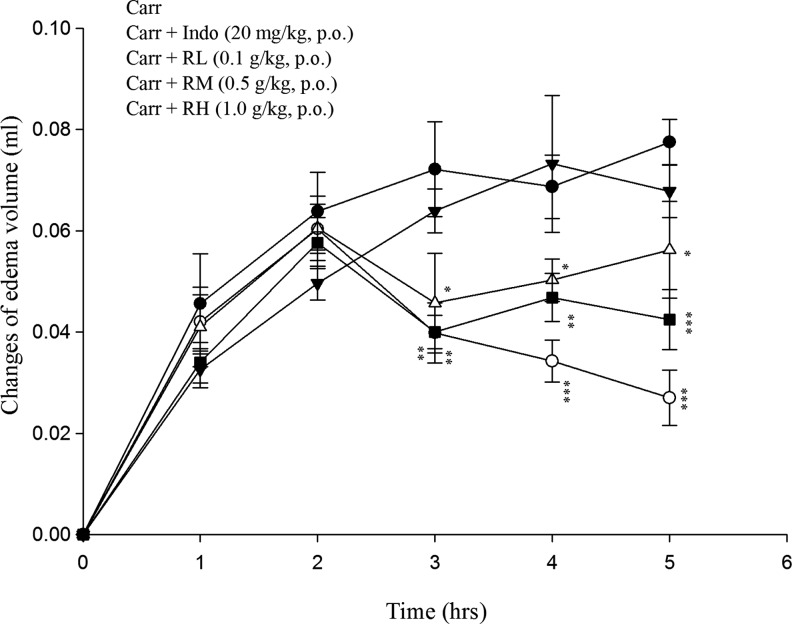
After the λ-carrageenan injection, the volume of mouse paw increased as edema developed, indicating inflammatory activities. It was observed that RTEtOH (0.5 and 1.0 g/kg) and indomethacin (20 mg/kg) significantly inhibited the development of paw edema at the 3rd, 4th, and 5th hours after the λ-carrageenan injection (P<.01–.001) (Fig. 4).
FIG. 4.
Effects of RTEtOH and indomethacin on hind paw edema induced by λ-carrageenan in mice. Each value was represented as mean±SEM (n=10). *P<.05, **P<.01 and ***P<.001 when compared with the λ-carrageenan (Carr) group (one-way ANOVA followed by Scheffe's multiple range test).
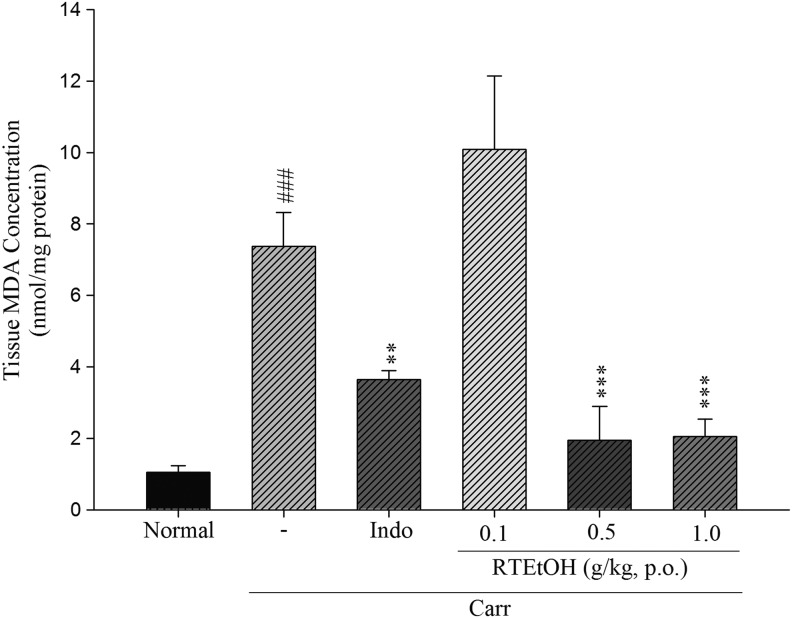
Effects of RTEtOH on MDA levels
MDA level was used to signify lipid peroxidation. As shown in Figure 5, the levels of MDA in the paw edema induced by λ-carrageenan in control animals were significantly elevated (7.26±0.95). However, MDA levels were reduced by pretreatment with RTEtOH 0.5 g/kg (1.95±0.93, P<.05) and 1.0 g/kg (2.05±0.48, P<.001), as well as indomethacin (20 mg/kg) (3.65±0.25, P<.001).
FIG. 5.
Effects of RTEtOH and indomethacin on the tissue malondialdehyde (MDA) concentration of paw edema in mice. Each value represents as mean±SEM (n=10). ###P<.001 as compared with the normal group. **P<.01 and ***P<.001 as compared with the λ-carrageenan (Carr) group (one-way ANOVA followed by Scheffe's multiple range test).
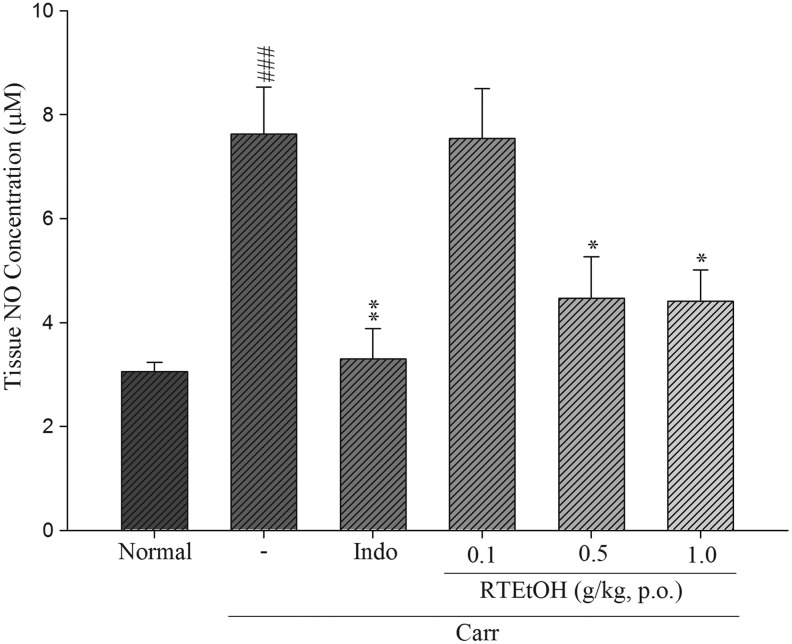
Effects of RTEtOH on NO levels
As shown in Figure 6. The NO level in the paw edema induced by λ-carrageenan was significantly raised (7.63±0.90). There is a significant reduction in the NO level on treatment with RTEtOH 0.5 g/kg (4.47±0.80, P<.05) and 1.0 g/kg (4.41±0.60, P<.05), as well as indomethacin at 20 mg/kg (3.30±0.58, P<.01).
FIG. 6.
Effects of RTEtOH and indomethacin on nitrate/nitrite concentration of paw edema in mice. Each value represents as mean±SEM (n=10). ###P<.001 as compared with the normal group. *P<.05 and **P<.01 as compared with the λ-carrageenan (Carr) group (one-way ANOVA followed by Scheffe's multiple range test).
Effects of RTEtOH on COX-2 levels
Results showed that treatment of RTEtOH (0.5 and 1.0 g/kg) inhibited COX-2 (71.6% and 67.6%) proteins expression in carrageenan-induced (P<.05) paw edema at the 5th hour (Fig. 7).
FIG. 7.
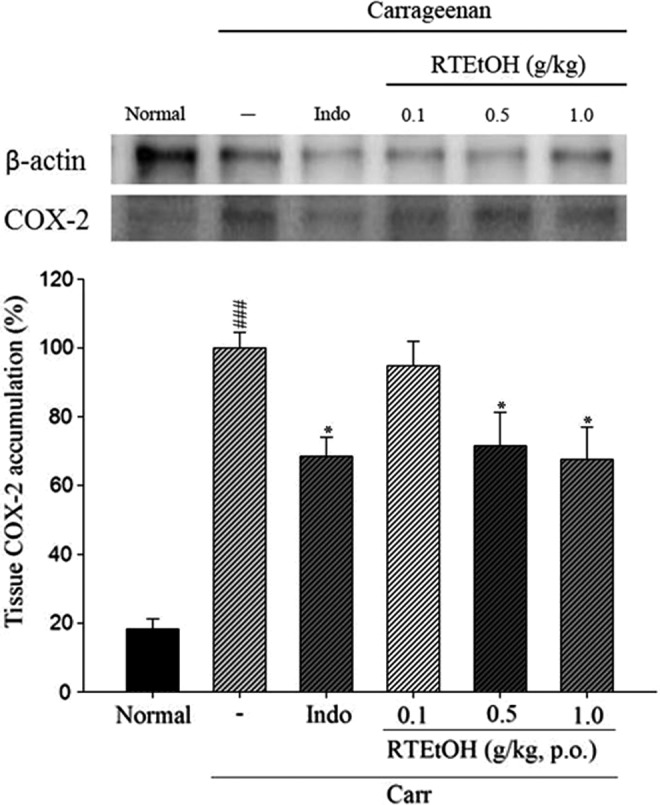
Inhibitions of RTEtOH and indomethacin on cyclooxygenase-2 (COX-2) protein expression of paw edema in mice. Tissue suspended were then prepared and subjected to western blotting using an antibody specific for COX-2. β-actin was used as an internal control. Each value was represented as mean±SEM for three different experiments performed in triplicate. ###P<.001 as compared with the normal group. *P<.05 as compared with the Carr group (one-way ANOVA followed by Scheffe's multiple range test).
Effects of RTEtOH on TNF-α, IL-1β, and IL-6 levels
The effects of RTEtOH on TNF-α, IL-1β, and IL-6 levels are shown in Table 1. TNF-α, IL-1β, and IL-6 levels in the λ-carrageenan-induced paw edema were significantly increased, respectively (51.56±7.23, 52.01±5.88, 663.39±32.74 pg/mg protein). The increased TNF-α levels were decreased by treatment with RTEtOH at doses of 0.5 g/kg (29.56±4.61 pg/mg protein, P<.01) and 1.0 g/kg (25.38±4.04 pg/mg protein, P<.001), as well as indomethacin at 20 mg/kg (23.66±3.17 pg/mg protein, P<.001). The IL-1β levels were decreased by treatment with RTEtOH 0.5 g/kg (28.93±2.84 pg/mg protein, P<.01), RTEtOH 1.0 g/kg (18.61±3.20 pg/mg protein, P<.001) and indomethacin 20 mg/kg (21.04±1.07 pg/mg protein, P<.001). The IL-6 levels were decreased by treatment with RTEtOH 0.5 g/kg (457.71±47.26 pg/mg protein, P<.05), RTEtOH 1.0 g/kg (412.32±23.48 pg/mg protein, P<.01) and indomethacin 20 mg/kg (305.01±41.44 pg/mg protein, P<.001).
Table 1.
Effect of RTEtOH and Indomethacin on TNF-α, IL-1β, and IL-6 Levels in the Paw Edema
| Groups | TNF-α (pg/mg protein) | IL-1β (pg/mg protein) | IL-6 (pg/mg protein) |
|---|---|---|---|
| Control | 16.71±2.63 | 10.31±3.72 | 5.70±1.93 |
| Carr | 51.56±7.23### | 52.01±5.88### | 663.39±32.74### |
| Carr+Indo | 23.66±3.17*** | 21.04±1.07*** | 305.01±41.44*** |
| Carr+RTEtOH 0.1 | 42.72±4.30 | 50.61±5.53 | 620.19±24.61 |
| Carr+RTEtOH 0.5 | 29.56±4.61** | 28.93±2.84** | 457.71±47.26* |
| Carr+RTEtOH 1.0 | 25.38±4.04*** | 18.61±3.20*** | 412.32±23.48** |
Each value represents as mean±SEM (n=10).
P<.001 as compared with the normal group.
P<.05, **P<.01, ***P<.001 as compared with the λ-carrageenan (Carr) group (one-way ANOVA followed by Scheffe's multiple range test).
ANOVA, analysis of variance; IL, interleukin; TNF-α, tumor necrosis factor-α.
Measurements of antioxidant enzymatic activities
The results of antioxidant enzymes such as SOD, GPx, and GRd at the 5th hour following the intrapaw injection of λ-carrageenan in mice are presented in Table 2. SOD, GPx, and GRd activities in liver tissue were decreased significantly after λ-carrageenan administration. Treatment with RTEtOH at doses of 0.5 and 1.0 g/kg and indomethacin at a dose of 20 mg/kg increased the levels of SOD and GRd activities significantly. Also, the GPx levels were significantly raised by treatment with RTEtOH at dose of 1.0 g/kg.
Table 2.
Effects of the RTEtOH and Indo on the Liver SOD, GPx, and GRd Activities in Mice
| Groups | SOD (U/mg protein) | GPx (U/mg protein) | GRd (U/mg protein) |
|---|---|---|---|
| Control | 6.68±0.28 | 0.307±0.014 | 0.117±0.006 |
| Carr | 4.55±0.14## | 0.167±0.003## | 0.069±0.002### |
| Carr+Indo | 5.86±0.49* | 0.292±0.025** | 0.081±0.003* |
| Carr+RTEtOH 0.1 | 4.681±0.19 | 0.186±0.003 | 0.075±0.002 |
| Carr+RTEtOH 0.5 | 5.73±0.48* | 0.228±0.015 | 0.082±0.002* |
| Carr+RTEtOH 1.0 | 5.98±0.24** | 0.276±0.029* | 0.086±0.003** |
Each value represents as mean±SEM (n=10).
P<.01, ###P<.001 as compared with the normal group.
P<.05, **P<.01 as compared with the Carr (λ-carrageenan) group (one-way ANOVA followed by Scheffe's multiple range test).
GPx, glutathione peroxidase; GRd, glutathione reductase; SOD, superoxide dismutase.
Discussion
The analgesic activity of RTEtOH was examined by acetic acid-induced abdominal writhing and formalin test. Acetic acid is known to cause pain by releasing endogenous mediators that stimulate the nociceptive neurons.20 Acetic acid-induced abdominal writhing is a visceral pain model and the nociceptive mechanism involves releasing of arachidonic acid metabolites. From our results, the numbers of the abdominal writhes induced by acetic acid were abated by treatment of RTEtOH, and this suggests that RTEtOH possessed analgesic effects, which might be due to participation in the inhibition of PG synthesis. The formalin test was selected in this study because it is relatively more specific to identify pain in central and/or peripheral affection. In addition, the formalin test involved a biphasic response: the early phase occurs because of the direct effect of formalin on nociceptors and the late phase reflects inflammatory pain, which appears to be related to PG synthesis.12 Previous studies illustrated that centrally acting drugs can inhibit both phases, whereas peripherally acting drugs can only inhibit the late phase.21 The treatments of RTEtOH and indomethacin could diminish the nociceptive response in the late phase induced by formalin. The results indicated that the analgesic effect of RTEtOH might be due to its peripheral anti-inflammatory effect.
The λ-carrageenan-induced paw edema is a widely used animal model and commonly employed for screening the anti-inflammatory activities of natural products.22 The cellular and molecular mechanism of the λ-carrageenan-induced inflammation is well characterized. The results showed that RTEtOH and indomethacin revealed anti-inflammatory effects. The development of λ-carrageenan-induced inflammation response has been characterized as a biphasic event, in which various mediators would be operated.23 In the first phase, several inflammatory mediators such as histamine, serotonin, and bradykinin were released, and then TNF-α, IL-1β, IL-6, and PGs are produced in the second phase.24,25 The expression of COX-2 is maximal at the late phase of λ-carrageenan-induced paw edema, which was responsible for the biosynthesis of PGs in inflammatory reactions.24 Our results also showed that λ-carrageenan could cause the productions of COX-2 in λ-carrageenan-induced paw edema model. The COX-2 levels in the paw edema tissues of mice were significantly diminished by treatment with RTEtOH. These findings demonstrated that the mechanisms of anti-inflammatory activities of RTEtOH might be related to the inhibition of COX-2 levels. Previous investigations indicated significant correlations between cytokine production, COX-2 protein expression, and PG synthesis in the λ-carrageenan-induced paw tissues of mice.26 Some inflammatory mediators, including IL-1β, IL-6, and TNF-α, are critical cytokines in the development of inflammatory response.27 In this study, IL-1β, IL-6, and TNF-α levels were significantly decreased in the paw edema tissues of mice by treatment of RTEtOH. These results supplied evidences that RTEtOH possessed anti-inflammatory activities.
Previous study indicated that λ-carrageenan-induced inflammation is concerned with free radical.28 The λ-carrageenan-induced inflammatory response would cause neutrophil infiltration and the production of neutrophil-derived free radicals, such as hydrogen peroxide, superoxide, and hydroxyl radicals, as well as the release of other neutrophil-derived mediators.29 NO is an important proinflammatory mediator in the pathogenesis of inflammation produced by inducible nitric oxide synthase during the conversion of l-arginine to l-citrulline.30 MDA formation was caused by free radicals attacking the plasma membrane and commonly used as a marker of free radical-mediated lipid peroxidation injury.31 Thus, the inflammatory effect would result in the accumulation of MDA. Our results indicated that the production of MDA and NO were both reduced by treatment of RTEtOH. SOD, GPx, and GRd are antioxidant enzymes and play important roles in cellular defense against reactive free radicals.32 The reaction of NO with superoxide anion forms peroxynitrite, a strong cytotoxic oxidant, which increases the production of PGs and causes lipid peroxidation and cellular damage.33 In this study, the liver SOD, GPx, and GRd activities were significantly raised with RTEtOH treatment. Therefore, these results suggest that the inhibition of MDA production was likely due to the increases of SOD, GPx, and GRd activities, and the increase of SOD not only enhanced the superoxide anion scavenging capacity, but also prevented the peroxynitrite-mediated tissue inflammatory response.
Phytochemical investigations have shown that RTEtOH contained betulinic acid and oleanolic acid.9 Both betulinic acid and oleanolic acid were triterpenoids and confirmed to express analgesic and anti-inflammatory activities.28,34 In this study, the contents of betulinic acid and oleanolic acid in RTEtOH were also analyzed by HPLC. Therefore, betulinic acid and oleanolic acid might be two of the important active constituents with anti-inflammatory activity of RTEtOH.
In conclusion, these results indicated that RTEtOH exhibited analgesic activity against acetic acid-induced writhing response and formalin test and anti-inflammatory activity against λ-carrageenan-induced paw edema. The anti-inflammatory mechanism of RTEtOH might be related to the inhibition of the formation of PGs by suppressing TNF-α, IL-1β, IL-6, and COX-2 levels and the reduction of MDA and NO productions by increasing the activities of SOD, GPx, and GRd activities. These findings supported that RTEtOH may be developed into a pharmacological agent for the prevention or treatment of inflammatory diseases.
Acknowledgments
The authors would like to thank Dr. Sheng-Yu Lu from Taipei Botanical Garden (Taipei, Taiwan) for plant identification. This study is supported, in part, by the National Science Council, Taiwan (NSC 101-2320-B-039-032-MY2), Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH100-TD-B-111-004), and the Committee on Chinese Medicine and Pharmacy, Department of Health, Executive Yuan (CCMP102-RD-104, CCMP102-RD-019).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Chao J, Lu TC, Liao JW, et al. : Analgesic and anti-inflammatory activities of ethanol root extract of Mahonia oiwakensis in mice. J Ethnopharmacol 2009;125:297–303 [DOI] [PubMed] [Google Scholar]
- 2.Akash MS, Rehman K, Chen S: Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J Cell Biochem 2013;114:525–531 [DOI] [PubMed] [Google Scholar]
- 3.Akash MS, Shen Q, Rehman K, Chen S: Interleukin-1 receptor antagonist: a new therapy for type 2 diabetes mellitus. J Pharm Sci 2012;101:1647–1658 [DOI] [PubMed] [Google Scholar]
- 4.Cirino G: Multiple controls in inflammation. Extracellular and intracellular phospholipase A2, inducible and constitutive cyclooxygenase, and inducible nitric oxide synthase. Biochem Pharmacol 1998;55:105–111 [DOI] [PubMed] [Google Scholar]
- 5.García Rodríguez L, Jick H: Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet 1994;343:769–772 [DOI] [PubMed] [Google Scholar]
- 6.Parveen A, Akash MS, Rehman K, Mahmood Q, Qadir MI: Analgesic, anti-inflammatory and anti-pyretic activities of Caesalpinia decapetala. Bioimpacts 2014;4:43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nwafor PA, Okwuasaba FK: Anti-nociceptive and anti-inflammatory effects of methanolic extract of Asparagus pubescens root in rodents. J Ethnopharmacol 2003;84:125–129 [DOI] [PubMed] [Google Scholar]
- 8.Prempeh A, Mensah-Attipoe J: Analgesic activity of crude aqueous extract of the root bark of zanthoxylum xanthoxyloides. Ghana Med J 2008;42:79–84 [PMC free article] [PubMed] [Google Scholar]
- 9.Yang SC, Fang JM, Cheng YS: chemical constituents from the root and aerial parts of Rosa taiwanensis. J Chin Chem Soc 1995;42:573–577 [Google Scholar]
- 10.Liao JW, Chung YC, Yeh JY, et al. : Safety evaluation of water extracts of Toona sinensis Roemor leaf. Food Chem Toxicol 2007;45:1393–1399 [DOI] [PubMed] [Google Scholar]
- 11.Koster R, Anderson M, De Beer E: Acetic acid for analgesic screening. Fed Proc 1959;18:412–416 [Google Scholar]
- 12.Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K: The formalin test: an evaluation of the method. Pain 1992;51:5–17 [DOI] [PubMed] [Google Scholar]
- 13.Winter CA, Risley EA, Nuss GW: Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med 1962;111:544–547 [DOI] [PubMed] [Google Scholar]
- 14.Draper HH, Hadley M: Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 1990;186:421–431 [DOI] [PubMed] [Google Scholar]
- 15.Moshage H, Kok B, Huizenga JR, Jansen PL: Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem 1995;41:892–896 [PubMed] [Google Scholar]
- 16.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–275 [PubMed] [Google Scholar]
- 17.Misra HP, Fridovich I: The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247:3170–3175 [PubMed] [Google Scholar]
- 18.Flohe L, Gunzler WA: Assays of glutathione peroxidase. Methods Enzymol 1984;105:114–121 [DOI] [PubMed] [Google Scholar]
- 19.Carlberg I, Mannervik B: Glutathione reductase. Methods Enzymol 1985;113:484–490 [DOI] [PubMed] [Google Scholar]
- 20.Collier HO, Dinneen LC, Johnson CA, Schneider C: The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother 1968;32:295–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibata M, Ohkubo T, Takahashi H, Inoki R: Modified formalin test: characteristic biphasic pain response. Pain 1989;38:347–352 [DOI] [PubMed] [Google Scholar]
- 22.Di Rosa M: Biological properties of carrageenan. J Pharm Pharmacol 1972;24:89–102 [DOI] [PubMed] [Google Scholar]
- 23.Vinegar R, Schreiber W, Hugo R: Biphasic development of carrageenin edema in rats. J Pharmacol Exp Ther 1969;166:96–103 [PubMed] [Google Scholar]
- 24.Seibert K, Zhang Y, Leahy K, et al. : Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci USA 1994;91:12013–12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spector WG, Willoughby DA: The inflammatory response. Bacteriol Rev 1963;27:117–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park WH, Park SY, Kim HM, Kim CH: Effect of a Korean traditional formulation, Hwaotang, on superoxide generation in human neutrophils, platelet aggregation in human blood, and nitric oxide, prostaglandin E2 production and paw oedema induced by carrageenan in mice. Immunopharmacol Immunotoxicol 2004;26:53–73 [DOI] [PubMed] [Google Scholar]
- 27.Dinarello CA: Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest 1997;112:321–329 [DOI] [PubMed] [Google Scholar]
- 28.Tsai JC, Peng WH, Chiu TH, Lai SC, Lee CY: Anti-inflammatory effects of Scoparia dulcis L. and betulinic acid. Am J Chin Med 2011;39:943–956 [DOI] [PubMed] [Google Scholar]
- 29.Dawson J, Sedgwick AD, Edwards JC, Lees P: A comparative study of the cellular, exudative and histological responses to carrageenan, dextran and zymosan in the mouse. Int J Tissue React 1991;13:171–185 [PubMed] [Google Scholar]
- 30.Salvemini D, Wang ZQ, Bourdon DM, Stern MK, Currie MG, Manning PT: Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur J Pharmacol 1996;303:217–220 [DOI] [PubMed] [Google Scholar]
- 31.Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P: Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem 1997;43:1209–1214 [PubMed] [Google Scholar]
- 32.Cuzzocrea S, Costantino G, Zingarelli B, Mazzon E, Micali A, Caputi AP: The protective role of endogenous glutathione in carrageenan-induced pleurisy in the rat. Eur J Pharmacol 1999;372:187–197 [DOI] [PubMed] [Google Scholar]
- 33.Salvemini D, Wang ZQ, Wyatt PS, et al. : Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br J Pharmacol 1996;118:829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasconcelos MA, Royo VA, Ferreira DS, et al. : In vivo analgesic and anti-inflammatory activities of ursolic acid and oleanoic acid from Miconia albicans (Melastomataceae). Z Naturforsch C 2006;61:477–482 [DOI] [PubMed] [Google Scholar]